Abstract
Objective:
To evaluate the insulin-only configuration of the iLet® bionic pancreas (BP) using insulin aspart or insulin lispro in adults with type 1 diabetes (T1D).
Methods:
In this multicenter, randomized, controlled trial, 161 adults with T1D (18–79 years old, baseline HbA1c 5.5%–13.1%, 32% using multiple daily injections, 27% using a pump without automation, 5% using a pump with predictive low glucose suspend, and 36% using a hybrid closed loop system before the study) were randomly assigned 2:1 to use the BP (N = 107) with insulin aspart or insulin lispro (BP group) or a standard-of-care (SC) control group (N = 54) using their usual insulin delivery plus continuous glucose monitoring (CGM). The primary outcome was HbA1c at 13 weeks.
Results:
Mean HbA1c decreased from 7.6% ± 1.2% at baseline to 7.1% ± 0.6% at 13 weeks with BP versus 7.6% ± 1.2% to 7.5% ± 0.9% with SC (adjusted difference = −0.5%, 95% confidence interval −0.6% to −0.3%, P < 0.001). Over 13 weeks, mean time in range 70–180 mg/dL (TIR) increased by 11% (2.6 h/d) and mean CGM glucose was reduced by 16 mg/dL with BP compared with SC (P < 0.001). Improvement in these metrics was seen during the first day of BP use and by the end of the first week reached levels that remained relatively stable through 13 weeks. Analyses of time >180 mg/dL, time >250 mg/dL, and standard deviation of CGM glucose all favored the BP group (P < 0.001). The CGM-measured hypoglycemia was low at baseline (median time <54 mg/dL of 0.21% [3 min/d] for the BP group and 0.11% [1.6 min/d] for the SC group) and not significantly different between groups over the 13 weeks (P = 0.51 for time <70 mg/dL and 0.33 for time <54 mg/dL). There were 7 (6.5% of 107 participants) severe hypoglycemic events in the BP group and 2 events in the SC group (1.9% of 54 participants, P = 0.40).
Conclusions:
In adults with T1D, use of the BP with insulin aspart or insulin lispro improved HbA1c, TIR, and hyperglycemic metrics without increasing CGM-measured hypoglycemia compared with standard of care. Clinical Trial Registry: clinicaltrials.gov; NCT04200313.
Keywords: Artificial pancreas, Bionic pancreas, Evaluation, Automated insulin delivery, Adult, type 1 diabetes
Introduction
The development and progression of complications of type 1 diabetes (T1D) can be reduced if glucose levels are kept near the normal range.1 This is difficult to achieve, and glycemic goals set by the American Diabetes Association2 are met in only ∼20% of adults with T1D.3,4 Insulin delivery systems that use algorithms to adjust insulin delivery in response to glucose levels measured with continuous glucose monitoring (CGM) have the potential to increase the number of people with diabetes who meet goals for therapy.5
Current commercially available systems that automate insulin delivery are referred to as hybrid closed loop (HCL) systems since they partially automate insulin delivery but still require actions on the part of the health care provider and user. This includes requiring the user to enter an estimate of the grams of carbohydrate in a meal and then initiate a meal bolus and to treat hyperglycemia as needed or desired by initiating correction doses of insulin.
These systems also require determination and programming of multiple settings before they are used, which typically include insulin basal rates, insulin-to-carbohydrates ratios, insulin sensitivity factors, glucose targets, active insulin time, and/or total daily dose (TDD) of insulin.
In contrast, the iLet® bionic pancreas (BP; Beta Bionics, Inc.) is an automated insulin delivery system initialized only with body weight and without requiring the input of any information about previous insulin dosing. All insulin titration, including for meals, is determined autonomously by the BP insulin-dosing algorithms and cannot be modified by the user or health care provider. These algorithms autonomously determine and continually adapt basal insulin doses, correction insulin doses, and meal-announcement doses to meet the individual's insulin needs in response to the CGM input signal to the BP.
Meals are announced by the user without carbohydrate counting as “Usual For Me,” “More” (around 50% more than usual), or “Less” (around 50% less than usual) than other meals of the same type (i.e., “Breakfast,” “Lunch,” “Dinner”). In response to these qualitative meal announcements, the system delivers ∼75% of the autonomously estimated insulin need immediately, and then will autonomously add or refrain from additional basal or correction insulin dosing post-prandially, as necessary.
When CGM data are not available, the BP continues to make all insulin-dosing decisions autonomously, based on a basal insulin profile determined, continually updated, and stored by the BP when CGM data are available, and in response to any entered blood glucose values obtained from a capillary glucometer. Insulin dosing can be maintained, increased, or temporarily suspended, autonomously by the BP, in response to the entered blood-glucose values. The BP has been developed both as an insulin-only system and as a bihormonal system that doses both insulin and glucagon.
We conducted a multicenter randomized trial of adults and youth 6–79 years old with T1D to evaluate the efficacy and safety of the insulin-only configuration of the BP, using insulin aspart or insulin lispro. The standard-of-care (SC) control group continued their pre-study subcutaneous insulin delivery (either multiple daily injections [MDI], an insulin pump without automation of insulin delivery, an insulin pump with predictive low glucose suspend feature, or an insulin pump as part of an HCL system) in conjunction with real-time CGM. Herein, we report the results of the trial in adults ≥18 years old.
Methods
This parallel group multi-center randomized trial enrolled adults (≥18 years old) with T1D at 13 diabetes centers in the United States.6 The protocol was approved by a central institutional review board, and written informed consent was obtained from each participant. An investigational device exemption for the conduct of the trial was approved by the US Food and Drug Administration. The full protocol is available at https://www.jaeb.org/finaliobp, and key aspects are summarized herein. The trial and the randomization also included an adult cohort using fast-acting insulin aspart, the results for which are reported elsewhere.7
Recruitment and screening
To be eligible for the trial, participants had to have T1D treated with insulin for at least 1 year by MDI or pump therapy with or without CGM or HCL. There was no restriction on HbA1c level and no exclusion for prior severe hypoglycemia events or prior diabetic ketoacidosis events. A complete list of inclusion and exclusion criteria is available in the protocol and at clinicaltrials.gov (NCT04200313). To enroll participants with characteristics as similar as possible to the general population of people with T1D, recruitment goals aimed for at least 33% using MDI therapy, at least 33% with HbA1c ≥8.0%, at least 33% with age ≥50 years; and at most 20% with HbA1c <7.0%.
Participants using a personal Dexcom G6 CGM System (Dexcom, Inc.) who had ≥85% of possible glucose data during the 14 days before the screening visit could proceed directly to randomization once eligibility was confirmed. All other participants completed a 14-day baseline data collection period using a Dexcom G6 CGM and were required to have at least 85% of CGM values during the 14 days before proceeding to randomization. Participants using a personal Dexcom G5 or G6 CGM used an unblinded G6 sensor, whereas all others wore a blinded G6 CGM. If participants used a non-Dexcom CGM, they were encouraged to continue its use during the baseline data collection period (while wearing the blinded Dexcom CGM).
Randomization and treatment groups
Randomization was performed on the study website using a computer-generated sequence with a permuted block design, stratified by site. Participants were randomly assigned in a 2:1 ratio to use of the BP with insulin lispro or insulin aspart (BP group) or standard care insulin delivery plus use of an unblinded real-time Dexcom G6 CGM (SC group).
Participants assigned to the BP group were provided with the iLet pump that is part of the BP system, Dexcom G6 sensors and transmitters, insulin infusion sets (Inset I, Unomedical) to deliver insulin subcutaneously, a Contour®Next One Blood Glucose Monitoring System (Ascensia Diabetes Care, Basel, Switzerland) and test strips, and a Precision Xtra ketone meter (Abbott Diabetes Care) and test strips. Participants already using insulin aspart or an insulin lispro filled 1.6 mL glass, ready-to-fill cartridges with their personal insulin vials whereas participants using pens or a different insulin were provided with insulin aspart or insulin lispro in 10 mL vials.
Participants were trained on the use of the BP system and given educational materials on use of the system, including specific written and video instructions for identifying and managing possible infusion set failures, which included a “ketone action plan” if instances of prolonged hyperglycemia arose. There were no restrictions on diet or exercise during the trial period.
The algorithms were initialized only by entering the participant's body weight; there was no run-in or warm-up period for the device before the automation of insulin delivery commenced. The default glucose target of “Usual” (120 mg/dL, 6.7 mmol/L) could be shifted by ±10 mg/dL (0.56 mmol/L), down to “Lower” or up to “Higher”; a different target from the default target could be set for part of the day.
Participants assigned to the SC group continued to use their pre-study personal insulin delivery method and insulin regimen, which for some was an FDA-approved/cleared HCL system. All participants used an unblinded Dexcom G6 CGM for real-time daily glucose monitoring, with study-provided sensors and transmitters. If they previously used a different CGM system, they could continue its use in addition to the Dexcom G6, at their discretion.
