Abstract
Significance:
Reactive oxygen species (ROS) contribute to multiple aspects of peripheral nervous system (PNS) biology ranging from physiological processes (e.g., axonal outgrowth and regeneration) to pathophysiology (e.g., nerve degeneration). Although ROS are derived from multiple sources, NADPH oxidase (Nox) family members are dedicated to ROS generation. Noxs are expressed in the PNS, and their overexpression is associated with detrimental effects on nerve function and contributes, at least in part, to peripheral neuropathies.
Recent Advances:
Of the seven members, studies mostly focused on Nox1, Nox2, and Nox4, which are expressed in the PNS in a cell-specific manner. We have also recently identified human Nox5 in sural nerve biopsies. When maintained at homeostatic levels, Noxs regulate several aspects of peripheral nerve health, most notably neurite outgrowth and axonal regeneration following nerve lesion. While Nox2 and Nox4 dysregulation is a major source of oxidative stress in PNS disorders, including neuropathic pain and diabetic peripheral neuropathy, recent evidence also implicates Nox1 and Nox5.
Critical Issues:
Although there is compelling evidence for a direct role of Noxs on nerve function, little is known about their subcellular localization, intercellular regulation, and interaction. These, together with redox signaling, are considered crucial components of nerve redox status. In addition, the lack of isoform-specific inhibitors limits conclusions about the physiological role of Noxs in the PNS and their therapeutic potential in peripheral neuropathies.
Future Directions:
Future research using isoform-specific genetic and pharmacological approaches are therefore needed to better understand the significance of Nox enzymes in PNS (patho) physiology.
Keywords: NADPH oxidases (Nox), neuron, neuropathy, peripheral nervous system (PNS), reactive oxygen species, Schwann cells
Introduction
The peripheral nervous system (PNS) refers to the portion of the nervous system that lies outside the central nervous system (CNS) and serves as a connecting point between the CNS and peripheral tissues. It consists of a complex network of cranial and spinal nerves, composed of neurons, their axons, and supporting Schwann cells. Afferent sensory neurons and their associated axons transmit information from sensory receptors in the PNS back to the CNS, whereas motor efferent neurons and their axonal extensions transmit information from the CNS to the muscles and glands (38). This back-and-forth communication between the PNS and the CNS is pivotal for the physiological regulation of the internal system as well as the interactions with the external environment.
The need to rapidly carry nerve impulses over long distances of up to 2 m or more in length poses a unique challenge to the PNS and makes it highly susceptible to metabolic, mechanical, toxic, and immune insults (141). When injured, the PNS exhibits abnormalities in nerve structure and function that disrupt the ability of the CNS to communicate via the PNS with effector organs and muscles.
Peripheral neuropathies are a heterogeneous group of disorders that occur secondary to peripheral nerve damage, leading to reduced quality of life (43). They affect more than 8% of the general population, and this number rises to 15% in patients 40 years or older (46). Peripheral neuropathies are often associated with axon degeneration, impaired regeneration, as well as disruptions in calcium (Ca2+) signaling, electrophysiological function, mitochondrial function, and substrate utilization (38, 135, 141). Redox signaling modulates many of these processes, and accumulating clinical and preclinical evidence has shown increased nerve reactive oxygen species (ROS) following peripheral nerve dysfunction. However, untargeted antioxidant therapies to treat PNS disorders have only exhibited limited therapeutic potential in the clinical setting (83, 110).
In addition to the incorrect selection of antioxidant dosages and treatment durations, these failures are mainly attributed to the lack of antioxidant specificity against the ROS source(s) altered in a disease- and tissue-specific manner. Untargeted antioxidant treatment can result in off-target effects or lead to global suppression of ROS-producing enzymes, including those required for normal physiology (133). Therefore, it is critical to abandon the previous conventional approach that “blindly” targets all antioxidant activity. The new and needed way forward is to identify specific ROS sources that are altered during disease course, so that targeted antioxidant therapies can be developed to treat PNS diseases.
The major sources of ROS in peripheral nerves include mitochondria, xanthine oxidase, and NADPH oxidases (Noxs) (Fig. 1) (38, 124, 143). Among these sources, the Nox family of enzymes consists of seven members (Nox1–5 and dual oxidases [Duox] 1 and 2) specialized for ROS production (103). Although the role of Noxs in the PNS is not completely understood, evidence implicates Nox family members in cellular functions ranging from the immune response and neuronal development to pathophysiological involvement and neurodegeneration (35, 73, 96, 143). In this review, we first provide a general overview of ROS and antioxidant generation in the PNS. We then focus on Nox expression in the PNS, their physiological roles, how Nox-derived ROS contribute to PNS disorders, and the novel concepts of Nox signaling, which may be relevant to nerve redox homeostasis and PNS function.
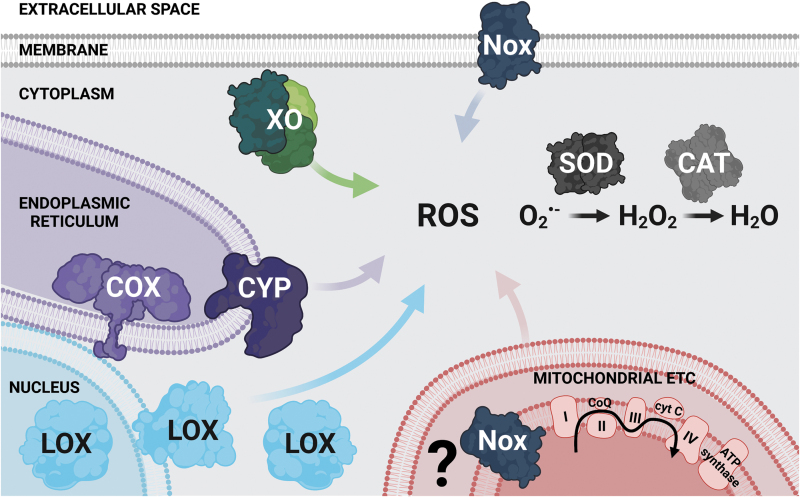
FIG. 1.
ROS sources and metabolism in the PNS. The mitochondrial ETC is considered a major source of ROS in the PNS and can inadvertently lead to O2−• generation under physiological conditions (38). Other sources that generate ROS as a by-product of metabolism include XO located in the cytoplasm (116, 127), CYP and COX (128) that reside in the ER, as well as LOX (147) found in the nuclear and cytosolic regions and membranes (not shown in the figure for simplicity) (124). Noxs are a specialized source of ROS (120). They localize to the plasma membrane and intracellular compartments, including the mitochondria (shown in the figure with a question mark that highlights a possible localization to the inner mitochondrial space), the ER, and the nuclear envelope (not shown in the figure for simplicity). Under physiological conditions, O2−• generated by these ROS-producing enzymes is rapidly converted to the more stable and easily diffusible H2O2 by SOD. H2O2 is then detoxified by CAT to form H2O (124). CAT, catalase; COX, cyclooxygenases; CYP, cytochrome P450 monooxygenases; ER, endoplasmic reticulum; ETC, electron transport chain; H2O, water; H2O2, hydrogen peroxide; LOX, lipoxygenases; Nox, NADPH oxidase; O2−•, superoxide anion; PNS, peripheral nervous system; ROS, reactive oxygen species; SOD, superoxide dismutase; XO, xanthine oxidase. Created with BioRender.com.
ROS and Antioxidants: General Overview
ROS are a family of oxygen containing molecules resulting from cellular metabolism, which can avidly react with biomolecules, including nitric oxide, proteins, lipids, carbohydrates, and DNA (119). Because of their ability to modify biomolecules, ROS generation was initially considered cytotoxic and believed to occur only under pathological conditions. However, it is now well established that homeostatic ROS levels are crucial for cellular physiology and regulate processes such as growth, apoptosis, signal transduction, cellular respiration, and host defense.
In the nervous system, ROS production is involved in blood pressure regulation, cognitive function, tissue repair, and immune response (103). However, when overproduced, these highly reactive molecules become deleterious and can promote cell damage in disease states. The increased understanding of both the beneficial and harmful effects of ROS is critical to the design of rationale, mechanism-based therapies for PNS disorders.
ROS are classified into two groups: radical and nonradical species. Radical ROS include superoxide anion (O2−•), hydroxyl radical (•OH), and nitrogen-based species such as the nitric oxide radical (NO•), whereas nonradical ROS include hydrogen peroxide (H2O2), singlet oxygen (1O2), and peroxynitrite (ONOO−) (119). Many ROS types are involved in redox signaling, defined as the reversible redox modifications exerted by ROS to a specific biomolecule (14). As an example, O2−• exerts its effects at the site of generation and is rapidly converted to the more stable and easily diffusible H2O2; both O2−• and H2O2 are considered major ROS involved in redox signaling through iron/sulfur cluster modification and cysteine oxidation.
More potent oxidants such as •OH, exert irreversible redox reactions (14). In peripheral nerves, redox signaling is implicated in physiological processes, including axonal outgrowth and regeneration, as well as pathophysiology, including pain processing and nerve degeneration (35, 72, 143).
Intracellular ROS levels in the PNS are maintained in check via antioxidant defense mechanisms, which consist of enzymes and nonenzymatic scavengers. Enzymes are mainly under the control of the transcription factor NF-E2-related factor 2 (Nrf2) and include superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (71, 88, 137). On the contrary, nonenzymatic scavengers are primarily of dietary origins and include α-tocopherol (vitamin E), β-carotene, and ascorbate (vitamin C).
Neurons and Schwann cells can initially increase Nrf2-dependent antioxidant signaling in the face of cellular stressors at early disease stages before progression to irreversible damage (53, 137). Interestingly, we have shown that Schwann cells have a high basal antioxidant potential under physiological conditions, which is further increased during metabolic stress. This feature is thought to confer resistance to oxidative damage relative to the more vulnerable neurons (137).
An imbalance between ROS generation and the ability of the antioxidant defense mechanisms to clear excess ROS, or effectively repair the resulting damage, is deleterious and is associated with irreversible oxidative modifications to macromolecules as well as impaired redox signaling. These processes eventually lead to uncontrolled ROS generation commonly referred to as oxidative stress (119), an effect that has been implicated in nerve degeneration and PNS disorders.
Sources That Generate ROS As a By-Product of Metabolism in the PNS
Multiple cellular sources generate ROS in the PNS as a by-product of oxidative phosphorylation and metabolism. The mitochondrial electron transport chain is perhaps the most studied ROS source in the PNS, which can generate O2−• as a result of an electron leak leading to a 1-electron reduction from oxygen to O2−•, instead of water (2).
Compared with other cell types, mitochondria make up half of the cytoplasmic volume of high energy consuming peripheral neurons, which require up to 4.7 billion adenosine triphosphate (ATP) molecules per second under normal physiologic conditions (153) to maintain their membrane potential across a large surface area (115). Because of this high mitochondrial content, the electron transport chain was long thought to be the major ROS source that sustains the oxidative potential in the PNS under physiological conditions besides being a substantial ROS source in PNS disorders (3, 16). Interestingly, Nox enzymes, mainly Nox4 and Nox5, might localize to mitochondria, turning the mitochondrial electron transport chain into both an oxidation target and an ROS source (10, 81, 93).
In addition to the mitochondrial electron transport chain, multiple enzymes in the PNS can produce ROS as by-products of their catalytic activities (Fig. 1). These include xanthine oxidase, located in the cytoplasm (116, 127), cytochrome P450 monooxygenases (unpublished data), and cyclooxygenases (128), residing in the endoplasmic reticulum (ER), as well as lipoxygenases (147), found in the nuclear and cytosolic regions and membranes (124). Studies have mostly evaluated the function of these ROS-generating enzymes in PNS pathophysiology and their role in normal PNS physiology is an avenue warranting further investigation.
