Abstract
Tenofovir disoproxil fumarate, lamivudine, and dolutegravir (TLD) as a safe and more effective single daily dose regimen is rolling out in Africa for people living with HIV. Although access to viral load (VL) testing is improving, patients may still be transitioned to TLD with virological failure and potential drug resistance. We reviewed annual VL test results of 390 children and adolescents who had enrolled in a community-based antiretroviral therapy program in rural Zimbabwe between 2018 and 2019. VL testing was done by the near point of care simplified amplification-based assays (Diagnostics for the Real World, Sunnyvale, CA, USA) at Chidamoyo Christian Hospital and rate of virological suppression (VS) on TLD (VL <1,000 copies/mL) was assessed. Overall, 184 children and adolescents on TLD were enrolled in this study. The median [interquartile range (IQR)] age was 15 (11–19) years, above half of the participants were female (57%). Before switching to TLD, rate of VS was 76% (139/184). After a median (IQR) duration of 6.9 (5.5–9.1) months on TLD, VS was observed in 95% (174/184) of the participants. Of the 10 participants with VL ≥1,000 copies/mL on TLD, 90% (9/10) were failing on their previous regimens, 6 of 9 (67%) having been on boosted protease inhibitor-based regimens. A high rate (95%) of VS was observed among children and adolescents on TLD in rural Zimbabwe. TLD may address the problems of virological failure and emergence of resistance in Africa. However, longer follow-up might be needed to ascertain sustained VS in this vulnerable population. Randomized Control Trial NCT03986099
Keywords: dolutegravir, viral suppression, rural Zimbabwe, children and adolescents, community-based antiretroviral therapy program
Introduction
The high levels of pretreatment and acquired HIV drug resistance (HIVDR) to non-nucleoside reverse transcriptase inhibitor (NNRTI) prompted the World Health Organization (WHO) to recommend dolutegravir (DTG)-based antiretroviral therapy (ART) as the preferred first-line and second-line treatment for all populations, including pregnant women and those of childbearing potential.1,2 DTG was found superior to ritonavir-boosted lopinavir and to raltegravir in the DAWNING and SAILING trials, respectively.3,4
The fixed dose combination of tenofovir disoproxil fumarate (TDF), lamivudine (3TC), and DTG (TLD) has become available in several low- and middle-income countries (LMICs). DTG is expected to address the concerning problems of virological failure (VF) and HIVDR in these regions because of its high genetic barrier to resistance, high potency, and good tolerability, alongside with its improved safety profile and low cost.5
Many LMICs reported to be transitioning to DTG-based regimens. Kenya, Nigeria, Botswana, and Uganda were among the first African countries to start pilot projects on procurement and rollout of DTG.6 A recent study by Nabitaka et al carried out in Uganda reported a very high level of acceptability of DTG-based regimens across both ART-naïve and experienced patients, with a viral suppression rate of 94%.7 The Zimbabwe National ART guidelines were revised to include DTG in the preferred first-line ART as of July 2019.8
DTG coverage in Zimbabwe varies by geographic area due to supply chain challenges. As of March 2021, the transition to TLD was at 78% in the country.9 This transition has been occurring in both rural and urban Zimbabwe. However, experience with DTG in Zimbabwe has been limited so far. There are no local data on virological suppression (VS) and emergence of HIVDR on DTG-based regimens. Therefore, we followed children, adolescents, and young adults enrolled in a community-based antiretroviral therapy (CBART) program in rural Zimbabwe.
Materials and Methods
Study design, setting, and population
This was a cohort study of children, adolescents, and young adults (CALWH) who enrolled between 2018 and 2019 in a randomized control trial, CBART at Chidamoyo Christian Hospital (CCH), rural Zimbabwe. The hospital is located in Hurungwe district, Mashonaland West, Zimbabwe, and provides ART to 2,206 patients with 95% (2,160/2,206) already on DTG-based regimens. The hospital also serves as a dispersed rural community, and the existing differentiated service delivery model bimonthly provides services (adherence counseling and ART refilling) to community care groups.10
The randomized control trial (CBART study)
The CBART study was a prospective randomized open label trial of two strategies for viral load (VL) differentiated care monitoring of virological outcome among CALWH receiving ART at eight treatment rural outreach sites near their homes provided by the CCH between February 2018 and July 2019. Participants were blinded and assigned (1:1) to either point of care or standard of care (SOC) VL testing. All participants had their VL done at enrollment, 6 and 12 months, respectively, consistently with the National ART SOC treatment guidelines.11
Similarly, VF (VL ≥1,000 copies/mL) was also managed as per the guidelines: Those with VL ≥1,000 copies/mL received enhanced adherence counseling for 3 months and a repeat VL testing at the end of those 3 months. ART was switched if repeat VL was still ≥1,000 copies/mL. For those on a protease inhibitor (PI)-based regimen, a genotypic resistance testing was done before ART switch.
