Abstract
The immunoregulatory cytokine interleukin 12 (IL-12) induces host resistance against experimental malaria. In this study, we tested the feasibility of using IL-12 in combination with chloroquine (CQ) to rescue susceptible A/J mice from lethal blood-stage Plasmodium chabaudi AS infection. Combined treatment with low doses of CQ and IL-12 resulted in a >15-fold reduction in the parasite load and 100% survival of A/J mice with established infections. Compared to control mice, which succumbed to severe anemia, CQ-plus-IL-12-treated mice had significantly higher early- and late-stage erythroid-cell progenitors in the bone marrow and spleen, resulting in significantly higher hematocrits, erythrocyte counts, and percentages of reticulocytes. Production of parasite-specific gamma interferon (IFN-γ) by splenocytes from these mice was upregulated >20-fold relative to controls in parallel with enhanced IFN-γ mRNA expression. Further, enhanced responsiveness to IL-12 and increased downstream IFN-γ production in CQ-plus-IL-12-treated mice was evident from increased mRNA expression for the β1 and β2 subunits of IL-12 receptor in the splenocytes. Moreover, this combined therapy induced higher levels of anti-malaria antibodies than did CQ alone as well as sterile immunity against reinfection. Because IL-12 can be used at low doses and is effective even in established infections, it may be feasible to use this immunochemotherapeutic approach in human malaria.
Malaria remains a major public health problem in most tropical countries, particularly sub-Saharan Africa. It has been estimated that between 300 million and 500 million individuals are infected annually and between 1.5 million and 2.7 million people die of malaria every year (2). Despite decades of frustrating research, an effective vaccine against this deadly disease is still not a reality (2, 5). In the meantime, however, we must rely on effective therapeutic strategies for treating acute infections to prevent malaria-associated complications and mortality, especially in patients with malaria due to Plasmodium falciparum. Chloroquine (CQ) has been both an affordable and well-tolerated drug for use in third-world countries, but this drug now faces severe limitations because of the widespread emergence of CQ-resistant P. falciparum strains and, more recently, P. vivax strains (20, 29). To overcome this problem, different combinations of antimalarial drugs have been used, but in most instances, multidrug-resistant P. falciparum strains have emerged (28). Thus, intensive investigations directed toward finding an effective method to successfully treat acute malaria infections are under way.
Interleukin 12 (IL-12), a potent immunomodulatory cytokine, has been proven to be effective in conferring protection against bacterial, viral, and intracellular parasitic infections (15, 27). This pleiotropic cytokine not only enhances cell-mediated immune responses but also influences humoral immunity by inducing isotype switching through both gamma interferon (IFN-γ)-dependent and -independent mechanisms (17). IL-12 also appears to stimulate enhanced antibody (Ab) production in switched B cells (17). Both mice and nonhuman primates can be protected against preerythrocytic malaria infections following IL-12 treatment (8, 24). Our laboratory has demonstrated the effectiveness of IL-12 in inducing protective immunity against blood-stage infection in the murine model of P. chabaudi AS malaria (26). In addition to its NK cell-activating, IFN-γ-stimulatory, and Th1-polarizing effects early during P. chabaudi AS blood-stage infection, IL-12 induces remarkable upregulation of splenic erythropoiesis, thereby preventing the fatal anemia associated with this infection (18, 19, 26). However, the dose of IL-12 appears to be critical, given the potential toxic effects of this cytokine (8, 22).
Although IL-12 can induce protective Th1-type immunity against experimental malaria infections, its therapeutic value is limited, given the need to begin treatment prior to or on the day of establishing infection (8, 24, 26). The main goal of this study was to improve the efficacy of IL-12 treatment, especially in terms of its efficacy in established infections. We examined the possibility of using IL-12 as a therapeutic agent, in combination with CQ, for treating established P. chabaudi AS infection in susceptible A/J mice. Our findings demonstrate that low-dose CQ plus IL-12 treatment of mice with established blood-stage infection induced a protective Th1 immune response and efficient upregulation of erythropoiesis during primary infection and higher anti-malaria Ab production following reinfection.
MATERIALS AND METHODS
Mice, parasites and infection protocol.
Male A/J mice, 8 to 12 weeks old, were purchased from Jackson Laboratory (Bar Harbor, Maine). The mice were infected intraperitoneally with 106 P. chabaudi AS parasitized red blood cells (PRBC) in pyrogen-free saline, and parasitemia and survival rate were monitored as described previously (26). To assess reinfection immunity, mice were challenged with the same dose of parasites 4 weeks after recovery from the primary infection and parasitemia was monitored for 2 weeks.
IL-12 and CQ treatment.
Murine recombinant IL-12 (rIL-12) was a gift from S. Wolf, Genetics Institute (Cambridge, Mass.). CQ diphosphate was purchased from Sigma (St. Louis, Mo.). To establish an optimal subcurative dose of CQ, mice were treated orally with 25 mg of CQ per kg of body weight (the therapeutic dose) or 12.5 and 6.25 mg/kg, divided according to World Health Organization recommendation (31). For the therapeutic dose, an initial dose of 10 mg/kg was given on day 3 postinfection (p.i.) followed by 5 mg/kg at 6, 24, and 48 h. For 12.5 and 6.25 mg/kg, the dose of CQ used was one-half and one-quarter of the therapeutic dose, respectively, at each treatment time point. Since our intention was to use lower doses of IL-12 in combination with a subcurative dose of CQ, the dose of IL-12 was decreased from our previously reported (26) protective dose of 0.6 μg/mouse (0.1 μg per day for 6 days) administered i.p. starting on the day of infection, to 0.3 μg/mouse (0.05 μg for 6 days). Further, to test the protective efficacy of IL-12 in an established infection in combination with a subcurative dose of CQ, the dose of IL-12 was further decreased to 0.2 μg/mouse (0.05 μg per day for 4 days) and treatment was started on day 3 p.i., once parasitemia was established at 0.5 to 1%. Infected mice receiving CQ alone, IL-12 alone, or CQ plus IL-12 combinations were monitored daily for parasitemia and survival.
