Abstract
Introduction:
Treatment of advanced stage non-small cell lung cancer (NSCLC) has changed dramatically due to immunotherapy. However, patients without Programmed Death-Ligand 1 (PD-L1) protein expression often benefit less from immunotherapy. This trial is designed to test if stereotactic body radiation therapy (SBRT) to a single tumor site can significantly enhance the outcome of patients with advanced stage PD-L1(−) NSCLC when added to systemic therapy including immunotherapy.
Materials and Methods:
Alliance A082002 is based on subgroup analysis from the randomized phase II PEMBRO-RT trial., PEMBRO-RT compared pembrolizumab alone or with SBRT and revealed improved progression-free and overall survival (PFS and OS, respectively) in PD-L1(−) patients when adding SBRT (8 Gy x 3 fractions). In A082002, patients without PD-L1 expression will be randomized to SBRT (8 Gy x3) plus systemic therapy versus systemic therapy alone. The primary endpoint of the phase II portion of the trial is PFS and will require 100 patients. The primary endpoint of the phase III portion of the trial is OS and will require an additional 284 patients. This trial will clarify whether adding SBRT to systemic therapy can improve PFS and OS in a larger multi-institutional cohort. Several systemic treatment options are allowed including either immunotherapy alone or chemo-immunotherapy.
Conclusions:
This phase II/III Alliance trial A082002 will test whether the addition of SBRT to a single tumor site will enhance the anti-tumor activity of systemic immunotherapy or chemo-immunotherapy in patients with stage IV PD-L1(−) NSCLC. It is now open in the National Clinical Trials Network (NCTN).
Keywords: Lung Cancer, stereotactic body radiation therapy, immunotherapy, chemotherapy
Introduction
Until recently, stage IV non-small cell lung cancer (NSCLC) was an invariably fatal disease. Although targeted therapies such as tyrosine kinase inhibitors have since improved outcomes in certain cases, most tumors lack targetable alterations. Immunotherapy has revolutionized therapy with the real prospect of achieving 5-year survival in 16 to 23% of select patients.1, 2 This study intends to see if we can enhance the outcome of immunotherapy in select patients who historically were felt to benefit less from immunotherapy, specifically patients with negative PD-L1 levels, defined as PD-L1 <1%.
Stereotactic body radiation therapy (SBRT) has shown promise in trials of select patients with low burden metastatic disease. The phase II SABR-COMET trial randomized 99 patients with various oligometastatic (≤5 metastases) tumors (including NSCLC) to conventional systemic therapy with or without SBRT to all metastases.3 The median survival was 28 months with conventional systemic therapy vs. 41 months with conventional systemic therapy plus SBRT (p=0.090). This met the primary statistical endpoint for success (p < 0.20).
The utility of local consolidative therapy in oligometastatic NSCLC patients was also tested by Gomez et al.4 Patients in this phase III trial were randomized to maintenance therapy/observation with or without local therapy (resection or radiation therapy: including both conventional RT or SBRT) to all active sites of disease. Forty-nine patients with oligometastatic NSCLC (≤3 metastases) without progression after systemic therapy were included. The trial was stopped early by the Data and Safety Monitoring Board when a significant survival benefit to the local therapy arm (median OS, 41.2 months vs. 17.0 months) (P=0.017) was seen. They concluded that patients with oligometastatic NSCLC who did not progress after front-line systemic therapy had better survival with the addition of local therapy. This strategy is being further evaluated in NRG-LU002, a phase III trial specifically for oligometastatic (≤3 metastases) NSCLC addressing the value of local consolidative therapy to each lesion.