Procedure for this study
We reviewed annual VL testing of 390 CALWH who enrolled in the CBART study. Review of clinic records in July 2020 identified 184 of 390 CALWH who had switched from either an NNRTI-based regimen or a PI-based regimen to TLD. VL test was carried out in real time at the hospital in contrast to genotyping resistance testing that was performed later. A confirmatory VL test was done before genotyping. Demographic (age and gender) and ART history data including prior treatment regimens before switching to TLD, and ART duration on TLD were extracted from the medical records.
Laboratory assessments
VL was measured by the near point of care simplified amplification-based assays, the SAMBA II semi-Q (Diagnostics for the Real World), with a VL detection limit of 1,000 copies/mL at CCH. Before genotyping, confirmatory VL testing was done at the Infectious Diseases Research Laboratory, University of Zimbabwe, using the Cobas/Ampliprep v2.0 (Roche, Indianapolis, IN, USA), with a VL detection limit of 20 copies/mL. Genotyping was only performed after confirmed VL failure (VL ≥1,000 copies/mL).
Viral HIV-1 RNA was isolated from plasma using a column-based extraction kit, the PureLink™ Mini Viral RNA/DNA Mini Kit (ThermoFisher Scientific, Carlsbad, CA, USA), in accordance with the manufacturer's instructions. Genotypic resistance testing was limited to the protease and reverse transcriptase regions of the HIV pol gene. Plasma samples were sequenced by Sanger sequencing at the Biomedical Research and Training Institute in Harare, Zimbabwe.
Statistical analysis
Descriptive statistics were used to summarize the baseline demographic and clinical characteristics. Rate of VS on TLD (VL <1,000 copies/mL) was determined. Fisher's exact test was used to determine any association between previous ART regimens and VF on TLD. All statistical analyses were done on Stata 14.
Ethics approval
This study was approved by the institutional review board of the Biomedical Research and Training Institute (AP143/2018) and the Medical Research Council of Zimbabwe (MRCZ/A/2269).
Results
Participant characteristics
A total of 184 children and adolescents on TLD were enrolled in this study. The median (IQR) age was 15 (11–19) years and above half of the participants were female (57%). Before switching to TLD, 62.5% (115/184) of the participants were receiving NNRTI-based first-line ART, of which the majority 83% (96/115) were on TDF/3TC/EFV and 17% (19/115) were on abacavir (ABC)/3TC + either efavirenz (18) or nevirapine (1) and 37% (68/184) were receiving a ritonavir boosted PI-containing regimen, of which the majority 81% (56/69) were on ATV/r/3TC + either ABC (53) or TDF (2) or zidovudine (1) and 17% (12/69) received LPV/r/3TC + either ABC (11) or TDF (1). One participant was already on third-line TDF/3TC/darunavir/DTG (Table 1).
Table 1.
Characteristics of the 184 Participants
| Characteristics | All participants (N = 184) |
|---|---|
| Age in years, median (IQR) | 15 (11–19) |
| Gender | |
| Female, n (%) | 104 (57) |
| Regimens before switch to TLD, n (%) | |
| NNRTI-based regimens | 115 (62.5) |
| PI-based regimens | 68 (37) |
| InSTi-based regimens | 1 (0.5) |
| VL before switch to TLD, n (%) | |
| VL <1,000 copies/mL | 139 (76) |
| VL ≥1,000 copies/mL | 45 (24) |
| VL after switch to TLD, n (%) | |
| VL <1,000 copies/mL | 174 (95) |
| VL ≥1,000 copies/mL | 10 (5) |
| Duration on TLD in months, median (IQR) | 6.9 (5.5–9.1) |
InSTi, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, non-nucleotide reverse transcriptase inhibitor; PI, protease inhibitor; TLD, tenofovir disoproxil fumarate/lamivudine/dolutegravir; VL, viral load.