Hematology and erythropoietic progenitor assays.
Hematocrit, total RBC counts, and percentage of reticulocytes were determined for heparinized blood from individual mice by using standard hematological procedures. Single-cell suspensions were obtained from the bone marrow and spleen, and the number of RBC bursts (BFU-E) and CFU (CFU-E) were determined in colony forming assays by using methylcellulose semisolid medium in Iscove modified Dulbecco minimal essential medium as previously described (19). The number of RBC progenitor colonies were counted after 48 h for CFU-E and after 7 days for BFU-E, and data are presented as mean ± standard error of the mean (SEM) per organ. The number of BFU-E in the peripheral blood was determined by using blood mononuclear cells separated by density gradient centrifugation and is expressed as mean ± SEM per 5 × 106 total cells.
Quantitation of IFN-γ and anti-malaria Abs.
Single-cell suspensions of unfractionated spleen cells were plated at 2 × 106 cells per well and incubated for 48 h with either medium alone, 5 μg of concanavalin A per ml, (ConA) or P. chabaudi AS antigen (mpAg) equivalent to 2 × 106 PRBC. A two-site sandwich enzyme-linked immunosorbent assay (ELISA) was used to measure IFN-γ in the culture supernatants as described previously (26). Malaria-specific, total immunoglobulin G (IgG) in sera obtained 2 weeks after reinfection was determined by ELISA. Total anti-malaria IgG was captured by using soluble mpAg-coated plates, and the level of malaria-specific IgG in the sera was estimated by using goat anti-mouse IgG-horseradish peroxidase conjugate (Bio-Rad Laboratories, Richmond, Calif.) and 2,2′-azinobis(3-ethylbenzthiazolinesulfonate) substrate (Boehringer Mannheim Canada, Laval, Canada). The levels of Ab are expressed as the optical density at 405 nm. Sera from uninfected, normal A/J mice served as negative controls.
RT-PCR for IFN-γ, IL-12Rβ1, and IL-12Rβ2 mRNA expression.
Reverse transcriptase PCR (RT-PCR) was performed essentially as described previously to determine the relative quantities of mRNA for IFN-γ and the two IL-12 receptors, β1 and β2 (11). The primers and probe used for IFN-γ have been described previously (1). For IL-12R subunits, primers, and probes were designed based on the recently cloned cDNA sequences, GenBank accession no. U23922 and U64199, for the β1 and β2 subunits, respectively (3, 23). The sequences of primers and probes used were as follows: IL-12Rβ1, sense primer, 5′-TGA-AGA-CGG-CGC-GTG-GGA-GTC-A-3′; antisense primer, 5′-TCG-CGG-GTA-CAA-CAC-CTC-CGG-G-3′; probe, 5′-GCG-AGC-GGA-CAC-TGC-GAG-CG-3′ (product size, 412 bp); IL-12Rβ2, sense primer, 5′-GGT-TGC-TGG-CTC-CTC-ACC-AGG-3′; antisense primer, 5′-ATG-CAG-CCC-CTT-TGC-TCC-GGG-3′; probe, 5′-TCC-CCC-ACA-CTG-GCT-GCG-GA-3′ (product size, 424 bp). Both positive and negative controls were included in each assay to ensure the efficacy of the reaction and to rule out possible genomic DNA contamination. The primers and probe for glyceraldehyde 6-phosphate dehydrogenase (G6PDH), the housekeeping gene used in this study, have been described previously (11). For RT-PCR, 1 μg of total RNA, isolated from unfractionated spleen cells with TRIzol reagent (Gibco BRL, Grand Island, N.Y.), was reverse transcribed with Moloney murine leukemia virus RT (Gibco). The reaction mixture was diluted 1:8, and 10 μl was used for PCR amplification of IFN-γ (30 cycles), IL-12 receptor subunit β1 and β2 mRNA (30 cycles), and G6PDH (26 cycles) with Taq DNA polymerase (Gibco). Following electrophoresis and Southern transfer onto nylon membranes (Hybond-N; Amersham, Arlington Heights, Ill.), PCR products were hybridized with internal cytokine-specific oligonucleotide probes labeled with [32P]ATP and visualized by autoradiography. The intensity of bands corresponding to specific cytokine-cytokine receptors was analyzed by high-resolution optical densitometry (SciScan; United States Biochemical, Cleveland, Ohio) and normalized to those of G6PDH.
RESULTS AND DISCUSSION
Effect of combined CQ-plus-IL-12 treatment on the course of infection and survival.
