Abstract
Members of the bHLH family of transcription factors play important roles in multiple aspects of plant biological processes, for instance, abiotic stress responses. Previously, we characterized CaNAC035, a gene that positively regulates stress tolerance and identified CabHLH035, a CaNAC035-interacting protein in pepper (Capsicum annuum L.). In this study, we describe the role of CabHLH035 in the response to salt stress. Our results show that the expression of CabHLH035 increased following salt treatment. Transient expression of CabHLH035 (CabHLH035-To) in pepper enhanced salt tolerance, ectopic expression of CabHLH035 in Arabidopsis increased the salt stress tolerance, whereas knocking down the expression of CabHLH035 in pepper plants resulted in decreased salt tolerance. Homologs of the Salt Overly Sensitive 1 (SOS1) and pyrroline-5-carboxylate acid synthetase (P5CS) genes showed drastically increased expression in transgenic Arabidopsis plants expressing CabHLH035 and CabHLH035-To plants, but expression decreased in CabHLH035-silenced plants. Our results also showed that CabHLH035 can directly bind to the CaSOS1 and CaP5CS gene promoters and positively activate their expression. We found that transgenic Arabidopsis plants, ectopic expression of CabHLH035 and pepper plants transiently overexpressing CabHLH035 (CabHLH035-To) showed lower Na+ and higher proline contents in response to NaCl treatment, while CabHLH035-silenced plants had higher Na+ and lower proline concentrations. Overall, CabHLH035 plays important roles in salt tolerance through its effects on the intracellular Na+ : K+ ratio and proline biosynthesis.
Introduction
Soil salinity negatively affects crop yields in many parts of the world through threatening crop growth and development. High concentrations of salt (NaCl) can perturb the ion balance in plant cells, causing rising in the concentrations of Na+ and Cl− ions. The damage caused by high salt concentrations can impair the ability of plant cells to absorb water and nutrients. High levels of salt lead to severe osmotic stress, which affects many physiological and metabolic processes in plants, causing serious damage and, ultimately, death of the plants. Saline soil has an adverse effect on crop growth by applying ionic toxicity and inhibiting important enzymes [1]. Ionic toxicity is believed to be caused by the Na+ transport from roots to shoots [2]. Proline plays pivotal roles in protein synthesis, and has been intensively studied in modulating salt stress. Proline plays pivotal roles and has been intensively studied in modulating salt stress. Proline as a key molecular switches to combat the salt stress, plant salt stress resistance can be effectively improved by higher proline content. [3]. For instance, the AtMYC2 gene in Arabidopsis is involved in salt tolerance by modulating proline biosynthesis [4]. FcWRKY40 contributes to salt tolerance by regulating the proline biosynthesis [5]. Basic helix–loop–helix (bHLH) proteins are members of a large super-family of transcription factors (TFs) that are found in eukaryotes from yeast to plants and animals. bHLH TFs play positive roles in regulating plant growth and development, metabolism, and the responses to various stresses. Plant resistance is a complex trait controlled by multiple genes, and it can be affected by other environmental factors. TFs bind directly to specific cis-acting elements in gene promoter regions and regulate the expression of downstream genes. TFs can also affect the expression of a series of downstream functional genes by regulating the expression of other transcription factor genes. Therefore, TFs function as key molecular switches in the plant responses to abiotic stresses. bHLHs contain a region of ~60 amino acids that encompasses two functional domains: a basic amino acid domain and a helix–loop–helix (HLH) domain [6]. The basic domain consists of approximately 15 amino acids at the N-terminal end of the 60-amino acid region that allows the TF to bind to G-box (5′-CACGTG-3′) or E-box (5′-CANNTG-3′) elements [7, 8]. The HLH domain is located at the carboy-terminal end of the region and consists of two hydrophobic residues in a helical-ring-helical structure that promote protein–protein interactions [9]. After the MYB TF gene family, bHLHs are the second largest TF gene family in plants. Although the functions of bHLH TFs have been intensively studied in many biological processes, the regulatory model of uncharacterized bHLH TFs remains to be explored.
Many bHLH transcription factor genes have been identified and cloned from different plants; for instance, Arabidopsis [10], tomato [11], rice [12], apple [13], and pepper [14]. It has been reported that bHLH TFs are involved in the growth and development of plants [15] and also modulate iron homeostasis. For example, SlbHLH068 positively regulated the iron homeostasis in tomato [16]. The tobacco NtbHLH123 gene is a bHLH-type transcription factor that has been reported to contribute to salt stress [17]. MfbHLH38, a bHLH transcription factor gene from the African medicinal shrub Myrothamnus flabellifolia, confers tolerance to salt stress tolerance in Arabidopsis [18]. Similarly, BvbHLH93 is involved in salt stress in Arabidopsis [19]. Arabidopsis AtUNE12 bHLH TF improves salt stress tolerance by modulating the expression of involved in ion homeostasis [20]. Pepper plants are susceptible to various abiotic (high temperature, cold, high humidity, drought, and salt) and biotic (bacterial wilt, phytophthora, and leaf rolling) stresses, which can result in reduced fruit yield. Thus, it is important to study the resistance mechanisms in pepper by analysing the signaling pathways for genetic improvement of stress tolerance. The functions of individual bHLH TFs in pepper plants are largely unknown. The pepper bHLH family has been characterized, and 122 pepper bHLH protein genes have been identified [14]. In a previously study, we characterized CaNAC035 in pepper, which positively regulates tolerance to cold, salt, and drought stress tolerance [21]. Using yeast one-hybrid, we identified CabHLH79 bound to the region upstream of the CaNAC035 promoter [22]; using yeast two-hybrid, we identified CabHLH035, a CaNAC035-interacting protein in pepper. Based on gene-specific expression patterns, the relative expression of CabHLH035 (LOC107866727) increased in response to salt treatment; therefore, we characterized the CabHLH035 gene and hypothesized that CabHLH035 plays a crucial role in salt stress tolerance. In the current study, we confirmed the important role of CabHLH035 in salt tolerance in pepper. These data provide a foundation for studies on the roles of bHLH TFs in pepper and other economically important plant species.
Results
Subcellular localization and expression of CabHLH035
To investigate the function of CabHLH035 under condition of salt stress (300 mM NaCl), the expression level of the CabHLH035 gene was measured using qRT-PCR. Intriguingly, CabHLH035 expression reached its peak of approximately (43-fold) at 6 h, after which expression rapidly decreased at 12 h (Fig. 1A). These results showed that transcription of CabHLH035 can rapidly respond to salt treatment. To explore the sub-cellular localization of the CabHLH035 protein, the Agrobacterium strains harboring the pVBG2307:CabHLH035:GFP and pVBG2307:GFP vectors were infiltrated into leaves of young Nicotiana benthamiana plants. The results showed that leaves infiltrated with pVBG2307::GFP had GFP signals in the nucleus and the cell membrane, whereas leaves infiltrated with pVBG2307:CabHLH035:GFP (expressing the CabHLH035:GFP fusion protein) had GFP signals localized to the nuclei only (Fig. 1B). The results of this experiment showed that the CabHLH035 protein localizes to the nucleus in N. benthamiana.