VL suppression
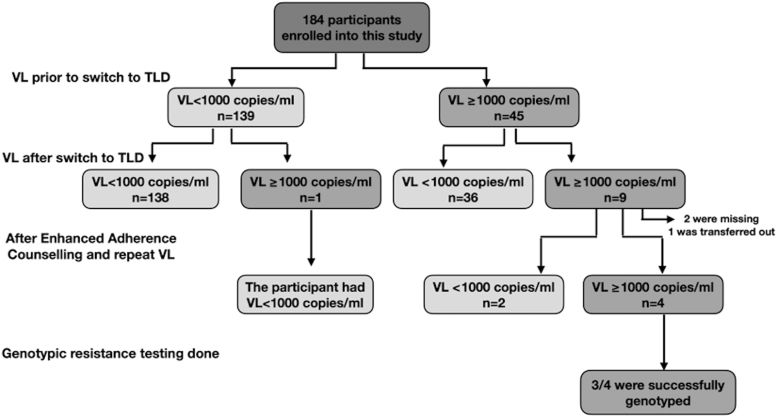
Before switching to TLD, the rate of VS was 76% (139/184) and the remaining 45 (24%) participants had VF. Of these 45 participants, the majority 80% (36/45) were suppressed on TLD after a median duration of 6.9 (5.5–9.1) months (Fig. 1).
FIG. 1.
Assessment of VL measures before switch and after switch to a DTG-based regimen among the 184 participants. DTG, dolutegravir; TDF, tenofovir disoproxil fumarate; TLD, TDF/lamivudine/dolutegravir; VL, viral load.
Overall, VS was observed in 95% (174/184) of the participants after a median (IQR) duration of 6.9 (5.5–9.1) months on TLD. Of the 10 participants with VL ≥1,000 copies/mL, 90% (9/10) were failing on their previous regimens. Participants with PI as prior ART regimens were more likely to fail on TLD compared with those with first-line NNRTI (10.1% vs. 2.6%, p = .042) despite the small sample size.
Genotyping resistance testing
A confirmatory VL test was done 3 months after an elevated VL reading before genotyping. Of the 10 participants failing, 7 (70%) had blood samples collected, 2 (20%) did not present for sample collection, and 1 (10%) was transferred out of the CCH. Among the seven with blood samples available, three resuppressed and the remaining four had confirmed VF (VL ≥1,000 copies/mL) with VL ranging from 1,047 to 111,527 copies/mL (Fig. 1).
Genotypic resistance testing of the reverse transcriptase and protease regions was attempted on the four samples with VL ≥1,000 copies/mL and three were successfully genotyped. Unsurprisingly, all three participants had no major resistance to their reverse transcriptase inhibitor drugs. In addition, these three participants were on a PI as prior regimens (ABC/3TC/ATV/r, TDF/3TC/ATV/r, and Zidovudine/3TC/ATV/r).
Discussion
Optimization of ART is a critical component to support country efforts to achieve the WHO treatment targets and eradication of HIV/AIDS by 2030. The fixed dose combination TLD has been adopted in Zimbabwe as part of its preferred first-line HIV treatment regimen. Here we evaluated the rate of virological suppression and emergence of drug resistance on TLD among CALWH in rural Zimbabwe.
After a median of 6.9 months of focused VL testing, 95% had viral suppression in this vulnerable population. Our result is consistent with previous studies that have shown high viral suppression rates on DTG-based regimens.7,12 The single dose tablet TLD has been proven to be more potent, suppressing VL more rapidly than efavirenz-based regimens. A WHO briefing note reported a VL suppression rate (VL <50 copies/mL) of 81% after 3 months of treatment among people who started with a DTG-based regimen compared with 61% among those on an EFV-based regimen.13 Similarly, in a recent study by Nabitaka et al conducted in Uganda, a viral suppression rate of 94% was observed among both ART-naïve and experienced patients on DTG-based regimens.7
Nonadherence to PI-based second-line regimens has been reported in previous studies and particularly in adolescents and young adults.14–17 In a study conducted in Zimbabwe, Chimbetete et al showed that only age >24 years was significantly associated with major PI mutations and concluded that adolescents and young adults had a lower risk of acquiring major PI resistance mutations, possibly due to poor adherence to ART.16 In a previous study, we showed that PI-based second-line therapies were poorly tolerated and adherence was reduced among adolescents and young adults in Zimbabwe.18
In line with the previous studies, we found in this study that of the 10 participants with VF on TLD, 90% (9/10) were already failing on their previous regimens, and of the participants confirmed VF and successfully genotyped, none had resistance mutations, suggesting behavioral tendencies and adherence problems. Participants who had previously been on a PI-based regimen were more likely to fail on TLD than those who had been on a NNRTI-based regimen. However, the high rate of viral suppression observed on TLD in this study cements the fact that TLD (as a smaller tablet taken once per day) is more effective than the current boosted PI-based regimens, particularly among this hard-to-treat population.