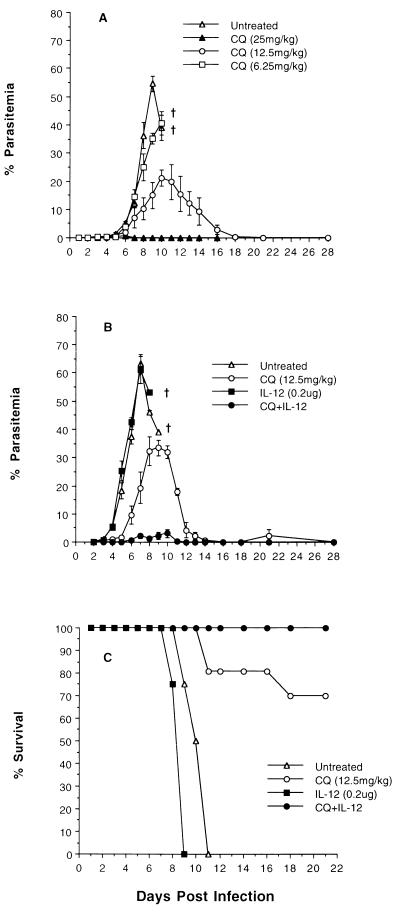
To determine a subcurative dose of CQ suitable for use in combination with IL-12 in later experiments, we first performed a CQ dose-response study in P. chabaudi AS-infected A/J mice. As demonstrated previously by our laboratory, mice of this strain are extremely susceptible to P. chabaudi AS infection and experience fulminant parasitemia and severe anemia with 100% mortality within a few days of peak parasitemia (26). Mice were treated beginning on day 3 p.i. with the curative dose of 25 mg of CQ per kg (body weight) or with two subcurative doses of 12.5 and 6.25 mg/kg. Since the parasite strain used is CQ sensitive, as expected, the curative dose of CQ did not allow the parasitemia to exceed 0.5% and no parasites were detectable from day 7 through day 28 p.i. (Fig. 1A). Untreated mice showed high parasitemia of more than 50%, and all the mice succumbed to infection by day 11 p.i. (Fig. 1C). Similarly, mice given 6.25 mg of CQ per kg of body weight developed mean peak parasitemia in excess of 40% and all the mice in this group succumbed to infection. On the other hand, mice receiving half the curative dose, that is, 12.5 mg/kg, showed a modest reduction in peak parasitemia to around 20% and the mortality rate was about 30%. We selected this latter dose of CQ to be used in combination with IL-12 to explore the possibility of reducing the parasite burden and rescuing all of the infected animals.
FIG. 1.
Dose response to CQ (A) and the effect of CQ plus IL-12 combined therapy (B) on the course of P. chabaudi AS infection (A and B) and survival rate (C) of A/J mice. Mice were infected i.p. with 106 PRBC and were treated with CQ alone (12.5 mg/kg) or IL-12 alone (0.05 μg/day for 4 days) or both, starting on day 3 p.i., after the parasitemia was established at 0.5 to 1.0%. Pooled parasitemia data represent mean ± SEM from 9 to 12 individual mice studied in two (A) or three (B) experiments. The mean percent survival data shown are pooled from 10 to 12 mice in each group. †, 100% mortality.
In the preliminary experiments, we identified the optimum dose of IL-12 suitable for use with half the therapeutic dose of CQ. Initially, 0.05 μg of IL-12 treatment per mouse was given for 6 days, starting on the day of infection (a total of 0.3 μg/mouse). Using this combination therapy, it was possible to reduce the peak parasitemia to about 3% and all the mice survived infection (data not shown), while the mice receiving 0.3 μg of IL-12 alone did not survive infection, suggesting the need for combined treatment for survival. Since our aim was to test the efficacy of the CQ plus IL-12 treatment combination in established infection, we conducted experiments with 0.05 μg of IL-12 per mouse per day starting on day 3 p.i. and continued until day 6 p.i., so as to deliver a total IL-12 dose of 0.2 μg/mouse along with 12.5 mg of CQ per kg. Untreated mice, mice treated with CQ or IL-12 alone, and CQ-plus-IL-12-treated mice had an initial mean parasitemia of 0.6% ± 0.08% (range, 0.3 to 1.6%) on day 3 p.i., before treatment was initiated. As shown in Fig. 1B, following CQ plus IL-12 treatment, parasite proliferation was markedly suppressed and the mean peak parasitemia was only 3.1% and was reached only on day 10 p.i. In contrast, untreated mice had a mean peak parasitemia of 63.5% by day 7 p.i. and all the mice were dead by day 11 p.i. (Fig. 1C). Untreated mice appeared severely anemic and hypothermic and were very lethargic for 3 to 4 days prior to death. Similarly, mice given a total dose of 0.2 μg of IL-12 alone had a mean peak parasitemia of >60% by day 7 p.i., and all the mice succumbed to infection by day 9 p.i. (Fig. 1B and C). Mice receiving 12.5 mg of CQ per kg of body weight alone had a mean peak parasitemia of 33.5% by day 9 p.i. (Fig. 1B), showed a survival rate of 70% (Fig. 1C), and were also lethargic, although these mice did not show signs of severe anemia or hypothermia. Parasites were detectable in these mice most of the time during the first 4 weeks of infection, with a recrudescent parasitemia on day 21 p.i. (Fig. 1B).
We also tested the curative efficacy of combining 6.25 mg of CQ per kg of body weight with or without a total IL-12 dose of 0.2 μg/mouse (days 3 through 6 p.i.). These mice developed severe infection and anemia and succumbed to infection during the second week of infection (data not shown). In contrast to mice receiving lower doses of CQ and IL-12, either alone or in combination, mice on combined therapy with 12.5 mg of CQ per kg of body weight and 0.2 μg of IL-12 per mouse remained healthy and active throughout the course of infection. Parasites were not detectable after day 12 p.i., and all the mice survived infection in repeated experiments (Fig. 1B and C). Thus, this dose of IL-12, in combination with half the curative dose of CQ, is as efficient as using 0.3 μg of IL-12 per mouse and is effective even in established infections. Moreover, this dose also represents the critical curative level required in combined therapy, since lowering the total dose of IL-12 further to 0.15 μg results in higher parasite burden (>30%), even when combined with half the curative dose of CQ (data not shown). Because a total IL-12 dose of 0.2 μg/mouse given along with 12.5 mg of CQ per kg markedly reduces the parasite load and rescues 100% of infected mice, we consider this combination therapy to be optimal for successful treatment of established P. chabaudi AS infection.
Effect of combination therapy on the development of malarial anemia.