Figure 1.

CabHLH035 sub-cellular localization and CabHLH035 gene expression patterns. A Relative transcription of CabHLH035 in response to 300 mM NaCl treatment at six time points from 0 to 24 hours. B CabHLH035 is localized to the nucleus N. benthamiana. Scale bars = 50 μm. Error bars indicate the standard deviations (SDs) in (A).
Transient expression of CabHLH035 enhanced pepper salt tolerance
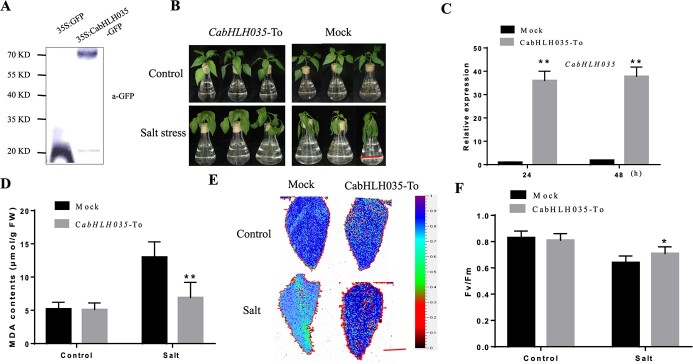
To explore the biological function of CabHLH035 under conditions of salt stress, transient expression of CabHLH035-GFP in pepper leaves was performed. Successful expression of CabHLH035-To in pepper was authorized by western blot (Fig. 2A). Under normal growth conditions, no significant phenotypic differences were observed between the transient overexpression (CabHLH035-To) plants and the 35S::GFP Mock (control) plants. However, after 48 h salt treatment, leaves of the Mock plants showed obvious symptoms of salt stress (wilted and distorted leaves) compared with the CabHLH035-To plants (Fig. 2B). In addition, the relative expression level of CabHLH035 was obviously up-regulated by CabHLH035-To compared with the control plant at 24 and 48 h (Fig. 2C). We next investigated the malondialdehyde (MDA) concentration in the CabHLH035-To and Mock plants. Without salt conditions, the CabHLH035-To and Mock plants showed no differences in MDA content. When the CabHLH035-To and Mock pepper plants were exposed to salt treatment for three days, the CabHLH035-To pepper plants showed significantly lower MDA content compared to the Mock plants (Fig. 2D). We also measured the photochemical efficiency of PSII (Fv/Fm) in the CabHLH035-To and Mock plants (Fig. 2E). Without salt condition, no conspicuous difference in Fv/Fm was detected in the CabHLH035-To and Mock plants. However, after salt stress the Fv/Fm of CabHLH035-To pepper plants was obviously higher than in the Mock pepper plants (Fig. 2F). These observations indicate that CabHLH035-To increased salt tolerance in pepper.
Figure 2.
The responses of CabHLH035-To and control pepper plants to treatment with 300 mM NaCl. ACabHLH035-GFP transient overexpression in pepper detected by immunoblotting. B Phenotypes of CabHLH035-To and Mock plants in response to 48 h salt treatment. Scale bars = 2 cm. C The expression of CabHLH035 in CabHLH035-To and Mock plants at 24 and 48 h. D MDA content in the Mock and CabHLH035-To plants after three days of NaCl treatment. E Chlorophyll fluorescence images. F Fv/Fm values. Scale bars = 1 cm. Error bars denote the standard deviation, and statistically significant differences are denoted by *P ≤ 0.05, **P ≤ 0.01.
Silencing the CabHLH035 gene decreased pepper salt tolerance
As shown in Fig. S1 (see online supplementary material), plants infected with the recombinant TRV2:CaPDS VIGS vector showed photo-bleaching in the leaves due to knocking down the expression of the phytoene desaturase gene, indicating that virus-induced gene silencing (VIGS) is effective. The silencing efficiency was then measured using qRT-PCR, and was found to be close to 85%. Leaves from the CabHLH035-silenced and control plants were immersed in 300 mM NaCl. No differences were observed under normal conditions between the CabHLH035-silenced and control plants. After salt stress, the CabHLH035-silenced leaves showed obvious wilting compared with leaves of the control pepper plants (Fig. 3A). Leaf disks from the silenced and control plants were floated on six concentrations of NaCl (0, 100, 200, 300, 400, and 500 mM) for three days. No differences were observed between the CabHLH035-silenced and the unsilenced control plants at 0 mM NaCl. At the higher NaCl concentrations, the CabHLH035-silenced leaf disks showed obvious chlorosis compared to the disks from the control plants (Fig. 3D). Accordingly, the chlorophyll content between the leaf disks from CabHLH035-silenced plants and the control plants were similar in the absence of salt. After three days of salt treatment, the chlorophyll contents in the leaf disks from CabHLH035-silenced plants were dramatically lower than in leaf disks from the control plants at NaCl concentrations >300 mM, and these differences were statistically significant (Fig. 3E). In addition, there was no evident change in the Fv/Fm of CabHLH035-silenced plants under control conditions, while after three days of NaCl treatment, the Fv/Fm of the CabHLH035-silenced plants were lower than in the absence of salt (Fig. 3F and G). We also measured the REL and MDA contents. Under normal conditions, the CabHLH035 VIGS plants and empty-vector control pepper plants showed no obvious differences in their REL and MDA contents. However, when the CabHLH035-silenced and the control pepper plants were treated with 300 mM NaCl forthree days, the CabHLH035-silenced pepper plants had increasedREL and MDA contents in comparison with the control plants (Fig. 3B and C). In addition, we performed NBT and DAB staining of the leaves to examine the accumulation of reactive oxygen species (ROS) in response to salt stress in the control and silenced pepper plants. Fig. 3H shows that the areas stained by DAB (diaminobenzidine tetrahydrochloride; for H2O2) andNBT (nitroblue tetrazolium; for superoxide anion) in CabHLH035-silenced plants were substantially greater than the control plants. These results show that CabHLH035-silenced plants had the highest levels of ROS than the control pepper plants. Overall,our results clearly demonstrate that knocking down CabHLH035 expression makes pepper plants more sensitive to salt stress.
Figure 3.
Assessment of CabHLH035-silenced pepper plants. A Phenotypes of the CabHLH035-silenced and empty-vector control plants were subjected to NaCl. Scale bars = 2 cm. B REL and C MDA content. D the phenotypes of leaf disks taken from CabHLH035-silenced and the control plants after exposure to different salt contents for three days. E Chlorophyll content, F Chlorophyll fluorescence images, and G Fv/Fm values in leaf disks after three days of treatment with 300 mM NaCl. Scale bars = 1 cm. H, DAB and NBT staining for H2O2 and superoxide anion, respectively. Values are means ± standard deviation (SD). Asterisks (*) indicates significant differences (*P ≤ 0.05, **P ≤ 0.01).
