Abstract
Background
Information regarding the protection conferred by vaccination and previous infection against infection with the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is limited.
Methods
We evaluated the protection conferred by mRNA vaccines and previous infection against infection with the omicron variant in two high-risk populations: residents and staff in the California state prison system. We used a retrospective cohort design to analyze the risk of infection during the omicron wave using data collected from December 24, 2021, through April 14, 2022. Weighted Cox models were used to compare the effectiveness (measured as 1 minus the hazard ratio) of vaccination and previous infection across combinations of vaccination history (stratified according to the number of mRNA doses received) and infection history (none or infection before or during the period of B.1.617.2 [delta]–variant predominance). A secondary analysis used a rolling matched-cohort design to evaluate the effectiveness of three vaccine doses as compared with two doses.
Results
Among 59,794 residents and 16,572 staff, the estimated effectiveness of previous infection against omicron infection among unvaccinated persons who had been infected before or during the period of delta predominance ranged from 16.3% (95% confidence interval [CI], 8.1 to 23.7) to 48.9% (95% CI, 41.6 to 55.3). Depending on previous infection status, the estimated effectiveness of vaccination (relative to being unvaccinated and without previous documented infection) ranged from 18.6% (95% CI, 7.7 to 28.1) to 83.2% (95% CI, 77.7 to 87.4) with two vaccine doses and from 40.9% (95% CI, 31.9 to 48.7) to 87.9% (95% CI, 76.0 to 93.9) with three vaccine doses. Incremental effectiveness estimates of a third (booster) dose (relative to two doses) ranged from 25.0% (95% CI, 16.6 to 32.5) to 57.9% (95% CI, 48.4 to 65.7) among persons who either had not had previous documented infection or had been infected before the period of delta predominance.
Conclusions
Our findings in two high-risk populations suggest that mRNA vaccination and previous infection were effective against omicron infection, with lower estimates among those infected before the period of delta predominance. Three vaccine doses offered significantly more protection than two doses, including among previously infected persons.
Evidence of the effectiveness of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) comes largely from studies of the original strain and of variants that emerged before the B.1.1.529 (omicron) variant.1-4 Previous infection confers protection against reinfection,1,5 but both infection-acquired and vaccine-acquired protection against infection wane over time.6-9
Recent studies indicate that vaccinations have continued to be effective against hospitalization and death10-13 but have had reduced effectiveness in protecting against confirmed infection11,14 and symptomatic illness10,15,16 with omicron. In some studies, estimates of hybrid immunity against infection with omicron have been reported.10,14 However, limited information is available with respect to the level of protection conferred by vaccine boosters and the timing of previous infection.
We analyzed data from the California Department of Corrections and Rehabilitation (CDCR), which operates the second largest state prison system in the United States. Prisons and jails are especially risky congregate settings for coronavirus disease 2019 (Covid-19), and many large outbreaks have occurred in these settings during the pandemic.1,17 The CDCR began offering a third (booster) mRNA vaccine dose to residents and staff in late August 2021; by the end of 2021, among persons in the eligible population, 77.9% of residents and 40.3% of staff had been vaccinated with a booster.18 The omicron variant was first identified within the CDCR system among assayed positive samples obtained from correctional staff on December 10, 2021. Substantial outbreaks occurred shortly thereafter among both residents and staff; these outbreaks were consistent with the timing of the worldwide wave of omicron infection.
We evaluated confirmed SARS-CoV-2 infections among nearly 60,000 incarcerated persons and 17,000 prison staff in California during the omicron outbreak period using data collected from December 24, 2021, through April 14, 2022. Our goal was to estimate the levels of protection conferred by mRNA vaccines against infection according to both the number of doses that persons had received and whether they had had a previous documented infection before the start of the observation period. We also estimated the effectiveness of a third mRNA vaccine dose among the persons who had been eligible to receive a booster.
Methods
Study Design and Population
We used a retrospective cohort study design. The period of interest was restricted to outbreak phases that occurred at each of the 35 prisons in the CDCR system between December 24, 2021 (approximately 2 weeks after the omicron variant was initially identified within the prison system), and April 14, 2022. The CDCR considered a prison to be in an outbreak phase if three of more related cases among residents were detected within a 14-day period at the prison. During the outbreak phases, the prisons implemented modified operations, programs, and services for residents and mandatory testing for staff to minimize the risk of ongoing Covid-19 transmission.19,20 The SARS-CoV-2 infections that were detected in the prison system and in the general population in California during this 16-week period were dominated by the omicron variant.21 Our study analyzed two high-risk populations: residents and correctional staff at these prisons.