Having established the protective efficacy of 12.5 mg of CQ per kg in combination with 0.2 μg of IL-12, we explored the possible mechanism(s) involved. Once infected, P. chabaudi AS-susceptible A/J mice suffer from severe anemia and shock prior to death (33). We previously showed that severe anemia is a main factor contributing to mortality, since blood transfusions given to these mice after the development of peak parasitemia rescued up to 90% of the mice (34). Our recent study examining the erythropoietic role of IL-12 in infected A/J mice showed that 0.1 μg of IL-12 for 6 days, starting on the day of infection, results in more than a sevenfold increase in splenic erythropoiesis (19). In the present study, we examined mice receiving CQ plus IL-12 therapy, using a substantially lower dose of IL-12 in an established infection, for possible inhibition of infection-induced development of anemia. The peripheral blood picture of CQ-plus-IL-12-treated animals was compared with that of untreated mice and mice receiving CQ or IL-12 alone. As shown in Table 1, mice receiving combined therapy had a significantly higher hematocrit and total RBC count on day 7 p.i. compared to untreated or mice treated with 12.5 mg of CQ per kg or 0.2 μg of IL-12 alone. Signs of severe anemia were apparent in untreated or IL-12-treated mice, while mice treated with 12.5 mg of CQ per kg retained hematocrit and RBC counts at significantly higher levels than did untreated mice. However, mice in the combined treatment group alone had significantly higher levels of both hematocrit and RBC counts after 7 days of infection compared to controls and showed no signs of anemia.
TABLE 1.
Effect of CQ and IL-12 treatment on hematological parameters in A/J mice with established P. chabaudi AS infectiona
| Treatment | Hematocrit (%) | RBC count (106/mm3) | % Reticulocytes on:
|
|
|---|---|---|---|---|
| Day 7 p.i. | Day 10 p.i. | |||
| None | 25.0 ± 2.2 | 5.5 ± 0.3 | 5.0 ± 1.0 | |
| CQ (12.5 mg/kg) | 44.0 ± 1.6a | 7.3 ± 0.3a | 9.0 ± 0.4a | 11.3 ± 1.7 |
| IL-12 (0.2 μg) | 29.0 ± 1.1 | 5.6 ± 0.3 | 6.0 ± 0.5 | |
| CQ plus IL-12 | 52.4 ± 0.9a,b,c | 8.6 ± 0.2a,b,c | 11.0 ± 1.0a,c | 25.3 ± 2.5b |
Mice were infected 7 days previously and were either untreated or treated with CQ (12.5 mg/kg), IL-12 (0.05 μg/day/mouse for 4 days), or both CQ and IL-12, starting on day 3 p.i. Heparinized peripheral blood was analyzed by standard hematological procedures. Data shown are mean ± SEM for six to eight individual mice. Significant differences shown by the unpaired-t-test are as follows: a, untreated versus treated mice; b, CQ-treated versus CQ-plus-IL-12-treated mice; c, IL-12 treated versus CQ-plus-IL-12-treated mice.
It is possible that since the mice receiving optimum combined therapy had >15-fold lower parasitemia, the absence of anemia was due to decreased direct RBC destruction by the developing parasites. On the other hand, it is also possible that the extent of upregulation of erythropoiesis following infection could have been of several magnitudes higher in the mice receiving combined therapy. To address this question, we first examined the percentage of reticulocytes in the peripheral blood. On day 7 p.i., mice treated with 12.5 mg of CQ per kg, with or without IL-12 treatment, showed significantly higher percentages of reticulocytes than did untreated mice (Table 1). However, mice treated with IL-12 alone failed to show a significantly higher percentage of reticulocytes compared to untreated mice. Since reticulocytosis is apparent during weeks 2 and 3 of infection in P. chabaudi AS-resistant C57BL/6 mice (33), we also examined blood films from surviving mice on day 10 p.i. At this time, A/J mice receiving CQ plus IL-12 treatment had more than a twofold increase in the percentage of reticulocytes compared to mice receiving 12.5 mg of CQ per kg alone.
Next, we examined the effect of CQ and IL-12 treatment on erythropoiesis by determining the number of erythroid progenitors in the bone marrow, spleen, and blood. Treatment of infected A/J mice with 0.05 μg of IL-12 per mouse for 4 days, starting on day 3 p.i., along with half the curative dose of CQ significantly enhanced bone marrow and splenic erythropoiesis compared to no treatment or treatment with CQ or IL-12 alone (Table 2). A marked increase was seen in the extent of splenic erythropoiesis in terms of both BFU-E and CFU-E. Mice receiving the combined treatment had a nearly twofold increase in the numbers of splenic BFU-E and more than a threefold increase in the numbers of splenic CFU-E compared to untreated animals or mice treated with CQ alone. Mice treated with 12.5 mg of CQ per kg alone showed a small but significant increase only in the bone marrow CFU-E compartment. However, mice receiving IL-12 monotherapy had significantly enhanced CFU-E numbers in bone marrow and spleen and higher BFU-E in the spleen compared to untreated controls.
TABLE 2.
CQ-plus-IL-12 combination therapy markedly upregulates erythropoiesis in A/J mice with established P. chabaudi AS infectiona
| Treatment | No. of cellsb in:
|
||||
|---|---|---|---|---|---|
| Bone marrow
|
Spleen
|
Peripheral blood
|
|||
| BFU-E (103) | CFU-E (103) | BFU-E (103) | CFU-E (104) | BFU-E/5 × 106 WBC | |
| None | 1.1 ± 0.2 | 3.2 ± 0.2 | 9.8 ± 0.6 | 5.6 ± 0.4 | 39 ± 6 |
| CQ (12.5 mg/kg) | 1.3 ± 0.1 | 5.3 ± 0.4a | 8.3 ± 0.6 | 5.8 ± 0.2 | 57 ± 6 |
| IL-12 (0.2 μg) | 1.2 ± 0.1 | 5.0 ± 0.6a | 11.4 ± 0.7a | 10.1 ± 1.1a | 60 ± 4a |
| CQ plus IL-12 | 1.8 ± 0.1a,b,c | 7.9 ± 0.2a,b,c | 16.3 ± 0.8a,b,c | 19.0 ± 1.3a,b,c | 111 ± 7a,b,c |
Mice were infected 7 days previously and were either untreated or treated with CQ (12.5 mg/kg), IL-12 (0.05 μg/day/mouse for 4 days), or both CQ and IL-12, starting on day 3 p.i. Single-cell suspensions from femur, spleen, and peripheral blood were cultured in methycellulose semisolid medium for either 7 days (BFU-E) or 48 h (CFU-E).