NADPH Oxidases of the Nox Family As a Dedicated Source of ROS
In contrast to the other ROS-generating systems, NADPH oxidases of the Nox family (Nox) are transmembrane proteins with no known metabolic function, except catalyzing ROS generation across biological membranes (120). This occurs via electron transfer from NADPH as an electron donor. Two NADPH molecules transfer two electrons to flavin adenine dinucleotide, which passes the electrons to two iron-containing heme groups. The electrons are then transferred to oxygen as an electron acceptor, thereby yielding O2−• largely thought to be the major product of the electron transfer (Fig. 2). In most mammals, the Nox enzyme family consists of seven members: Nox1 through 5 and Duox 1 and 2. Interestingly, the Nox5 isoform is only expressed in higher mammals and is absent in rats and mice, which limits our understanding of its physiological and pathophysiological significance in humans (39).
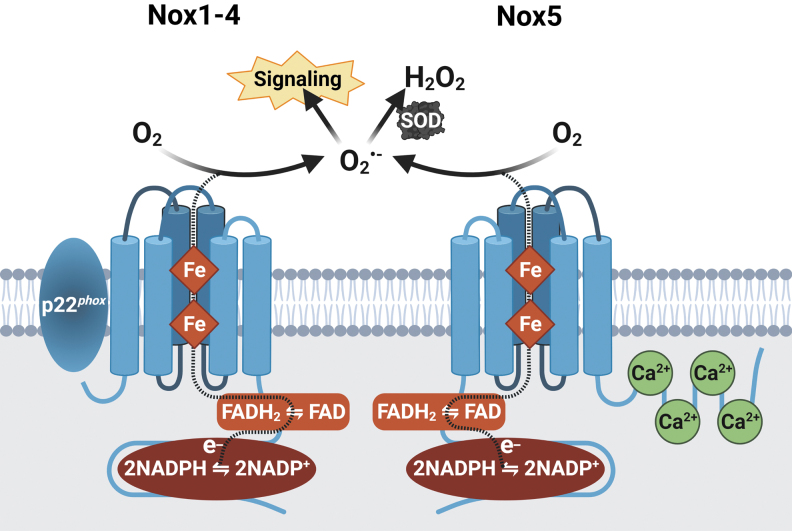
FIG. 2.
NADPH oxidase family of enzymes as a dedicated source of ROS. Nox enzymes are a family of transmembrane proteins. They bind within their C-terminus, NADPH, which acts as the electron (e-) donor. Two NADPH molecules transfer two e- to FAD, also bound within the C-terminus, which passes the e- to two Fe-containing heme groups. Finally, the e- are transferred to O2, the terminal acceptor, catalyzing the transformation to O2−•, although Nox4 and Nox5 release H2O2. Nox1–4 share a certain extent of structural homology and all require interaction with the regulatory subunit p22phox to produce ROS. Nox5 does not require p22phox, but is Ca2+-dependent and thus contains N-terminal EF-hand domains with four Ca2+-binding sites (103). Ca2+, calcium; FAD, flavin adenine dinucleotide; Fe, iron; O2, molecular oxygen. Created with BioRender.com.
The prototype NADPH oxidase, Nox2, originally discovered in neutrophils, is one of the best-characterized members of the Nox family and is critical for innate immunity (25). It consists of two transmembrane catalytic subunits (gp91phox [commonly referred to as Nox2], and the regulatory subunit p22phox), three cytosolic subunits (p47phox, p67phox, and p40phox), and a small Rho GTP-binding protein (Rac1 or Rac2). These subunits are disassociated in the inactive state, but assemble upon enzyme stimulation to produce O2−• (112).
While other Nox members share a certain extent of structural homology with Nox2, the mechanisms by which they are activated may vary; for example, similar to Nox2, Nox1–4 full activation also requires interaction with p22phox to produce ROS (112). Nox1 through 3 enzymes require cytosolic subunits (p47phox and p67phox, or homologues) to form a fully functional enzyme complex (112). However, unlike the other isoforms, Nox4 is constitutively active and does not require any cytosolic subunits for full activation (92). Nox5 and Duox enzymes contain N-terminal EF-hand domains with four binding sites for Ca2+, which is required for enzyme activation (103).
Under physiological conditions, Noxs are maintained at a relatively low level of constitutive activity to regulate redox signaling in the vicinity of target molecules. While Nox-dependent redox signaling mainly regulates cell differentiation, proliferation, and apoptosis, some Nox isoforms exert cell-specific functions. For example, as mentioned above, phagocyte Nox2 is heavily involved in the innate immune response, while Nox3 is highly expressed in the inner ear and plays a role in otoconia biogenesis (84).
With respect to the ROS type, Nox isoforms generally produce O2−• as their primary product. However, data have demonstrated that Nox4, Duox1, and Duox2 generate H2O2 (26, 105). Evidence also shows that H2O2 can be detected following Nox5 activation (20, 118). While the biochemical mechanism underlying H2O2 generation is unknown, a rapid conversion of O2−• into H2O2 before release from the enzyme has been suggested as a contributing factor. It has been further demonstrated that Nox4-dependent H2O2 generation relies on a histidine (His-222) residue, localized in the extracellular loop of the enzyme (105, 126), a mechanism that requires validation in the other isoforms.
In the following sections, we review Nox cellular distribution, as well as their physiological and pathophysiological involvement in the PNS. Because the roles of Nox3 and Duox1-2 in the PNS are unclear, we focus this review on Nox1, Nox2, Nox4, and Nox5.
Nox Expression in the PNS
Nox subcellular localization
Nox enzymes reside both at the plasma membrane and in intracellular compartments, including the ER, mitochondria, and the nuclear envelope (103). Under resting conditions, Nox2 is closely associated with p22phox and together primarily localize to intracellular and plasma membranes, while cytosolic subunits are typically located in the cytoplasm (102). Upon cell activation, the cytosolic subunits translocate to membrane-bound Nox2 to form a fully functional enzyme complex (102). Nox4 on the contrary is expressed intracellularly and can be found in the ER (146), the nuclear envelope (22), and the mitochondria (10). While both Nox1 and Nox5 have been identified in the plasma membrane (49, 130), reports have also detected Nox5 at several intracellular sites, including the perinuclear area, the mitochondria, and the ER (130).
Because of their high reactivity, this diverse subcellular localization of Noxs has several consequences, including how Nox isoforms influence redox signaling as well as how Nox isoforms function under normal and disease states. For example, Nox2 assembly and activation on the plasma membrane of neutrophils are essential for ROS generation and pathogen elimination at the injury site (36). A recent report identified mitochondrial Nox4 as an energetic sensor, whose activity is directly regulated by ATP in renal cells (117). These results suggest a close connection between ATP turnover and Nox4 redox signaling and could be of relevance to the PNS.
With respect to the PNS, axons are intimately associated with Schwann cells, and growing evidence highlights the importance of the Schwann cell/axon cross talk in peripheral nerve metabolic support (11). This in turn raises the possibility that ROS release from axons into the extracellular space may directly influence the surrounding Schwann cells and vice versa. Specifically, Nox4-derived oxidative stress in sensory dorsal root ganglion neurons is accompanied by Schwann cell injury and dysmyelination in a mouse model of neuropathic pain (72). While the underlying mechanism is unknown, one can speculate that Nox4-derived ROS in axons could damage Schwann cells in a paracrine manner, similar to what has been observed in the vasculature (12, 13, 98). This novel aspect of Nox signaling is discussed in detail below.
Nox cellular distribution in the PNS
Relative to the better characterized CNS (103), the cellular distribution of Nox enzymes in the PNS has not been comprehensively analyzed (Table 1). Data, however, demonstrate that Nox isoforms can be expressed simultaneously at multiple PNS sites (28, 73). In addition, it is generally thought that Nox expression in the PNS is maintained at low basal levels under physiological conditions, and upregulated in disease states (103).
Table 1.
Nox Cellular Distribution in the Peripheral Nervous System
| PNS cellular localization | Nox isoforms | Accessory proteins | References |
|---|---|---|---|
| Dorsal root ganglion neurons | Nox1, Nox2, and Nox4 | p22phox, p47phox, and Rac1 | (17, 59, 72, 111, 136) |
| Schwann cells | Nox1 and Nox4 | Not identified | (28, 35) |
| Macrophages | Nox2 and Nox4 | p22phox and p47phox | (9, 28, 70) |
Nox, NADPH oxidase; PNS, peripheral nervous system.
Dorsal root ganglion neurons express Nox1, Nox2, and Nox4 (59, 72, 136). Studies have also reported the expression of Nox accessory proteins p22phox, p47phox, and Rac1 in rodent primary cultures of dorsal root ganglion neurons (17, 111, 136).
As we mentioned above, Nox subcellular localization in dorsal root ganglion neurons is a crucial determinant of the downstream signaling effects of intracellular versus extracellular Nox-derived ROS. While this is still an area requiring further research, multiple studies have used dihydroethidium (DHE) for detecting O2−• in neuronal cultures or whole peripheral nerve samples (35, 57), which suggests a role for Nox-derived ROS in intracellular redox signaling. Increasing studies are, however, assessing ROS release into the extracellular space using cytochrome c assay and Amplex Red (23, 72), which may reflect a role in cell-to-cell communication and in the propagation of ROS signals into neighboring cells.
Schwann cells mainly express Nox1 and Nox4 (28, 35), with no available reports on Nox2 protein expression.
As a whole, Nox subcellular localization in Schwann cells remains largely unexplored. Recent data, however, have shown that Nox1 can generate both intra- and extracellular oxidants in Schwann cells in a neuropathic pain model. The authors suggest that intracellular ROS maintain mechanical allodynia, while extracellular H2O2 promotes Nox2-expressing macrophage recruitment to the perineurial space (28). Simultaneous expression of Schwann cell Nox1 and macrophage Nox2 in the PNS leads to a sustained feed-forward loop of oxidative injury. These results suggest a potential interaction between different Nox isoforms to regulate redox signaling, a novel concept that is further discussed below.
Macrophages mostly express Nox2 (28, 70), which is low in the absence of a stimulus (77). Once activated, reports demonstrate the Nox2-mediated oxidative burst at the injury site in rodent models of PNS disorders (28, 70). Besides Nox2, Nox4 is upregulated in spinal cord macrophages at late stages of neuropathic pain and, together with Nox2, modulates macrophage polarization (9). Whether Nox2 and Nox4 are simultaneously coexpressed in PNS macrophages and the significance of this coexpression on peripheral nerve health are areas requiring additional studies.
Nox Signaling in the PNS Under Physiological Conditions
Under physiological conditions, Nox enzymes regulate redox signaling by generating low ROS levels, in a spatially confined manner, which induces conformational changes in the target molecule, in turn impacting its interactions and downstream function (14). Signaling proteins containing active-site and structural cysteine residues are perhaps the most susceptible targets for redox modifications and include kinases, phosphatases, ion channels, and transcription factors (108). Oxidation targets that may be especially important for peripheral nerve function include redox switches Nrf2 and NFκB (69, 82), as well as sensory neuron ion transient receptor potential (TRP) channels (40).
The direct contributions of Nox enzymes to these processes in the PNS remain unclear and are not discussed in this review. Instead, we consider examples of cellular functions of Nox enzymes in neurite outgrowth and axon regeneration (Fig. 3).
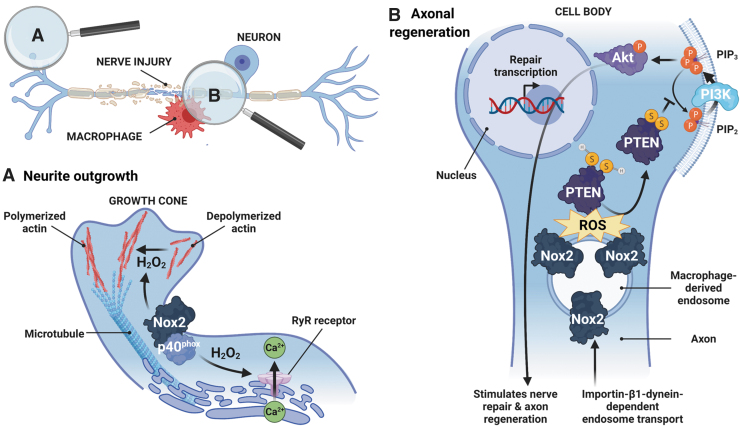
FIG. 3.