The potential synergy of immunotherapy, chemotherapy, and radiation therapy is one of the greatest areas of interest in NSCLC research. For example, the current standard of care for stage III NSCLC is chemoradiation followed by a year of durvalumab immunotherapy. This is based on the results of the PACIFIC trial, which demonstrated median OS for durvalumab arm of 47.5 months vs. 29.1 months without durvalumab. The 5-year OS rates were 42.9% versus 33.4% for durvalumab versus placebo arms.5, 6
In the setting of immune checkpoint inhibition, preclinical and clinical data suggests that radiation alone can induce a T-cell mediated systemic response enhancing the anti-tumor effect of systemic therapy.7-9 The synergistic effect of SBRT and immunotherapy was studied in PEMBRO-RT trial,10 a randomized phase II study of 92 stage IV NSCLC patients that assessed whether adding SBRT to a single lesion prior to pembrolizumab enhanced response. In contrast to the prior trials mentioned above, PEMBRO-RT was not limited to patients with oligometastatic disease . Patients who had previously received prior chemotherapy were randomly assigned to pembrolizumab with or without SBRT. In the SBRT arm, the first pembrolizumab dose was given < 7 days after SBRT. SBRT included three doses of 8 Gy delivered on alternate days to a single, safe, and convenient tumor site. A significant benefit of SBRT with respect to progression-free survival (PFS) was seen in the PD-L1(−) subgroup (HR 0.49; 95% CI, 0.26-0.94; P = .03). For the entire group, the median OSwas 7.6 months vs. 15.9 months (HR, 0.66; 95% CI, 0.37-1.18, P = 0.16, NS) for pembrolizumab vs. pembrolizumab plus SBRT. Unplanned subgroup analyses found a significant benefit for SBRT with respect to OS but only in the PD-L1(−)patients (HR, 0.48: 95% CI, 0.24-0.99; P = .046). No significant increase in toxicity was observed in the SBRT arm. Despite a doubling of response rate, this outcome did not meet the prespecified criteria for meaningful clinical benefit. Positive results were largely influenced by the PD-L1(−)patients, who had significantly improved survival. The authors concluded that a larger trial would be needed to determine whether SBRT potentiates the outcome of immunotherapy for stage IV NSCLC patients.
The PEMBRO-RT trial was important in identifying a patient subgroup (those with PD-L1(−)tumors) that appeared to benefit from the use of SBRT to alter the tumor microenvironment in a manner potentiating pembrolizumab immunotherapy and improving survival. It also demonstrated that the addition of SBRT did not result in increased adverse events.
As summarized above, there are data from randomized trials supporting the role of local consolidative therapy in oligometastatic NSCLC.3,4 For patients with PD-L1(−)with more widespread stage IV NSCLC, a benefit was observed when SBRT to a single lesion was added to pembrolizumab.10 Additionally, there are randomized data that shows immunotherapy with nivolumab plus ipilimumab yielded improved OS over chemotherapy alone in PD-L1(−) patients.11 A similar benefit for OS was seen with the addition of both pembrolizumab as well as ipilimumab/nivolumab to a chemotherapy backbone.7-10 Therefore, we hypothesized that the addition of SBRT to immunotherapy, with or without chemotherapy, will improve OS for patients with PD-L1(−) treated with immunotherapy or chemo-immunotherapy.
Mechanistically, the benefit appears due to the ability of radiation to lyse tumor cells releasing antigens, raise PD-L1 levels, and enhance the immunologic response and outcomes of patients treated with PD-1/PD-L1 inhibitors.12, 13 In this respect, we are hoping to see the combination act analogously to a vaccine, converting an immune-tolerant state (immunologically “cold”) to an anti-tumor activated state (immunologically “hot”). Thus, we proposed to test an immune-priming dose of SBRT in this subgroup in this randomized phase II/III trial.
