To the Editor: We previously reported that the incidence of myocarditis in Israel after receipt of the BNT162b2 messenger RNA vaccine (Pfizer–BioNTech) against coronavirus disease 2019 (Covid-19) was highest among males between the ages of 16 and 29 years (10.7 cases per 100,000 persons).1 BNT162b2 vaccination has since been approved for adolescents between the ages of 12 and 15 years, and initial evidence from this age group in Israel suggests a similar incidence and mild course of myocarditis, although follow-up was limited to 30 days.2 A study from Hong Kong showed an incidence of 28.7 cases per 100,000 persons, but cases were not adjudicated beyond the discharge diagnosis code of the International Classification of Diseases, Ninth Revision (ICD-9).3 Our aim was to provide further evidence regarding the incidence of myocarditis after vaccination among adolescents and data regarding follow-up of 6 months or more.
We collected information about patients who were listed in the database of Clalit Health Services (the largest health care organization in Israel) from June 2 to November 30, 2021. We used ICD-9 codes to identify cases of myocarditis, which were adjudicated with the use of all available data in each patient’s electronic medical record. In-hospital and follow-up data were collected until February 26, 2022, as reported previously.1 Detailed methods and definitions are provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
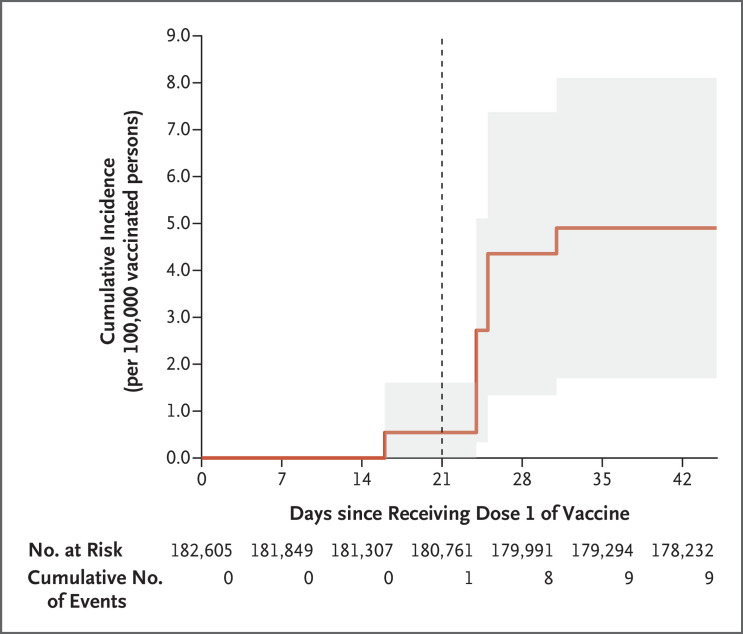
Of 182,605 adolescents who had been vaccinated during this period, 20 potential cases of myocarditis were identified. A total of 9 cases were adjudicated as probable or definite myocarditis, according to the case definition of the Centers for Disease Control and Prevention. This finding translated to an incidence of 4.8 cases (95% confidence interval, 1.7 to 7.9) per 100,000 persons (Figure 1); 8 cases occurred after the second vaccine dose (Fig. S1 and Table S1 in the Supplementary Appendix). Incidence values that are stratified according to sex and age are provided in Table S2.
Figure 1. Cumulative Incidence of Myocarditis after BNT162b2 Vaccination among Israeli Adolescents 12 to 15 Years of Age.
Shown is the cumulative incidence of myocarditis during a 42-day period after the receipt of the first dose of the BNT162b2 vaccine in Israel among 182,605 vaccinated adolescents between the ages of 12 and 15 years of age who were enrolled in the Clalit Health Services. A diagnosis of myocarditis was made in 9 patients. The vertical dashed line at 21 days indicates the median day of administration of the second vaccine dose. The shaded area represents the 95% confidence interval.
All cases of myocarditis were classified as mild,4 and the condition of patients was hemodynamically stable at presentation. Abnormal electrocardiographic (ECG) results were reported in 67% of the patients, and cardiac and inflammatory markers were elevated in all the patients. The in-hospital course of the patients was uneventful, and the median duration of admission was 3 days (interquartile range, 2 to 4 days) (Table S3). Eight patients had a normal ejection fraction, and four had a pericardial effusion (Table S4).
Echocardiographic findings were available for 8 of 9 patients after hospital discharge (median interval, 10 days). All echocardiograms showed a normal ejection fraction and resolution of pericardial effusion (in cases in which the latter had previously been identified). Five patients underwent cardiac magnetic resonance imaging (including three scans that were performed at a median of 104 days after discharge) with minimal evidence of myocardial scarring or fibrosis, with evidence of late gadolinium enhancement ranging from 0 to 2%. At a median follow-up of 206 days (interquartile range, 192 to 229 days) after hospital discharge, all the patients were alive and none had been readmitted to the hospital.
Our study indicates that BNT162b2 vaccine–induced myocarditis in adolescents appears to be a rare adverse event that occurs predominantly in males after the second vaccine dose. The clinical course appears to be mild and benign over a follow-up period of 6 months, and cardiac imaging findings suggest a favorable long-term prognosis.
Supplementary Appendix
Disclosure Forms
This letter was published on October 19, 2022, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 vaccination in Israeli adolescents. N Engl J Med 2022;386:998-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Lai FTT, Chua GT, et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr 2022;176:612-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law YM, Lal AK, Chen S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation 2021;144(6):e123-e135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.