Ectopic expression of CabHLH035 enhanced Arabidopsis salt tolerance
To further establish the function of CabHLH035, CabHLH035 was ectopically expressed in Arabidopsis. The 35S::CabHLH035-GFP vector was generated and introduced into Agrobacterium strain GV3101, which was then used to transform Arabidopsis. We selected three CabHLH035 transgenic lines of Arabidopsis for our experiments, all three lines (#1, #2, and #3) showed higher for the relative expression and survival rate, in comparison with wild-type (WT) plants (Fig. S2, see online supplementary material). We first measured the seed emergence rates of transgenic and WT seeds on 0.5 Murashige and Skoog (MS) solid medium after 7 days with or without added NaCl. After exposure to 100 mM NaCl, the CabHLH035 transgenic lines showed obviously higher seed emergence rates than did the WT (Fig. 4A and B). Second, we measured the root lengths of seedlings on MS medium containing 0, 100, and 150 mM NaCl (Fig. 4C and J), and no obvious changes were observed between the WT and transgenic plants without salt stress. After treatment with 100 and 150 mM NaCl, the roots of the CabHLH035 transgenic lines were substantially longer than in WT seedlings. Third, we checked the response of mature plants to salt stress. As shown in Fig. 4D, after 300 mM salt treatment for seven days, the leaves of the transgenic line seedlings showed little yellowing, while WT leaves wilted and the plants ultimately died. Similarly, 15 days after NaCl treatment (300 mM), all the WT plants showed severe wilting compared to the CabHLH035 transgenic plants, as indicated by the higher survival rate of the CabHLH035 transgenic plants (Fig. 4E). Meanwhile, the chlorophyll content of CabHLH035 transgenic Arabidopsis plants was prominently higher than that of WT plants (Fig. 4I). Following salt treatment, the CabHLH035 transgenic lines exhibited markedly increased salt tolerance, accompanied by lower REL and MDA contents (Fig. 4F and G). To further determine whether the enhanced response to salt stress in CabHLH035 transgenic Arabidopsis plants is due to decreased transpiration, we measured the rate of water loss in 3-week-old transgenic and WT plants. As shown in Fig. 4H, in comparison with WT, the CabHLH035 transgenic lines had substantially lower water loss rates. Overall, these results show that the CabHLH035 gene positively regulates salt stress tolerance.
Murashige and Skoog (MS) solid medium after 7 days with or without added NaCl. After exposure to 100 mM NaCl, the CabHLH035 transgenic lines showed obviously higher seed emergence rates than did the WT (Fig. 4A and B). Second, we measured the root lengths of seedlings on MS medium containing 0, 100, and 150 mM NaCl (Fig. 4C and J), and no obvious changes were observed between the WT and transgenic plants without salt stress. After treatment with 100 and 150 mM NaCl, the roots of the CabHLH035 transgenic lines were substantially longer than in WT seedlings. Third, we checked the response of mature plants to salt stress. As shown in Fig. 4D, after 300 mM salt treatment for seven days, the leaves of the transgenic line seedlings showed little yellowing, while WT leaves wilted and the plants ultimately died. Similarly, 15 days after NaCl treatment (300 mM), all the WT plants showed severe wilting compared to the CabHLH035 transgenic plants, as indicated by the higher survival rate of the CabHLH035 transgenic plants (Fig. 4E). Meanwhile, the chlorophyll content of CabHLH035 transgenic Arabidopsis plants was prominently higher than that of WT plants (Fig. 4I). Following salt treatment, the CabHLH035 transgenic lines exhibited markedly increased salt tolerance, accompanied by lower REL and MDA contents (Fig. 4F and G). To further determine whether the enhanced response to salt stress in CabHLH035 transgenic Arabidopsis plants is due to decreased transpiration, we measured the rate of water loss in 3-week-old transgenic and WT plants. As shown in Fig. 4H, in comparison with WT, the CabHLH035 transgenic lines had substantially lower water loss rates. Overall, these results show that the CabHLH035 gene positively regulates salt stress tolerance.
Figure 4.
Ectopic expression of CabHLH035 in Arabidopsis enhances resistance to salt stress. A, B Germination rates of CabHLH035 transgenic lines and WT containing 100 mM NaCl. B, C Greening cotyledon from transgenic and WT containing NaCl. D Phenotypes of transgenic Arabidopsis and WT under salt stress. E survival rate. F REL, G MDA contents. H Water loss. I Chlorophyll contents. J Root length. Error bars denote the standard deviation, and statistically significant differences are denoted by *P ≤ 0.05, **P ≤ 0.01.
The transcripts of genes related to intracellular Na+ : K+ ratio and proline synthesis
To elucidate the function of CabHLH035 in response to salt tolerance, we studied the expression of salt stress-related genes, including SALT OVERLY SENSITIVE 1–3 (SOS1, SOS2, and SOS3) and HIGH-AFFINITY K+ TRANSPORTER 2–1 (HKT2–1), the defense-related genes AMINOCYCLOPROPANE-1-CARBOXYLATE OXIDASE (ACO), NONEXPRESSER of PR GENES 1 (NPR1), CaABR1, and DEFENSIN 1 (DEF1), and the proline synthesis-related gene pyrroline-5-carboxylate acid synthetase (P5CS) in CabHLH035 transgenic Arabidopsis plants, CabHLH035-To transient expression plants, and CabHLH035-silenced plants. These genes were related to ion equilibrium or proline concentration, and have been identified as being positive regulators of salt tolerance [23–25]. We found that among the tested genes, the expression patterns of SOS2, SOS3, HKT2–1, ACO, NPR1, ABR1, and DEF1 did not change in the CabHLH035 transgenic Arabidopsis, CabHLH035-To, and CabHLH035-silenced plants. However, the relative expression of SOS1 and P5CS was prominently lower in the CabHLH035 VIGS plants than in the control pepper plants. In contrast, the SOS1 and P5CS mRNA levels of CabHLH035-overexpressing and CabHLH035-To plants were dramatically higher than the control plants (Fig. 5).
Figure 5.

Relative transcription of genes related to intracellular Na+ : K+ ratio and proline synthesis. A Expression of ion homeostasis and proline synthesis genes in CabHLH035-To and control pepper plants. B The expression of ion homeostasis and proline synthesis genes in CabHLH035-silenced and control plants. C The expression of ion homeostasis and proline synthesis genes in three transgenic CabHLH035-overexpressing Arabidopsis lines and WT plants. Error bars show ± SD (n = 3). Asterisks indicate significant differences (*P ≤ 0.05, **P ≤ 0.01).
The accumulation of Na+, K+ and proline concentration
Previous studies have shown that the SOS pathway plays a central role in maintaining cellular ion homeostasis; the SOS pathway is responsible for Na+ homeostasis in plants [26]. Proline has been shown to modulate abiotic stresses, especially salt stress [27]. We determined the Na+ and K+ concentrations in CabHLH035-To and CabHLH035-silenced plants, but no clear differences in the Na+ and K+ concentrations were found between them and the control pepper plants. However, salt stress caused severe leaf wilting and resulted in higher Na+ concentrations and lower K+ concentrations in CabHLH035-silenced plants compared to the controls (Fig. 6D and E). In addition, the Na+ : K+ ratios were markedly increased in the CabHLH035-silenced plants (Fig. 6F). In contrast, the CabHLH035-To plants had lower Na+ and higher K+ contents than the control pepper plants (Fig. 6A and B). In addition, the Na+ : K+ ratios were significantly down-regulated in the CabHLH035-To plants (Fig. 6C). Next, we measured the proline contents, and found that the proline content of CabHLH035-silenced plants showed decreased proline content compared with the control plants (Fig. 6H), and the CabHLH035-To plants had higher proline contents than did the control plant under salt stress conditions (Fig. 6G). These results show that the tolerance of CabHLH035-To plants and the susceptibility of CabHLH035-silenced plants to salt stress were associated with the intracellular Na+ : K+ ratio and proline concentration.