Before switching to TLD, 45 (24%) participants had virological failure. Of these participants, the majority 80% (36/45) suppressed on TLD after a median duration of 6.9 months. Our finding is consistent with results from the recent Paton19 randomized control study (NADIA) carried out in three African countries (Uganda, Kenya, and Zimbabwe). The NADIA study reported the efficacy of DTG in second-line therapy, even in the presence of NRTI resistance, suggesting that NRTI (TDF and 3TC) could be recycled in second-line treatment even in the presence of prior VF and high-level HIVDR to these NRTIs without compromising viral suppression.19
These results indeed support and cement the fact that DTG has a high potency as previously reported5 compared with NNRTIs and older integrase inhibitors. In contrast to these observations, Rhee et al in a systematic review of DTG genetic mechanisms of resistance reported the risk of functional monotherapy, suggesting that a fully active NRTI backbone may be required to sustain complete effectiveness of first-line DTG-based regimens (TLD).20 Similarly, up to 31% and 47.6% of patients after VL failure and ART switch to a DTG-based or blind switching without prior VL testing, respectively, were estimated to be on functional DTG monotherapy in a recent study by Salou et al carried out in Togo.21
Although we found high rate of VS on TLD, one of the limitations of our study is that the study follow-up period was short (median = 6.9 months). Therefore, the long-term effect of DTG-based regimens on viral suppression in this cohort is yet to be evaluated. Another limitation was the absence of measures of adherence such as tenofovir drug levels in hair or blood cells.22 Genotyping of the integrase region of the pol gene was not done, yet the participants were failing on TLD. However, this is balanced by a real-world observation of the low frequency of resistance mutations to DTG as previously reported.12,23–26 The detection limit of SAMBA (<1,000) is also a limitation as it does not provide information on low-level viremia that is known to progress to VF.
Conclusions
Overall, we found a high rate (95%) of VS among children and adolescents on TLD in rural Zimbabwe. DTG may addresses the problems of VL failure and HIVDR resistance in LMICs. However, VL monitoring and more effective adherence support for young people with history of treatment failure need to be reinforced to maximize the efficacy of DTG.
Supplementary Material
Acknowledgments
The authors are grateful to all study participants, nurses, clinicians, and staff at Chidamoyo Christian Hospital. We also thank staff who contributed to this research at the Biomedical Research and Training Institute.
Authors' Contributions
D.K. and V.K. conceived the study. K.M., D.K., V.K., and T.M. supervised data collection. T.M. and V.K. performed data analysis. R.M., T.M., C.M., S.M., J.M., T.S. and J.M. critically reviewed and approved the final article.
Sequence Data
HIV-1 drug resistance sequence data of the three participants were provided as Supplementary Data. The sequences have been submitted to GenBank and the accession numbers will be provided at a later date.
Author Disclosure Statement
All authors have no reported conflicts of interest. All authors have submitted the ICMJE Form for disclosure of potential conflicts of interest.
Funding Information
This study was supported by the Gilead Sciences, Inc., and was made possible through various local collaborations including the Biomedical Research and Training Institute and the Infectious Diseases Research Laboratory (Grant number: ISR-17-10142).
Supplementary Material
References
- 1. World Health Organization. Guidelines on the Public Health Response to Pre-treatment HIV Drug Resistance. Switzerland; 2017. [Google Scholar]
- 2. World Health Organization. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Switzerland; 2018. [Google Scholar]
- 3. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019;19(3):253–264; doi: 10.1016/S1473-3099(19)30036-2. [DOI] [PubMed] [Google Scholar]
- 4. Underwood M, DeAnda F, Dorey D, et al. Resistance Post Week 48 in ART-Experienced, Integrase Inhibitor-Naive Subjects with Dolutegravir (DTG) vs. Raltegravir (RAL) in SAILING (ING111762). 13th European Meeting on HIV & Hepatitis Treatment Strategies & Antiviral Drug Resistance. Barcelona, Spain; 2015. [Google Scholar]
- 5. Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in Sub-Saharan Africa: A modelling study. Lancet HIV 2019;6(2):e116–e127; doi: 10.1016/S2352-3018(18)30317-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Transition to New Antiretroviral Drugs in HIV Programmes: Clinical and Programmatic Considerations. Switzerland; 2017. [Google Scholar]
- 7. Nabitaka VM, Nawaggi P, Campbell J, et al. High acceptability and viral suppression of patients on dolutegravir-based first-line regimens in pilot sites in Uganda: A mixed-methods prospective cohort study. PLoS One 2020;15(5):e0232419; doi: 10.1371/journal.pone.0232419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The National Medicines and Therapeutics Policy Advisory Committee (NMTPAC) and AIDS and TBDirectorate of the Ministry of Health and Child Care. Final Addendum to the 2016 ART Guidelines. Zimbabwe; 2019. [Google Scholar]
- 9. HIV/TB Care and Treatment Partnership Forum. Final 4 _Rapid COVID 19 Impact Assessment on HIV Service Delivery_CareTreatment. Zimbabwe; 2021. [Google Scholar]
- 10. Mapangisana T, Machekano R, Kouamou V, et al. Viral load care of HIV-1 infected children and adolescents: A longitudinal study in rural Zimbabwe. PLoS One 2021;16(1):e0245085; doi: 10.1371/journal.pone.0245085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ministry of Health and Child Care. Operational and Service Delivery Manual for the Prevention, Care and Treatment of HIV in Zimbabwe. Zimbabwe; 2017. [Google Scholar]
- 12. Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013;382(9893):700–708; doi: 10.1016/S0140-6736(13)61221-0 [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. Dolutegravir (DTG) and the Fixed Dose Combination of Tenofovir/Lamivudine/Dolutegravir (TLD). Briefing Note, April 2018. Google Search; 2018. Available from: https://www.fhi360.org/sites/default/files/media/documents/linkages-tld-transition-information.pdf [Last accessed: June 6, 2021].