Progenitor cell numbers are expressed as number of BFU or CFU per organ. Data shown are mean ± SEM for six to eight individual mice. Significant differences shown by unpaired t test are as follows: a, untreated versus treated mice; b, CQ-treated versus CQ-plus-IL-12-treated mice; c, IL-12-treated versus CQ-plus-IL-12-treated mice.
Our earlier study showed that IL-12 treatment of normal, but not infected, A/J mice results in bone marrow suppression (19), possibly due to the ability of this cytokine to enhance the mobilization of bone marrow precursors to the spleen (9). These studies suggest a role for IL-12 in enhancing extramedullary erythropoiesis, especially in the spleen. To examine the mobilization of bone marrow erythroid precursors to the spleen following low-dose CQ plus IL-12 treatment, we investigated the frequency of BFU-E in the peripheral blood. We observed a nearly threefold increase in the blood BFU-E numbers in mice treated with CQ plus IL-12 compared to untreated mice and nearly a twofold increase compared to mice receiving either CQ or IL-12 alone (Table 2).
It is interesting that even though the dose of IL-12 used was similar in mice receiving either 12.5 or 6.25 mg of CQ per kg, the extent of erythropoiesis was higher in the former group of mice, which had a lower parasite load. This indirectly suggests that high parasitemia may inhibit efficient erythropoietic upregulation, possibly due to impaired levels of other hematopoietic cofactors such as IL-3, IL-6, IL-11, and steel factor in mice with high parasite burdens. Thus, the use of IL-12 treatment along with half the curative dose of CQ not only suppresses parasitemia and limits the extent of direct RBC destruction by the parasites but also initiates a more efficient erythropoietic response. The erythropoietic stimulatory effect of IL-12 is not restricted to the combination of CQ plus IL-12, since we observed similarly enhanced RBC genesis when we used IL-12 in combination with clindamycin to treat infected A/J mice (unpublished observations). This finding raises the possibility of using IL-12 in combination with other antimalarial drugs to prevent fatal anemia. In human situations, this is particularly important in areas with widespread CQ resistance, where one has to rely on other antimalaria drugs for the treatment of acute infections. However, this strategy should be tested in infections due to drug-resistant parasite strains, because such strains are usually resistant to higher-than-therapeutic doses of the drugs.
Development of a novel therapeutic strategy to prevent malarial anemia is critical, considering the importance of anemia in malaria morbidity and mortality. Studies of Gambian children with severe P. falciparum malaria and presenting with severe anemia showed marked dyserythropoietic changes, including erythroblast multinuclearity, karyorrhexis, incomplete and unequal mitosis, and cytoplasmic bridging (30). While blood transfusion has been proven to be beneficial in correcting malarial anemia (12), the risks involved are considerable, especially because of the high levels of human immunodeficiency virus infection in many malaria-endemic populations. This calls for a method of treatment which can correct hyperparasitemia as well as infection-induced anemia without the need for transfusion. The combination therapy used in this study appears to be promising in this direction. Endogenous upregulation of erythropoiesis enhances reticulocyte numbers in the peripheral blood and prevents the risk of prolonged parasite patency observed following transfusion (34), since reticulocytes are refractory to infection by most normophilic malaria parasites.
Combination therapy significantly upregulates parasite-specific IFN-γ production and IL-12R expression.
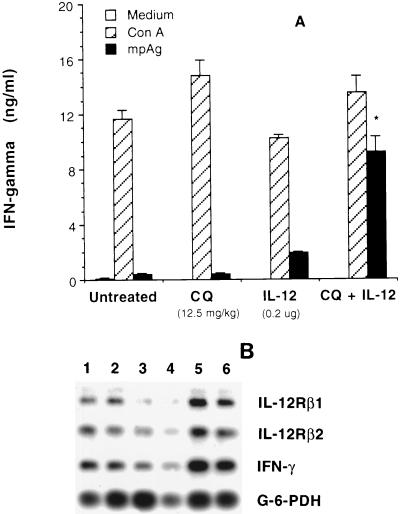
We next examined the level of IFN-γ production by splenocytes, in terms of both protein and gene expression, following combination therapy of infected A/J mice. We have previously demonstrated the essential role of IFN-γ in the protective host response against P. chabaudi AS in resistant C57BL/6 mice (10, 18, 25) as well as in IL-12-treated, susceptible A/J mice (18, 26). Anti-IFN-γ treatment of IL-12-treated A/J mice resulted in a significant increase in parasite load and high mortality (26). Our recent study also suggested that a defect in the production of IFN-γ by NK cells early during infection contributes to the extreme susceptibility of A/J mice to blood-stage malaria (18). This finding is consistent with an earlier observation in resistant C57BL/6 mice that tissue-specific expression of IFN-γ mRNA in the spleen is significantly higher by day 3 p.i. compared to that in A/J mice, and higher expression of this cytokine mRNA persists for 1 week p.i. (10). Furthermore, both in vivo IL-12 treatment of A/J mice and in vitro IL-12 supplementation of enriched NK cell cultures from infected A/J mice significantly enhance the levels of spontaneous NK cell-derived IFN-γ production (18). To achieve a protective response in A/J mice, our previous study used 0.1 μg of IL-12/mouse/day for 6 days starting on the day of infection, for a total dose of 0.6 μg of IL-12 (26), while the present study used 0.05 μg of IL-12 daily for only 4 days starting on day 3 p.i., for a total dose of 0.2 μg of IL-12, in combination with CQ, once the parasitemia was established between 0.5 and 1.0%. Hence, we asked whether inclusion of low, subprotective doses of IL-12 in combined treatment influences the immune response in A/J mice by inducing a protective Th1 response. In response to nonspecific stimulation with ConA, splenocytes from untreated mice, mice treated with CQ or IL-12 alone, or those receiving CQ plus IL-12 combined treatment secreted comparable amounts of IFN-γ on day 7 p.i. (Fig. 2A). In contrast, spleen cells from mice treated with both CQ and IL-12 secreted significantly higher levels of IFN-γ in response to specific stimulation with mpAg than did those from untreated mice or mice treated with CQ or IL-12 alone. Following mpAg stimulation, splenocytes from mice receiving IL-12 alone produced more than a fourfold-higher concentration of IFN-γ than did those from untreated or CQ-treated mice. The magnitude of upregulation of IFN-γ in mice treated with CQ plus IL-12 was more than 20-fold higher than in untreated or CQ-treated groups of mice and was nearly 5-fold higher than in mice given IL-12 alone. In parallel, on day 7 p.i., IFN-γ mRNA expression was also upregulated in mice receiving combined treatment (Fig. 2B). This suggests the development of efficient antiparasitic immunity in mice given combination therapy during the early phase of infection by an enhanced IFN-γ production. Further, this finding has an important bearing on the development of the adaptive immune response, since it has been shown that during murine Leishmania major infection, IFN-γ, along with IL-12, acts as an essential cofactor in the development of parasite-specific, Th1 type protective immunity (7).