The CGM-naive participants in the SC group were trained on the insertion and maintenance of the Dexcom G6 CGM and in the interpretation and use of CGM data. Participants in the SC group were not provided with a study blood glucose meter or ketone meter and were not provided with, or trained on, the ketone action plan for the management of potential infusion set failures that was provided to the BP group. Diabetes management for participants in the SC group, including any adjustments to their insulin regimen and management of problems such as infusion set failures, was done by their own diabetes care providers, not study staff.
Visit schedule and testing
After randomization, participants in both groups were contacted by phone after 1–2 days and 1 week. Follow-up visits occurred at 2, 6, 10, and 13 weeks. Some visits were completed remotely via video conference due to the COVID pandemic. Data from the BP were downloaded at weeks 6 and 13 when these were in-person, or when the BP was shipped back to the study site whenever the week-13 visit was done by video conference.
Blood samples from venipuncture or fingerstick were collected at randomization and after 6 and 13 weeks for measurement of HbA1c by a central laboratory at the University of Minnesota Advanced Research and Diagnostic Laboratory (measured with a Tosoh BioScience instrument).8 Participants completed a questionnaire weekly, with each day of the week sampled equally throughout the trial that queried them about episodes of hypoglycemia and treatment of such events with carbohydrate during the prior 24 h. Quality-of-life questionnaires were completed at baseline, 6 weeks, and 13 weeks, the results from which will be reported separately.
Reporting of adverse events was solicited throughout the trial. Severe hypoglycemia was defined as hypoglycemia requiring assistance because of an altered cognitive state. Diabetic ketoacidosis was defined by the criteria established by the Diabetes Control and Complications Trial (DCCT).1
Statistical methods
The study was planned to include ∼110 adults assigned to the BP group and 55 to the SC group. HbA1c was the primary outcome and CGM metrics were secondary outcomes including mean glucose, time in range 70–180 mg/dL (TIR), time >180 mg/dL, time >250 mg/dL, time <70 mg/dL, time <54 mg/dL, standard deviation, and coefficient of variation. Statistical analyses were performed on an intention-to-treat basis. Continuous outcomes were compared between groups using linear mixed-effects regression models and binary outcomes with logistic regression models, adjusting for the baseline value of the metric, age, and site (random effect).
Modification of the treatment effect by baseline variables was assessed by including an interaction term in the models described earlier. For key safety outcomes (when at least five events occurred combined between groups), treatment group comparisons were made using a Poisson regression model adjusting for age and HbA1c at randomization, and site (random effect), and for severe hypoglycemia, adjusting for prior severe hypoglycemia events. All analyses were pre-specified except for the treatment group comparisons in the subgroup with baseline HbA1c ≥8.0%, the subgroup using an HCL system before the study, and treatment group comparisons for the variance of HbA1c, mean glucose, and TIR.
Across all outcomes, the type I error was controlled with the use of the adaptive Benjamini-Hochberg false discovery rate correction procedure.9 Descriptive statistics include means with standard deviations and medians with interquartile ranges (IQRs), depending on the distribution of data. All P values are two-tailed except as noted. Analyses were performed with SAS software, version 9.4 (SAS Institute).
Results
There were 161 adult participants included in the analyses: 107 randomly assigned to the BP group and 54 to the SC group. Participant age ranged from 18 to 79 (mean 44 ± 15), with 48% being female. Race/ethnicity distribution was 82% non-Hispanic White, 10% non-Hispanic Black, 6% Hispanic/Latino, and 2% other race or more than one race. Baseline HbA1c ranged from 5.5% to 13.1% (mean 7.6% ± 1.2%). At study entry, participants were using a variety of insulin delivery modalities: 32% MDI therapy, 27% a pump without automation, 5% a pump with predictive low glucose suspend, and 36% an HCL system. Characteristics according to treatment group are shown in Table 1.
Table 1.
Participant Characteristics by Treatment Group
| BP (N = 107) | SC (N = 54) | |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 44 ± 15 | 44 ± 16 |
| 18 to <25, n (%) | 16 (15) | 7 (13) |
| 25 to <50, n (%) | 50 (47) | 28 (52) |
| 50 to <65, n (%) | 32 (30) | 12 (22) |
| ≥65, n (%) | 9 (8) | 7 (13) |
| Range | 18 − 73 | 18 − 79 |
| Diabetes duration (years) | ||
| Mean ± SD | 26 ± 14 | 29 ± 14 |
| Range | 2 − 59 | 5 − 66 |
| HbA1c level at randomization (%)a | ||
| Mean ± SD | 7.6 ± 1.2 | 7.6 ± 1.2 |
| ≤7.0, n (%) | 37 (35) | 18 (34) |
| 7.1 − 7.9, n (%) | 33 (31) | 17 (32) |
| 8.0 − 8.9, n (%) | 25 (23) | 12 (23) |
| ≥9.0, n (%) | 12 (11) | 6 (11) |
| Range | 5.5 − 13.1 | 5.5 − 11.3 |
| Sex, female, n (%) | 52 (49) | 26 (48) |
| Race/Ethnicity group, n (%) | ||
| White non-Hispanic | 85 (79) | 47 (87) |
| Black non-Hispanic | 14 (13) | 2 (4) |
| Hispanic or Latino | 7 (7) | 3 (6) |
| Asian | 0 (0) | 1 (2) |
| American Indian/Alaskan Native | 0 (0) | 1 (2) |
| More than one race | 1 (<1) | 0 (0) |
| Unknown/not reported | 0 (0) | 0 (0) |
| Annual household income, n (%) | ||
| <$25,000 | 3 (3) | 1 (2) |
| $25,000 to <$35,000 | 3 (3) | 5 (9) |
| $35,000 to <$50,000 | 4 (4) | 2 (4) |
| $50,000 to <$75,000 | 18 (17) | 4 (7) |
| $75,000 to <$100,000 | 9 (8) | 8 (15) |
| $100,000 to <$200,000 | 41 (38) | 12 (22) |
| ≥$200,000 | 17 (16) | 12 (22) |
| Unknown/Does not wish to provide | 12 (11) | 10 (19) |
| Education, n (%) | ||
| <Bachelor's | 35 (33) | 21 (39) |
| Bachelor's | 40 (37) | 22 (41) |
| >Bachelor's | 30 (28) | 10 (19) |
| Unknown/Does not wish to provide | 2 (2) | 1 (2) |
| Health insurance, n (%) | ||
| Private | 94 (88) | 45 (83) |
| Medicare/Medicaid | 9 (8) | 6 (11) |
| Other Government Insurance | 2 (2) | 2 (4) |
| None | 0 (0) | 1 (2) |
| Did not provide/Unknown | 2 (2) | 0 (0) |
| BMI (kg/m2) | ||
| Mean ± SD | 28.9 ± 5.5 | 29.1 ± 6.9 |
| <18.5, n (%) | 0 (0) | 2 (4) |
| 18.5–24.9, n (%) | 29 (27) | 16 (30) |
| 25.0–29.9, n (%) | 40 (37) | 14 (26) |
| ≥30.0, n (%) | 38 (36) | 22 (41) |
| Insulin/CGM device use, n (%) | ||
| MDI without CGM | 13 (12) | 6 (11) |
| MDI with CGM | 21 (20) | 12 (22) |
| Pump without CGM | 5 (5) | 3 (6) |
| Pump with CGM (without automation) | 21 (20) | 14 (26) |
| Pump with predictive low glucose suspend | 6 (6) | 2 (4) |
| HCL System | 41 (38) | 17 (31) |
| Currently using CGM, n (%) | 89 (83) | 45 (83) |
| c-Peptide, ng/mLa | ||
| Mean ± SDb | 0.046 ± 0.185 | 0.009 ± 0.023 |
| <0.007, n (%) | 77 (78) | 46 (92) |
| Total daily insulin [units/(kg·d)], median (IQR) | 0.60 (0.47, 0.76) | 0.65 (0.50, 0.83) |
| Time since most recent SH event,c n (%) | ||
| Never had an event | 48 (45) | 17 (31) |
| <3 Months ago | 4 (4) | 0 (0) |
| 3 to <6 Months ago | 0 (0) | 1 (2) |
| ≥6 Months ago | 55 (51) | 36 (67) |
| Time since last DKA event, n (%) | ||
| Never had an event | 57 (53) | 22 (41) |
| <3 Months ago | 1 (<1) | 0 (0) |
| 3 to <6 Months ago | 0 (0) | 0 (0) |
| ≥6 Months ago | 49 (46) | 32 (59) |
| Non-insulin blood sugar control medications taken, n (%) | ||
| None | 98 (92) | 52 (96) |
| Metformin | 7 (7) | 2 (4) |
| GLP-1 agonist | 2 (2) | 0 (0) |
HbA1c at randomization missing for one SC participant. c-Peptide at randomization missing for eight BP participants and four SC participants.