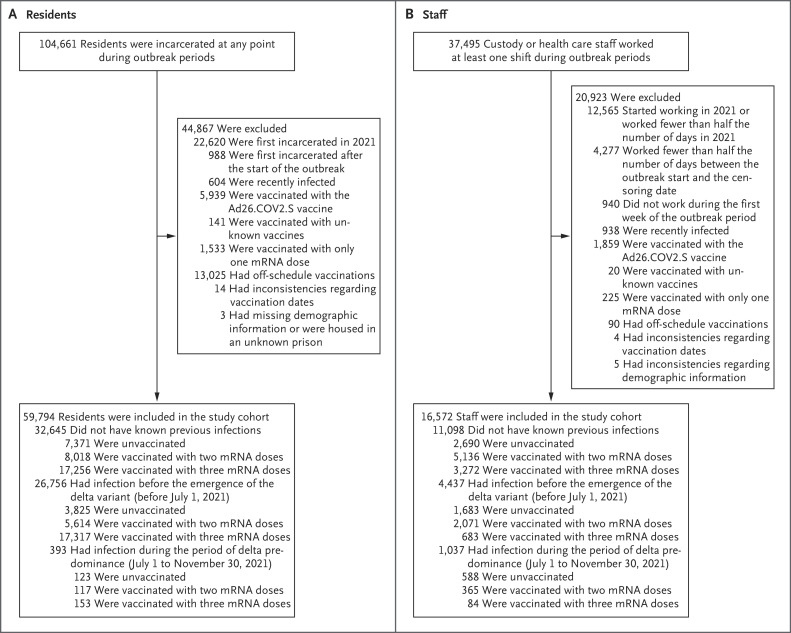
Residents were eligible for inclusion in the study cohort if they were incarcerated at the start of the outbreak phase at their prison. To focus on correctional workers who had the highest risk of workplace exposure, staff were eligible for inclusion if they worked in custody or health care positions (excluding contract employees), if they had regular direct contact with residents, if they had worked during the first week of the outbreak phase at their prison, and if they had worked at least half the number of days between the start of an outbreak and their data censoring date. In addition, to reduce the potential for misclassification of previous infection and vaccination status, we restricted our study cohort to residents who were incarcerated in a CDCR prison before January 1, 2021, and staff who were employed before January 1, 2021, and had worked consistently during 2021 (details are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Next, residents and staff who met the above criteria were excluded if they had received the adenovirus vector–based vaccine Ad26.COV2.S (Johnson & Johnson–Janssen), if they had received only one mRNA vaccine dose, if they had received an unknown vaccine or a vaccine that was not approved or authorized by the Food and Drug Administration, if they had inconsistencies regarding vaccination dates, if they had off-schedule vaccination,22 if they had incomplete demographic data, or if the prison where they were housed or where they worked was not known. To avoid possible misclassification of reinfections, we excluded persons who had had a recent SARS-CoV-2 infection (i.e., persons who had had an infection that was first detected ≤90 days before the start of the outbreak phase at their prison or persons who continued to test positive ≤30 days before the start of the outbreak phase). The observation time began on the start date of the outbreak and ended on the earliest of the following time points: the day that the sample from a positive test was obtained, the day before new vaccination, or the day before transfer (for residents) or a work-shift change (for staff) to a different prison.
Data Collection and Key Measures
CDCR collects and stores deidentified data on all residents and staff on a daily basis. For residents, detailed information from reverse-transcriptase–polymerase-chain-reaction (RT-PCR) and antigen SARS-CoV-2 testing came from a multilayered, voluntary testing program (in which 99.9% of the tests performed were RT-PCR assays) that included risk-based routine testing, surveillance testing, and testing in response to detected outbreaks (see Table S1 in the Supplementary Appendix). During outbreaks, all staff were tested at least once weekly through a mandatory RT-PCR testing program, and staff who worked in prison health care facilities were tested at least twice weekly.23 All staff could undergo testing voluntarily, and close contacts with active cases triggered a compulsory test.24 By January 1, 2022, more than half the prisons had entered an outbreak phase, with the phase of the last prison commencing on January 18, 2022.
All tests and vaccinations that were administered by or reported to the CDCR since March 2020 were used to derive each person’s previous infection status and vaccination status. We defined a previous SARS-CoV-2 infection by the presence of at least one positive test in the CDCR records before the start of the observation period. Previous infections were categorized according to whether they occurred before July 1, 2021, which reflected the period before the emergence of the B.1.617.2 (delta) variant, or whether they occurred on or after July 1, 2021, which corresponded to the period of high prevalence of the delta variant.1,21 Persons infected during both periods were assigned to the latter period.
In addition to detailed information on testing and vaccination (e.g., dates and vaccine brand), the study data included demographic characteristics of the cohort members (sex or gender identity, age, and race or ethnic group), carceral characteristics of the residents (prison, room type, and security level), and carceral characteristics of the staff (prison and type of position). For residents, a documented history of 17 possible coexisting conditions (e.g., hypertension, chronic kidney disease, and asthma) and a composite risk score designed by the CDCR to grade the risk of severe illness from SARS-CoV-2 infection (see Table S2) were provided. Risk scores were top-coded to a score of 2 in accordance with the CDCR definition of residents at moderate to high risk. Residents with scores of 2 or higher were younger than 65 years of age with coexisting conditions or were 65 years of age or older. This risk score was used to guide Covid-19 mitigation policies, including those involving vaccination, testing, and housing. Information on severe outcomes of infection (i.e., hospitalization and death) was available only for residents.
Study Oversight
The study was approved by the institutional review board at Stanford University. Results are reported in accordance with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines25; a checklist of STROBE recommendations is provided in the Supplementary Appendix. The authors vouch for the accuracy and completeness of the data.
Statistical Analysis
The goal of our analysis was to estimate the effectiveness of vaccination and previous infection against omicron infection. In our primary analysis, we used multivariate Cox models to examine the incidence of confirmed infections in groups defined according to combinations of vaccination and previous infection histories during the relevant omicron outbreak phase. The models allowed the baseline hazard to vary according to prison. We used inverse-probability weighting to reduce the effects of confounding due to differences in baseline characteristics among cohort members. Covariate-balancing propensity scores26 were calculated according to demographic, clinical, and carceral characteristics. Specifically, residents were weighted according to prison, Covid-19 risk score (0, 1, or ≥2), room type (cell or dormitory), sex (male or female), and age (as a continuous variable). Staff were weighted according to prison, position (custody or health care), age group (18 to 39, 40 to 54, or ≥55 years), and gender identity (man or woman); details are provided in Section 3.1 in the Supplementary Appendix. Stabilized weights were trimmed to reduce instability and bias from extreme scores.27
We estimated vaccine- and infection-acquired protection with a set of indicator variables that described combinations of vaccination and previous infection status. Effectiveness was expressed as a percent reduction in the hazard rate of omicron infection relative to the group that had not had previous documented infection or been vaccinated (measured as 1 minus the hazard ratio). We also repeated the analyses using a modified set of indicators to estimate the additional effectiveness of three doses of vaccine as compared with two doses. We calculated 95% confidence intervals using standard errors clustered according to prison. Analyses were not adjusted for multiple comparisons. We report the results for residents and staff separately.