NADPH oxidase signaling in the PNS under physiological conditions. Nox-derived ROS promote neurite outgrowth (A) and axon regeneration (B) under physiological conditions. (A) Nox2 and its cytosolic subunit p40phox localize in growth cones, dynamic structures composed of an actin and microtubule cytoskeleton located at the tip of elongating neurites, which allows outgrowth and guidance to the proper target. Nox2-derived H2O2 oxidizes the actin cytoskeleton, changing its polymerization state and favoring neurite outgrowth (101). Nox2-derived H2O2 can also oxidize RyR localized in the ER, releasing Ca2+. This Ca2+-dependent process regulates different aspects of neurite outgrowth, including axonal development and polarization (144). (B) Following peripheral nerve lesion, macrophages release exosomal Nox2, which is taken up by injured axons via endocytosis and retrogradely transported to the cell body in an importin-β1–dynein-dependent mechanism. Endosomal Nox2 oxidizes PTEN, triggering a disulfide bond (SS) formation, which inactivates PTEN. PTEN inactivation favors PIP2 phosphorylation (P) to PIP3 and PI3K-Akt pathway activation, promoting nerve repair and axon regeneration (50). PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PTEN, phosphatase and tensin homologue; RyR, ryanodine receptors. Created with BioRender.com.
Neurite outgrowth
Redox signaling is implicated in the regulation of neurite outgrowth, a highly coordinated process that allows exact pathfinding for developing and regenerating neurons in response to environmental cues (150). In this context, an early study showed that exposing cultured neurons to nerve growth factor (NGF) induces neurite outgrowth in an ROS-dependent manner (125). More recent data revealed that Rac1, the Nox cytosolic subunit, and increased Nox activity are both required for neurite outgrowth in vitro (78).
When evaluating the contribution of specific Nox isoforms to this process, Ibi et al. found that Nox1-dependent ROS suppress neurite outgrowth (58). However, studies in Aplysia bag cell neurons showed that Nox2 and its cytosolic subunit p40phox localize in growth cones, dynamic structures composed of an actin and microtubule cytoskeleton located at the tip of elongating neurites, which allows outgrowth and guidance to the proper target.
The authors suggest that Nox2/p40phox may modulate neurite outgrowth by oxidizing the actin cytoskeleton and changing its polymerization state (101). Findings in cultured hippocampal neurons further demonstrated that Nox2-derived H2O2 can also oxidize ryanodine receptors (RyR) localized in the ER, releasing Ca2+. This Ca2+-dependent process regulates the different aspects of neurite outgrowth, including axonal development and polarization (144). Together, these data suggest that physiological Nox levels are required for neurite outgrowth during development (Fig. 3A). Moreover, Nox isoforms (e.g., Nox1 vs. Nox2) may have differential effects on neurite outgrowth, an idea that requires further research.
Tissue repair and axonal regeneration
As we mentioned above, neurons and their long axonal processes have high energy demands with considerable ATP consumption. Neuron maintenance and repair require efficient signaling over long distances, which can be achieved through bidirectional protein and organelle transport between cell body and axons (114).
Indeed, lesions to peripheral nerves trigger well-orchestrated cellular and molecular events, including an inflammatory response at the injury site to induce axonal regeneration (7). This inflammatory response favors a highly oxidizing environment, and accumulating evidence suggests that ROS are in turn essential for axonal regeneration and functional recovery after peripheral nerve injury (29, 50, 113). Earlier studies mainly examined axonal regeneration in Drosophila and zebrafish [reviewed in Terzi and Suter (129)], which consistently pointed to a key role of Duox-mediated ROS in axon reinnervation and wound healing (76, 104, 113).
More recently, studies in genetically modified mouse models revealed that macrophage-derived Nox2 promotes axon growth and regeneration in mouse dorsal root ganglion neurons (50). Hervera et al. found that exosomal Nox2 is taken up by injured axons via endocytosis and retrogradely transported to the cell body in endosomes in an importin-β1–dynein-dependent mechanism. Endosomal Nox2 oxidizes phosphatase and tensin homologue (PTEN), triggering a disulfide bond formation, which inactivates PTEN. PTEN inactivation favors phosphatidylinositol 4,5-bisphosphate (PIP2) phosphorylation to phosphatidylinositol (3,4,5)-trisphosphate (PIP3) and PI3K-Akt pathway activation, promoting nerve repair and axon regeneration (50).
The same team went on to show that in vivo activation of neuronal Nox2 promotes axonal regeneration and partial restoration of sensory nerve function after spinal cord injury (29).
Overall, these studies strongly support a role for Nox-dependent ROS as signaling molecules required for tissue regeneration after PNS injury (Fig. 3B). The idea of Nox shuttling from macrophages to damaged axons is a novel concept, which advocates a new way forward in our understanding of physiological redox signaling in the PNS (51). In addition, these results are particularly exciting in light of the growing interest in axo-glial metabolic communication (6) and require further investigation in in vitro and in vivo models of PNS disorders.
Nox Pathophysiological Involvement in PNS
Table 2 outlines the involvement of major Nox isoforms and their potential roles in PNS disorders detailed below.
Table 2.
Nox Isoform Involvement in Peripheral Nervous System Disorders
| PNS disorder | Nox isoform(s) involved | Potential role(s) | References |
|---|---|---|---|
| Neuropathic pain | (a) Nox1 (b) Nox2 (c) Nox4 |
(a) Macrophage infiltration; thermal and mechanical hyperalgesia (b) Central and peripheral immune regulation in pain sensitization after peripheral nerve injury (c) Peripheral pain processing, neuroinflammation, and dysmyelination |
(28, 42, 59, 70, 72) |
| CIDP | Nox2 | Unclear | (91) |
| CIPN | Nox4 | Pain hypersensitivity; increased proinflammatory mediators | (96, 97) |
| DPN | (a) Nox2 (b) Nox4 |
(a) Pain processing and allodynia (b) Schwann cell injury; neurophysiological defects |
(21, 35, 136) |
CIDP, chronic inflammatory demyelinating polyneuropathy; CIPN, chemotherapy-induced peripheral neuropathy; DPN, diabetic peripheral neuropathy.
Neuropathic pain
Neuropathic pain is a common and debilitating complication associated with a range of PNS disorders, including diabetic neuropathy, amputation, acute peripheral nerve injury, and chemotherapy-induced neuropathy (64). It presents as hypersensitivity with allodynia and hyperalgesia or continuous pain sensations (38). Current therapies only marginally provide relief due to the lack of understanding of pain processing (19).
Preclinical studies in animal models of neuropathic pain implicate oxidative stress as a prominent pathogenic factor in pain sensitization (73). Specifically, increasing evidence suggests that Nox enzymes contribute to pain processing (42, 70, 140), and the most studied isoforms are Nox1, Nox2, and Nox4 (Fig. 4). Nox2-dependent ROS are a key mediator of oxidative stress in pain sensitization after peripheral nerve injury (28, 70, 77, 86). Kallenborn-Gerhardt et al. also demonstrated that increased Nox2 signaling in infiltrating macrophages promotes dorsal root ganglion damage and neuropathic pain after peripheral nerve injury, an observation not present in Nox2-deficient mice (70). Besides its action on sensory neurons, Nox2 induction in infiltrating macrophages can also target Schwann cells, which in turn activate Nox1, as we discussed above (28).
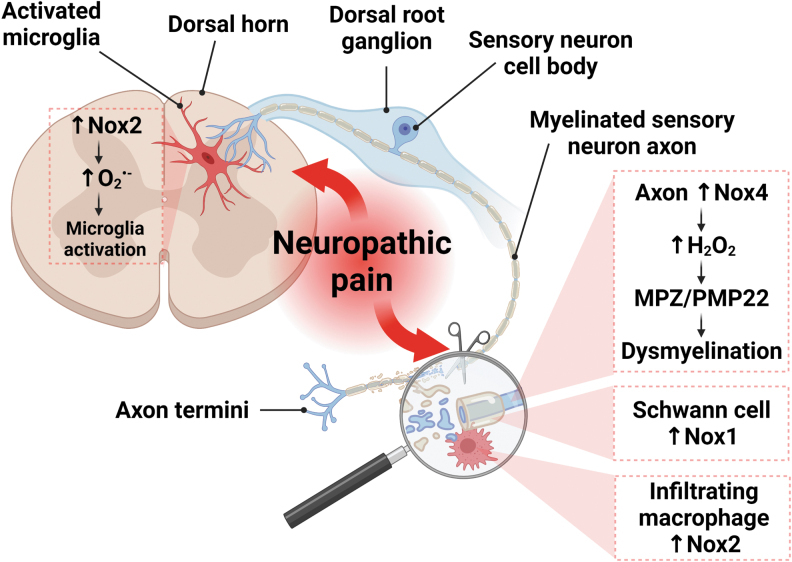
FIG. 4.
Role of NADPH oxidases in pain processing. Nox2 induction in both central and peripheral immune cells contributes to neuropathic pain. In the dorsal horn of the spinal cord, Nox2 activation following peripheral nerve injury leads to O2−• generation, microglial activation, and pain hypersensitivity (77, 86). In addition, Nox2 upregulation in infiltrating macrophages promotes sensory neuron damage and neuropathic pain after peripheral nerve injury (70). Besides its action on sensory neurons, Nox2 induction in infiltrating macrophages can also target Schwann cells, which in turn activate Nox1 (28). This initiates a pro-oxidative feed-forward loop, leading to sustained macrophage infiltration to the damaged area, further exacerbating neuroinflammation and neuropathic pain (not shown in the figure). Nox4 is also involved in peripheral pain processing through H2O2 release in nociceptive primary afferent neurons, which is associated with neuropathic pain and dysmyelination, as evidenced by peripheral myelin protein MPZ and PMP22 degradation (72). Created with BioRender.com.
In addition to its effect on peripheral immune cells, Nox2 can modulate neuropathic pain after peripheral nerve injury by its actions on microglia located in the dorsal horn of the spinal cord, a mechanism that requires Toll-like receptor 2 (TLR2)-dependent activation of the inflammatory response (77, 86). These findings suggest that Nox2-derived ROS in central and peripheral immune cells mediate, at least in part, neuropathic pain. This raises the possibility of inhibiting excess Nox2 activity as a meaningful therapeutic strategy. The caveat with this approach lies in the critical roles of Nox2-derived ROS in the innate immune response (133). In addition, we have discussed in a previous section the emerging role of Nox2 in axonal regeneration following PNS injury (50).
These results conflict with the findings that Nox2 mediates pain processing and suggest that the role of Nox2 may differ at different stages of peripheral nerve injury, which calls for caution in the use of Nox2 inhibitors as these may limit regeneration. Furthermore, genetic deletion of Nox2 functional subunits p47phox−/− and gp91phox−/− leads to arthritis, joint inflammation, and increased bone destruction (134). Thus, inhibiting Nox2 could be of limited therapeutic use for treating neuropathic pain due to its physiological roles in the immune response and axonal outgrowth (50).
Nox4 is also implicated in pain processing after peripheral nerve injury (42, 72, 140). Geis et al. found that Nox4 upregulation contributes to early neuropathic pain and increases proinflammatory cytokine release at the lesion site (42), worsening the pain sensation (121). While Nox4 genetic deletion prevented this acute neuropathic state, the authors found that pharmacological inhibition using the dual Nox1/4 inhibitor GKT136901 did not prevent pain in the later stages of neuropathy (42), suggesting that targeting Nox4 should occur early in the disease course.