One BP participant had an outlier c-peptide value of 1.6 ng/mL. With this outlier removed, mean c-peptide is 0.031 ± 0.095 ng/mL for BP group.
An SH event is defined as a hypoglycemic event that (1) required assistance of another person due to altered consciousness, and (2) required another person to actively administer carbohydrate, glucagon, or other resuscitative actions.
BMI, body mass index; BP, bionic pancreas; CGM, continuous glucose monitoring; DKA, diabetic ketoacidosis; HCL, hybrid closed loop; IQR, interquartile range; MDI, multiple daily injections; SC, standard-of-care; SD, standard deviation; SH, severe hypoglycemic.
The trial was completed by 104 (97%) of 107 participants in the BP group and all 54 participants in the SC group (Supplementary Fig. S1). The overall visit and phone contact completion rate was 99%. In addition to the three participants who withdrew before the end of the trial, use of the BP was discontinued early by eight participants who remained in the trial (two after a severe hypoglycemia event; four due to dissatisfaction with glucose control related to hyperglycemia, hypoglycemia, or both; and two related to pump/infusion set issues).
Over the 13 weeks of the trial, the BP was autonomously dosing insulin a median of 96% (IQR 93%, 98%) of the time, with CGM input available for 90% (IQR 83%, 93%) of the time. When the BP was in use, median percent time BP was autonomously dosing insulin was 97% (IQR 94%, 99%), with CGM data available for autonomous dosing a median of 91% (IQR 86%, 93%) of the time (Supplementary Table S1). In the SC group, CGM use was very high, with median usage over the 13 weeks of the trial being 97% (IQR 95%, 98%).
Efficacy outcomes
Mean HbA1c decreased from 7.6% ± 1.2% at baseline to 7.1% ± 0.7% at 6 weeks and to 7.1% ± 0.6% at 13 weeks in the BP group and from 7.6% ± 1.2% at baseline to 7.5% ± 1.0% at 6 weeks and to 7.5% ± 0.9% at 13 weeks in the SC group (adjusted difference in mean change in HbA1c from baseline to 13 weeks −0.5%, 95% confidence interval [CI] −0.6% to −0.3%, P < 0.001) (Table 2 and Supplementary Fig. S2). HbA1c improved by >0.5% in 43% of the BP group versus 17% of the SC group (P < 0.001) and by >1.0% in 23% versus 4%, respectively (P = 0.009, Table 3).
Table 2.
Key Efficacy Outcomes
| Baseline |
Follow-up (at or over 13 weeks) |
Adjusted difference, BP minus SC (95% CI)b | P b | |||
|---|---|---|---|---|---|---|
| BP (N = 107)a | SC (N = 54)a | BP (N = 107)a | SC (N = 54)a | |||
| Overall | ||||||
| HbA1c (%), mean ± SD | 7.6 ± 1.2 | 7.6 ± 1.2 | 7.1 ± 0.6 | 7.5 ± 0.9 | −0.5 (−0.6 to −0.3) | <0.001 |
| Mean glucose (mg/dL), mean ± SD | 179 ± 41 | 186 ± 42 | 157 ± 12 | 174 ± 30 | −16 (−20 to −11) | <0.001 |
| Time 70–180 mg/dL, mean ± SD | 56% ± 19% | 53% ± 21% | 69% ± 8% | 58% ± 17% | 11% (8% to 13%) | <0.001 |
| Time >180 mg/dL, mean ± SD | 42% ± 20% | 45% ± 21% | 28% ± 9% | 40% ± 18% | −11% (−13% to −8%) | <0.001 |
| Time >250 mg/dL, median (IQR) | 11.0% (4.9%, 23.0%) | 13.2% (3.8%, 32.0%) | 5.4% (3.4%, 7.7%) | 10.4% (4.3%, 23.6%) | −3.6% (−5.5% to −2.1%) | <0.001 |
| Time <70 mg/dL, median (IQR) | 1.7% (0.5%, 2.8%) | 1.3% (0.4%, 2.6%) | 1.9% (1.1%, 2.8%) | 1.5% (0.6%, 2.7%) | 0.1% (−0.2% to 0.4%) | 0.51 |
| Time <54 mg/dL, median (IQR) | 0.21% (0.02%, 0.57%) | 0.11% (0.00%, 0.37%) | 0.33% (0.14%, 0.52%) | 0.18% (0.08%, 0.58%) | 0.02% (−0.04% to 0.08%) | 0.33 |
| SD (mg/dL), mean ± SD | 62 ± 16 | 65 ± 18 | 54 ± 9 | 61 ± 14 | −7 (−9 to −4) | <0.001 |
| Coefficient of variation (%), mean ± SD | 35% ± 6% | 35% ± 5% | 34% ± 4% | 35% ± 5% | −0.8% (−1.9% to 0.4%) | 0.17 |
| Daytime (06:00 − 23:59) | ||||||
| Mean glucose (mg/dL), mean ± SD | 181 ± 40 | 186 ± 41 | 159 ± 12 | 175 ± 30 | ||
| Time 70–180 mg/dL, mean ± SD | 55% ± 19% | 53% ± 20% | 67% ± 8% | 57% ± 17% | ||
| Time >180 mg/dL, mean ± SD | 43% ± 20% | 45% ± 21% | 30% ± 8% | 41% ± 18% | ||
| Time >250 mg/dL, median (IQR) | 11.8% (4.9%, 24.2%) | 14.1% (4.8%, 31.8%) | 6.0% (4.1%, 9.2%) | 10.9% (3.3%, 22.8%) | ||
| Time <70 mg/dL, median (IQR) | 1.6% (0.4%, 3.0%) | 1.6% (0.4%, 2.5%) | 1.8% (0.9%, 3.0%) | 1.4% (0.6%, 2.5%) | ||
| Time <54 mg/dL, median (IQR) | 0.13% (0.00%, 0.60%) | 0.10% (0.00%, 0.43%) | 0.29% (0.10%, 0.53%) | 0.16% (0.05%, 0.46%) | ||
| SD (mg/dL), mean ± SD | 63 ± 16 | 65 ± 18 | 55 ± 8 | 61 ± 14 | ||
| Coefficient of variation (%), mean ± SD | 35% ± 6% | 35% ± 6% | 35% ± 4% | 35% ± 5% | ||
| Nighttime (00:00 − 05:59) | ||||||
| Mean glucose (mg/dL), mean ± SD | 174 ± 45 | 185 ± 51 | 150 ± 17 | 173 ± 33 | ||
| Time 70–180 mg/dL, mean ± SD | 59% ± 23% | 55% ± 26% | 75% ± 12% | 59% ± 20% | ||
| Time >180 mg/dL, mean ± SD | 38% ± 23% | 44% ± 26% | 23% ± 12% | 39% ± 20% | ||
| Time >250 mg/dL, median (IQR) | 7.4% (1.7%, 22.6%) | 10.8% (2.5%, 31.8%) | 3.4% (1.2%, 6.2%) | 9.5% (3.3%, 20.4%) | ||
| Time <70 mg/dL, median (IQR) | 1.2% (0.1%, 3.5%) | 0.6% (0.0%, 1.9%) | 1.9% (1.2%, 2.9%) | 1.1% (0.5%, 3.0%) | ||
| Time <54 mg/dL, median (IQR) | 0.00% (0.00%, 0.84%) | 0.00% (0.00%, 0.20%) | 0.38% (0.17%, 0.63%) | 0.19% (0.05%, 0.45%) | ||
| SD (mg/dL), mean ± SD | 57 ± 19 | 60 ± 19 | 49 ± 11 | 59 ± 15 | ||
| Coefficient of variation (%), mean ± SD | 33% ± 7% | 32% ± 7% | 32% ± 5% | 34% ± 6% | ||
One SC participant missing baseline HbA1c. Five BP participants and one SC participant missing 13-week HbA1c. One BP participant with missing follow-up CGM data. Median amount of CGM data for analyses was 334 (IQR 321, 336) h at baseline and 2022 (IQR 1887, 2062) during follow-up in the BP group and 334 (IQR 326, 336) and 2114 (IQR 2063, 2136), respectively in the SC group.
All statistical testing is for superiority. P-values and 95% CIs are from mixed-effect models adjusting for baseline value of the metric, age at randomization, and site (random effect). Missing data were handled using direct likelihood analyses. Due to a skewed distribution, % time >250, <70, and <54 mg/dL were transformed using a rank normal transformation. Multiple comparisons were adjusted using the Benjamini-Hochberg adaptive false discovery rate correction procedure.
CI, confidence interval.
Table 3.