- 14. Abreu JC de, Vaz SN, Netto EM, et al. Virological suppression in children and adolescents is not influenced by genotyping, but depends on optimal adherence to antiretroviral therapy. Braz J Infect Dis 2017;21(3):219–225; doi: 10.1016/j.bjid.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boerma RS, Bunupuradah T, Dow D, et al. Multicentre analysis of second-line antiretroviral treatment in HIV-infected children: Adolescents at high risk of failure. J Int AIDS Soc 2017;20(1):21930; doi: 10.7448/IAS.20.1.21930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chimbetete C, Katzenstein D, Shamu T, et al. HIV-1 drug resistance and third-line therapy outcomes in patients failing second-line therapy in Zimbabwe. Open Forum Infect Dis 2018;5(2):ofy005; doi: 10.1093/ofid/ofy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindsey JC, Bosch RJ, Rudy BJ, et al. Early patterns of adherence in adolescents initiating highly active antiretrovial therapy predict long-term adherence, virologic, and immunologic control. AIDS Patient Care STDs 2009;23(10):799–801; doi: 10.1089/apc.2009.0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kouamou V, Manasa J, Katzenstein D, et al. Drug resistance and optimizing dolutegravir regimens for adolescents and young adults failing antiretroviral therapy. AIDS 2019;33 (11):1729–1737; doi: 10.1097/QAD.0000000000002284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paton NI. Nucleosides and Darunavir/Dolutegravir in Africa (NADIA) Trials: 48 Wks Primary Outcome. Conference on Retroviruses and Opportunistic Infections, Abstract 94, 2021; 2021. Available from: https://www.croiconference.org/abstract/nucleosides-and-darunavir-dolutegravir-in-africa-nadia-trial-48wks-primary-outcome/ [Last accessed: March 18, 2021].
- 20. Rhee S-Y, Grant PM, Tzou PL, et al. A systematic review of the genetic mechanisms of dolutegravir resistance. J Antimicrob Chemother 2019;74(11):3135–3149; doi: 10.1093/jac/dkz256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salou M, Butel C, Comlan AS, et al. Challenges of scale-up to dolutegravir-based regimens in Sub-Saharan Africa. Aids 2020;34(5):783–787; doi: 10.1097/QAD.0000000000002470 [DOI] [PubMed] [Google Scholar]
- 22. Adams JL, Sykes C, Menezes P, et al. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: A new measure of antiretroviral adherence? J Acquir Immune Defic Syndr 1999 2013;62(3):260; doi: 10.1097/QAI.0b013e3182794723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): Week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019;393(10167):143–155; doi: 10.1016/S0140-6736(18)32462-0 [DOI] [PubMed] [Google Scholar]
- 24. Joly V, Burdet C, Landman R, et al. Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: Results of the ANRS 167 Trial (LAMIDOL). J Antimicrob Chemother 2019;74(3):739–745; doi: 10.1093/jac/dky467 [DOI] [PubMed] [Google Scholar]
- 25. Maggiolo F, Gulminetti R, Pagnucco L, et al. lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis 2017;17(1):215; doi: 10.1186/s12879-017-2311-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide–based 3-or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: Phase 3, Randomized, Noninferiority TANGO Study. Clin Infect Dis 2020;71(8):1920–1929; doi: 10.1093/cid/ciz1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.