The approach that was used in our primary analysis, described above, enabled the estimation and comparison of infection risks across various strata that were defined according to combinations of vaccination and previous infection histories. However, we hypothesized that an alternative study design — a rolling matched-cohort design28 — might be better suited to controlling for potential confounding when estimating vaccine effectiveness within each previous-infection stratum. Instead of weighting exposure groups on the basis of characteristics at the start of the omicron outbreak, as in our primary analysis, this alternative approach matched boosted persons with persons who had been eligible for a booster but had been vaccinated with only two doses. Persons were matched on the basis of previous infection status and other covariates relevant to vaccination according to the date that the boosted person was vaccinated with the third dose. Details are provided in Section 3.2 in the Supplementary Appendix. In this secondary analysis, Cox models were used to estimate the association between vaccination status (two doses or three doses) and omicron infection according to previous infection status. These models allowed the baseline hazard to vary according to prison, with adjustment for calendar week.
We performed five sets of sensitivity analyses. In the first set, we assessed the sensitivity of the results from our primary analysis to extreme weights by comparing estimates from a range of analyses that varied the extent to which large weights were trimmed downward. In the second set, we modified the primary analysis to adjust for the type of mRNA vaccine (BNT162b2 [Pfizer–BioNTech], mRNA-1273 [Moderna], or both). In the third set, we examined the sensitivity of estimates to our specification of reinfections by narrowing the exclusion criterion regarding recent infections, whereby the maximum number of days between the date that an infection was first detected and the start of the outbreak phase was shortened from 90 days to 30 days. In the fourth set, given that our primary analysis included both persons who had been eligible for boosters and those who had not been eligible, we excluded tests from persons who had been vaccinated with two doses but were not yet eligible for a third dose. In the fifth set of sensitivity analyses, to assess the sensitivity of our propensity-score model, we expanded the set of demographic and carceral characteristics that had previously been identified as being predictive of vaccine acceptance among incarcerated persons and staff.29,30
All the analyses were performed with the use of R software, version 3.5.2 (R Foundation for Statistical Computing). Additional details regarding model and variable specifications are provided in the Supplementary Appendix.
Results
Study Population
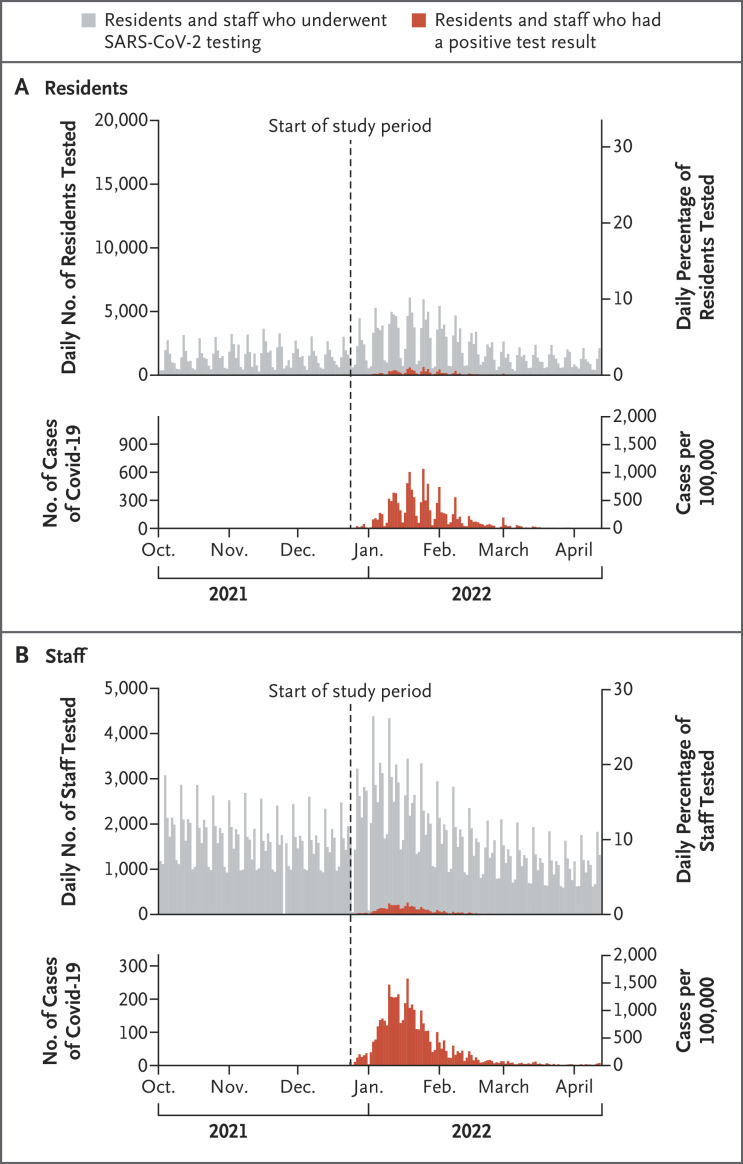
The weighted characteristics of the study population at baseline are shown in Table 1. Among 59,794 residents who met the inclusion criteria for the study cohort, 16.7% tested positive during the study period, and among 16,572 staff who met the inclusion criteria, 30.3% tested positive during the study period (Figure 1 and Table S4). The persons who were included in the study cohort were broadly representative of the overall population of residents who were incarcerated at any point during the study period and the overall population of staff who worked at any time during the study period (Table S3). The mean (±SD) number of times per week that persons were tested was 0.6±0.6 among residents and 1.5±1.0 among staff (Figure 2 and Fig. S2). A total of 96 hospitalizations and 1 death that were assessed as being related to SARS-CoV-2 infection were documented among residents. Data on hospitalizations and deaths were not available for staff.