Figure 6.

Analysis of Na+, K+, and proline contents in CabHLH035-silenced and CabHLH035-To pepper plants after NaCl treatment. Contents of Na+ (A, D), K+ (B, E), Na+ : K+ ratios (C, F) and endogenous proline concentrations (G, H) in the leaves of CabHLH035-silenced, CabHLH035-To pepper, and control pepper plants. Error bars denote the standard deviation, and statistically significant differences are denoted by (*P ≤ 0.05, **P ≤ 0.01).
CaSOS1 is a direct target gene of CabHLH035
We observed that the transcription of the endogenous SOS1 genes were dramatically changed in CabHLH035 transgenic Arabidopsis plants and CabHLH035-To and CabHLH035-silenced pepper plants. Previous studies have shown that G-box or E-box motifs bound by bHLH proteins. We found that the sequences of the promoter regions upstream of the pepper CaSOS1 gene contain four cis-acting G-box elements (Fig. 7A). The ChIP-qPCR results showed that the P2 fragment of the CaSOS1 promoter had a higher relative enrichment, which suggested that the CabHLH035 protein is able to bind the P2 fragment of the CaSOS1 promoter region (Fig. 7B). To show that the CabHLH035 protein can directly bind to the CaSOS1 P2 promoter fragment, yeast one-hybrid (Y1H) was carried out. The Y1H results showed that yeast cells co-expressing the prey and bait plasmids grew normally on the selective media, suggesting that CaSOS1 interacts with CabHLH035 (Fig. 7C and D). We then performed Dual LUC (luciferase) assays to test the interaction between CaSOS1 and CabHLH035. The LUC/REN ratio of the a, b:CabHLH035 + paCaSOS1 sample was higher than other samples, which strongly suggests that the CabHLH035 TF protein binds to the CaSOS1 gene promoter region (Fig. 7E and F). An electrophoretic mobility shift assay (EMSA) also indicated that CaSOS1 is a direct target of CabHLH035, as revealed by the CabHLH035-probe DNA complex on the polyacrylamide gel (Fig. 7G). Based on the LUC/REN, ChIP-qPCR, Y1H, and EMSA results, when the G-box 2 was mutated, CaSOS1 not interacts with CabHLH035. These results CabHLH035 bind to the CaSOS1 G-box 2 promoter fragment. As was evident when the two genes were co-expressed, the CaSOS1 mRNA level decreased in CabHLH035-silenced pepper plants exposed to salt stress and was obviously higher in plants in which CabHLH035 was transiently overexpressed (Fig. 7H and I). Similarly, in comparison with WT, the AtSOS1 mRNA level was higher in three transgenic CabHLH035-OE Arabidopsis lines (Fig. 7J). These results indicate that CaSOS1 is a direct target that is positively regulated by CabHLH035 under condition of salt stress.
Figure 7.
CaSOS1 is a target gene of CabHLH035 under salt stress conditions. A Diagrams showing the CaSOS1 promoter and the four G-box elements. B ChIP assays of CabHLH035 binding to CaSOS1 promoter. C the bait and prey DNA constructs used in the Y1H assays. D Results of the Y1H experiment. E Schematic diagrams of the effector and reporter constructs used in the luciferase experiment. F LUC/REN ratios. G EMSA analysis of CabHLH035 binding to the CaSOS1 promoter. H, ICaSOS1 expression is up-regulated by transient overexpression but is down-regulated when CabHLH035 is silenced in pepper leaves exposed to salt stress. JAtSOS1 was up-regulated in transgenic Arabidopsis lines overexpressing CabHLH035. REN is the luciferase gene from Renilla. P1–P4 is the fragment amplified by primer pairs and CK is the G-box-free fragments. Error bars show the standard deviation (SD). Asterisks indicate statistical significance (*P ≤ 0.05, **P ≤ 0.01).
CabHLH035 interacts with the promoter of CaP5CS
We examined the expression level of the proline synthesis gene P5CS in pepper plants exposed to NaCl. The P5CS changed in CabHLH035-expressing transgenic Arabidopsis plants and CabHLH035-To and CabHLH035-silenced pepper plants. We hypothesized that CabHLH035 directly regulates CaP5CS gene transcription. Our analysis of the DNA sequence upstream of CaP5C revealed the presence of an E-box element (Fig. 8A). A higher relative enrichment of the E-box DNA fragment from the CaP5CS promoter was found, followed the ChIP-qPCR, suggesting that CabHLH035 binds to the E-box element present in the CaP5CS gene promoter (Fig. 8B). To further determine whether CabHLH035 can directly bind to the CaP5CS promoter region, we performed a Y1H assay. The results showed that yeast cells in which the prey and bait plasmids were co-expressed grew normally on the selective media. Nevertheless, when the E-box was mutated, the yeast did not grow on the selective media (Fig. 8C and D). To further establish this relationship, we measured the LUC/REN ratios, and tested whether CaP5CS is a direct target gene of CabHLH035. The results strongly suggest that the CabHLH035 TF binds to the CaP5CS gene promoter region (Fig. 8E and F). EMSA analysis revealed that CaP5CS is a direct target of CabHLH035, as revealed by the protein DNA complex between CabHLH035 and the CaP5CS probe observed on a polyacrylamide gel (Fig. 8G). Based on the LUC/REN, ChIP-qPCR, Y1H, and EMSA results, when the E-box was mutated, CaP5CS does not interact with CabHLH035. These results show CabHLH035 binds to the CaP5CS E-box promoter fragment. To explore the relationship between CabHLH035 and CaP5CS, we measured the mRNA level of P5CS in CabHLH035-overexpressing transgenic Arabidopsis plants and CabHLH035-To and CabHLH035-silenced pepper plants. We found that the P5CS mRNA level was higher in CabHLH035 transgenic Arabidopsis and CabHLH035-To pepper plants compared to the control plants (Fig. 8H and J). In contrast, the CabHLH035 VIGS plants decreased expression of CaP5CS (Fig. 8I). These results indicate that CabHLH035 interacts with the promoter of CaP5CS.
Figure 8.
CaP5CS is a target gene of CabHLH035. A, C Schematic diagrams of the CaP5CS gene promoter region showing the position and sequence of the E-box element. B A ChIP analysis of CabHLH035 to the CaP5CS promoter. D Yeast-one hybrid (Y1H) analysis of the physical interaction between CabHLH035 and the CaP5CS gene promoter. E Diagrams showing the maps of the effector and reporter plasmid constructs. F LUC/REN ratios. G EMSA showing that CabHLH035 binds to the CaP5CS promoter region. H–J Relative expression levels of CaP5CS in pepper leaves transiently overexpressing CabHLH035, in pepper leaves in which CabHLH035 was silenced using VIGS, and expression of AtP5CS in three transgenic Arabidopsis lines overexpressing CabHLH035. P1 is the fragment amplified by primer pairs and CK is the E-box-free fragments. Error bars show the standard deviation (SD). Asterisks indicate statistical significance (*P ≤ 0.05, **P ≤ 0.01).