Additional Efficacy Binary Outcomes
| Follow-up (at or over 13 weeks) |
Adjusted difference, BP minus SC (95% CI)b | P b | ||
|---|---|---|---|---|
| BP (N = 107),a N (%) | SC (N = 54),a N (%) | |||
| HbA1c <7.0% | 43 (42) | 15 (28) | 14% (4% to 27%) | 0.008 |
| HbA1c <7.5% | 76 (75) | 23 (43) | 28% (18% to 38%) | <0.001 |
| HbA1c <8.0% | 97 (95) | 41 (77) | 16% (8% to 24%) | <0.001 |
| HbA1c >9.0% | 0 (0) | 6 (11) | 36% (−23% to 91%) | 1.00 |
| HbA1c improvement from baseline >0.5% | 44 (43) | 9 (17) | 25% (19% to 30%) | <0.001 |
| HbA1c improvement from baseline >1.0% | 23 (23) | 2 (4) | 18% (8% to 24%) | 0.009 |
| HbA1c relative improvement from baseline >10% | 32 (31) | 2 (4) | 26% (17% to 33%) | 0.002 |
| HbA1c improvement from baseline >1.0% or HbA1c <7.0% | 58 (57) | 16 (31) | 26% (11% to 39%) | <0.001 |
| Time 70–180 mg/dL >70% | 50 (47) | 17 (31) | 12% (3% to 21%) | 0.01 |
| Time 70–180 mg/dL improvement from baseline ≥5% | 78 (74) | 28 (52) | 26% (17% to 36%) | <0.001 |
| Time 70–180 mg/dL improvement from baseline ≥10% | 72 (68) | 24 (44) | 29% (20% to 39%) | <0.001 |
| Time <70 mg/dL <4% | 93 (88) | 43 (80) | 10% (2% to 19%) | 0.01 |
| Time <54 mg/dL <1% | 92 (87) | 44 (81) | 9% (2% to 16%) | 0.01 |
| Mean glucose <154 mg/dL and time <54 mg/dL <1% | 42 (40) | 12 (22) | 15% (8% to 22%) | <0.001 |
| Time 70–180 mg/dL >70% and time <54 mg/dL <1% | 46 (43) | 12 (22) | 20% (11% to 28%) | <0.001 |
| HbA1c <7.0% for participants with baseline HbA1c >7.5% | N = 44, 9 (20) | N = 26, 1 (4) | 28% (−1% to 53%) | 0.06 |
| Improvement in HbA1c >0.5% without an increase in time <54 mg/dL by >0.5% or improvement in time <54 mg/dL by >0.5% without an increase in HbA1c by >0.5% | 47 (46) | 11 (21) | 20% (11% to 28%) | <0.001 |
| Improvement in time 70–180 mg/dL by >10% without an increase in time <54 mg/dL by >0.5% or improvement in time <54 mg/dL by >0.5% without a decrease in time 70–180 mg/dL by >10% | 55 (52) | 13 (24) | 27% (17% to 37%) | <0.001 |
One SC participant missing baseline HbA1c. Five BP participants and one SC participant missing 13-week HbA1c. One BP participant with missing follow-up CGM data.
P-values are from a logistic regression model adjusting for the baseline value of the metric, age at randomization, and site (random effect). A 95% CI for the treatment group adjusted risk difference (BP minus SC) was produced using parametric bootstrapping. Multiple comparisons were adjusted using the Benjamini-Hochberg adaptive false discovery rate correction procedure.
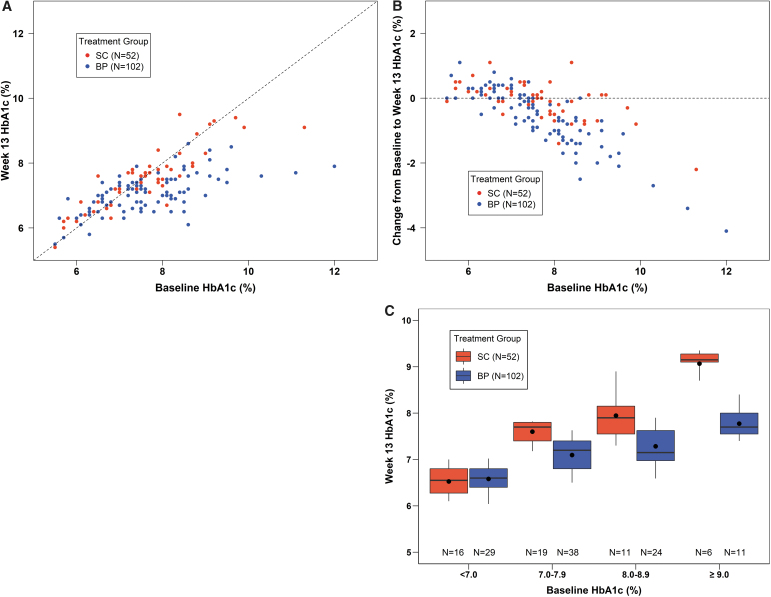
A treatment effect on HbA1c was evident for participants with baseline HbA1c ≥7.0%, which was particularly prominent at high baseline HbA1c levels (Fig. 1). For participants with baseline HbA1c ≥8.0% (N = 55), mean HbA1c decreased from 8.9% ± 1.1% at baseline to 7.4% ± 0.6% at 13 weeks with BP compared with 8.8% ± 0.8% to 8.3% ± 0.8% with SC (difference = −0.9, 95% CI −1.3 to −0.6, P < 0.001) (Supplementary Table S2).
FIG. 1.
HbA1c at 13 weeks. (A) Scatter plot of 13-week HbA1c versus baseline HbA1c with the line of identity. (B) Scatter plot of change in HbA1c from baseline to 13 weeks versus baseline HbA1c, with the horizontal line representing zero change. (C) Box plots of 13-week HbA1c in subgroups based on baseline HbA1c. Black dots indicate the mean values, horizontal bars in the boxes indicate the medians, the bottom and top of each box represent the 25th and 75th percentiles, respectively, and the bottom and top whiskers represent the 10th and 90th percentiles, respectively.
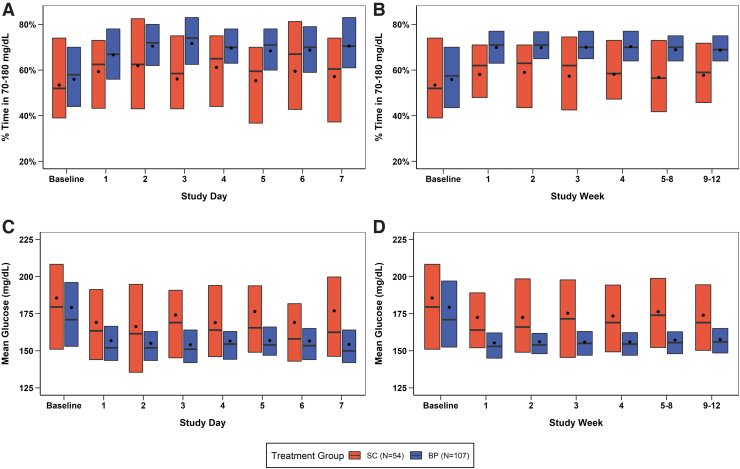
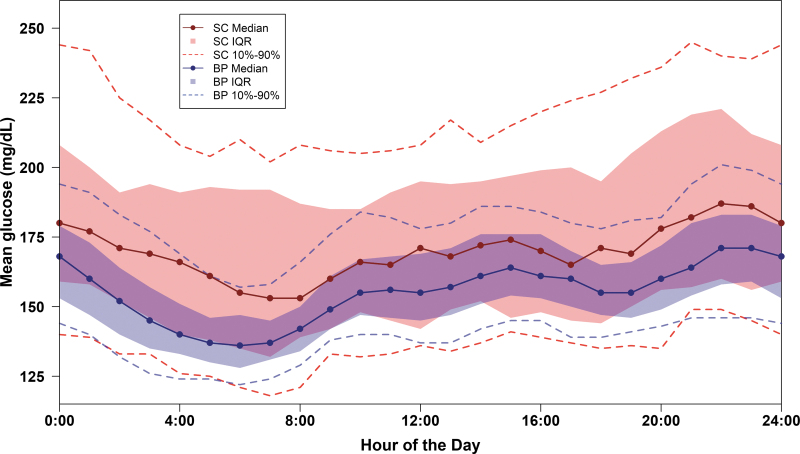
Improvement in mean TIR and mean CGM glucose was seen during the first day of BP use and by the end of the first week reached levels that remained relatively stable through 13 weeks (Fig. 2 and Supplementary Table S3). Over 13 weeks, mean TIR was increased by 11% (2.6 h/d) and mean CGM glucose was reduced by 16 mg/dL in the BP group compared with the SC group (P < 0.001) (Table 2 and Supplementary Figs. S3–S5). As seen in Figure 3, mean CGM glucose was substantially lower with BP than SC throughout the 24 h of the day, particularly overnight, with the largest difference seen at 6 a.m. to 7 a.m.
FIG. 2.