Yet, another study showed that Nox4 upregulation in nociceptive primary afferent neurons maintains neuropathic pain in both the subacute and late phases after peripheral nerve injury by mechanisms involving Schwann cell injury and dysmyelination, as evidenced by peripheral myelin protein MPZ and PMP22 degradation (72). This group went on to demonstrate, using mice with sensory neuron-specific Nox4 deletion, that the Ca2+-binding protein S100A4 is an oxidation target of Nox4, which mediates hypersensitivity downstream of Nox4 (140).
These studies suggest that Nox4 signaling may vary during different stages of neuropathic pain. Future studies are needed to identify Nox4 signaling kinetics in neuropathic pain by direct comparisons across different clinically relevant animal models, on different genetic backgrounds, using pharmacological inhibitors and global and tissue-specific genetic manipulations (60) to identify potential therapeutic windows for pharmacological intervention.
In addition to the role of Nox1 in Schwann cells (28), Ibi et al. have demonstrated that Nox1-derived ROS mediate thermal and mechanical hyperalgesia in dorsal root ganglia using Nox1 knockout mice (59). Nox1-dependent pain processing is thought to involve the enhanced activity of the inflammatory sensor transient receptor potential vanilloid 1 (TRPV1) (59).
Chronic inflammatory demyelinating polyneuropathy
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an autoimmune disease of the PNS (85), which typically presents as a slowly progressive and symmetric neuropathy, with impaired sensorimotor function (85). While immune therapies are generally effective, at least half of CIDP patients require prolonged treatment to prevent disease relapse (45). Identifying targetable pathways in CIDP is therefore needed to develop more effective therapies and to improve disease outcomes.
Numerous pathogenic mechanisms are implicated in human and mouse CIDP, including neuroinflammation, Schwann cell dysfunction, and oxidative stress (74). Because of its known role in neuroinflammation (122), it is perhaps not surprising that Nox2 activity is increased in granulocytes and monocytes of CIDP patients (91). Interestingly, treatment with intravenous immunoglobulin increases this activity compared with pretreatment values. The significance of this increased Nox2-mediated ROS following treatment remains unclear. However, another study found that Nox2 may play a role in combating neuroinflammation in multiple sclerosis (100). While a similar mechanism may occur in CIDP, future studies are required to validate this hypothesis in clinically relevant mouse models and determine the precise mechanisms downstream of Nox2.
Chemotherapy-induced peripheral neuropathy
Chemotherapy-induced peripheral neuropathy (CIPN), a disabling consequence of cancer therapies (109), is predominantly a sensory and painful neuropathy (89). Currently, there are no effective CIPN treatments beyond symptomatic relief, and a better understanding of disease pathogenesis is essential for developing much needed mechanism-based neuroprotective therapies.
One of the established mechanisms by which chemotherapeutic agents induce cancer cell apoptosis is via ROS generation (148). Yet, ROS production is not only restricted to the tumor environment, but can diffuse to the neighboring healthy cells and induce damage as well (79). Importantly, sensory dorsal root ganglion neurons and their axons, which are the first to be affected during CIPN, have low antioxidant potential and high mitochondrial content, and, unlike the CNS, lack a protective vascular barrier, rendering them more susceptible to oxidative damage (38, 137). While increased ROS levels and lipid peroxidation are present in the peripheral nerves of multiple experimental CIPN models (31, 33, 149), much less is known about the exact source of ROS in CIPN.
While some studies report ROS overproduction secondary to mitochondrial dysfunction (75, 94), there remains little direct evidence on the primary ROS source in the context of disease. With respect to Noxs, increased NADPH oxidase activity in the spinal cord of a rat model of CIPN results in neurotoxic peroxynitrite accumulation and CIPN development (32, 62). Miao et al. reported a role for Nox4 signaling in the dorsal root ganglion and the dorsal horn of the spinal cord during painful CIPN (96, 97). These findings suggest that Nox4 may be a primary source of ROS in CIPN. However, the lack of studies assessing changes in other Nox subunits, both in the CNS and PNS in the presence or absence of specific inhibitors, limits conclusions about the therapeutic potential of Noxs in CIPN.
Diabetic peripheral neuropathy
Diabetic neuropathy is a common and debilitating complication affecting more than 50% of all diabetic patients. While diabetes-induced nerve damage can present in multiple forms, the most common form is diabetic peripheral neuropathy (DPN), a length-dependent and symmetric peripheral nerve degeneration (37). There are no disease-modifying therapies for DPN beyond glycemic control, which often fails to slow or reverse progression, especially in prediabetes and type 2 diabetes (T2D) (15). It is therefore critical to identify specific pathogenic factors contributing to DPN development to develop targeted mechanism-based therapies.
Experimental and clinical data, including our own, have shown that oxidative stress is a major component of cellular and molecular injury in DPN (4, 35, 56, 136, 137, 154). Mechanisms of injury downstream of hyperglycemia converge and lead to oxidative stress. Moreover, high-fat diet (HFD)-fed mice, which develop robust peripheral neuropathy closely resembling human disease, have increased nerve oxidative stress (136). These findings indicate that other metabolic stressors, such as dyslipidemia, an independent DPN risk factor, may also induce ROS and contribute to DPN at early disease stages before progressing to overt T2D. However, untargeted antioxidant therapy, including our own clinical trial of allopurinol, α-lipoic acid, and nicotinamide (110), has only exhibited limited therapeutic potential (154).
Among the sources of ROS in the PNS, we have previously shown that Nox activity is increased in dorsal root ganglion neurons following multiple metabolic stressors, including hyperglycemia and hyperlipidemia (136, 138), implicating Nox enzymes in DPN pathogenesis. What is more important, however, is the specific Nox isoform altered in DPN-relevant cell types, which may underlie oxidative damage in DPN (summarized in Fig. 5).
FIG. 5.
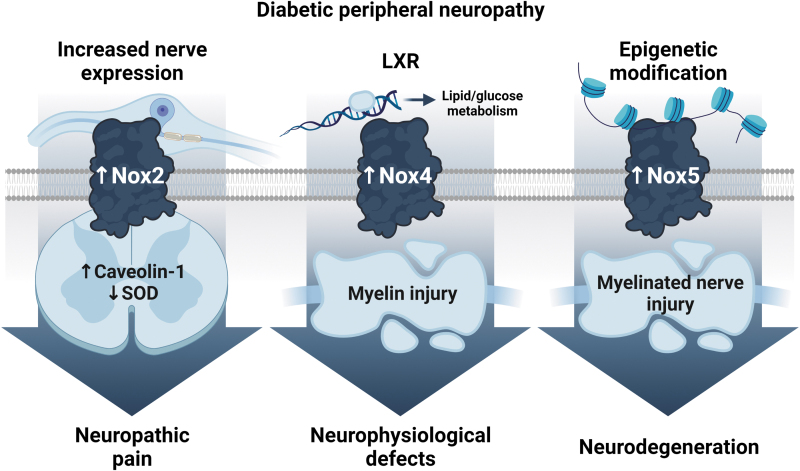
Schematic summary of Nox-dependent mechanisms involved in diabetic peripheral neuropathy. Nox2, 4, and 5 are induced in peripheral nerves under diabetic conditions. Nox2 is activated in both dorsal root ganglion neurons and spinal cord. Increased spinal cord Nox2 is associated with reduced SOD activity and neuropathic pain (152). Nox2 is thought to mediate pain hypersensitivity by interacting with caveolin-1, a regulatory protein involved in lipid homeostasis (21). The LXR, a master regulator of lipid and glucose homeostasis, is inhibited in diabetes. This inhibition is accompanied by Nox4 induction in the sciatic nerves of type 1 diabetic mice and in cultured Schwann cells exposed to high glucose conditions. Nox4-derived ROS results in myelin injury and neurophysiological defects in hyperglycemia-induced peripheral neuropathy (35). The human Nox5 isoform is hypomethylated at the promoter region (47), which leads to increased Nox5 gene and protein expression in sural nerve biopsies of type 2 diabetic subjects with peripheral neuropathy (34). LXR, liver X receptor. Created with BioRender.com.
Studies show that Nox2 functional subunits p47phox and gp91phox are upregulated in the spinal cord of streptozotocin (STZ)-induced type 1 diabetic (T1D) rats, which develop neuropathic pain (152). These changes are associated with increased ROS generation and lipid peroxidation, and reduced SOD activity, ultimately resulting in tactile allodynia (152). Nox2-derived ROS also contributed to neuropathic pain in a T2D rat model rendered diabetic through an HFD and a single low STZ dose (21). Mechanistic analyses further showed that Nox2 induction in spinal cord microglia leads to chronic pain through a direct interaction with caveolin-1 (Cav-1), a regulatory protein involved in lipid homeostasis (21).
In line with these findings, we have previously shown that p47phox is increased in dorsal root ganglion neurons treated with oxidized low-density lipoproteins to mimic the dyslipidemic milieu in DPN (136). Taken together, these results provide supporting evidence for a role for Nox2 in the development of DPN and neuropathic pain, which may be conserved across diabetes type.
In addition to Nox2, we were the first to show that Nox4 is a major ROS source in peripheral nerves of STZ-induced T1D mice and that its pharmacological inhibition using GKT137831 prevents hyperglycemia-induced nerve dysfunction (35). Interestingly, we also showed that Nox4 mRNA levels were significantly increased in skin biopsies of T2D patients without clinical signs of DPN, an effect that was further enhanced in T2D patients with DPN (35). These clinical data are complemented by preclinical findings, which show increased Nox4-derived ROS in sciatic nerves from prediabetic HFD-fed mice even in the absence of hyperglycemia (unpublished data) and hyperglycemic T2D db/db mice (151).
Overall, these results support the hypothesis that Nox4-derived ROS are instrumental for human and murine DPN progression in prediabetes, type 1 diabetes, and T2D. Thus, in addition to hyperglycemia, additional studies exploring the link between Nox4 and different metabolic drivers of prediabetes, T2D, and DPN, such as dyslipidemia and insulin resistance, will be critical to validate Nox4 therapeutic efficacy in DPN.
Beyond Nox4, the human Nox5 isoform has emerged as a pathogenic factor in diabetic complications (30, 66). Particularly, data from humanized transgenic mice expressing Nox5 in different kidney cell populations have identified a role for Nox5 in promoting diabetic kidney disease, even in the absence of the Nox4 effect (66, 67). Consistent with these findings, our preliminary observations in sural nerve biopsies from T2D participants with DPN indicate that the human Nox5 promoter is hypomethylated (47), which promotes increased Nox5 gene and protein expression (34).
These data suggest that in addition to Nox2 and Nox4, Nox5-derived ROS may play a key role in human DPN development. We are currently examining the selective expression of Nox5 in Schwann cells and dorsal root ganglion neurons in transgenic mice to evaluate cell-specific Nox5 effects in the presence or absence of diabetes. These data will also allow us to evaluate the relative effects and the oxidation targets of Nox4 versus Nox5 in DPN.
Nox Inhibition As a Therapeutic Target for PNS Diseases
Given the emerging evidence implicating specific Nox isoforms as critical mediators of oxidative stress and nerve injury, Nox inhibition could be a promising therapeutic strategy to treat PNS diseases. Below we discuss these advantages.
Antioxidants
In the past two decades, untargeted antioxidant therapy aimed at neutralizing ROS overproduction was considered the only approach to reduce oxidative stress in the PNS. Although promising results were obtained using antioxidant therapy to treat PNS diseases in the experimental setting (18, 38), clinical trials, especially for DPN, were either of limited efficacy or were inconclusive (83, 110). This failure was mainly attributed to the lack of antioxidant specificity against the ROS source altered in a disease- and tissue-specific manner. In fact, it is thought that antioxidant supplementation could result in off-target effects or lead to global suppression of other ROS-generating enzymes or Nox isoforms, including those required for normal physiology (133).