Materials and Methods:
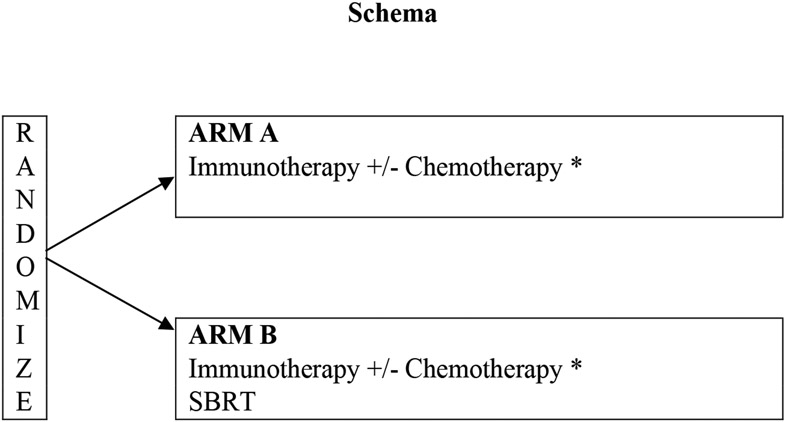
This trial will test if SBRT (8 Gy x 3 fractions) to a single tumor site can “jumpstart” the systemic immunotherapy of stage IV NSCLC patients and improve patient outcome for those with PD-L1(−) tumors. The dose and fractionation of SBRT is similar to that used in the PEMBRO-RT trial, and is intended to prime the immune system. See table 1 and figure 1 for the eligibility criteria, schema and systemic therapy options employed. Stratification factors include Eastern Cooperative Oncology Group Performance Status (0-1 vs. 2) and systemic therapy (Immunotherapy (IO) alone vs. IO plus chemotherapy). Pragmatically, to accrue an adequate number of patients, multiple US Food and Drug Administration-approved systemic options were allowed (Figure 1).
Table 1:
Eligibility Criteria for ALLIANCE A082002 Trial:
| 1. | Histologic/cytologic documentation of stage IV NSCLC or stage III in patients who are not candidates for standard chemo-radiotherapy |
| 2. | PD-L1 Tumor Proportion Score (TPS) TPS < 1% based on local testing |
| 3. | EGFR, ALK and ROS1 (−)* based on local testing |
| 4. | Measurable disease with at least one lesion amenable to SBRT |
| 5. | ECOG Performance Status 0-2 |
| 6. | No Prior Treatment for this malignancy |
| 7. | No comorbid conditions that would preclude immunotherapy |
| 8. | Age ≥18 years |
| 9. | No active other malignancies |
| 10. | No live vaccines within 30 days |
| 11. | Organ function criteria: |
| Absolute neutrophil count ≥1500/mm3 | |
| Platelet count ≥100,000 | |
| Creatinine Clearance ≥45 mL/minute | |
| Total Bilirubin ≤1.5 x Normal | |
| AST/ALT ≤2.5 x Normal | |
| 12. | Treated or small asymptomatic brain metastasis patients are eligible |
epidermal growth factor receptor, anaplastic lymphoma kinase
Figure 1: Schema and Systemic Option for ALLIANCE A082002 Trial:
Treatment is to continue for up to 24 months or until disease progression or unacceptable adverse event. Patients will be followed for 5 years or until death, whichever comes first.
*Nivolumab/Ipilimumab (for squamous or non-squamous)
*Pembrolizumab/Carboplatin/Pemetrexed (for non-squamous)
*Nivolumab/Ipilimumab/Carboplatin/Pemetrexed (for non-squamous)
*Pembrolizumab/Carboplatin /Paclitaxel (for squamous)
*Pembrolizumab/Carboplatin/Nab-paclitaxel (for squamous)
*Nivolumab/Ipilimumab/Carboplatin/Paclitaxel (for squamous)
Study design:
The primary endpoint of the phase II portion of the trial is PFS and will require 100 patients. The sample size of the phase II portion is 100 patients with 50 on each arm. With at least 74 PFS events for Arm A (SBRT + systemic therapy) and Arm B (systemic therapy alone) combined, the phase II portion has approximately 90% power to detect a HR of 0.55, corresponding to an 81.8% improvement in median PFS for Arm A over Arm B from 6 months to 10.9 months, at a one-sided significance level of 0.10.
The primary endpoint of the phase III portion of the trial is OS and will require an additional 284 patients. We are interested in testing a HR of 0.70 for Arm A over Arm B, corresponding to approximately a 42.8% increase in median OS from 17 months to 24.3 months. A total of 298 deaths, observed in Arm A and Arm B combined, provides approximately 85% power to detect a HR of 0.70 with a type I error of 0.025 (one-sided) after adjusting for early stopping due to interim analyses. Assuming approximately 8 PD-L1 negative patients per month are randomized, it will take approximately 72 months (48 months for accrual and additional 24 months for follow-up after last enrollment) from study activation to observe 298 deaths for the final analysis.