TIR and mean glucose over first 7 days of BP use and over 13 weeks of trial. (A, B) TIR data and (C, D) Mean glucose data for each day during the first 7 days of BP use and then in weekly intervals. Black dots indicate the mean values, horizontal bars in the boxes indicate the medians, and the bottom and top of each box represent the 25th and 75th percentiles, respectively. BP, bionic pancreas; SC, standard-of-care; TIR, time in range 70–180 mg/dL.
FIG. 3.
Mean glucose by hour of the day over 13 weeks. Dots represent the median mean glucose. The shaded area represents the interquartile range and dashed curves represent the 10th and 90th percentiles over each hour of the day.
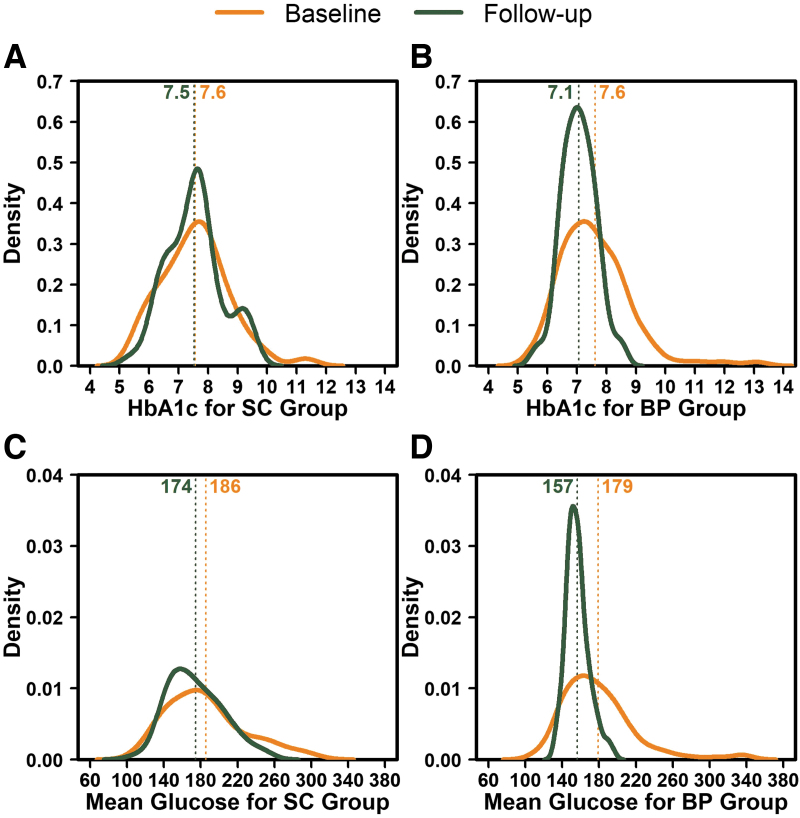
Statistically significant differences favoring the BP group also were present for mean time CGM glucose was >180 mg/dL and >250 mg/dL, and mean CGM glucose standard deviation (Table 2). Additional HbA1c and CGM outcomes reflective of hyperglycemia indicated a strong treatment benefit for the BP group compared with the SC group (Table 4). In addition to the improvement in the mean of the key metrics, the between participant variance for HbA1c, mean CGM glucose, and mean TIR was substantially smaller with BP compared with SC (P < 0.001) (Supplementary Table S4 and Fig. 4).
Table 4.
Additional Efficacy Continuous Glucose Monitoring Outcomes
| Baseline |
Follow-up (at or over 13 weeks) |
Adjusted difference, BP minus SC (95% CI)a | P a | |||
|---|---|---|---|---|---|---|
| BP (N = 107) | SC (N = 54) | BP (N = 106) | SC (N = 54) | |||
| Hours of CGM data, median (IQR) | 334 (321, 336) | 334 (326, 336) | 2022 (1887, 2062) | 2114 (2063, 2136) | ||
| Time 70–140 mg/dL, mean ± SD | 33% ± 15% | 32% ± 16% | 42% ± 7% | 34% ± 14% | 7% (5% to 9%) | <0.001 |
| Time 70–120 mg/dL, mean ± SD | 21% ± 12% | 20% ± 12% | 26% ± 5% | 22% ± 10% | 3% (2% to 5%) | <0.001 |
| Time <60 mg/dL, median (IQR) | 0.5% (0.1%, 1.1%) | 0.3% (0.1%, 0.8%) | 0.7% (0.4%, 1.0%) | 0.5% (0.2%, 1.0%) | 0.0% (−0.1% to 0.2%) | 0.46 |
| Area over the curve <70 mg/dL,b median (IQR) | 0.15 (0.03, 0.29) | 0.08 (0.02, 0.25) | 0.18 (0.10, 0.27) | 0.13 (0.05, 0.24) | 0.01 (−0.02 to 0.04) | 0.49 |
| Low blood glucose index, median (IQR) | 0.49 (0.20, 0.86) | 0.39 (0.18, 0.66) | 0.58 (0.42, 0.80) | 0.43 (0.27, 0.79) | 0.05 (−0.03 to 0.13) | 0.22 |
| Hypoglycemic event rate per week,c median (IQR) | 0.50 (0.00, 1.08) | 0.00 (0.00, 1.02) | 0.66 (0.25, 1.14) | 0.36 (0.16, 1.01) | 0.00 (−0.07 to 0.16) | 0.34 |
| Time >300 mg/dL, median (IQR) | 3.1% (0.6%, 9.8%) | 4.0% (0.5%, 14.1%) | 1.2% (0.6%, 2.4%) | 2.6% (0.7%, 9.0%) | −1.2% (−1.9% to −0.6%) | <0.001 |
| Area under the curve >180 mg/dL,b median (IQR) | 20.8 (11.3, 37.7) | 24.9 (10.1, 47.8) | 12.1 (8.6, 16.0) | 20.7 (11.0, 38.5) | −6.1 (−8.9 to 3.6) | <0.001 |
| High blood glucose index, median (IQR) | 8.8 (5.6, 13.3) | 10.2 (5.1, 15.9) | 6.1 (5.0, 7.3) | 8.8 (5.6, 13.3) | −2.0 (−2.8 to −1.3) | <0.001 |
| Hyperglycemic event rate per week (≥15 min >300 mg/dL),d median (IQR) | 3.0 (0.6, 6.0) | 3.8 (0.5, 7.6) | 1.4 (0.9, 2.4) | 2.5 (0.7, 6.7) | −0.7 (−1.3 to −0.3) | 0.002 |
| Hyperglycemic event rate per week (≥90 min >300 mg/dL in 120 min),e median (IQR) | 1.0 (0.0, 3.1) | 1.5 (0.0, 5.0) | 0.5 (0.2, 0.9) | 0.9 (0.3, 3.1) | −0.5 (−0.8 to −0.2) | <0.001 |
| Mean of daily difference, mean ± SD | 27 ± 12 | 28 ± 12 | 17 ± 6 | 25 ± 9 | −8 (−9 to −6) | <0.001 |
| Blood glucose risk index,f median (IQR) | 9.4 (6.5, 14.1) | 10.5 (5.5, 16.2) | 6.9 (5.5, 7.7) | 9.3 (6.2, 13.9) | −2.0 (−2.8 to −1.3) | <0.001 |
P-values and 95% CIs are from mixed-effect models adjusting for baseline value of the metric, age at randomization, and site (random effect). Missing data were handled using direct likelihood analyses. Multiple comparisons were adjusted using the Benjamini-Hochberg adaptive false discovery rate correction procedure.
Area over the curve 70 mg/dL is calculated as the average magnitude of CGM glucose values below 70 mg/dL. Area over the curve 180 mg/dL is calculated as the average magnitude of CGM glucose values above 180 mg/dL.
A CGM-measured hypoglycemic event is defined as ≥15 consecutive minutes with a CGM sensor value <54 mg/dL. The hypo event ends when there are ≥15 consecutive minutes with a CGM sensor value ≥70 mg/dL, at which point the participant becomes eligible for another hypoglycemic event.
A CGM-measured hyperglycemic event is defined as ≥15 consecutive minutes with a CGM glucose value >300 mg/dL. The hyper event ends when there are ≥15 consecutive minutes with a CGM glucose value ≤250 mg/dL, at which point the participant becomes eligible for another hyper event.
A CGM-measured hyperglycemic event is defined as ≥90 cumulative minutes with a CGM sensor value >300 mg/dL within a 120-min period. The hyper event ends when there are ≥15 consecutive minutes with a CGM sensor value ≤180 mg/dL, at which point the participant becomes eligible for another hyperglycemic event.
Blood glucose risk index = low blood glucose index + high blood glucose index.
FIG. 4.
Distribution of HbA1c and mean glucose at baseline and outcome for the BP and SC groups. (A, B) HbA1c data for baseline and 13 weeks for the SC group and BP group, respectively. (C, D) Mean glucose measured with CGM over 13 weeks for the SC group and BP group, respectively. The curves represent the distribution of values at baseline and outcome. The dotted lines represent the mean values that are indicated numerically at the top of each line. BP, bionic pancreas; CGM, continuous glucose monitoring; SC, standard-of-care.