Another limitation is related to the rapid oxidization by ROS, which leads to cellular damage even before initiation of antioxidant beneficial effects (103).
Nonspecific Nox inhibition
Diphenyleneiodonium (DPI) and apocynin are the two most commonly used nonspecific Nox inhibitors. Besides Nox inhibition, DPI inhibits other flavoproteins, such as xanthine oxidase and nitric oxide synthase (145). DPI as a therapy has been primarily studied in diabetic complications where it has cytoprotective effects in complication-prone tissues (61), including peripheral nerves (61, 68). However, its use in vivo is associated with insolubility and toxicity issues (133), rendering DPI a poor therapeutic option.
Apocynin may interfere with ROS detection by chemiluminescence and displays variable efficacy and potency (145). Unlike DPI, apocynin inhibition efficiency in vitro is low and very high concentrations are required for the antioxidant effect (52), which explains why it is more suitable and more commonly used in vivo (133, 145). Experimental evidence shows that apocynin alleviates neuropathic pain in the presence or absence of diabetes (48, 107). Apocynin treatment restored nerve conduction velocity and corrected blood flow and vascular conductance deficits in STZ-induced T1D rats (24). Although available safety data show low toxicity (123), to our knowledge, there are no studies addressing apocynin efficacy in patients with PNS disorders.
Specific Nox inhibition
A specific Nox inhibitor is essential to determine the therapeutic potential of targeting Noxs in PNS diseases. As opposed to global genetic deletion, administering a specific Nox inhibitor at a particular dose in vivo will be crucial for restoring Nox activity back to the homeostatic levels, rather than completely abolishing enzyme activity (134). Accordingly, recent high-throughput screening campaigns have effectively identified compound classes with enhanced selectivity against Nox enzymes, including the orally available, small-molecule Nox1/Nox4 allosteric inhibitors of the pyrazolopyridine chemical series: GKT136901 and its close analogue GKT137831. GKT compounds preferentially inhibit Nox1 and Nox4, and to a lesser extent Nox5 (1).
These dual Nox1/Nox4 inhibitors have gained considerable attention mainly because of their ability to prevent the development of diabetic complications, including DPN in the preclinical setting (30, 44). Specifically, we have shown that GKT137831 treatment improves nerve conduction velocity, sensorimotor deficits, and thermal sensitivity in neuropathic STZ-induced T1D mice, effects attributed to Nox4 inhibition (35). Experimental advances using GKT137831, particularly in the area of diabetic kidney disease, led to a randomized phase II trial in T2D participants with advanced diabetic kidney disease treated with a renin/angiotensin/aldosterone system inhibitor for 12 weeks (Genkyotex Innovation SAS; NCT02010242, GSN000200, completed).
The dual Nox1/Nox4 inhibitor had a favorable safety profile and improved several secondary outcome measures. Unfortunately, it did not effectively improve albuminuria, the primary outcome measure. Many reasons for drug failure were cited, such as inclusion of participants with very advanced kidney disease, short trial duration, low drug dose, as well as the heterogeneous nature of T2D-induced kidney disease relative to type 1 diabetes (133).
Accordingly, a new ongoing clinical trial addressing many of these concerns will shed light on the efficacy of GKT137831 in T1D patients with persistent albuminuria, using a longer treatment duration and a higher drug dose (27) (Genkyotex Innovation SAS; ACTRN12617001187336, UTN U1111-1187-2609). While DPN pathogenesis differs significantly between type 1 diabetes and T2D (15), experimental evidence suggests that Nox4 may be a viable therapeutic target for DPN conserved across diabetes type. It would therefore be interesting to evaluate the therapeutic efficacy of Nox4 inhibition using GKT137831 on DPN in type 1 diabetes, T2D, or preferably both.
In addition to DPN, the neuroprotective effects of the GKT compounds were tested in a rodent model of CIPN (97). GKT137831 improved mechanical and thermal sensitivity in CIPN rats, which was accompanied by reduced neuronal oxidative stress, proinflammatory cytokines, and increased Nrf2 signaling (97).
VAS2870 is a less specific pan-Nox inhibitor with a slight preference for Nox2 inhibition (5). While the therapeutic potential of VAS2870 is unknown for PNS dysfunction, reports show that VAS2870 treatment reduces neurodegeneration and improves neural function in a mouse model of acute ischemic stroke, an effect possibly mediated by Nox2 and/or Nox4 (80, 132). The therapeutic potential of VAS2870 is an area worthy of further investigation in PNS diseases, particularly in the context of neuropathic pain and CIDP, where Nox2 has emerged as a prominent pathogenic factor.
Novel Aspects of Nox Signaling: Potential Relevance to PNS Diseases?
Nox-derived ROS production in microparticles
Over the past decade, there has been a paradigm shift in PNS research, from focusing solely on neurons and their axonal extensions as an isolated system, to studying the interactions between axons and other nerve cell populations, namely Schwann cells and macrophages. Indeed, growing evidence points to the importance of Schwann cells and the Schwann cell/axon cross talk in axon viability and function (38, 135), such as through energy substrate transfer from Schwann cells to axons during periods of high energy demand and ROS scavenging by Schwann cells (6, 137).
Under conditions of metabolic dysfunction, however, studies show that Schwann cells can transfer lipotoxic species into the axon, promoting neurodegeneration (55, 135).
Interestingly, this shuttling activity can be mediated by Schwann cell-derived extracellular vesicles (EVs) under both normal and stress conditions (87, 142). EVs are a heterogenous group of membranous vesicles, released by all cells into body fluids or tissues (41). They mediate intercellular communication by transferring molecular cargo enriched with enzymes, nucleic acids, and metabolites to recipient cells (90). According to their biogenesis and size, EVs are commonly classified into three main types: exosomes (40–150 nm), originating from the endocytic pathway, microparticles (MPs; 100–1000 nm), derived from plasma membranes, and apoptotic bodies (50–5000 nm), released following apoptosis. EVs are implicated in neurodegeneration in disorders of the CNS [reviewed in Hill (54)].
In the PNS, most studies to date focus on the contribution of Schwann cell-derived exosomes to nerve regeneration after axonal injury and DPN through microRNA (miRNA), growth factor, and metabolite transfer (87, 142). Yet, the role of EVs in nerve redox signaling and the effect of oxidative stress on EV molecular cargo remain understudied.
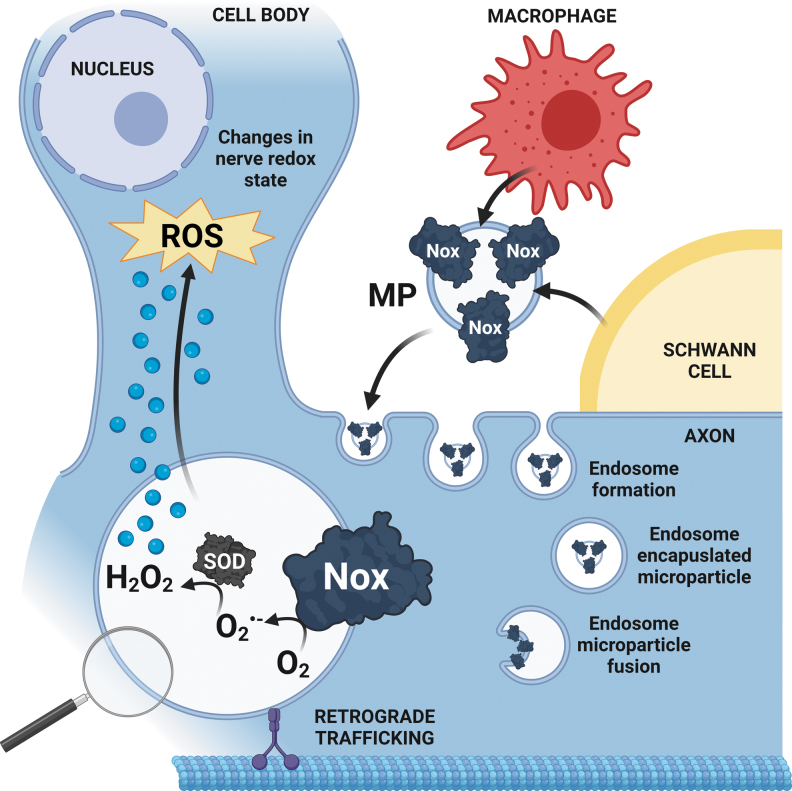
Newly emerging ideas in the Nox field include the concept of intercellular ROS shuttling in MPs (98). The role of MPs has been generally studied in the context of vascular dysfunction, and increased MP levels positively correlate with adverse cardiovascular events and dyslipidemia (8, 106). Interestingly, increasing data implicate MPs in processes such as angiogenesis, vasorelaxation, inflammation, as well as oxidative stress (12, 95, 131), and further suggest that MPs may themselves be metabolically active and generate ROS (12).
Relevant to this review, multiple studies report that endothelium-derived MPs contain the Nox4 isoform as well as the regulatory subunit p22phox and produce Nox-dependent ROS (13, 63, 98). Secreted MPs can disrupt endothelial and vascular smooth muscle cell function in a paracrine/autocrine manner via a pro-oxidative feed-forward loop of injury, leading to apoptosis, inflammation, and impaired vascular tone (12, 13, 98).
Similar to the vasculature, it is tempting to speculate that disruption of the redox status between glial cells and axons, or the transfer of Nox-derived ROS from glia to axons via MPs, may contribute to nerve degeneration and PNS disorders (Fig. 6).
FIG. 6.
Diagram summarizing our proposed mechanism of Nox-containing MPs in PNS diseases. We speculate that Nox-derived ROS are released from macrophages and/or Schwann cells via MPs, and engulfed by axons by endocytosis at the injury site. This ROS-producing MP transfer may disrupt the axo-glial redox state, contributing to nerve degeneration and PNS diseases. MP, microparticle. Created with BioRender.com.
Nox isoform interaction: is it biologically relevant?
As detailed above, PNS cell types simultaneously express several Nox isoforms, each exerting a distinct role (28). Emerging data suggest that these isoforms can interact and regulate ROS generation promoting sustained oxidative stress in disease states (98). Indeed, previous data reported the ability of Nox homologues such as Nox2 and Nox4 to dimerize (139). Furthermore, in cultured human endothelial cells with normal Nox2 and Nox4 expression, Nox5 knockdown is sufficient to abolish Nox-dependent ROS generation (99). In addition, this response is not accompanied by compensatory Nox2 or Nox4 increases, suggesting a potential regulatory effect of Nox5 on ROS generation.
More recently, Jha et al. reported similar results in transgenic models expressing human Nox5 in kidney mesangial cells, highlighting the ability of Nox5 to promote kidney disease progression, even in the absence of Nox4 upregulation (66). Perhaps more importantly, the same group showed that Nox5 may interact with Nox4 to regulate redox signaling in cultured renal cells exposed to metabolic stressors (65). While the precise mechanisms underlying these observations remain unclear, the findings do suggest, at least partly, an interdependent effect of Nox4 and Nox5 on the cellular oxidative response.
Thus, future studies are warranted to determine the relative Nox isoform expression and function in PNS cell types, their interactions, and their independent and interdependent effects on ROS levels. These findings will in turn be critical to understanding the cellular oxidative response and damage in PNS diseases.
Conclusion
In summary, oxidative stress research has evolved from the traditional view that ROS are exclusively harmful with focus on untargeted antioxidant therapies, to new advances centered on understanding the intricacies of redox signaling and the regulation of ROS sources in a cell- and disease-specific manner. The NADPH oxidase family, specialized for ROS generation, appears to be particularly important in the PNS for multiple cellular functions, ranging from neurite outgrowth and tissue repair to pathophysiological implications and neurodegeneration.