FIG. 2.
Effect of CQ plus IL-12 combined therapy on the levels of IFN-γ secretion (A) and the expression of mRNA for IFN-γ, IL-12Rβ1, and IL-12Rβ2 (B) by splenocytes obtained from P. chabaudi AS-infected A/J mice on day 7 p.i. IFN-γ secretion by unfractionated spleen cells was measured in the culture supernatants by ELISA after 48 h of stimulation with ConA or mpAg as indicated. Data represent mean ± SEM from four individual mice from two experiments. Levels of IFN-γ and IL-12R mRNA expression were measured by RT-PCR, and the results shown are from two representative mice in each group. Lanes 1 and 2, untreated mice; lanes 3 and 4, mice given 12.5 mg of CQ per kg alone; lanes 5 and 6, mice given CQ+IL-12 combined therapy. ∗, P < 0.001 by Student’s unpaired t test.
Responsiveness to IL-12 and subsequent downstream signal transduction events, IFN-γ production, and Th-cell development has recently been shown to be dependent on the levels of IL-12Rβ1 and IL-12Rβ2 expression on the responder cells (6, 32). Coexpression of these two IL-12R subunits results in the expression of both high- and low-affinity IL-12 binding sites and confers IL-12 responsiveness in transfected cells (23). Hence, we questioned whether enhanced IFN-γ production and development of a protective Th1 immune response in mice receiving combined therapy correlate with enhanced IL-12R expression. For this, we analyzed the expression of β1 and β2 IL-12R subunit mRNA expression in splenocytes isolated on day 7 p.i. from mice given CQ plus IL-12 combined treatment. Compared to untreated mice, CQ-plus-IL-12-treated mice had a higher expression of both IL-12Rβ1 and β2 (Fig. 2B), indicating a role for IL-12 in upregulating its own receptors on responder cells during P. chabaudi AS infection. Higher levels of IL-12R expression on responder cells may explain why spleen cells from mice receiving combined treatment produced significantly higher levels of IFN-γ than did cells from control mice following specific Ag stimulation. We recently observed that on day 7 p.i., spleens from IL-12-treated, infected A/J mice contain more than fourfold greater absolute numbers of asialo-GM1+ NK cells and more than twofold greater numbers of B cells than do spleens from uninfected mice (18). While NK cells expressing increased IL-12R could contribute to higher levels of protective IFN-γ secreted during the early course of infection, IL-12 may also influence B-cell production of parasite-specific Ab. To test this latter possibility, we quantitated anti-malaria Ab levels as well as the development of immunity against reinfection in the surviving A/J mice in CQ-treated and CQ-plus-IL-12-treated groups.
CQ plus IL-12 treatment results in higher anti-malaria Ab levels and protects against reinfection.
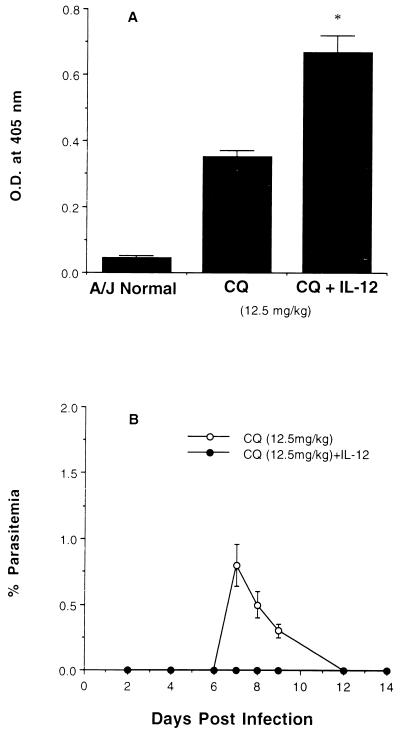
Mice surviving primary infection were reinfected after 4 weeks of parasite clearance. The course of infection was monitored, and sera were collected 14 days after reinfection. Anti-malaria Ab in terms of total IgG in serum was significantly higher in mice receiving combined therapy than in those treated with CQ alone (Fig. 3A). High anti-malaria Ab levels correlated with protection, since sterile immunity was found after reinfection of mice treated with CQ plus IL-12 during primary infection whereas low-grade parasitemia was observed in mice given CQ alone (Fig. 3B).
FIG. 3.