The amount of CGM-measured hypoglycemia was low during both day and night in both groups at baseline and during the 13 weeks of the trial. Median time <54 mg/dL was 0.21% at baseline and 0.33% during the 13 weeks of the trial in the BP group and 0.11% at baseline and 0.18% during the trial in the SC group (adjusted difference = 0.02%, 95% CI −0.04% to −0.08%, P = 0.33).
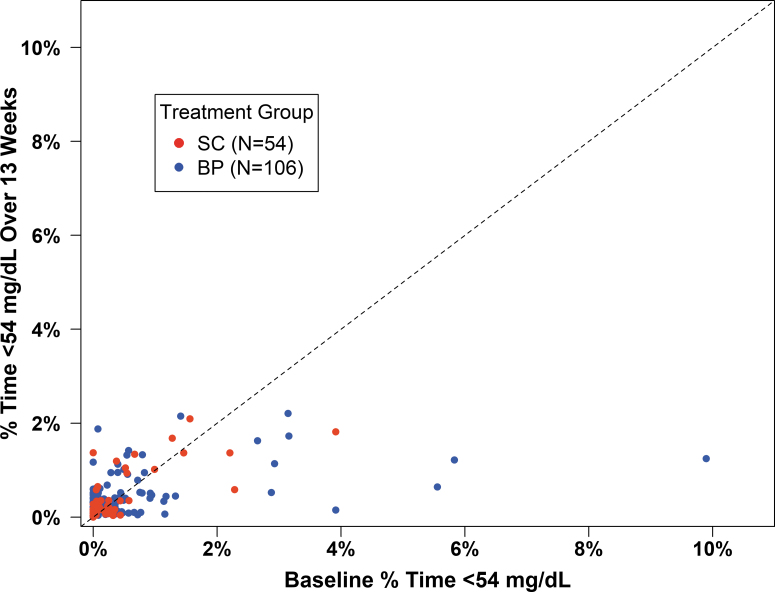
The distribution of time <70 mg/dL also was not significantly different between groups (P = 0.51) (Table 2). The frequency of hypoglycemia reported during the 24 h before the completion of each weekly questionnaire was similar in the two groups (Supplementary Table S5). Although there was no significant change in time in hypoglycemia for the cohort as a whole, some individual participants in the BP group who had high percentages of time <54 mg/dL at baseline experienced large reductions in hypoglycemia during 13 weeks on the BP (Fig. 5).
FIG. 5.
Time <54 mg/dL over 13 weeks versus baseline time <54 mg/dL. Scatter plot of time <54 mg/dL at baseline versus baseline time <54 mg/dL with the line of identity.
Analyses restricted to participants with baseline HbA1c >7.0% (Supplementary Table S6) and analyses restricted to participants not using HCL before the study (Supplementary Table S7) demonstrated a larger treatment effect on HbA1c than the primary analysis, with adjusted mean treatment group differences of −0.7% (95% CI −1.0% to −0.5%, P < 0.001) and −0.6% (95% CI −0.8% to −0.4%, P < 0.001), respectively. Among the 58 users of an HCL system prestudy (and in the SC group, used during the study), mean HbA1c was 7.2% ± 0.8% at baseline and 7.0% ± 0.6% at 13 weeks in the BP group versus 7.0% ± 0.7% and 7.1% ± 0.8%, respectively, in the SC group (adjusted difference −0.2, 95% CI −0.5 to 0.0, P = 0.08) (Supplementary Table S8).
In subgroup analyses, the HbA1c and TIR benefits of BP compared with SC were evident across participant age range, for higher and lower education and income levels, and for both MDI and pump (without automation) users (Supplementary Tables S9 and S10). Similar to the greater effect seen with higher baseline HbA1c, the treatment effect on HbA1c was also greater with lower baseline TIR, higher baseline mean CGM glucose, and higher baseline time in hyperglycemia.
There were no significant differences between the BP group and the SC group in the mean TDD of insulin, change in body weight, or body mass index (Supplementary Tables S11–S13). For participants with baseline HbA1c ≥9.0%, there was little change in the TDD of insulin in both groups [0.00 ± 0.26 units/(kg·d) with BP and 0.04 ± 0.12 units/(kg·d) with SC].
Adverse events and device issues
There were 7 severe hypoglycemia events (as defined in footnote to Table 5) in 7 participants in the BP group (6.5% of 107 participants) and 2 events in 1 participant in the SC group (1.9% of 54 participants). The rates of severe hypoglycemia were 25.5 and 14.2 per 100 person-years, respectively (P = 0.40). An evaluation of the seven events in the BP group revealed that the BP functioned as intended with no indication of a device malfunction. There was no observed commonality among the participants experiencing a severe hypoglycemia event with respect to their characteristics or possible precipitating factors for the event (Supplementary Table S14). There were no cases of diabetic ketoacidosis.
Table 5.
Adverse Events and Safety Outcomes
| BP (N = 107 randomized) | SC (N = 53 randomized) | P | |
|---|---|---|---|
| All AEs N events | 63 | 6 | |
| Number of AEs per participant, n (%) | |||
| 0 | 64 (60) | 49 (91) | |
| 1 | 31 (29) | 4 (7) | |
| 2 | 7 (7) | 1 (2) | |
| 3 | 3 (3) | 0 (0) | |
| 4 | 1 (<1) | 0 (0) | |
| 5 | 1 (<1) | 0 (0) | |
| SH eventsa | 0.40 | ||
| Number of SH events per participant, n (%) | |||
| 0 | 100 (93) | 53 (98) | |
| 1 | 7 (7) | 0 (0) | |
| 2 | 0 (0) | 1 (2) | |
| Incidence rate per 100 person-years | 25.5 | 14.2 | |
| DKA eventsb | |||
| Number of DKA events per participant, n (%) | |||
| 0 | 107 (100) | 54 (100) | |
| Other SAEsc | |||
| Number of SAEs per participant, n (%) | |||
| 0 | 106 (>99) | 53 (98) | |
| 1 | 1 (<1) | 1 (2) | |
| Incidence rate per 100 person-years | 3.6 | 7.1 | |
| Participants with worsening of HbA1c from baseline to 13 weeks by >0.5%, n (%) | 4 (4) | 4 (8) | 0.42d |
| Other AEs N events/N participants | |||
| Hyperglycemia with or without ketosis related to study devicee | 34/27 | NA | |
| Hyperglycemia with or without ketosis not related to study device | 13/12 | 0/0 | |
| Non-severe hypoglycemia | 1/1 | 0/0 | |
| Other reportable AEs | 7/7 | 3/3 | |
An SH event is defined as a hypoglycemic event that (1) required the assistance of another person due to altered consciousness, and (2) required another person to actively administer carbohydrate, glucagon, or other resuscitative actions. P-value for number of SH events per subject is produced from a Poisson regression model adjusting for age at randomization, central lab HbA1c at randomization, whether or not participant had at least one SH event before randomization, and site (random effect).
As reported in a separate manuscript,7 in the group using the BP with fast-acting insulin aspart, a DKA event occurred in 2 out of 114 (1.8%) study patients (both events were caused by an infusion set failure).
Hypoglycemia (one) in BP group and epiglottitis (one) in SC group. The hypoglycemic event did not meet the criteria for severe hypoglycemia related to cognitive impairment but was considered an SAE (significant medical event) as judged by the investigator.
P-value produced from a logistic regression model adjusting for age at randomization, central lab HbA1c at randomization, and site (random effect).
Among the 34 hyperglycemia events related to the study device, 30 were due to infusion set failure, 2 due to cartridge issues, 1 CGM issue, and 1 motor issue.
AEs, adverse events; NA, not applicable; SAEs, serious adverse events.
Among the other reportable adverse events in the BP groups, most were related to hyperglycemia with or without ketosis and were attributed to infusion set failure (Table 5). A summary of BP group device issues is provided in Supplementary Table S15.
Discussion
This multicenter, randomized controlled trial evaluated the insulin-only BP using insulin aspart or insulin lispro in comparison with SC, which included CGM for all participants. The study cohort comprised racially and socioeconomically diverse adults with T1D ranging in age from 18 to 79 years who had varying levels of glycemic control with baseline HbA1c values ranging from 5.5% to 13.1% and were using either an HCL system, a pump with a predictive low glucose suspend feature, a pump without automation, or MDI for insulin delivery.
A statistically significant and clinically meaningful 0.5% reduction in HbA1c was found with BP use compared with SC without an increase in CGM-measured hypoglycemia, which was low at baseline and remained low over the 13 weeks of the trial. There was also a statistically significant 11% increase in TIR, which equates with 2.6 h/d greater TIR on average, and a statistically significant 16 mg/dL decrease in mean CGM glucose as well as statistically significant decreases in hyperglycemia. This increase in TIR and decrease in mean CGM glucose was seen as early as the first day of BP use, and after the first week, remained reasonably constant through the 13 weeks. Beneficial effects were seen during both daytime and nighttime. The improvement in glycemic metrics occurred without an increase in the TDD of insulin.