Effect of CaNAC035 silencing on the binding of CabHLH035 to the G-or E-box containing promoters of CaSOS1 and CaP5CS
In this study we found that CabHLH035 can bind to the promoter motifs of the CaSOS1 and CaP5CS genes. To determine whether CaSOS1 and CaP5CS are directly targeted by CabHLH035, and if so, if the targeting is regulated by CaNAC035, the chromatin from 35S:CabHLH035:GFP in CaNAC035-silenced and TRV2:00 (control) plants were assayed by ChIP-PCR and qPCR. The DNA of ChIP was used as the template in PCR amplification containing the G-and E-box sequences (Fig. 9A). The ChIP-PCR results indicated that CabHLH035 could bind to the CaSOS1 and CaP5CS promoter regions (Fig. 9B). CaNAC035 silencing reduced CabHLH035 binding to the CaSOS1 and CaP5CS promoters (Fig. 9C and D). The results show that CaNAC035 is requisitioned for the targeting of CabHLH035 binding to G-box or E-box elements in the CaSOS1 and CaP5CS promoters under salt stress.
Figure 9.

Effect of silencing the CaNAC035 gene on CabHLH035 binding to the CaSOS1 and CaP5CS gene promoter regions. A Schematic diagrams of the CaSOS1 and CaP5CS gene promoters showing the locations of the G- and E-box elements. P1–P5 are the primers used to amplify the specific DNA sequences. BChIP-PCR analysis of the binding of CabHLH035 to the G-box- or E-box-containing promoters of CaSOS1 and CaP5CS. C, DChIP-qPCR analysis of CaNAC035 silencing on the binding of CabHLH035 to the G-boxes in the CaSOS1 promoter and the E-box in the CaP5CS promoter. Error bars show the standard deviation (SD). Asterisks indicate statistical significance (*P ≤ 0.05, **P ≤ 0.01).
CabHLH035-mediated salt tolerance via proline accumulation pathway
The relative expression of P5CS changed in CabHLH035-To (transiently overexpressing) and CabHLH035-silenced pepper plants (Fig. 5), indicating that CabHLH035-mediated salt tolerance via proline accumulation. We therefore measured the endogenous proline contents of CabHLH035-To plants treated with 0.05 μM 24-epi-brassinolide (EBL), a proline biosynthesis inhibitor that has been shown to reduce proline content [28] and CabHLH035-silenced plants that were sprayed with a 5 mM proline solution, and observed their responses to salt stress. No dramatic differences in proline, REL, and MDA contents were found in the CabHLH035-silenced and control plants under normal conditions. Under salt stress, the proline, REL, and MDA contents were increased; however, plants treated with exogenous proline had significantly lower REL and MDA contents and higher proline contents (Fig. 10B–D). These results suggest that exogenous application of proline restored salt tolerance to the CabHLH035-silenced plants, further illustrating the importance of proline in CabHLH035-mediated salt tolerance in pepper. There were no differences in proline, REL, and MDA contents in the CabHLH035-To and control plants before exposure to salt stress. However, the proline, REL, and MDA contents were markedly increased in response to salt stress, and plants treated with EBL had higher REL and MDA levels and lower proline contents than the water-treated controls (Fig. 10E–H). These results show that a reduction in the proline content in CabHLH035-To pepper plants significantly decreased their salt tolerance.
Figure 10.
Phenotypes of plants sprayed with exogenous proline and treated with 24-epibrassinolide (EBL). A Phenotypes of water- or proline-pretreated CabHLH035-silenced and control pepper plants. Scale bars = 2 cm. B, F proline concentrations in CabHLH035-silenced VIGS plants treated with water or proline (B) and plants transiently overexpressing CabHLH035 treated with water or EBL (F). C, H REL in CabHLH035-silenced VIGS plants treated with water or proline (C) and plants transiently overexpressing CabHLH035 treated with water or EBL (H). D, G MDA contents in CabHLH035-silenced VIGS plants treated with water or proline (D) and plants transiently overexpressing CabHLH035 treated with water or EBL (G). E Phenotypes of water- or EBL-treated CabHLH035-To and control pepper plants. Scale bars = 2 cm. Error bars show the standard deviation, the small letters show significant differences at P ≤ 0.05.
Discussion
Because of the sessile life habit of plants and variable environmental conditions, plants must adapt to a range of environmental challenges. Saline soils are a major abiotic stress that impedes plant growth, crop yields, and farmers’ incomes [29]. Members of the bHLH family of transcription factors (TFs) are involved in many biological processes and the response to abiotic stress in plants. A recent study identified 122 bHLH TF family members in pepper (Capiscum annuum) [14], but only a few that regulate plant responses to various stresses have been characterized. In a previous study we characterized the pepper gene CaNAC035, which positively regulates stress tolerance. We showed that CabHLH79 binds to the CaNAC035 promoter, and CabHLH79 could enhance cold tolerance [21]. We also identified CabHLH035, a CaNAC035-interacting protein in pepper [22]. Based on mRNA expression levels, we found that transcription of CabHLH035 is induced by NaCl treatment (Fig. 1). In our previous study, CaNAC035 was a positive regulator for cold, mannitol, and salt stress. Many studies have reported that NAC TFs play important roles in plants in processes that include leaf senescence, secondary metabolism, and responses to various unfavorable environmental conditions [30–33]. In this study, we characterized the role of CabHLH035 TF gene from pepper leaves, and show that it responds to salt stress (Fig. 1). The results of our study provide new insight into CabHLH035 in response to salt stress.
Reactive oxygen species (ROS) is associated with many abiotic stresses in plants [34]. Production of high levels of ROS leads to oxidative damage in cells, especially under abiotic stress conditions [35]. We found that CabHLH035-silenced plants had substantially higher MDA levels than in control plants (Fig. 3). A better explanation might be that CabHLH035 protects plants from oxidative damage by scavenging ROS. Overall, our findings suggest that CabHLH035 contributes to salt tolerance.