Effect of CQ plus IL-12 combined treatment on the levels of anti-malaria Ab (total IgG) production (A) and the course of P. chabaudi AS reinfection (B) in A/J mice. Mice were reinfected with 106 PRBC 4 weeks after recovery from primary infection, and the total anti-malaria IgG level was measured in 1:256-diluted sera by ELISA 2 weeks after reinfection. (A) Mean ± SEM data for optical densities (O.D.) representing Ab levels from six individual mice; and (B) mean ± SEM percent parasitemia from six individual mice. ∗, P < 0.001, by Student’s unpaired t test.
Our present understanding of immunity against blood-stage P. chabaudi AS infection in resistant mice is that control of primary infection is dependent on an early Th1 response involving NK-cell and macrophage activation, followed by a Th2 response involving protective Ab during the chronic phase (10, 13, 18, 25, 26), which eventually clears parasites from the circulation. The present study revealed that IL-12 used in the combined therapy of A/J mice, in addition to providing protection against acute infection, induces better Ab responses during reinfection. Although both cell- and Ab-mediated immune mechanisms are believed to be important in protection against malaria, the results of the present study do not distinguish between the relative importance of the two arms of the immune system, since we had to evaluate IFN-γ and antibody production at different phases of infection. In practice, unlike in murine malaria, “semi-immune” or nonimmune humans are vulnerable to reinfection after successful treatment of primary infection with antimalaria drugs. By using CQ plus IL-12 combination treatment, it may be possible not only to reduce the primary parasite load and the associated complications but also to induce a protective Ab response and immunity to reinfection. Our findings raise the question of the role of IL-12 and IFN-γ in influencing Ab responses during human malaria infections where long-lasting immunity in populations in areas of endemic infection is associated primarily with anti-malaria Ab levels (16).
In areas with endemic malaria infection, the use of antimalarial drugs has an inhibitory effect on the acquisition of anti-malaria Ab, both in children and, more particularly, in adults on chemoprophylaxis (16). It is not clear yet whether this is due to direct immunosuppressive effects of drugs like CQ or to decreased exposure to malaria parasite antigens. In the present study, we observed lower levels of expression of both IFN-γ and IL-12R mRNA in splenocytes of mice treated with CQ alone compared to untreated mice. These mice had lower anti-malaria Ab levels than did CQ-plus-IL-12-treated mice, although it was not possible to compare the Ab levels in mice treated with CQ alone with those in untreated mice, which did not survive till our reinfection studies. However, the issue of possible immunosuppression by CQ could be resolved by studying the effect of CQ treatment in P. chabaudi AS-resistant C57BL/6 mice. In any event, as shown by the present study, any immunosuppressive effects of CQ can be effectively overcome by using CQ plus IL-12 combined treatment, which resulted in marked upregulation of IFN-γ and IL-12R mRNA levels as well as that of anti-malaria Ab levels.
IL-12, in combination with amphotericin B, has been used as immunotherapy in Histoplasma capsulatum-infected SCID mice (35) and, more recently, in combination with antibiotics for bacterial clearance in Mycobacterium avium-infected SCID mice (4). Furthermore, successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with the combination of sodium stibogluconate and IFN-γ was found to be dependent on continued production of IL-12 (14). Cure of established Leishmania major infection in mice following combined therapy with Pentostam and IL-12 involves a switch from a Th2- to a Th1-type immune response (21). However, in this study, IL-12 alone appeared unable to enhance Th1 cell expansion in vivo, which was thought to be due to high parasite loads in mice receiving only IL-12 compared to mice given combined treatment with the drug and IL-12. Similarly, A/J mice could be rescued from lethal malaria by using IL-12 when the treatment was given on the day of infection (26) but not after the infection was established (unpublished observations). In contrast, as observed in the present study, IL-12-induced development of an early Th1 response appears possible even in established infections when CQ is given along with IL-12 to substantially reduce the parasite load. Moreover, in this treatment regimen, doses of both CQ and IL-12 could be reduced to one-half and one-third, respectively, compared to the doses required to induce parasite suppression when either of these is used alone. In particular, cure of established infections with this combined treatment and the feasibility of using IL-12 at low, possibly nontoxic, doses suggest the usefulness of IL-12 in combination with antimalarial drugs in treating human malaria.
ACKNOWLEDGMENTS
This work received grant support from the National Institutes of Health (AI35955) and the Medical Research Council of Canada (MT12638 and MT14663). H. Sam is a recipient of an M.D./Ph.D. studentship from Medical Research Council of Canada.