Table 1. Population Counts and Weighted Characteristics of Residents and Staff at Baseline According to Previous Infection and Vaccination Status.*.
| Characteristic | No Known Previous Infection | Infection before the Period of Delta Predominance | Infection during the Period of Delta Predominance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated | Received Two Doses | Received Three Doses | Unvaccinated | Received Two Doses | Received Three Doses | Unvaccinated | Received Two Doses | Received Three Doses | |
| Residents (N=59,794) | |||||||||
| No. of residents | 7371 | 8018 | 17,256 | 3825 | 5614 | 17,317 | 123 | 117 | 153 |
| Confirmed infection (%) | 24.2 | 20.5 | 17.5 | 20.4 | 14.1 | 13.0 | 20.3 | 10.3 | 5.2 |
| Vaccine type (%)† | |||||||||
| mRNA-1273 | — | 99.9 | 99.6 | — | >99.9 | 99.5 | — | >99.9 | >99.9 |
| Both mRNA-1273 and BNT162b2 | — | 0.1 | 0.4 | — | <0.1 | 0.5 | — | <0.1 | <0.1 |
| Age (yr) | 41.3±12.2 | 42.1±12.2 | 43.6±12.7 | 41.7±12.4 | 42.9±12.6 | 43.9±12.7 | 35.1±9.5 | 36.4±10.0 | 43.7±12.6 |
| Age group (%) | |||||||||
| 18–39 yr | 51.6 | 47.8 | 43.8 | 49.6 | 46.6 | 42.7 | 76.4 | 70.9 | 40.5 |
| 40–54 yr | 31.9 | 34.9 | 35.4 | 32.1 | 33.5 | 35.4 | 18.7 | 23.1 | 37.9 |
| ≥55 yr | 16.5 | 17.3 | 20.8 | 18.3 | 19.9 | 21.9 | 4.9 | 6.0 | 21.6 |
| Sex (%)‡ | |||||||||
| Female | 3.7 | 3.9 | 3.5 | 3.4 | 3.4 | 3.6 | 0 | 2.6 | 3.3 |
| Male | 96.3 | 96.1 | 96.5 | 96.6 | 96.6 | 96.4 | 100 | 97.4 | 96.7 |
| Race or ethnic group (%)§ | |||||||||
| Hispanic or Latinx | 31.1 | 42.3 | 50.1 | 36.5 | 45.5 | 53.3 | 35.8 | 55.6 | 60.8 |
| Black, non-Hispanic | 48.0 | 35.0 | 24.8 | 37.8 | 31.3 | 20.5 | 39.8 | 19.7 | 17.0 |
| White, non-Hispanic | 15.2 | 16.4 | 18.5 | 18.9 | 17.3 | 18.8 | 17.1 | 16.2 | 19.0 |
| Other non-Hispanic or unknown | 5.6 | 6.3 | 6.7 | 6.7 | 5.9 | 7.5 | 7.3 | 8.5 | 3.3 |
| Covid-19 risk score (%)¶ | |||||||||
| 0 | 44.0 | 41.9 | 40.3 | 43.1 | 41.1 | 40.1 | 63.4 | 62.4 | 45.1 |
| 1 | 30.0 | 28.6 | 28.2 | 30.4 | 28.7 | 28.2 | 25.2 | 23.1 | 27.5 |
| ≥2 | 26.0 | 29.5 | 31.4 | 26.5 | 30.2 | 31.7 | 11.4 | 14.5 | 27.5 |
| Room type (%) | |||||||||
| Cell | 76.9 | 72.0 | 68.5 | 71.3 | 68.4 | 67.7 | 77.2 | 68.4 | 70.6 |
| Dormitory | 23.1 | 28.0 | 31.5 | 28.7 | 31.6 | 32.3 | 22.8 | 31.6 | 29.4 |
| Staff (N=16,572) | |||||||||
| No. of staff | 2690 | 5136 | 3272 | 1683 | 2071 | 683 | 588 | 365 | 84 |
| Confirmed infection (%) | 47.8 | 30.6 | 17.1 | 43.7 | 23.9 | 14.0 | 30.7 | 10.2 | 7.1 |
| Vaccine type (%)† | |||||||||
| mRNA-1273 | — | 66.3 | 85.4 | — | 62.1 | 84.6 | — | 46.2 | 72.6 |
| BNT162b2 | — | 33.5 | 10.5 | — | 37.6 | 11.6 | — | 53.3 | 22.6 |
| Both mRNA-1273 and BNT162b2 | — | 0.2 | 4.0 | — | 0.3 | 3.8 | — | 0.6 | 4.8 |
| Age (yr) | 42.0±9.9 | 42.4±10.0 | 44.9±9.4 | 40.6±9.6 | 41.8±10.1 | 46.3±9.1 | 39.7±9.0 | 40.1±9.1 | 48.2±10.2 |
| Age group (%) | |||||||||
| 18–39 yr | 42.8 | 41.3 | 34.6 | 47.6 | 43.6 | 24.8 | 50.1 | 48.4 | 20.2 |
| 40–54 yr | 44.6 | 46.1 | 51.1 | 44.0 | 44.8 | 57.6 | 45.1 | 45.8 | 51.2 |
| ≥55 yr | 12.6 | 12.6 | 14.3 | 8.4 | 11.6 | 17.7 | 4.8 | 5.8 | 28.6 |
| Gender identity (%)‡ | |||||||||
| Woman | 26.5 | 27.5 | 27.4 | 20.6 | 25.7 | 29.9 | 18.9 | 29.1 | 29.8 |
| Man | 73.5 | 72.5 | 72.6 | 79.4 | 74.3 | 70.1 | 81.1 | 70.9 | 70.2 |
| Race or ethnic group (%)§ | |||||||||
| Hispanic or Latinx | 32.8 | 34.8 | 35.3 | 39.6 | 37.2 | 41.6 | 34.0 | 42.1 | 32.1 |
| Black, non-Hispanic | 8.5 | 10.2 | 10.8 | 4.8 | 10.2 | 9.5 | 4.5 | 9.4 | 13.1 |
| White, non-Hispanic | 32.7 | 23.1 | 23.3 | 28.7 | 21.4 | 19.3 | 39.4 | 21.7 | 21.4 |
| Other non-Hispanic or unknown | 26.0 | 31.9 | 30.6 | 27.0 | 31.2 | 29.6 | 22.1 | 26.9 | 33.3 |
| Type of position (%) | |||||||||
| Custody | 79.9 | 77.0 | 75.0 | 89.6 | 79.1 | 70.0 | 94.2 | 81.8 | 58.3 |
| Health care | 20.1 | 23.0 | 25.0 | 10.4 | 20.9 | 30.0 | 5.8 | 18.2 | 41.7 |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
The mRNA-1273 vaccine was developed by Moderna, and BNT162b2 by Pfizer–BioNTech.