Chlorophyll content is reduced when plants are subjected to adverse conditions [36]. In our study, the chlorophyll contents of CabHLH035-silenced pepper plants were lower compared to non-silenced plants at NaCl concentrations >200 mM (Fig. 3). In plants, MDA content is an indicator of ROS-mediated damage to cell membranes. Ion leakage is another significant physiological indicator of membrane damage [37]. Following salt treatment, in comparison with WT, the transgenic CabHLH035 Arabidopsis lines showed significantly lower REL and MDA levels (Fig. 4). These data reveal that CabHLH035 plays a role in mediating salt stress tolerance. Compared with CabHLH035-silenced plants, CabHLH035-To plants affected less gene expression in Fig. 5; perhaps ectopic expression of CabHLH035 in Arabidopsis was not high enough. The amino acid proline has been reported to modulate abiotic stresses in plants, especially salt stress. Thus, the proline accumulation is considered to be an important mechanism in plant salt stress tolerance [38]. The proline content of CabHLH035-silenced plants was dramatically reduced after salt treatment, however, and the CabHLH035-To plants had higher proline contents than the control plants (Fig. 6). It is worth noting that the proline content showed a positive correlation with salt tolerance. Thus, we speculated that the proline concentration had an effect on the response of the CabHLH035 gene to salt stress. CaP5CS is a proline biosynthesis gene, and we found that that after salt treatment, the CabHLH035-silenced plants showed lower CaP5CS gene expression levels than did the control plants (Fig. 8). However, the CabHLH035 transgenic Arabidopsis lines showed higher AtP5CS gene expression levels compared to WT (Fig. 8). We should note that the expression pattern of P5CS was consistent with the proline contents in the plants, suggesting that the free proline content may depend on the relative level of P5CS gene expression. To further explore how CabHLH035 positively regulates salt stress, expression of the proline synthesis gene P5CS was measured, and we found that P5CS mRNA levels increased in CabHLH035 transgenic and CabHLH035-To lines but decreased in CabHLH035-silenced plants (Fig. 5). To investigate whether CabHLH035 binds to the CaP5CS promoter region to directly regulate salt resistance, we performed Y1H, Dual LUC, and EMSA assays. The results showed that CabHLH035 binds to the CaP5CS promoter, resulting in increased salt tolerance (Fig. 8). We therefore hypothesize that the bHLH-P5CS pathway mediates and the CabHLH035 gene contributes to salt.
Salt stress severely limits plant growth, development, and yield, and it has two main adverse effects on plants: ion toxicity and osmotic stress [39]. Maintaining an optimal Na+ : K+ ratio is an important way to alleviate ion toxicity and is necessary to maintain plant natural habitats [40], which suggests that ion homeostasis is the main determinant of salt tolerance. In this study, SOS genes, especially SOS1, showed higher expression levels in the CabHLH035 transgenic and CabHLH035-To plants, but lower expression in the CabHLH035-silenced plants (Fig. 5). We found that the CabHLH035-silenced pepper had higher Na+ and lower K+ contents than did the control pepper plants under salt stress, which led to increased Na+ : K+ ratios. The CabHLH035-To lines had lower Na+ and higher K+ contents, along with lower Na+ : K+ ratios, than the control pepper plants (Fig. 6). These results show that salt tolerance in the CabHLH035 transgenic and CabHLH035-To plants and the increased salt susceptibility of CabHLH035-silenced plants is primarily due to the intracellular ion balance, therefore lower Na+ : K+ ratios confer increased salt tolerance. Thus, it is conceivable that changes in the SOS genes are key to the accumulation of intracellular Na+ and the growth performance of plants under salt stress. We observed that there is a positive correlation between the CabHLH035 response to salt tolerance and the expression levels of CaSOS1. Based on the Y1H, LUC/REN ratios, and EMSA test assays, we conclude that CaSOS1 expression is regulated by CabHLH035, which indicates that CaSOS1 is a target gene of CabHLH035 (Fig. 7). Therefore, CabHLH035 plays an important role in maintaining the intracellular Na+ balance, which indicates that CaSOS1 is critical for growth in plants exposed to salt stress. In summary, we have shown that CabHLH035 regulates CaSOS1 expression and thus acts as a major regulator of salt tolerance.
Conclusion
We identified CabHLH035 was induced by salt treatment. Silencing the CabHLH035 gene through VIGS decreased the pepper salt tolerance. However, transient expression of CabHLH035 enhanced pepper salt tolerance and ectopic expression of CabHLH035 enhanced Arabidopsis salt tolerance. CaSOS1 and CaP5CS are the direct target genes of CabHLH035. Herein, we proposed the model for CabHLH035 in response to salt stress (Fig. 11). Our study provides a basis for further study to unravel the molecular mechanism of CabHLH035 in response to salt stress and the stress-related candidate genes to utilize it in molecular breeding to enhance the salt tolerance in pepper and other important crops.
Figure 11.

A proposed model showing how CabHLH035 functions in the plant response to salt stress. Upon salt stress, CabHLH079 is activated, CaNAC035 is a direct target gene of CabHLH079, and CaNAC035 interact with CabHLH035, to regulate CaSOS1 and CaP5CS by binding to G-Box or E-Box motifs. Up-regulation of the CabHLH035-CaSOS1/CaP5CS modulates ion homeostasis and proline biosynthesis to achieve salt tolerance.
Materials and methods
Plant material and growth conditions
In this study, pepper cultivar P70 and Arabidopsis thaliana (ecotype Columbia-0) seedlings were used in this study. The test plants were grown at 25°C in daylight for 16 h and 18°C in dark for 8 h with 80% humidity. In order to examine the expression of CabHLH035, one-month-old uniform size pepper seedlings were transferred to 300 mM NaCl solution for 24 h. The tissues were collected at 0, 1, 3, 6, 12, and 24 h of 300 mM NaCl treatment. All materials were independently harvested at the specified time.
RNA extraction and qRT-PCR analysis
Total RNA was extracted as described by Prime Script Kit (Takara, Dalian, China). First-strand cDNA was obtained following the manufacturer protocol of Prime Script Kit (Takara). qRT-PCR was measured using the iCycleriQ™ (Bio-Rad, Hercules, CA, USA) machine. The gene expressions were quantified by the 2-ΔΔCt method, all the primers used for this study are listed in Table S1 (see online supplementary material).
Subcellular localization and transient expression of CabHLH035
The coding sequence of CabHLH035 was amplified using gene-specific primers, then introduced into the pVBG2307::GFP vector to construct the pVBG2307:CabHLH035:GFP plasmid; the pVBG2307::GFP vector without CabHLH035 was used as control. The designed construct was infiltrated into Nicotiana benthamiana leaves and were grown at 22/18°C (day/night) for two days and the CabHLH035 was visualized through a fluorescent microscope (Olympus, Tokyo, Japan). The transient expression in pepper was carried out as described by Cai et al. (2021) [ 41].
Virus-induced gene silencing (VIGS)
Through VIGS the CabHLH035 was knocked-down in pepper as previously described by Lim et al. (2015) [42]. A 300-bp fragment of CabHLH035 was introduced into the vector pTRV2, which is driven by a CaMV 35S promoter. The pTRV1, pTRV2:00, and the fusion plasmids of pTRV2:CabHLH035 were separately transformed into Agrobacterium strain GV3101 and co-infiltrated into the leaves of pepper seedlings. One month later, fully expanded uniformly sized leaves were harvested from the infected plants for silencing efficiency by qRT-PCR and other biochemical analyses.
Generation of transgenic Arabidopsis
To generate CabHLH035-overexpressing Arabidopsis, the coding sequence (CDS) of CabHLH035 was introduced into the pVBG2307-GFP vector. The fusion constructs of 35S:CabHLH035-GFP were transformed into GV3101. The floral dip method was used for genetic transformation [43]. For the selection of CabHLH035 transgenic Arabidopsis lines, seeds were selected on MS medium containing 50 mg/L kanamycin. The T3 plants were selected for further study.