The largest reduction in HbA1c occurred in participants who had the highest baseline HbA1c levels. This is an important finding, with the potential for substantial public health benefit, since these individuals are at the greatest risk for developing chronic diabetic micro- and macrovascular complications.10 There was no upper limit for baseline HbA1c in this study, and recruitment goals were specifically designed to enroll at least one-third of the cohort with HbA1c ≥8.0%. We found that participants with high HbA1c levels not only used the BP safely, but they also achieved dramatic improvements in glycemic control.
A beneficial treatment effect was consistently observed across a wide range of other baseline characteristics, including participants of racial/ethnic minority groups or lower socio-economic status as well as in both MDI and pump users without automation. As anticipated, there was little further improvement observed in glycemia and CGM outcomes in participants using an HCL system before the study.
Similar to our trial results, the pivotal trial testing the t:slim X2 insulin pump with Control-IQ® Technology, which included 168 individuals with T1D from 14 to 71 years in age, also found an 11% mean improvement in TIR compared with sensor-augmented pump therapy but only a 0.3% treatment group difference in HbA1c11 versus 0.5% in our trial. Of note, mean baseline HbA1c was lower in the Control-IQ trial than our trial (7.6% vs. 7.4%), and the Control-IQ pivotal trial did not include HCL users in the control arm, whereas 31% of the control arm in our trial used an HCL system. It is notable that similar improvements in glycemic and CGM outcomes were observed with the BP relative to the results of the Control-IQ pivotal trial, despite no carbohydrate quantification for meal boluses, no setting or adjusting basal insulin, and no user-initiated correction boluses. The Medtronic Minimed™ 670G12 and 780G13 pivotal trials and the Insulet Omnipod® 5 pivotal trial14 did not include a control arm; thus, a direct comparison with our trial is not possible.
With respect to safety, the rate of severe hypoglycemia events was nominally greater in the BP group compared with the SC group, but this was not statistically significant, although the study was not powered to detect a difference. The observed rate using the BP of 25.5 events per 100 person-years using a definition of cognitive impairment requiring assistance for treatment is similar to the rate of 25 events per 100 person-years estimated from T1D Exchange data based on a much stricter definition requiring seizure or loss of consciousness to have occurred.3
The findings that the amount of time <54 mg/dL and time <70 mg/dL were not significantly different between the groups (with minimal nominal differences of 0.02% and 0.1%, respectively), and that secondary hypoglycemia metrics (percentage of participants with time <54 mg/dL <1% and time <70 mg/dL <4%) were statistically significant in favor of the BP group suggest that the BP is not overly aggressive in dosing insulin.
The observed frequency of infusion set failures may be no higher than what would be expected with any pump. Assuming that infusion sets were changed on average every 3 days per participant instructions, the 30 hyperglycemia adverse events associated with infusion set failures in the BP group represent a failure rate of 0.9% for 3203 infusion sets. A recent analysis of infusion set changes associated with prolonged hyperglycemia from a different automated insulin delivery system suggests that the infusion set failure rate in our study is likely no higher than it is with other systems.15
Since infusion set failures with hyperglycemia were only reportable adverse events in the BP group, the greater number of infusion set failures reported in the BP group than in the SC group is explained by differential adverse event reporting between the groups rather than the BP group actually having a higher rate of infusion set failure than the pump users in the SC Group.
In addition, the BP group received specific written instructions on identifying and managing potential infusion set failures, which included contacting the clinical site, whereas the SC group was instructed to follow their routine diabetes management and contact their diabetes health care provider with any concerns or questions. It is also noteworthy that there were significantly fewer episodes of prolonged hyperglycemia (defined as CGM glucose >300 mg/dL for at least 90 min during a 120-min period) with the BP than SC.
Strengths of the trial include the participation by individuals with T1D across a wide range of baseline characteristics, which enhances the generalizability of the results, a participant retention rate of 98%, high adherence to use of the assigned devices in both treatment groups, and inclusion of an SC control group using real-time CGM for the duration of the trial plus insulin delivery by MDI, insulin pump, or an HCL system.
The main limitation of the trial is that the low amount of baseline hypoglycemia precluded an evaluation as to whether the insulin-only BP system can reduce hypoglycemia, but it was clear from the results that it does not increase hypoglycemia as measured with CGM. More unscheduled contacts occurred in the BP group than the SC group, but this is inherent in the study design in which one group uses an investigational device and the other group follows their usual care and contacts their own health care providers with questions.
In conclusion, the BP using insulin aspart or insulin lispro substantially improves HbA1c and CGM metrics of TIR, mean CGM glucose, and hyperglycemia, without increasing CGM-measured hypoglycemia, in comparison with standard care insulin delivery plus CGM. The trial included a more diverse population than studies of other systems with respect to minority representation, method of insulin delivery, and HbA1c levels. The BP therapy is initialized by entering only the user's body weight, and as such the BP differs from the current FDA-approved/cleared HCL systems in not requiring any information about the previous insulin regimen or a quantitative estimate of carbohydrates at mealtimes or manually adjusting or titrating insulin doses.
These features may facilitate the adoption of an automated insulin delivery system by a wide spectrum of people with T1D and a broad spectrum of health care providers, and by improving glycemic levels has the potential to reduce long-term diabetic complications.
Supplementary Material
Acknowledgments
A complete listing of the Bionic Pancreas Research Group appears in the Supplementary Appendix SA1. Below is a listing of authors and non-author contributors.
Authors: Massachusetts General Hospital, Boston, MA: Steven J. Russell, Jordan S. Sherwood, Luz E. Castellanos, Mallory A. Hillard, Marwa Tuffaha, Melissa S. Putman, Mollie Y. Sands, Courtney A. Balliro. Barbara Davis Center for Diabetes, University of Colorado, Aurora, CO: R. Paul Wadwa, Gregory Forlenza, Robert Slover, Laurel H. Messer, Erin Cobry, Viral N. Shah, Sarit Polsky. Stanford University School of Medicine, Palo Alto, CA: Bruce Buckingham, Rayhan Lal, Laya Ekhlaspour, Michael S. Hughes, Marina Basina. Cleveland Clinic, Cleveland, OH: Betul Hatipoglu, MD, Keren Zhou, MD, Leann Olansky. Children's Hospital of Orange County: Mark Daniels, MD, Amrit Bhangoo, Nikta Forghani, Himala Kashmiri, Francoise Sutton. Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX: Philip Raskin. Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX: Perrin White, Abha Choudhary, Jimmy Penn. University of Texas Health Science Center, San Antonio, San Antonio, TX: Jane Lynch, Rabab Jafri, Maria Rayas, Elia Escaname, Ruby Favela-Prezas. University of California, San Diego, CA: Jeremy Pettus, Schafer Boeder. University of Washington, Seattle, WA: Irl B. Hirsch, Subbulaxmi Trikudanathan. Naomi Berrie Diabetes Center, Columbia University, New York City, NY: Robin Goland, Jacqueline Lonier, Kristen Williams, Natasha Leibel. University of North Carolina, Chapel Hill, NC: John B. Buse, Alex Kass, M. Sue Kirkman, Kate Bergamo, Klara R. Klein, Jean M. Dostou, Sriram Machineni, Laura A. Young, Jamie C. Diner. Henry Ford Health System, Detroit, MI: Davida Kruger, Arti Bhan, J. Kimberly Jones. Nemours Children's Health Jacksonville, Jacksonville, FL: Nelly Mauras, Matthew Benson, Keisha Bird, Kimberly Englert, Joe Permuy. Emory University, Atlanta, GA: Andrew Muir, MD, Kristina Cossen, Eric Felner. Washington University, St. Louis, MO: Janet B. McGill, Maamoun Salam, Julie M. Silverstein, Samantha Adamson, Andrea Cedeno. Children's National Hospital, Washington, DC: Fran Cogen, Seema Meighan, Andrew Dauber. Ann and Robert Lurie Children's Hospital, Pritzker Department of Psychiatry and Behavioral Health, Chicago, IL: Jill Weissberg-Benchell. Boston University, Boston, MA and Beta Bionics, Inc., Concord, MA: Edward R. Damiano. Beta Bionics, Inc., Concord, MA: Firas H. El-Khatib. Jaeb Center for Health Research, Tampa, FL: Roy Beck, Katrina Ruedy, Zoey Li, Peter Calhoun, Martin Chase Marak.