Data on sex were obtained for residents only and reflect information recorded on legal-identity documents. Data on gender identity were obtained for staff only and were reported by the staff.
The race and ethnic group of the residents were derived from a combination of administrative records and reports by the residents, and the race and ethnic group of the staff were reported by the staff.
The coronavirus disease 2019 (Covid-19) risk score was developed by the California Department of Corrections and Rehabilitation (CDCR) to grade a resident’s risk of severe outcomes after severe acute respiratory syndrome coronavirus 2 infection. Risk scores were top-coded to a score of 2 in accordance with the CDCR definition of residents at moderate to high risk. Residents with scores of 2 or higher were younger than 65 years of age with coexisting conditions or were 65 years of age or older. Details are provided in Table S2.
Figure 1. Study Population.
The Ad26.COV2.S vaccine, an adenovirus vector–based vaccine, was developed by Johnson & Johnson–Janssen. The delta variant is also known as B.1.617.2.
Figure 2. Testing and Cases in the Study Cohort.
The upper graphs of Panels A and B show the daily numbers and percentages of residents and staff who underwent testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and who had a positive test result. There was reduced staff testing on federal holidays. The lower graphs of Panels A and B show the daily numbers of positive cases of coronavirus disease 2019 (Covid-19). Testing and case series were extended over the historical period that began 2.5 months before the start of the study period.
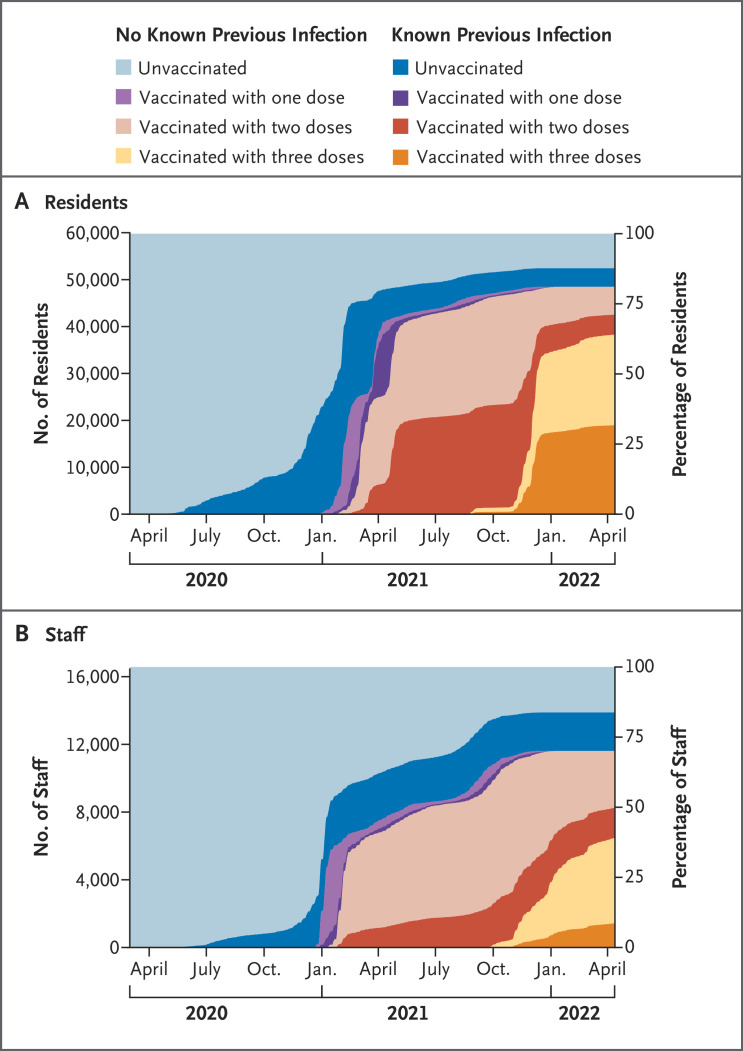
Nearly half the residents and one third of the staff had previous confirmed infections, most of which had occurred before the delta variant became the dominant strain (Figure 3). Among residents, the median interval between the start of their last infection and the start of the outbreak phase in their prison was 393 days (interquartile range, 372 to 435); among staff, the median interval was 367 days (interquartile range, 163 to 400).
Figure 3. Vaccination and Previous Infection Status of the Study Cohort over Time.
The California Department of Corrections and Rehabilitation began their vaccination program for the mRNA primary series at the end of 2020 and began offering boosters at the end of August 2021. A high incidence of previous infection was detected in the study cohort during periods of high incidence of Covid-19 in the general population in California.
Among persons who had received two vaccine doses only, the median time since receipt of the second dose was 246 days (interquartile range, 127 to 294) for residents and 205 days (interquartile range, 90 to 324) for staff. Among those who had received three doses, the median time since receipt of the third dose was 35 days (interquartile range, 27 to 48) for residents and 37 days (interquartile range, 13 to 55) for staff.