Salt tolerance assay
To analyse the salt tolerance in pepper plants, leaf samples of the transient expression CabHLH035 (CabHLH035-To), 35S::GFP (Mock), CabHLH035-silenced pepper plants were immersed in 300 mM NaCl concentrations for three days. To further identify the role of CabHLH035 in salt stress tolerance of the transgenic Arabidopsis, the three-week-old WT and T3 transgenic lines were treated with 300 mM NaCl. The samples for gene expression analyses were taken on the seventh day and the phenotypic changes were observed and photographed at the seventh and fifteenth days. Samples for the stress-related genes expression analysis and physiological indices were determined after treatment with salt. The control plants were grown in distilled water. Each line contained 30 plants for every test.
Biochemical analyses and histochemical staining
The relative electrolyte leakage (REL) was assessed according to the method of Cao et al. (2007) [44]. The MDA content was measured using the thiobarbituric acid (TBA) method as described by Heath and Packer (1968) [45]. Briefly 0.5 g leaves were grounded in 1 mL 10% trichloroacetic acid (TCA) then diluted with TCA to 10 mL, 12 000 g, for 10 min at 4°C, the supernatant was mixed with 0.6% 2-thiobarbituric acid (TBA) and boiled for 15 min. Absorbance was measured at 600, 532, and 450 nm.
Chlorophyll fluorescence was determined using an IMAGING-PAM chlorophyll fluorimeter, and Fv/Fm ratios were estimated using Imaging software. The total chlorophyll content was determined using the methods of Arkus et al. (2005) [46]. Histochemical staining of O2− and H2O2 was executed with NBT and DAB, respectively, following the methods of Huang et al. (2013) [47]. For water loss rate, the leaves from 4 weeks of CabHLH035 transgenic and WT plants were collected and respectively weighed. At least 30 leaves of each CabHLH035 transgenic and WT plants were weighed every 30 minutes, the water loss rate was assessed following the method of Wei et al. (2018) [48]. Proline contents were quantified according to the method of Zhuo et al. (2018) [49]. The leaves of ectopic expression of CabHLH035 in Arabidopsis plants, CabHLH035-To transient expression plants, CabHLH035-silenced plants, and control plants were collected, then dried at 80°C for 48 h, and the Na+ and K+ contents were examined as described by the method of Li (2015) [50].
Yeast-one-hybrid assay
Promoter sequences (1500 bp upstream of ATG) were acquired from the pepper genome platform (PGP). The CDS of CabHLH035 was inserted into the pGADT7, and promoter sequence fragments of CaP5CS and CaSOS1 were inserted into the pAbAi vector. Recombinant vectors were co-transformed into Y1H and the Y1H experiment was performed following the Clontech method. The bait yeast strain was coated on the SD/−Leu/ medium, and incubated at 30°C for 5–7 days.
Dual luciferase and electrophoretic mobility shift (EMSA) assay
Dual luciferase was calculated by determining the ratio of luciferase (LUC) and Renilla (REN) using the Dual-Luciferase® machine (Promega, WI, USA). The coding sequence of CabHLH035 was inserted into the pMAL-c5x vector and then transformed into Escherichia coli BL21. The mixture was incubated with binding buffer at 24°C for 30 minutes. Protein-probe was separated by the PAGE method. EMSA was performed following the methods of Xie et al. (2018) [51].
Chromatin immunoprecipitation (ChIP) assay
ChIP experiment was carried out according to the method of Khan et al. (2018) [52]. The 35S:CabHLH035-GFP was transformed into GV3101 then infected the pepper leaves. The infected pepper leaves were collected at 48 hpi and cross-linked with 1% formaldehyde and the chromatin was divided into 500 bp fragments. DNA-protein complexes were immunoprecipitated with GFP antibodies, and DNA fragments were decross-linked and purified, the template was used for ChIP-qPCR using specific primer of the G-box-or E-box fragments in CaSOS1 and CaP5CS promoter (Table S1, see online supplementary material).
Statistical analysis
The statistical analyses were performed using one-way analysis of variance (ANOVA) tests with least significant differences at P ≤ 0.05 (*) and P ≤ 0.01 (**) by the statistical package SPSS 17.0 (IBM, Chicago, IL, USA).
Acknowledgments
This research was supported by the National Natural Science Foundation of China (32172582, 31672146), Agricultural Key Science and Technology Program of Shaanxi Province (2021NY-086), Scientific & Technological Innovative Research Team of Shaanxi Province (2021TD-34), and the Natural Science Foundation of Shaanxi Province (2018JM3023).
Author contributions
H.Z., R.C., and H.G. conceived the experiments; H.Z., Y.P., M.L., and L.C. performed the experiments; U.S., J.G., Y.Z., and X.C. analysed the data; H.Z. wrote the paper.
Data availability
The data that support the results are included in this article and its supplementary materials.
Conflict of interest
The authors declare no conflict of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Huafeng Zhang, College of Horticulture, Northwest A&F University, Yangling 712100, China.
Jiangbai Guo, College of Horticulture, Northwest A&F University, Yangling 712100, China.
Xiaoqing Chen, College of Horticulture, Northwest A&F University, Yangling 712100, China.
Yunyun Zhou, College of Horticulture, Northwest A&F University, Yangling 712100, China.
Yingping Pei, College of Horticulture, Northwest A&F University, Yangling 712100, China.
Lang Chen, College of Horticulture, Northwest A&F University, Yangling 712100, China.
Saeed ul Haq, College of Horticulture, Northwest A&F University, Yangling 712100, China; Department of Horticulture, The University of Agriculture Peshawar, Peshawar 25130, Pakistan.
Minghui Lu, College of Horticulture, Northwest A&F University, Yangling 712100, China.
Haijun Gong, College of Horticulture, Northwest A&F University, Yangling 712100, China; Shaanxi Engineering Research Center for Vegetables, Yangling 712100, China.
Rugang Chen, College of Horticulture, Northwest A&F University, Yangling 712100, China; Shaanxi Engineering Research Center for Vegetables, Yangling 712100, China.