REFERENCES
- 1.Allen R D, Stanley T A, Sidman C L. Differential cytokine expression in acute and chronic murine graft-versus-host disease. Eur J Immunol. 1993;23:333–339. doi: 10.1002/eji.1830230205. [DOI] [PubMed] [Google Scholar]
- 2.Butler D. Time to put malaria control on the global agenda. Nature. 1997;386:535–541. doi: 10.1038/386535a0. [DOI] [PubMed] [Google Scholar]
- 3.Chua A O, Willinson V L, Presky D H, Gubler U. Cloning and characterization of a mouse IL-12 receptor-beta component. J Immunol. 1995;155:4286–4294. [PubMed] [Google Scholar]
- 4.Doherty T M, Sher A. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J Immunol. 1998;160:5428–5435. [PubMed] [Google Scholar]
- 5.Facer C A, Tanner M. Clinical trials of malaria vaccines: progress and prospects. Adv Parasitol. 1997;39:1–68. doi: 10.1016/s0065-308x(08)60044-5. [DOI] [PubMed] [Google Scholar]
- 6.Guler M L, Jacobson N G, Gubler U, Murphy K M. T cell genetic background determines maintenance of IL-12 signaling: effect on BALB/c and B10.D2 T helper cell type 1 phenotype development. J Immunol. 1997;159:1767–1774. [PubMed] [Google Scholar]
- 7.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman S L, Crutcher J M, Puri S K, Ansari A A, Villinger F, Franks E D, Singh P P, Finkelman F, Gately M K, Dutta G P, Sedegah M. Sterile protection of monkeys against malaria after administration of interleukin-12. Nat Med. 1997;3:80–83. doi: 10.1038/nm0197-80. [DOI] [PubMed] [Google Scholar]
- 9.Jackson J D, Yan Y, Brunda M J, Kelsey L S, Talmadge J E. Interleukin-12 enhances peripheral hematopoiesis in vivo. Blood. 1995;85:2371–2376. [PubMed] [Google Scholar]
- 10.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kichian K, Nestel F P, Kim D, Ponka P, Lapp W S. IL-12 p40 messenger RNA expression in target organs during acute graft-versus-host disease. J Immunol. 1996;157:2851–2856. [PubMed] [Google Scholar]
- 12.Lackritz E M, Campbell C C, Ruebush T K, Hightower A W, Wakube W, Steketee R W, Were J B O. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet. 1989;340:524–528. doi: 10.1016/0140-6736(92)91719-o. [DOI] [PubMed] [Google Scholar]
- 13.Langhorne J. The role of CD4+ T cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989;5:362–365. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Sutterwala S, Farrell J P. Successful therapy of chronic, nonhealing murine cutaneous leishmaniasis with sodium stibogluconate and gamma interferon depends on continued interleukin-12 production. Infect Immun. 1997;65:3225–3230. doi: 10.1128/iai.65.8.3225-3230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locksley R M. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci USA. 1993;90:5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGregor I A, Wilson R J M. Specific immunity: acquired in man. In: Wernsdorfer W H, McGregor I A, editors. Malaria: principles and practice of malariology. Edinburgh, United Kingdom: Churchill Livingstone, Ltd.; 1988. p. 559. [Google Scholar]
- 17.Metzger D W, McNutt R M, Collins J T, Buchanan J M, Van Cleave V H, Dunnick W A. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-γ-dependent and -independent mechanisms. Eur J Immunol. 1997;27:1958–1965. doi: 10.1002/eji.1830270820. [DOI] [PubMed] [Google Scholar]
- 18.Mohan K, Moulin P, Stevenson M M. NK cell cytokine production not cytotoxicity contribute to resistance against blood-stage Plasmodium chabaudi AS infection. J Immunol. 1997;159:4990–4998. [PubMed] [Google Scholar]
- 19.Mohan K, Stevenson M M. Interleukin 12 corrects severe anemia during blood-stage Plasmodium chabaudi AS in susceptible A/J mice. Exp Hematol. 1998;26:45–52. [PubMed] [Google Scholar]
- 20.Murphy G S, Basri H, Purnomo, Andersen E M, Bangs M J, Mount D L, Gorden J, Lal A A, Purwokusumo A R, Harjosuwarno S, Sorensen K, Hoffman S L. Vivax malaria resistant to treatment and prophylaxis with chloroquine. Lancet. 1993;341:96–100. doi: 10.1016/0140-6736(93)92568-e. [DOI] [PubMed] [Google Scholar]
- 21.Nabors G S, Afonso L C C, Farrell J P, Scott P. Switch from a type 2 to type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin 12 and Pentostam. Proc Natl Acad Sci USA. 1995;92:3142–3146. doi: 10.1073/pnas.92.8.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orange J A, Salazar-Mather T P, Opal S M, Spencer R L, Miller A H, McEwen B S, Biron C A. Mechanisms of interleukin 12-mediated toxicities during experimental viral infections: Role of tumor necrosis factor and glucocorticoides. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Presky D H, Yang H, Minetti L J, Chua A O, Nabavi N, Wu C Y, Gately M K, Gubler U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedegah M, Finkelman F, Hoffman S L. Interleukin 12 induction of interferon γ-dependent protection against malaria. Proc Natl Acad Sci USA. 1994;91:10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson M M, Tam M F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 27.Trinchieri G, Scott P. The role of interleukin 12 in the immune response, disease and therapy. Immunol Today. 1994;15:460–463. doi: 10.1016/0167-5699(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 28.White N J. Antimalarial drug resistance: the pace quickens. J Antimicrob Chemother. 1992;30:571–585. doi: 10.1093/jac/30.5.571. [DOI] [PubMed] [Google Scholar]
- 29.White N J. Treatment of malaria. N Engl J Med. 1996;335:801–806. doi: 10.1056/NEJM199609123351107. [DOI] [PubMed] [Google Scholar]
- 30.Wickramasinghe S N, Abdalla S, Weatherall D J. Cell cycle distribution of erythroblasts in P. falciparum malaria. Scand J Hematol. 1982;29:83–87. doi: 10.1111/j.1600-0609.1982.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. Practical chemotherapy of malaria. W H O Tech Rep Ser. 1990;805:1–124. [PubMed] [Google Scholar]
- 32.Wu C Y, Ferrante J, Gately M K, Magram J. Characterization of IL-12 receptor β1 chain (IL-12Rβ1)-deficient mice: IL-12Rβ1 is an essential component of the functional mouse IL-12 receptor. J Immunol. 1997;159:1658–1665. [PubMed] [Google Scholar]
- 33.Yap G S, Stevenson M M. Plasmodium chabaudi AS: erythropoietic responses in resistant and susceptible mice. Exp Parasitol. 1992;75:340–352. doi: 10.1016/0014-4894(92)90219-z. [DOI] [PubMed] [Google Scholar]
- 34.Yap G S, Stevenson M M. Blood transfusion alters the course and outcome of Plasmodium chabaudi AS infection in mice. Infect Immun. 1994;62:3761–3765. doi: 10.1128/iai.62.9.3761-3765.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou P, Sieve M C, Tewari R P, Seder R A. Interleukin-12 modulates the protective immune response in SCID mice infected with Histoplasma capsulatum. Infect Immun. 1997;65:936–942. doi: 10.1128/iai.65.3.936-942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]