Effectiveness of Vaccination and Previous Infection against Omicron Infection
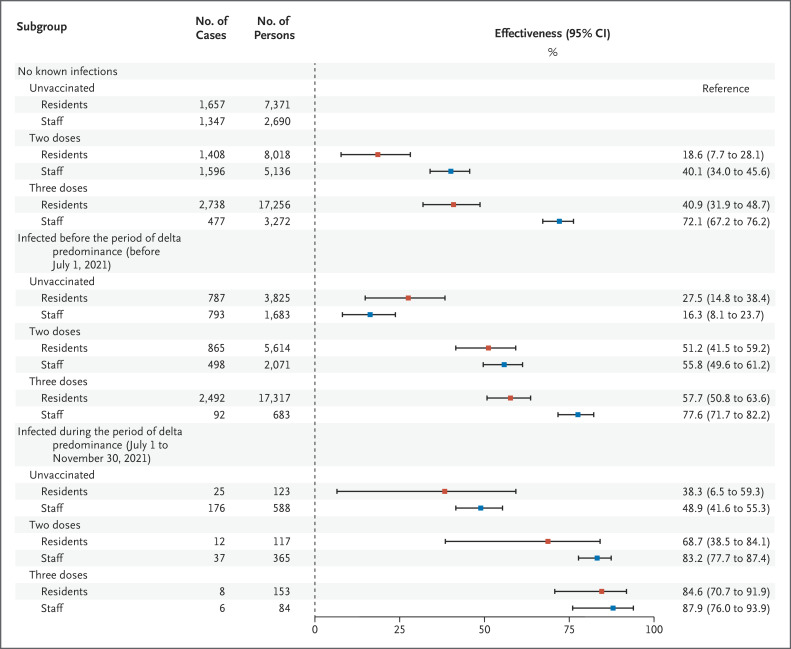
Within the resident population, the estimated effectiveness (measured as 1 minus the hazard ratio) of vaccination against confirmed SARS-CoV-2 infection during the period of omicron predominance among persons who had not had known previous infection was 18.6% (95% confidence interval [CI], 7.7 to 28.1) for those who had received only two vaccine doses and 40.9% (95% CI, 31.9 to 48.7) for those who had received three doses (Figure 4). Among the residents who had not been vaccinated, the estimated infection-conferred effectiveness against infection was 27.5% (95% CI, 14.8 to 38.4) among persons who had been infected before the period of delta predominance and 38.3% (95% CI, 6.5 to 59.3) among those who had been infected during the period of delta predominance. Among the residents who had been infected before the period of delta predominance, the estimated effectiveness of vaccination was 51.2% (95% CI, 41.5 to 59.2) with two vaccine doses and 57.7% (95% CI, 50.8 to 63.6) with three doses; among those infected during the period of delta predominance, the estimated effectiveness was 68.7% (95% CI, 38.5 to 84.1) and 84.6% (95% CI, 70.7 to 91.9), respectively.
Figure 4. Adjusted Estimates of Effectiveness of Vaccination and Previous Infection against Omicron Infection among Residents and Staff in California State Prisons.
Shown are unadjusted case counts, total counts, and adjusted estimates of the effectiveness of vaccination and previous infection against omicron infection outbreaks that occurred between December 24, 2021, and April 14, 2022. Persons who had been vaccinated had received two or three doses of mRNA vaccines only. The red squares denote residents, and the blue squares denote staff. 𝙸 bars denote 95% confidence intervals.
Analyses of data from residents and staff produced estimates that indicated that the relative levels of effectiveness from vaccination and previous infection were generally consistent (Figure 4 and Table S5A). However, the estimated levels of effectiveness from vaccination among staff who had not had known previous infection were higher than those among residents without known previous infection, with estimates of 40.1% (95% CI, 34.0 to 45.6) among staff who had received two vaccine doses and 72.1% (95% CI, 67.2 to 76.2) among those who had received three doses.
Among the staff who had not been vaccinated, the estimated effectiveness of infection that had occurred before the period of delta predominance was 16.3% (95% CI, 8.1 to 23.7), and the estimated effectiveness of infection that had occurred during the period of delta predominance was 48.9% (95% CI, 41.6 to 55.3). Among the staff who had been infected before the period of delta predominance, the estimated effectiveness of vaccination was 55.8% (95% CI, 49.6 to 61.2) with two doses and 77.6% (95% CI, 71.7 to 82.2) with three doses; among those infected during the period of delta predominance, the estimated effectiveness was 83.2% (95% CI, 77.7 to 87.4) and 87.9% (95% CI, 76.0 to 93.9), respectively.
Effectiveness of a Third Dose among Booster-Eligible Persons
In our primary analysis, protection against infection was estimated relative to an individual person’s status at the start of the outbreak phase, and the analysis included both persons who had been eligible to receive a booster and those who had not been eligible to receive a booster. In this analysis, the estimated effectiveness of a third dose, as compared with two doses, among residents who were eligible to receive a booster was 27.4% (95% CI, 19.9 to 34.2) among those who had not had known previous infection, 13.3% (95% CI, 3.2 to 22.3) among those who had been infected before the period of delta predominance, and 50.7% (95% CI, −42.0 to 82.9) among those who had been infected during the period of delta predominance. Among staff, the estimated effectiveness of a third dose was 53.4% (95% CI, 46.7 to 59.3) among those who had not had known previous infection, 49.2% (95% CI, 35.0 to 60.3) among those who had been infected before the period of delta predominance, and 28.0% (95% CI, −33.0 to 61.0) among those who had been infected during the period of delta predominance.
In our secondary analysis, in which boosted persons were matched according to the date of vaccination with the third dose with controls who had been eligible for a third dose but had not received a third dose, the estimated incremental effectiveness of the third dose among residents was 25.0% (95% CI, 16.6 to 32.5) among those who had not had known previous infection and 46.4% (95% CI, 38.3 to 53.4) among those who had been infected before the period of delta predominance (Table S5B). Among staff, the estimated incremental effectiveness of the third dose was 57.9% (95% CI, 48.4 to 65.7) among those who had not had known previous infection and 57.7% (95% CI, 46.2 to 66.7) among those who had been infected before the period of delta predominance. Incremental effectiveness could not be estimated for persons infected during the period of delta predominance because no infections were documented among those who had received a third dose.