References
- 1. Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–81. [DOI] [PubMed] [Google Scholar]
- 2. Maathuis FJ. Sodium in plants: perception, signalling, and regulation of sodium fluxes. J Exp Bot. 2014;65:849–58. [DOI] [PubMed] [Google Scholar]
- 3. Huang Z, Zhao L, Chen Det al. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS One. 2013;8:e62085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verma D, Jalmi SK, Bhagat PKet al. A bHLH transcription factor, MYC2, imparts salt intolerance by regulating proline biosynthesis in Arabidopsis. FEBS J. 2020;287:2560–76. [DOI] [PubMed] [Google Scholar]
- 5. Dai W, Wang M, Gong Xet al. The transcription factor FcWRKY40 of Fortunella crassifolia functions positively in salt tolerance through modulation of ion homeostasis and proline biosynthesis by directly regulating SOS2 and P5CS1 homologs. New Phytol. 2018;219:972–89. [DOI] [PubMed] [Google Scholar]
- 6. Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atchley WR, Fitch WM. A natural classification of the basic helix-loop-helix class of transcription factors. Proc Natl Acad Sci. 1997;94:5172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–83. [DOI] [PubMed] [Google Scholar]
- 10. Bailey PC, Martin C, Toledo-Ortiz Get al. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell. 2003;15:2497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun H, Fan HJ, Ling HQ. Genome-wide identification and characterization of the bHLH gene family in tomato. BMC Genomics. 2015;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Duan X, Jiang Het al. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006;141:1167–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao K, Dong Q, Li Cet al. Genome wide identification and characterization of apple bHLH transcription factors and expression analysis in response to drought and salt stress. Front Plant Sci. 2017;8:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Chen J, Liang Cet al. Genome-wide identification and characterization of the bHLH transcription factor family in pepper (Capsicum annuum L.). Front Genet. 2020;11:570156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nesi N, Debeaujon L, Jond Cet al. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell. 2000;12:1863–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Du J, Huang Z, Wang Bet al. SlbHLH068 interacts with FER to regulate the iron-deficiency response in tomato. Ann Bot. 2015;116:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu D, Li YY, Zhou ZCet al. Tobacco transcription factor bHLH123 improves salt tolerance by activating NADPH oxidase NtRbohE expression. Plant Physiol. 2021;186:1706–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Wang S, Tian Yet al. Functional characterization of a sugar beet BvbHLH93 transcription factor in salt stress tolerance. Int J Mol Sci. 2021;22:3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He Z, Wang Z, Nie Xet al. UNFERTILIZED EMBRYO SAC 12 phosphorylation plays a crucial role in conferring salt tolerance. Plant Physiol. 2022;188:1385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu JR, Huang Z, Xiang XYet al. MfbHLH38, a Myrothamnus flabellifolia bHLH transcription factor, confers tolerance to drought and salinity stresses in Arabidopsis. BMC Plant Biol. 2020;20:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang HF, Ma F, Wang Xet al. Molecular and functional characterization of Canac035, an NAC transcription factor from pepper (Capsicum annuum L.). Front Plant Sci. 2020;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Z, Zhang Y, Hu Het al. CabHLH79 acts upstream of CaNAC035 to regulate cold stress in pepper. Int J Mol Sci. 2022;23:2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nanjo T, Kobayashi M, Yoshiba Yet al. Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana. Plant J. 1999;18:185–93. [DOI] [PubMed] [Google Scholar]
- 24. Qiu QS, Guo Y, Quintero FJet al. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J Biol Chem. 2004;279:207–15. [DOI] [PubMed] [Google Scholar]
- 25. Asif MA, Zafar Y, Iqbal Jet al. Enhanced expression of AtNHX1, in transgenic groundnut (Arachis hypogaea L.) improves salt and drought tolerence. Mol Biotechnol. 2001;49:250–6. [DOI] [PubMed] [Google Scholar]
- 26. Mansour MMF, Ali EF. Evaluation of proline functions in saline conditions. Phytochemistry. 2017;140:52–68. [DOI] [PubMed] [Google Scholar]
- 27. Yang X, Ren Y, Cai Yet al. Overexpression of OsbHLH107, a member of the basic helix-loop-helix transcription factor family, enhances grain size in rice (Oryza sativa L.). Rice (N Y). 2018;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozdemir F, Bor M, Demiral Tet al. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul. 2004;42:203–11. [Google Scholar]
- 29. Deinlein U, Stephan AB, Horie Tet al. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014;19:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cramer GR, Urano K, Delrot Set al. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol. 2011;11:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puranik S, Sahu PP, Srivastava PSet al. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17:369–81. [DOI] [PubMed] [Google Scholar]
- 32. Nakashima K, Takasaki H, Mizoi Jet al. NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819:97–103. [DOI] [PubMed] [Google Scholar]
- 33. Zhu M, Chen G, Zhou Set al. A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol. 2014;55:119–35. [DOI] [PubMed] [Google Scholar]
- 34. Choudhury FK, Rivero RM, Blumwald Eet al. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–67. [DOI] [PubMed] [Google Scholar]
- 35. Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–10. [DOI] [PubMed] [Google Scholar]
- 36. Mafakheri A, Siosemardeh A, Bahramnejad B. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci. 2010;4:580–5. [Google Scholar]
- 37. Bajji M, Kinet JM, Lutts S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 2002;36:61–70. [Google Scholar]
- 38. Shelden MC, Dias DA, Jayasinghe NSet al. Root spatial metabolite profiling of two genotypes of barley (Hordeum vulgare L.) reveals differences in response to short-term salt stress. J Exp Bot. 2016;67:3731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang WW, Meng JJ, Xing JYet al. The K+/H+ antiporter AhNHX1 improved tobacco tolerance to NaCl stress by enhancing K+ retention. Journal of Plant Biology. 2017;60:259–67. [Google Scholar]
- 41. Cai W, Yang S, Wu Ret al. Pepper NAC-type transcription factor NAC2c balances the trade-off between growth and defense responses. Plant Physiol. 2021;186:2169–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim CW, Yang SH, Shin KHet al. The AtLRK10L1.2, Arabidopsis ortholog of wheat LRK10, is involved in ABA-mediated signaling and drought resistance. Plant Cell Rep. 2015;34:447–55. [DOI] [PubMed] [Google Scholar]
- 43. Clough SJ, Bent AF. Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–43. [DOI] [PubMed] [Google Scholar]
- 44. Cao WH. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007;143:707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–98. [DOI] [PubMed] [Google Scholar]
- 46. Arkus KA, Cahoon EB, Jez JM. Mechanistic analysis of wheat chlorophyllase. Arch Biochem Biophys. 2005;438:146–55. [DOI] [PubMed] [Google Scholar]
- 47. Huang XS, Wang W, Zhang Qet al. A basic helix-loop-helix transcription factor, PtrbHLH, of Poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol. 2013;162:1178–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wei W, Cui MY, Hu Yet al. Ectopic expression of FvWRKY42, a WRKY transcription factor from the diploid woodland strawberry (Fragaria vesca), enhances resistance to powdery mildew, improves osmotic stress resistance, and increases abscisic acid sensitivity in Arabidopsis. Plant Sci. 2018;275:60–74. [DOI] [PubMed] [Google Scholar]
- 49. Zhuo C, Liang L, Zhao Yet al. A cold responsive ethylene responsive factor from Medicago falcata confers cold tolerance by up-regulation of polyamine turnover, antioxidant protection, and proline accumulation. Plant Cell Environ. 2018;41:2021–32. [DOI] [PubMed] [Google Scholar]
- 50. Li HL. The Effect and Mechanism of Exogenous Silicon on Salt Resistance of Tomato Seedlings. Yangling, China: Institute of Horticulture, Northwest A&F University; 2015. [Google Scholar]
- 51. Xie YP, Chen P, Yan Yet al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2018;218:201–18. [DOI] [PubMed] [Google Scholar]
- 52. Khan MI, Zhang Y, Liu Zet al. CaWRKY40b in pepper acts as a negative regulator in response to Ralstonia solanacearum by directly modulating defense genes including CaWRKY40. Int J Mol Sci. 2018;19:1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the results are included in this article and its supplementary materials.