How specific Nox isoforms mediate peripheral nerve damage has fostered preclinical research examining the effect of Nox genetic and pharmacological manipulation on nerve function. While Nox inhibitors are currently being tested in the context of PNS diseases, these are not isoform specific and may simultaneously inhibit several Nox isoforms. Thus, the development of isoform-specific Nox inhibitors with improved specificity is more promising for a better understanding of Nox biology in PNS health and disease.
The growing appreciation of the importance of axo-glial metabolic cross talk on nerve health is a recent area of interest among PNS researchers and may be of potential relevance to nerve redox status. Indeed, more research is needed in the area of Nox shuttling in MPs and how Nox isoforms interact to regulate nerve redox status and function in the axo-glial milieu. While much remains to be elucidated, increased experimental and clinical knowledge in the Nox field may facilitate the development of much needed mechanism-based therapies for the treatment of peripheral neuropathies.
Abbreviations Used
- ATP
adenosine triphosphate
- Ca2+
calcium
- CAT
catalase
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CIPN
chemotherapy-induced peripheral neuropathy
- CNS
central nervous system
- COX
cyclooxygenases
- CYP
cytochrome P450 monooxygenases
- DPI
diphenyleneiodonium
- DPN
diabetic peripheral neuropathy
- Duox
dual oxidases
- ER
endoplasmic reticulum
- ETC
electron transport chain
- EV
extracellular vesicle
- FAD
flavin adenine dinucleotide
- Fe
iron
- H2O
water
- H2O2
hydrogen peroxide
- HFD
high-fat diet
- LOX
lipoxygenases
- LXR
liver X receptor
- MP
microparticle
- Nox
NADPH oxidase
- Nrf2
NF-E2-related factor 2
- O2
molecular oxygen
- O2−•
superoxide anion
- •OH
hydroxyl radical
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol (3,4,5)-trisphosphate
- PNS
peripheral nervous system
- PTEN
phosphatase and tensin homologue
- ROS
reactive oxygen species
- RyR
ryanodine receptors
- SOD
superoxide dismutase
- STZ
streptozotocin
- T1D
type 1 diabetic
- T2D
type 2 diabetes
- XO
xanthine oxidase
Authors' Contributions
S.A.E. and E.L.F. contributed equally to the concept and design of the review. S.A.E. had final responsibility for the decision to submit for publication. S.A.E. completed the literature search, drafted, and, with E.L.F., finalized the review. M.G.S., A.A.E., and E.L.F. provided critical review of the article. M.G.S. created the figures, and, with S.A.E., conducted the literature search associated with the figures. All authors have reviewed and approved the article before submission. The review has been submitted solely to Antioxidant & Redox Signaling and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
All authors declare no competing interests.
Funding Information
Funding support is provided by the National Institutes of Health (R24 DK082841 and R21NS102924 to E.L.F.); Novo Nordisk Foundation (NNF14OC0011633 to E.L.F.); Nathan and Rose Milstein Research Fund to S.A.E.; Sinai Medical Staff Foundation Neuroscience Scholar Fund to E.L.F.; NeuroNetwork for Emerging Therapies and the A. Alfred Taubman Medical Research Institute to S.A.E. and E.L.F.
References
- 1. Altenhofer S, Radermacher KA, Kleikers PW, Wingler K, and Schmidt HH. Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement. Antioxid Redox Signal 23: 406–427, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angelova PR and Abramov AY. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett 592: 692–702, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Areti A, Yerra VG, Komirishetty P, and Kumar A. Potential therapeutic benefits of maintaining mitochondrial health in peripheral neuropathies. Curr Neuropharmacol 14: 593–609, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Askwith T, Zeng W, Eggo MC, and Stevens MJ. Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human Schwann cells: implications for pathogenesis of diabetic neuropathy. Am J Physiol Endocrinol Metab 297: E620–E628, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Augsburger F, Filippova A, Rasti D, Seredenina T, Lam M, Maghzal G, Mahiout Z, Jansen-Durr P, Knaus UG, Doroshow J, Stocker R, Krause KH, and Jaquet V. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol 26: 101272, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Babetto E, Wong KM, and Beirowski B. A glycolytic shift in Schwann cells supports injured axons. Nat Neurosci 23: 1215–1228, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benowitz LI and Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol 24: 577–583, 2011. [DOI] [PubMed] [Google Scholar]
- 8. Berezin A, Zulli A, Kerrigan S, Petrovic D, and Kruzliak P. Predictive role of circulating endothelial-derived microparticles in cardiovascular diseases. Clin Biochem 48: 562–568, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Bermudez S, Khayrullina G, Zhao Y, and Byrnes KR. NADPH oxidase isoform expression is temporally regulated and may contribute to microglial/macrophage polarization after spinal cord injury. Mol Cell Neurosci 77: 53–64, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Block K, Gorin Y, and Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boucanova F and Chrast R. Metabolic interaction between Schwann cells and axons under physiological and disease conditions. Front Cell Neurosci 14: 148, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burger D, Montezano AC, Nishigaki N, He Y, Carter A, and Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 31: 1898–1907, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Burger D, Turner M, Munkonda MN, and Touyz RM. Endothelial microparticle-derived reactive oxygen species: role in endothelial signaling and vascular function. Oxid Med Cell Longev 2016: 5047954, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burgoyne JR, Mongue-Din H, Eaton P, and Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 111: 1091–1106, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Callaghan BC, Hur J, and Feldman EL. Diabetic neuropathy: one disease or two? Curr Opin Neurol 25: 536–541, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canta A, Pozzi E, and Carozzi VA. Mitochondrial dysfunction in chemotherapy-induced peripheral neuropathy (CIPN). Toxics 3: 198–223, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao X, Demel SL, Quinn MT, Galligan JJ, and Kreulen D. Localization of NADPH oxidase in sympathetic and sensory ganglion neurons and perivascular nerve fibers. Auton Neurosci 151: 90–97, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carvalho LF, Silva AMF, and Carvalho AA. The use of antioxidant agents for chemotherapy-induced peripheral neuropathy treatment in animal models. Clin Exp Pharmacol Physiol 44: 971–979, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Cavalli E, Mammana S, Nicoletti F, Bramanti P, and Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol 33, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen F, Haigh S, Yu Y, Benson T, Wang Y, Li X, Dou H, Bagi Z, Verin AD, Stepp DW, Csanyi G, Chadli A, Weintraub NL, Smith SM, and Fulton DJ. Nox5 stability and superoxide production is regulated by C-terminal binding of Hsp90 and CO-chaperones. Free Radic Biol Med 89: 793–805, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen JL, Lu JH, Xie CS, Shen YJ, Wang JW, Ye XY, Zhang MB, Jia GL, Tao YX, Li J, and Cao H. Caveolin-1 in spinal cord modulates type-2 diabetic neuropathic pain through the Rac1/NOX2/NR2B signaling pathway. Am J Transl Res 12: 1714–1727, 2020. [PMC free article] [PubMed] [Google Scholar]
- 22. Chen K, Kirber MT, Xiao H, Yang Y, and Keaney JF Jr. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowdhury SK, Zherebitskaya E, Smith DR, Akude E, Chattopadhyay S, Jolivalt CG, Calcutt NA, and Fernyhough P. Mitochondrial respiratory chain dysfunction in dorsal root ganglia of streptozotocin-induced diabetic rats and its correction by insulin treatment. Diabetes 59: 1082–1091, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cotter MA and Cameron NE. Effect of the NAD(P)H oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci 73: 1813–1824, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Dahan I, Smith SM, and Pick E. A Cys-Gly-Cys triad in the dehydrogenase region of Nox2 plays a key role in the interaction with p67phox. J Leukoc Biol 98: 859–874, 2015. [DOI] [PubMed] [Google Scholar]
- 26. De Deken X, Corvilain B, Dumont JE, and Miot F. Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid Redox Signal 20: 2776–2793, 2014. [DOI] [PubMed] [Google Scholar]
- 27. De Livera AM, Reutens A, Cooper M, Thomas M, Jandeleit-Dahm K, Shaw JE, and Salim A. Evaluating the efficacy and safety of GKT137831 in adults with type 1 diabetes and persistently elevated urinary albumin excretion: a statistical analysis plan. Trials 21: 459, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Logu F, Nassini R, Materazzi S, Carvalho Goncalves M, Nosi D, Rossi Degl'Innocenti D, Marone IM, Ferreira J, Li Puma S, Benemei S, Trevisan G, Souza Monteiro de Araujo D, Patacchini R, Bunnett NW, and Geppetti P. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat Commun 8: 1887, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Virgiliis F, Hutson TH, Palmisano I, Amachree S, Miao J, Zhou L, Todorova R, Thompson R, Danzi MC, Lemmon VP, Bixby JL, Wittig I, Shah AM, and Di Giovanni S. Enriched conditioning expands the regenerative ability of sensory neurons after spinal cord injury via neuronal intrinsic redox signaling. Nat Commun 11: 6425, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deliyanti D, Alrashdi SF, Touyz RM, Kennedy CR, Jha JC, Cooper ME, Jandeleit-Dahm KA, and Wilkinson-Berka JL. Nox (NADPH oxidase) 1, Nox4, and Nox5 promote vascular permeability and neovascularization in retinopathy. Hypertension 75: 1091–1101, 2020. [DOI] [PubMed] [Google Scholar]
- 31. Di Cesare Mannelli L, Zanardelli M, Failli P, and Ghelardini C. Oxaliplatin-induced oxidative stress in nervous system-derived cellular models: could it correlate with in vivo neuropathy? Free Radic Biol Med 61: 143–150, 2013. [DOI] [PubMed] [Google Scholar]
- 32. Doyle T, Chen Z, Muscoli C, Bryant L, Esposito E, Cuzzocrea S, Dagostino C, Ryerse J, Rausaria S, Kamadulski A, Neumann WL, and Salvemini D. Targeting the overproduction of peroxynitrite for the prevention and reversal of paclitaxel-induced neuropathic pain. J Neurosci 32: 6149–6160, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duggett NA, Griffiths LA, McKenna OE, de Santis V, Yongsanguanchai N, Mokori EB, and Flatters SJ. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 333: 13–26, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eid S. NOX, NOX, are you here? The emerging role of NOX5 in diabetic neuropathy. Orlando, FL: American Diabetes Association, 2018. [Google Scholar]
- 35. Eid SA, El Massry M, Hichor M, Haddad M, Grenier J, Dia B, Barakat R, Boutary S, Chanal J, Aractingi S, Wiesel P, Szyndralewiez C, Azar ST, Boitard C, Zaatari G, Eid AA, and Massaad C. Targeting the NADPH oxidase-4 and liver X receptor pathway preserves Schwann cell integrity in diabetic mice. Diabetes 69: 448–464, 2020. [DOI] [PubMed] [Google Scholar]
- 36. El-Benna J, Dang PM, and Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 30: 279–289, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, and Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers 5: 41, 2019. [DOI] [PubMed] [Google Scholar]
- 38. Feldman EL, Nave KA, Jensen TS, and Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93: 1296–1313, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fulton DJR. The molecular regulation and functional roles of NOX5. Methods Mol Biol 1982: 353–375, 2019. [DOI] [PubMed] [Google Scholar]
- 40. Gamper N and Ooi L. Redox and nitric oxide-mediated regulation of sensory neuron ion channel function. Antioxid Redox Signal 22: 486–504, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gangoda L, Boukouris S, Liem M, Kalra H, and Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15: 260–271, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geis C, Geuss E, Sommer C, Schmidt HH, and Kleinschnitz C. NOX4 is an early initiator of neuropathic pain. Exp Neurol 288: 94–103, 2017. [DOI] [PubMed] [Google Scholar]