Sensitivity Analyses
Estimates that were derived with the use of untrimmed weights and weights with higher degrees of trimming were not appreciably different from those of the primary analysis, nor were estimates that were derived from alternative model specifications that were adjusted for the type of mRNA vaccine. Analyses of the expanded sample that included more recent previous infections produced slightly higher estimates of effectiveness than those of the primary analysis among persons whose previous infection occurred during the period of delta predominance. When persons who had not been eligible for a third dose were excluded, the estimates of effectiveness were slightly lower than those of the primary analysis among persons who had been vaccinated with two doses. Estimates that were derived with the use of the propensity-score model in which an expanded set of covariates was included produced estimates of effectiveness that were similar to those of the primary analysis. The results of the sensitivity analyses are provided in Tables S5A, S5C, S5D, and S5E.
Discussion
This study involving two high-risk populations evaluated the effectiveness of two or three doses of BNT162b2 and mRNA-1273 vaccines, and of previous infection before and during the period of delta predominance, in reducing the risk of infection with the SARS-CoV-2 omicron variant. Our findings add to the evidence base for vaccine effectiveness and immunity conferred by previous infection by providing estimates of effectiveness that were specific to different combinations of vaccination and previous infection histories. These estimates were based on two vulnerable populations in which reliable ascertainment of infection during the study period was likely, owing to high levels of testing.
Vaccination with three mRNA doses was associated with a reduced risk of infection with the omicron variant. Our estimates among persons who had not had known previous infection were similar to those of a convenience-sample study of the effectiveness of two or three mRNA-1273 doses involving data from a health care system that serves southern California.11 A cohort study that was conducted with the use of data from a health care system that serves Connecticut did not show significant additional protection from vaccination with a third dose among persons with previous infection.14 Our primary analysis included both persons who had been eligible to receive a third dose and those who had not been eligible to receive a third dose, an approach that potentially biased estimates of the effectiveness of a third dose downward. The higher risk of infection among persons who had received two or fewer vaccine doses could have induced selection bias if the most susceptible persons had been infected during the period before omicron predominance, which would also have biased estimates of effectiveness downward. As expected, the estimates of effectiveness were similar but slightly higher in the secondary analysis than in the primary analysis; the secondary analysis used an alternative design that probably corrected for these biases more effectively and provided estimates among persons who survived long enough to become eligible for a third dose. However, the conservative estimates from the primary analysis, as well as those from the secondary and sensitivity analyses, all indicate substantial, additional protection from a third mRNA dose against confirmed infection, irrespective of previous infection history. Our findings suggest that a third dose is beneficial, even in persons with previous confirmed infection.
The absolute levels of effectiveness against infection in our study are substantially lower than those estimated in earlier studies8,31-33 that were conducted before the omicron outbreaks occurred; furthermore, we observed that the estimated protection conferred by previous infection varied substantially with respect to timing, with considerably lower estimates among persons infected before the emergence of the delta variant. We estimated that vaccination augmented protection among persons who had had previous infection, a finding that is consistent with findings reported in other studies.11,14 Three doses of vaccine in combination with previous infection resulted in the highest estimates of effectiveness, and these levels approached those reported in studies involving vaccination with the primary series that were conducted before the occurrence of the omicron outbreaks.
For several combinations of exposure history, the resident population had lower estimates of effectiveness than the staff population. Although the CDCR has implemented an extensive testing program in both populations, testing among residents was neither routine nor compulsory. Undetected previous infections could have contributed to dilution of the estimates of effectiveness among residents, as could underascertainment of infections during the study period. Staff also have a greater ability to engage in protective behaviors, such as masking and social distancing, both at work and outside of work. Staff who remained uninfected before the emergence of the omicron variant may reflect a subpopulation that engages in a higher degree of protective behavior; this situation could also explain some of the differences between population estimates.
In this study, vaccine effectiveness was examined in the context of information about infection- and vaccine-acquired immunity against the omicron variant and information about the timing of previous infections. The study has notable strengths. First, we used detailed daily information regarding vaccination status and key Covid-19 outcomes for each member of the high-risk populations studied. These data allowed us to adjust for key potential confounders, as well as for demographic and exposure-related characteristics. Second, the large sample sizes of the two distinct populations enabled relatively high precision in our estimates, and the fact that estimates of the relative levels of effectiveness across the two populations were similar is notable despite the differences between the populations with respect to living situations, testing programs, and demographic characteristics.
Our study also has several limitations. Although a variety of covariates — including those related to vaccine acceptance and the risk of previous infection — were used to balance baseline characteristics, residual confounding could remain. Vaccine uptake and the occurrence of previous infections varied between residents and staff. Differences in infection risks between the two populations may in part reflect complex interactions of vaccine and previous infection levels and timing. Furthermore, the CDCR conducted limited viral sequencing and molecular testing, so we cannot disentangle the effects of variants from temporal waning, nor can we confirm that all cases that were observed during the study period were omicron infections.
Distinct testing programs and exposures for residents and staff during the study period may also have introduced confounding, although several results provide reassurances. First, although staff testing was inconsistent across vaccination and previous infection statuses, nearly all staff were tested at least once weekly (range of means, 0.9 to 2.0 tests per week), which provided relatively complete case detection. Second, although testing for residents was not routine, random, or compulsory, testing was relatively consistent across vaccination and previous infection statuses (range of means, 0.5 to 0.7 tests per week). Third, it is important to note that the consistency between the relative levels of effectiveness in the stratified analyses in these two populations provides some confidence that bias related to variation in testing practices may not be of major concern.