Study treatment and assessments
The SBRT will include delivery of 8 Gy x 3 fractions to a single disease lesion. Treatment sites will be credentialed by the Imaging and Radiation Oncology Core (IROC) and SBRT plans checked prior to delivery to ensure the quality of treatment. SBRT will be delivered every other day during the first 60 days after registration but not on the days systemic therapy is administered. Systemic therapy will consist of a total of 18 cycles administered every 42 days, for a total of 2 years of therapy; options are provided in figure 1. Brain imaging will be repeated if brain metastasis were present at the time of registration. Other endpoints evaluated will include objective responses, severe toxicity, and quality of life. We also plan to collect tumor tissue and blood in order to determine if pretreatment tissue-based immune-related biomarkers and intratreatment blood-based immune-related biomarkers predict for response. Quality of life will be assessed using the European Organization for the Research and Treatment of Cancer(EORTC) QLQ 30 and LC-13 surveys and compared between the treatment arms. Post-treatment follow-up will be performed every 3 months for up to 5 years following randomization or until disease progression, and will include chemistry panels, complete blood counts, history and physical examinations and computed tomography of the chest and upper abdomen.
CONCLUSION:
Advanced stage NSCLC carries a poor prognosis, and approximately half of these patients demonstrate PD-L1 levels of <1%. These patients appear to benefit from immunotherapy to a lesser degree than those with higher levels of PD-L1. While immunotherapy has improved the survival of advanced stage NSCLC patients, the majority still succumb to this disease. We hypothesize the addition of moderate, non-ablative doses of SBRT to immunotherapy will improve PFS and OS for PD-L1(−) patients. If positive, this trial will establish SBRT as an important immunomodulatory factor in improving PFS and OS in approximately half of all advanced stage NSCLC patients (those with PD-L1(−) tumors). We are hoping to establish whether SBRT to a single tumor lesion can “jumpslart” immune checkpoint blockade-based systemic treatments and ultimately improve outcomes. Alliance A082002 is now open in the National Clinical Trials Network (NCTN).
Support:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA232760, UG1CA233180, UG1CA233191, UG1CA233253, UG1CA233277, U10CA180820 (ECOG-ACRIN), U10CA180888 (SWOG), and U10CA180868 (NRG). https://acknowledgments.alliancefound.org.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
ClinicalTrials.gov Identifier: NCT04929041
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Topalian SL, Hodi FS, Brahmer JR, et al. Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced NonSmall-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol. 2019;37:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. [DOI] [PubMed] [Google Scholar]
- 4.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol. 2019;37:1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol. 2021;16:860–867. [DOI] [PubMed] [Google Scholar]
- 6.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. Journal of Clinical Oncology. 2021;39:8511–8511. [Google Scholar]
- 7.IMPORTANT DRUG WARNING Regarding AVASTIN® (bevacizumab). http://www.fda.gov/medwatch/safety/2007/Avastin_DHCP_TEF_Final_April2007.pdf.
- 8.Chow J, Hoffend NC, Abrams SI, Schwaab T, Singh AK, Muhitch JB. Radiation induces dynamic changes to the T cell repertoire in renal cell carcinoma patients. Proc Natl Acad Sci U S A. 2020;117:23721–23729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh AK, Winslow TB, Kermany MH, et al. A Pilot Study of Stereotactic Body Radiation Therapy Combined with Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2017;23:5055–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non–Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378:2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoneda K, Kuwata T, Kanayama M, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer. 2019;121:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang WL, Niemierko A, Hwang KL, et al. Clinical Outcomes in Patients With Metastatic Lung Cancer Treated With PD-1/PD-L1 Inhibitors and Thoracic Radiotherapy. JAMA Oncol. 2018;4:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]