- 43. Girach A, Julian TH, Varrassi G, Paladini A, Vadalouka A, and Zis P. Quality of life in painful peripheral neuropathies: a systematic review. Pain Res Manag 2019: 2091960, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, Fanti P, Szyndralewiez C, Barnes JL, Block K, and Abboud HE. Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am J Physiol Renal Physiol 308: F1276–F1287, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gorson KC, van Schaik IN, Merkies IS, Lewis RA, Barohn RJ, Koski CL, Cornblath DR, Hughes RA, Hahn AF, Baumgarten M, Goldstein J, Katz J, Graves M, Parry G, and van Doorn PA. Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst 15: 326–333, 2010. [DOI] [PubMed] [Google Scholar]
- 46. Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, and Geiss L; 1999–2000 National Health and Nutrition Examination Survey. Prevalence of lower-extremity disease in the US adult population > = 40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care 27: 1591–1597, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Guo K, Elzinga S, Eid S, Figueroa-Romero C, Hinder LM, Pacut C, Feldman EL, and Hur J. Genome-wide DNA methylation profiling of human diabetic peripheral neuropathy in subjects with type 2 diabetes mellitus. Epigenetics 14: 766–779, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hassler SN, Johnson KM, and Hulsebosch CE. Reactive oxygen species and lipid peroxidation inhibitors reduce mechanical sensitivity in a chronic neuropathic pain model of spinal cord injury in rats. J Neurochem 131: 413–417, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Helmcke I, Heumuller S, Tikkanen R, Schroder K, and Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009. [DOI] [PubMed] [Google Scholar]
- 50. Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, Hutson T, Danzi MC, Perry RB, Santos CXC, Kapustin AN, Fleck RA, Del Rio JA, Carroll T, Lemmon V, Bixby JL, Shah AM, Fainzilber M, and Di Giovanni S. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol 20: 307–319, 2018. [DOI] [PubMed] [Google Scholar]
- 51. Hervera A, Santos CX, De Virgiliis F, Shah AM, and Di Giovanni S. Paracrine mechanisms of redox signalling for postmitotic cell and tissue regeneration. Trends Cell Biol 29: 514–530, 2019. [DOI] [PubMed] [Google Scholar]
- 52. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 53. Hichor M, Sundaram VK, Eid SA, Abdel-Rassoul R, Petit PX, Borderie D, Bastin J, Eid AA, Manuel M, Grenier J, and Massaad C. Liver X receptor exerts a protective effect against the oxidative stress in the peripheral nerve. Sci Rep 8: 2524, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hill AF. Extracellular vesicles and neurodegenerative diseases. J Neurosci 39: 9269–9273, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hinder LM, Figueroa-Romero C, Pacut C, Hong Y, Vivekanandan-Giri A, Pennathur S, and Feldman EL. Long-chain acyl coenzyme A synthetase 1 overexpression in primary cultured Schwann cells prevents long chain fatty acid-induced oxidative stress and mitochondrial dysfunction. Antioxid Redox Signal 21: 588–600, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hinder LM, Vivekanandan-Giri A, McLean LL, Pennathur S, and Feldman EL. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J Endocrinol 216: 1–11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hong S, Agresta L, Guo C, and Wiley JW. The TRPV1 receptor is associated with preferential stress in large dorsal root ganglion neurons in early diabetic sensory neuropathy. J Neurochem 105: 1212–1222, 2008. [DOI] [PubMed] [Google Scholar]
- 58. Ibi M, Katsuyama M, Fan C, Iwata K, Nishinaka T, Yokoyama T, and Yabe-Nishimura C. NOX1/NADPH oxidase negatively regulates nerve growth factor-induced neurite outgrowth. Free Radic Biol Med 40: 1785–1795, 2006. [DOI] [PubMed] [Google Scholar]
- 59. Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango K, Shirai Y, Yokoyama T, Kaneko S, Saito N, and Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci 28: 9486–9494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jaggi AS, Jain V, and Singh N. Animal models of neuropathic pain. Fundam Clin Pharmacol 25: 1–28, 2011. [DOI] [PubMed] [Google Scholar]
- 61. Jaimes EA, Hua P, Tian RX, and Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol 298: F125–F132, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Janes K, Esposito E, Doyle T, Cuzzocrea S, Tosh DK, Jacobson KA, and Salvemini D. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain 155: 2560–2567, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J, Nickenig G, and Werner N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res 98: 94–106, 2013. [DOI] [PubMed] [Google Scholar]
- 64. Jensen TS and Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 13: 924–935, 2014. [DOI] [PubMed] [Google Scholar]
- 65. Jha JC. The relative roles of pro-oxidant enzymes Nox4 and Nox5 in diabetic kidney disease. San Francisco, CA: American Diabetes Association, 2019. [Google Scholar]
- 66. Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, Thallas-Bonke V, De Vos L, Holterman CE, Coughlan MT, Power DA, Skene A, Ekinci EI, Cooper ME, Touyz RM, Kennedy CR, and Jandeleit-Dahm K. NADPH oxidase Nox5 accelerates renal injury in diabetic nephropathy. Diabetes 66: 2691–2703, 2017. [DOI] [PubMed] [Google Scholar]
- 67. Jha JC, Dai A, Holterman CE, Cooper ME, Touyz RM, Kennedy CR, and Jandeleit-Dahm KAM. Endothelial or vascular smooth muscle cell-specific expression of human NOX5 exacerbates renal inflammation, fibrosis and albuminuria in the Akita mouse. Diabetologia 62: 1712–1726, 2019. [DOI] [PubMed] [Google Scholar]
- 68. Ji ZH, Liu ZJ, Liu ZT, Zhao W, Williams BA, Zhang HF, Li L, and Xu SY. Diphenyleneiodonium mitigates bupivacaine-induced sciatic nerve damage in a diabetic neuropathy rat model by attenuating oxidative stress. Anesth Analg 125: 653–661, 2017. [DOI] [PubMed] [Google Scholar]
- 69. Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, and Chen PC. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci 1147: 61–69, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kallenborn-Gerhardt W, Hohmann SW, Syhr KM, Schroder K, Sisignano M, Weigert A, Lorenz JE, Lu R, Brune B, Brandes RP, Geisslinger G, and Schmidtko A. Nox2-dependent signaling between macrophages and sensory neurons contributes to neuropathic pain hypersensitivity. Pain 155: 2161–2170, 2014. [DOI] [PubMed] [Google Scholar]
- 71. Kallenborn-Gerhardt W, Lu R, Syhr KM, Heidler J, von Melchner H, Geisslinger G, Bangsow T, and Schmidtko A. Antioxidant activity of sestrin 2 controls neuropathic pain after peripheral nerve injury. Antioxid Redox Signal 19: 2013–2023, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kallenborn-Gerhardt W, Schroder K, Del Turco D, Lu R, Kynast K, Kosowski J, Niederberger E, Shah AM, Brandes RP, Geisslinger G, and Schmidtko A. NADPH oxidase-4 maintains neuropathic pain after peripheral nerve injury. J Neurosci 32: 10136–10145, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kallenborn-Gerhardt W, Schroder K, Geisslinger G, and Schmidtko A. NOXious signaling in pain processing. Pharmacol Ther 137: 309–317, 2013. [DOI] [PubMed] [Google Scholar]
- 74. Kamil K, Yazid MD, Idrus RBH, Das S, and Kumar J. Peripheral demyelinating diseases: from biology to translational medicine. Front Neurol 10: 87, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kelley MR, Jiang Y, Guo C, Reed A, Meng H, and Vasko MR. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS One 9: e106485, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khan SJ, Abidi SNF, Skinner A, Tian Y, and Smith-Bolton RK. The Drosophila Duox maturation factor is a key component of a positive feedback loop that sustains regeneration signaling. PLoS Genet 13: e1006937, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim D, You B, Jo EK, Han SK, Simon MI, and Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A 107: 14851–14856, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim du S, An JM, Lee HG, Seo SR, Kim SS, Kim JY, Kang JW, Bae YS, and Seo JT. Activation of Rac1-dependent redox signaling is critically involved in staurosporine-induced neurite outgrowth in PC12 cells. Free Radic Res 47: 95–103, 2013. [DOI] [PubMed] [Google Scholar]
- 79. Kim SJ, Kim HS, and Seo YR. Understanding of ROS-inducing strategy in anticancer therapy. Oxid Med Cell Longev 2019: 5381692, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, Barthel K, Schuhmann MK, Herrmann AM, Meuth SG, Stoll G, Meurer S, Schrewe A, Becker L, Gailus-Durner V, Fuchs H, Klopstock T, de Angelis MH, Jandeleit-Dahm K, Shah AM, Weissmann N, and Schmidt HH. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8: e1000479, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Koziel R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, and Jansen-Durr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J 452: 231–239, 2013. [DOI] [PubMed] [Google Scholar]
- 82. Kratsovnik E, Bromberg Y, Sperling O, and Zoref-Shani E. Oxidative stress activates transcription factor NF-kB-mediated protective signaling in primary rat neuronal cultures. J Mol Neurosci 26: 27–32, 2005. [DOI] [PubMed] [Google Scholar]
- 83. Laczy B, Cseh J, Mohas M, Marko L, Tamasko M, Koszegi T, Molnar GA, Wagner Z, Wagner L, and Wittmann I. Effects of pentoxifylline and pentosan polysulphate combination therapy on diabetic neuropathy in type 2 diabetes mellitus. Acta Diabetol 46: 105–111, 2009. [DOI] [PubMed] [Google Scholar]
- 84. Lavinsky J, Crow AL, Pan C, Wang J, Aaron KA, Ho MK, Li Q, Salehide P, Myint A, Monges-Hernadez M, Eskin E, Allayee H, Lusis AJ, and Friedman RA. Genome-wide association study identifies nox3 as a critical gene for susceptibility to noise-induced hearing loss. PLoS Genet 11: e1005094, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lehmann HC, Burke D, and Kuwabara S. Chronic inflammatory demyelinating polyneuropathy: update on diagnosis, immunopathogenesis and treatment. J Neurol Neurosurg Psychiatry 90: 981–987, 2019. [DOI] [PubMed] [Google Scholar]
- 86. Lim H, Kim D, and Lee SJ. Toll-like receptor 2 mediates peripheral nerve injury-induced NADPH oxidase 2 expression in spinal cord microglia. J Biol Chem 288: 7572–7579, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lopez-Leal R and Court FA. Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol 36: 429–436, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lv W, Deng B, Duan W, Li Y, Liu Y, Li Z, Xia W, and Li C. Schwann cell plasticity is regulated by a weakened intrinsic antioxidant defense system in acute peripheral nerve injury. Neuroscience 382: 1–13, 2018. [DOI] [PubMed] [Google Scholar]
- 89. Maihofner C, Diel I, Tesch H, Quandel T, and Baron R. Chemotherapy-induced peripheral neuropathy (CIPN): current therapies and topical treatment option with high-concentration capsaicin. Support Care Cancer 29: 4223–4238, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Margolis L and Sadovsky Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol 17: e3000363, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Marrali G, Salamone P, Casale F, Fuda G, Cugnasco P, Caorsi C, Amoroso A, Calvo A, Lopiano L, Cocito D, and Chio A. NADPH oxidase 2 (NOX2) enzyme activation in patients with chronic inflammatory demyelinating polyneuropathy. Eur J Neurol 23: 958–963, 2016. [DOI] [PubMed] [Google Scholar]
- 92. Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. [DOI] [PubMed] [Google Scholar]
- 93. Marzaioli V, Hurtado-Nedelec M, Pintard C, Tlili A, Marie JC, Monteiro RC, Gougerot-Pocidalo MA, Dang PM, and El-Benna J. NOX5 and p22phox are 2 novel regulators of human monocytic differentiation into dendritic cells. Blood 130: 1734–1745, 2017. [DOI] [PubMed] [Google Scholar]
- 94. McCormick B, Lowes DA, Colvin L, Torsney C, and Galley HF. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. Br J Anaesth 117: 659–666, 2016. [DOI] [PubMed] [Google Scholar]
- 95. Mezentsev A, Merks RM, O'Riordan E, Chen J, Mendelev N, Goligorsky MS, and Brodsky SV. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol 289: H1106–H1114, 2005. [DOI] [PubMed] [Google Scholar]