Several additional limitations warrant mention. Our estimates of effectiveness focused on confirmed infections and not on other important outcomes such as symptomatic infections or severe disease. The incidence of hospitalization and death in our sample was too low to support rigorous analysis of those outcomes according to the combinations of vaccine and previous infection histories analyzed, and symptom reporting was unreliable during the study period.34 In the secondary analysis, we were also unable to estimate the effectiveness of a third dose among persons who had had infection during the period of delta predominance. Finally, the generalizability of our results to jails, other prisons, other high-risk populations (e.g., residents of nursing homes and health care workers), and lower-risk populations is unknown.
The findings from this study suggest that, although mRNA vaccines and previous infection provide less protection against infection with the omicron variant than they did against earlier variants, boosters continue to provide substantial additional effectiveness, including among previously infected persons. Continued emphasis on vaccination and other ongoing mitigation practices is essential in preventing transmission, especially in highly vulnerable populations that have already borne a disproportionately large burden of disease and disruption during the Covid-19 pandemic.
Acknowledgments
We thank Joseph Bick, John Dunlap, Heidi Bauer, and the other staff members at the California Department of Corrections and Rehabilitation for providing data and assistance with interpretation of the study results, and members of the Stanford–Center for Research and Teaching in Economics (CIDE) Coronavirus Simulation Model modeling consortium and the Stanford Health Justice Coalition for their support.
Supplementary Appendix
Disclosure Forms
This article was published on October 26, 2022, at NEJM.org.
Footnotes
Supported in part by the Covid-19 Emergency Response Fund at Stanford University, which was established with a gift from the Horowitz Family Foundation; the Stanford Impact Labs Design Fellowship; and by grants from the National Institute on Drug Abuse at the National Institutes of Health (R37-DA15612), the Centers for Disease Control and Prevention through the Council of State and Territorial Epidemiologists (NU38OT000297-02), and the National Science Foundation Graduate Research Fellowship Program (DGE-1656518).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Chin ET, Leidner D, Zhang Y, et al. Effectiveness of the mRNA-1273 vaccine during a SARS-CoV-2 delta outbreak in a prison. N Engl J Med 2021;385:2300-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin ET, Leidner D, Zhang Y, et al. Effectiveness of coronavirus disease 2019 (COVID-19) vaccines among incarcerated people in California state prisons: retrospective cohort study. Clin Infect Dis 2022;75(1):e838-e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 2021;385:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med 2022;386:1091-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordström P, Ballin M, Nordström A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet 2022;399:814-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med 2022;386:1207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med 2021;385(24):e83-e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med 2022;387:21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med 2022;28:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AG, Amin AB, Ali AR, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence — 25 U.S. jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep 2022;71:132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind ML, Robertson AJ, Silva J, et al. Effectiveness of primary and booster COVID-19 mRNA vaccination against infection caused by the SARS-CoV-2 omicron variant in people with a prior SARS-CoV-2 infection. April 21, 2022. (https://www.medrxiv.org/content/10.1101/2022.04.19.22274056v2). preprint.
- 15.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 2022;386:1532-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA 2022;327:639-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chin ET, Ryckman T, Prince L, et al. COVID-19 in the California state prison system: an observational study of decarceration, ongoing risks, and risk factors. J Gen Intern Med 2021;36:3096-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.California Department of Corrections and Rehabilitation. Population COVID-19 tracking. December 31, 2021. (https://www.cdcr.ca.gov/covid19/population-status-tracking).
- 19.California Department of Corrections and Rehabilitation. COVID-19 mandatory 15-day modified program. January 6, 2022. (https://www.cdcr.ca.gov/covid19/covid-19-mandatory-15-day-modified-program/).
- 20.California Department of Corrections and Rehabilitation. Institutional roadmap to reopening. February 16, 2022. (https://www.cdcr.ca.gov/covid19/institutional-roadmap-to-reopening-february-16-2022/).
- 21.California ALL. Variants. May 1, 2022. (https://covid19.ca.gov/variants/).
- 22.Centers for Disease Control and Prevention. COVID-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. September 7, 2022. (https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf).
- 23.California Department of Corrections and Rehabilitation. Mandatory COVID-19 vaccination, booster and testing for institution/facility staff. August 19, 2021. (https://www.cdcr.ca.gov/covid19/mandatory-covid-19-vaccination-booster-and-testing-for-institution-facility-staff/).
- 24.California Department of Corrections and Rehabilitation. COVID-19 response efforts. February 2, 2022. (https://www.cdcr.ca.gov/covid19/covid-19-response-efforts/).
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453-1457. [DOI] [PubMed] [Google Scholar]
- 26.Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc B 2014;76:243-263. [Google Scholar]
- 27.Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS One 2011;6(3):e18174-e18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Witman A, Beadles C, Liu Y, et al. Comparison group selection in the presence of rolling entry for health services research: rolling entry matching. Health Serv Res 2019;54:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin ET, Leidner D, Ryckman T, et al. Covid-19 vaccine acceptance in California state prisons. N Engl J Med 2021;385:374-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prince L, Long E, Studdert DM, et al. Uptake of COVID-19 vaccination among frontline workers in California state prisons. JAMA Health Forum 2022;3(3):e220099-e220099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gazit S, Shlezinger R, Perez G, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity versus vaccine-induced immunity, reinfections versus breakthrough infections: a retrospective cohort study. Clin Infect Dis 2022;75(1):e545-e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrestha NK, Burke PC, Nowacki AS, Terpeluk P, Gordon SM. Necessity of coronavirus disease 2019 (COVID-19) vaccination in persons who have already had COVID-19. Clin Infect Dis 2022;75(1):e662-e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheehan MM, Reddy AJ, Rothberg MB. Reinfection rates among patients who previously tested positive for coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis 2021;73:1882-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryckman T, Chin ET, Prince L, et al. Outbreaks of COVID-19 variants in US prisons: a mathematical modelling analysis of vaccination and reopening policies. Lancet Public Health 2021;6(10):e760-e770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.