Non-Author Contributors: Massachusetts General Hospital, Boston, MA: Evelyn Greaux, Barbara Steiner, Sarah Gaston, Rachel Bartholomew, Kim Martin. Barbara Davis Center for Diabetes, University of Colorado, Aurora, CO: Emily Jost, Cari Berget, Lindsey Towers, Samantha Lange, Estella Escobar, Christie Beatson, Sonya Walker, Angela Karami, Emily Boranian. Stanford University School of Medicine, Palo Alto, CA: Liana Hsu. Cleveland Clinic, Cleveland, OH: Ana Surckla, Laura Lomeli, Diana Isaacs, Shannon Knapp, Andrea Debs, Tracy Tomaro, Julia Blanchette. Children's Hospital of Orange County: Heather Speer, Marissa Erickson, Samantha Thompson, Allyson McDaniel. Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX: Suzanne Strowig, Lin Jordan. Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX: Michael Henson, Yasmin Molina, Chantal Nwosu, Vandana Kumar, Angie Burris, Kim Jernigan. University of Texas Health Science Center, San Antonio, San Antonio, TX: Sara Olivarri. University of California, San Diego, CA: Todd May, Adrienne Armstrong, Erin Giovanetti. University of Washington, Seattle, WA: Nancy Sanborn, Xenia Averkiou. Naomi Berrie Diabetes Center, Columbia University, New York City, NY: Jamie Hyatt, Sarah Pollak, Elizabeth Robinson, Emily Casciano, Analia Alvarez, Eleanor Zagoren, Jaclynn Johnson, Silpa Sharma. University of North Carolina, Chapel Hill, NC: Virginia Purrington, Rachel Fraser, Julie Uehling. Henry Ford Health System, Detroit, MI: Terra Cushman, Heather Hunter, Natalie Corker, Shereen Mukhashen. Nemours Children's Health Jacksonville, Jacksonville, FL: Kimberly Ponthieux, Albina Tarko. Emory University, Atlanta, GA: Amber Antich, Wanda Sanchez, Mone Anzai, Kathryn Lucas, Catherine Simpson. Washington University, St. Louis, MO: Mary Jane Clifton, Toni Schweiger, Traci Bell. Children's National Hospital, Washington, DC: Meryll Castro, Tara McCarthy, Kimberly Boucher. Jaeb Center for Health Research: Sarah Borgman, Sydnee Bradshaw, Paige Miller, Rosa Pritchard, Elizaveta Dolzhenko. University of Minnesota Advanced Research and Diagnostic Laboratory: Deanna Gabrielson, Julie Idzorek, Anne Elstrom-Park.
Contributor Information
Collaborators: Bionic Pancreas Research Group
Authors' Contributions
D.K.: conceptualization, methodology, investigation, and writing—original draft. A.K.: investigation, project administration, and writing—review and editing. J.L.: investigation, project administration, and writing—review and editing. P.R.: investigation, project administration, and writing—review and editing. M.S.: investigation, project administration, and writing—review and editing. S.T.: investigation, project administration, and writing—review and editing. K.Z.: investigation, project administration, and writing—review and editing. S.J.R.: conceptualization, investigation, project administration, and writing—review and editing. E.R.D.: conceptualization, investigation, project administration, and writing—review and editing. F.H.E.-K.: conceptualization, investigation, project administration, and writing—review and editing. K.J.R.: methodology, data curation, resources, and writing—review and editing. C.B.: investigation, project administration, and writing—review and editing. Z.L.: formal analysis, validation, and writing—original draft. M.C.M.: formal analysis, validation, and writing—original draft. P.C.: formal analysis, validation, and writing—original draft.
Author Disclosure Statement
D.K. receives research funding from Abbott Diabetes Care, Dexcom, Inc., Novo Nordisk, and Beta Bionics, Inc.; advisory board funding from Abbott Diabetes Care, Novo Nordisk, Sanofi-aventis, Pendulum, and Medical Module; and speaker bureau from Dexcom, Inc., Novo Nordisk, Eli Lilly, Sanofi-aventis, BI Lilly, and Xeris. A.K. has no personal financial disclosures but reports that his employer's efforts were supported in part by grants from the NIH: UL1TR002489, P30DK124723. J.P. receives consulting fees from Sanofi, Novo Nordisk, Mannkind, Diasome, and Lilly and reports that his employer receives grant funding from Dexcom, Inc. and Eli Lilly. M.S. receives consulting fees from Eli Lilly and Neurocrine Biosciences and grant funding to his institution from Neurocrine Biosciences, Inc., and Mylan. S.T. receives research support from Insulet and Beta Bionics, Inc. S.J.R. has issued patents and pending patents on aspects of the BP that are assigned to Massachusetts General Hospital and licensed to Beta Bionics, Inc., has received honoraria and/or travel expenses for lectures from Novo Nordisk, Roche, and Ascensia Diabetes Care, serves on the scientific advisory boards of Unomedical, served on scientific advisory boards and had stock in Companion Medical that was bought out by Medtronic, has received consulting fees from Beta Bionics, Inc., Novo Nordisk, Senseonics, and Flexion Therapeutics, has received grant support from Zealand Pharma, Novo Nordisk, and Beta Bionics, Inc., and has received in-kind support in the form of technical support and/or donation of materials from Zealand Pharma, Ascencia, Senseonics, Adocia, and Tandem Diabetes. E.R.D. has issued patents and pending patents on aspects of the BP, and is an employee, the Executive Chair of the Board of Directors, and shareholder of Beta Bionics, Inc. F.H.E.-K. has issued patents and pending patents on aspects of the BP, and is an employee and shareholder of Beta Bionics, Inc. K.J.R. has no personal financial disclosures but reports that her employer has received grant support from Beta Bionics, Inc., Dexcom, Inc., and Tandem Diabetes Care. C.B. reports receiving consulting payments from Beta Bionics, Inc., Novo Nordisk, and Zealand Pharma. Z.L. has no personal financial disclosures but reports that her employer has received grant support from Beta Bionics, Inc., Dexcom, Inc., and Tandem Diabetes Care. M.C.M. has no personal financial disclosures but reports that his employer has received grant support from Beta Bionics, Inc., Dexcom, Inc., and Tandem Diabetes Care. P.C. is a former Dexcom, Inc. employee and his current employer has received consulting payments on his behalf from vTv Therapeutics, Beta Bionics, Inc., Dexcom, Inc., and Diasome. R.W.B. reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding and study supplies from Tandem Diabetes Care, Beta Bionics, Inc., and Dexcom, Inc.; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome. All other authors have no personal financial disclosures to report.
Funding Information
Study funding was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (grant number 1UC4DK108612-01), by an Investigator-Initiated Study award from Novo Nordisk, and by Beta Bionics, Inc., which also provided the experimental BP devices used in the study. Fast-acting insulin aspart and insulin aspart were provided by Novo Nordisk, and insulin lispro was provided by Eli Lilly. Blood glucose meters and test strips (Contour Next One Blood Glucose Monitoring System) were provided by Ascensia Diabetes Care. Continuous glucose monitor sensors and transmitters were purchased from Dexcom, Inc., at a discounted price.
Supplementary Material
References
- 1. The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, et al. : 6. Glycemic targets: standards of medical care in diabetes-2022. Diabetes Care 2022;45(Suppl 1):S83–S96. [DOI] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pettus JH, Zhou FL, Shepherd L, et al. : Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–2227. [DOI] [PubMed] [Google Scholar]
- 5. Forlenza GP, Lal RA: Current status and emerging options for automated insulin delivery systems. Diabetes Technol Ther 2022;24:362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bionic Pancreas Research Group; Russell SJ, Beck RW, Damiano ER, et al. : Multicenter, randomized trial of a bionic pancreas in type 1 diabetes. N Engl J Med 2022;387:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bionic Pancreas Research Group; Beck RW, Russell SJ, Damiano E, et al. . A multicenter randomized trial evaluating fast-acting insulin aspat in the bionic pancreas in adults with type 1 diabetes. Diabetes Technol Ther 2022;24:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck RW, Bocchino LE, Lum JW, et al. : An evaluation of two capillary sample collection kits for laboratory measurement of HbA1c. Diabetes Technol Ther 2021;23:537–545. [DOI] [PubMed] [Google Scholar]
- 9. Benjamini Y, Hochberg Y: On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 2000;25:60–83. [Google Scholar]
- 10. The Diabetes Control and Complications Trial Research Group: The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes 1995;44:968–983. [PubMed] [Google Scholar]
- 11. Brown SA, Kovatchev BP, Raghinaru D, et al. : Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408. [DOI] [PubMed] [Google Scholar]
- 13. Carlson AL, Sherr JL, Shulman DI, et al. : Safety and glycemic outcomes during the MiniMed™ advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown SA, Forlenza GP, Bode BW, et al. : Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44:1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanapka LG, Lum JW, Beck RW: Insulin pump infusion set failures associated with prolonged hyperglycemia: frequency and relationship to age and type of infusion set during 22,741 infusion set wears. Diabetes Technol Ther 2022;24:396–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.