- 96. Miao F, Wang R, Cui G, Li X, Wang T, and Li X. Engagement of microRNA-155 in exaggerated oxidative stress signal and TRPA1 in the dorsal horn of the spinal cord and neuropathic pain during chemotherapeutic oxaliplatin. Neurotox Res 36: 712–723, 2019. [DOI] [PubMed] [Google Scholar]
- 97. Miao H, Xu J, Xu D, Ma X, Zhao X, and Liu L. Nociceptive behavior induced by chemotherapeutic paclitaxel and beneficial role of antioxidative pathways. Physiol Res 68: 491–500, 2019. [DOI] [PubMed] [Google Scholar]
- 98. Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, and Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 120: 131–141, 2011. [DOI] [PubMed] [Google Scholar]
- 99. Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, and Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106: 1363–1373, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mossberg N, Movitz C, Hellstrand K, Bergstrom T, Nilsson S, and Andersen O. Oxygen radical production in leukocytes and disease severity in multiple sclerosis. J Neuroimmunol 213: 131–134, 2009. [DOI] [PubMed] [Google Scholar]
- 101. Munnamalai V, Weaver CJ, Weisheit CE, Venkatraman P, Agim ZS, Quinn MT, and Suter DM. Bidirectional interactions between NOX2-type NADPH oxidase and the F-actin cytoskeleton in neuronal growth cones. J Neurochem 130: 526–540, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nauseef WM. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr Opin Immunol 60: 130–140, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Nayernia Z, Jaquet V, and Krause KH. New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal 20: 2815–2837, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Niethammer P, Grabher C, Look AT, and Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459: 996–999, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nisimoto Y, Diebold BA, Cosentino-Gomes D, and Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry 53: 5111–5120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nomura S, Inami N, Shouzu A, Omoto S, Kimura Y, Takahashi N, Tanaka A, Urase F, Maeda Y, Ohtani H, and Iwasaka T. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets 20: 16–22, 2009. [DOI] [PubMed] [Google Scholar]
- 107. Olukman M, Onal A, Celenk FG, Uyanikgil Y, Cavusoglu T, Duzenli N, and Ulker S. Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats. Neural Regen Res 13: 1657–1664, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Paulsen CE and Carroll KS. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev 113: 4633–4679, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, and Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract 2012: 913848, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pop-Busui R, Stevens MJ, Raffel DM, White EA, Mehta M, Plunkett CD, Brown MB, and Feldman EL. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia 56: 1835–1844, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Puntambekar P, Mukherjea D, Jajoo S, and Ramkumar V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem 95: 1689–1703, 2005. [DOI] [PubMed] [Google Scholar]
- 112. Rastogi R, Geng X, Li F, and Ding Y. NOX activation by subunit interaction and underlying mechanisms in disease. Front Cell Neurosci 10: 301, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rieger S and Sagasti A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol 9: e1000621, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Saito A and Cavalli V. Signaling over distances. Mol Cell Proteomics 15: 382–393, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sasaki Y. Metabolic aspects of neuronal degeneration: from a NAD(+) point of view. Neurosci Res 139: 9–20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schmidt AP, Bohmer AE, Antunes C, Schallenberger C, Porciuncula LO, Elisabetsky E, Lara DR, and Souza DO. Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: role of A1 adenosine receptors. Br J Pharmacol 156: 163–172, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shanmugasundaram K, Nayak BK, Friedrichs WE, Kaushik D, Rodriguez R, and Block K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat Commun 8: 997, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Si J, Fu X, Behar J, Wands J, Beer DG, Souza RF, Spechler SJ, Lambeth D, and Cao W. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-kappaB in Barrett's esophageal adenocarcinoma cells. J Biol Chem 282: 16244–16255, 2007. [DOI] [PubMed] [Google Scholar]
- 119. Sies H and Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 21: 363–383, 2020. [DOI] [PubMed] [Google Scholar]
- 120. Sirokmany G, Donko A, and Geiszt M. Nox/Duox family of NADPH oxidases: lessons from knockout mouse models. Trends Pharmacol Sci 37: 318–327, 2016. [DOI] [PubMed] [Google Scholar]
- 121. Sommer C and Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361: 184–187, 2004. [DOI] [PubMed] [Google Scholar]
- 122. Sorce S, Krause KH, and Jaquet V. Targeting NOX enzymes in the central nervous system: therapeutic opportunities. Cell Mol Life Sci 69: 2387–2407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stefanska J and Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm 2008: 106507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sun Y, Lu Y, Saredy J, Wang X, Drummer Iv C, Shao Y, Saaoud F, Xu K, Liu M, Yang WY, Jiang X, Wang H, and Yang X. ROS systems are a new integrated network for sensing homeostasis and alarming stresses in organelle metabolic processes. Redox Biol 37: 101696, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, and Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem 275: 13175–13178, 2000. [DOI] [PubMed] [Google Scholar]
- 126. Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, and Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Takahashi K, Mizukami H, Osonoi S, Ogasawara S, Hara Y, Kudoh K, Takeuchi Y, Sasaki T, Daimon M, and Yagihashi S. Inhibitory effects of xanthine oxidase inhibitor, topiroxostat, on development of neuropathy in db/db mice. Neurobiol Dis 155: 105392, 2021. [DOI] [PubMed] [Google Scholar]
- 128. Takahashi M, Kawaguchi M, Shimada K, Konishi N, Furuya H, and Nakashima T. Cyclooxygenase-2 expression in Schwann cells and macrophages in the sciatic nerve after single spinal nerve injury in rats. Neurosci Lett 363: 203–206, 2004. [DOI] [PubMed] [Google Scholar]
- 129. Terzi A and Suter DM. The role of NADPH oxidases in neuronal development. Free Radic Biol Med 154: 33–47, 2020. [DOI] [PubMed] [Google Scholar]
- 130. Touyz RM, Anagnostopoulou A, Camargo LL, Rios FJ, and Montezano AC. Vascular biology of superoxide-generating NADPH oxidase 5-implications in hypertension and cardiovascular disease. Antioxid Redox Signal 30: 1027–1040, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tripathi D, Biswas B, Manhas A, Singh A, Goyal D, Gaestel M, and Jagavelu K. Proinflammatory effect of endothelial microparticles is mitochondria mediated and modulated through MAPKAPK2 (MAPK-activated protein kinase 2) leading to attenuation of cardiac hypertrophy. Arterioscler Thromb Vasc Biol 39: 1100–1112, 2019. [DOI] [PubMed] [Google Scholar]
- 132. Tuo YH, Liu Z, Chen JW, Wang QY, Li SL, Li MC, Dai G, Wang JS, Zhang YL, Feng L, and Shi ZS. NADPH oxidase inhibitor improves outcome of mechanical reperfusion by suppressing hemorrhagic transformation. J Neurointerv Surg 9: 492–498, 2017. [DOI] [PubMed] [Google Scholar]
- 133. Urner S, Ho F, Jha JC, Ziegler D, and Jandeleit-Dahm K. NADPH oxidase inhibition: preclinical and clinical studies in diabetic complications. Antioxid Redox Signal 33: 415–434, 2020. [DOI] [PubMed] [Google Scholar]
- 134. van de Loo FA, Bennink MB, Arntz OJ, Smeets RL, Lubberts E, Joosten LA, van Lent PL, Coenen-de Roo CJ, Cuzzocrea S, Segal BH, Holland SM, and van den Berg WB. Deficiency of NADPH oxidase components p47phox and gp91phox caused granulomatous synovitis and increased connective tissue destruction in experimental arthritis models. Am J Pathol 163: 1525–1537, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Viader A, Sasaki Y, Kim S, Strickland A, Workman CS, Yang K, Gross RW, and Milbrandt J. Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 77: 886–898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vincent AM, Hayes JM, McLean LL, Vivekanandan-Giri A, Pennathur S, and Feldman EL. Dyslipidemia-induced neuropathy in mice: the role of oxLDL/LOX-1. Diabetes 58: 2376–2385, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Vincent AM, Kato K, McLean LL, Soules ME, and Feldman EL. Sensory neurons and Schwann cells respond to oxidative stress by increasing antioxidant defense mechanisms. Antioxid Redox Signal 11: 425–438, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vincent AM, McLean LL, Backus C, and Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J 19: 638–640, 2005. [DOI] [PubMed] [Google Scholar]
- 139. von Lohneysen K, Noack D, Wood MR, Friedman JS, and Knaus UG. Structural insights into Nox4 and Nox2: motifs involved in function and cellular localization. Mol Cell Biol 30: 961–975, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wack G, Metzner K, Kuth MS, Wang E, Bresnick A, Brandes RP, Schroder K, Wittig I, Schmidtko A, and Kallenborn-Gerhardt W. Nox4-dependent upregulation of S100A4 after peripheral nerve injury modulates neuropathic pain processing. Free Radic Biol Med 168: 155–167, 2021. [DOI] [PubMed] [Google Scholar]
- 141. Wang JT, Medress ZA, and Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol 196: 7–18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wang L, Chopp M, Szalad A, Lu X, Zhang Y, Wang X, Cepparulo P, Lu M, Li C, and Zhang ZG. Exosomes derived from Schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes 69: 749–759, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wilson C, Munoz-Palma E, and Gonzalez-Billault C. From birth to death: a role for reactive oxygen species in neuronal development. Semin Cell Dev Biol 80: 43–49, 2018. [DOI] [PubMed] [Google Scholar]
- 144. Wilson C, Munoz-Palma E, Henriquez DR, Palmisano I, Nunez MT, Di Giovanni S, and Gonzalez-Billault C. A feed-forward mechanism involving the NOX complex and RyR-mediated Ca2+ release during axonal specification. J Neurosci 36: 11107–11119, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, and Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wu RF, Ma Z, Liu Z, and Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol Cell Biol 30: 3553–3568, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wu Y, Xu D, Zhu X, Yang G, and Ren M. MiR-106a associated with diabetic peripheral neuropathy through the regulation of 12/15-LOX-meidiated oxidative/nitrative stress. Curr Neurovasc Res 14: 117–124, 2017. [DOI] [PubMed] [Google Scholar]
- 148. Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, and Liang X. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res 37: 266, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yang Y, Luo L, Cai X, Fang Y, Wang J, Chen G, Yang J, Zhou Q, Sun X, Cheng X, Yan H, Lu W, Hu C, and Cao P. Nrf2 inhibits oxaliplatin-induced peripheral neuropathy via protection of mitochondrial function. Free Radic Biol Med 120: 13–24, 2018. [DOI] [PubMed] [Google Scholar]
- 150. Ye X, Qiu Y, Gao Y, Wan D, and Zhu H. A subtle network mediating axon guidance: intrinsic dynamic structure of growth cone, attractive and repulsive molecular cues, and the intermediate role of signaling pathways. Neural Plast 2019: 1719829, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhang Y, Song C, Liu J, Bi Y, and Li H. Inhibition of miR-25 aggravates diabetic peripheral neuropathy. Neuroreport 29: 945–953, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhao WC, Zhang B, Liao MJ, Zhang WX, He WY, Wang HB, and Yang CX. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett 560: 81–85, 2014. [DOI] [PubMed] [Google Scholar]
- 153. Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, Ugurbil K, and Chen W. Quantitative imaging of energy expenditure in human brain. Neuroimage 60: 2107–2117, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, Schutte K, and Dyck PJ. Efficacy and safety of antioxidant treatment with alpha-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care 34: 2054–2060, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]