Abstract
F4 receptor-positive (F4R+) and F4 receptor-negative (F4R−) pigs were orally vaccinated with purified F4 fimbriae of enterotoxigenic Escherichia coli (ETEC). Serum immunoglobulin G (IgG) and IgA responses were readily detected in F4R+ animals, whereas immune responses were not detected in F4R− animals. Even after a subsequent oral infection with virulent F4+ ETEC and a booster immunization with F4, the F4R− animals remained F4 seronegative whereas the unvaccinated F4R+ pigs exhibited clear IgA and IgG responses. These results clearly demonstrate that F4Rs are a prerequisite for an immune response following oral immunization. Furthermore, indications that oral F4 vaccination can induce mucosal protection were obtained, since the experimental ETEC infection did not induce a systemic booster response or fecal ETEC excretion in orally vaccinated F4R+ pigs, in contrast to the clear immune response and ETEC excretion of unvaccinated F4R+ animals. F4-specific IgA antibodies could be found in the feces of the vaccinated F4R+ pigs. They are secreted at the intestinal mucosal surface and appear to prevent ETEC infection. The F4R-dependent induction of a mucosal immune response can be used as a model to better understand mucosal immunization and mucosal immune responses and can contribute to the development of oral vaccines in veterinary as well as in human medicine.
The intestines of humans and animals constitute large mucosal surfaces which come daily in contact with huge amounts of pathogenic as well as nonpathogenic antigens (20, 31). To deal with this continuous input of antigens, the gut-associated lymphoid tissues have a variety of effector mechanisms. Activation of these mechanisms results either in immunostimulation, leading to protection against or rejection of antigens (e.g., enteropathogenic microorganisms), or in immunosuppression, resulting in a tolerance for these antigens (e.g., food antigens) (25, 26). So, the intestinal immune system can discriminate between nonpathogenic and pathogenic antigens (16). The mechanisms responsible for both opposite effects are not well understood and form one of the important obstacles for the development of mucosal vaccines. Indeed, vaccines should activate the immunostimulating mechanisms but not the immunosuppressive ones. The importance of receptor-mediated antigen uptake in the induction of immune responses has been hypothesized before (9), but real evidence for this possibility is still missing.
Enterotoxigenic Escherichia coli (ETEC) is an important cause of diarrhea and mortality in neonatal (22) and recently weaned (19) piglets. Some ETEC strains bear F4 fimbriae, which allow these microorganisms to adhere to F4-specific receptors (F4R) present on brush borders of villous enterocytes. Consequently, colonization of the small intestine can occur. Vaccination of a sow during pregnancy leads to secretion of antigen-specific antibodies in colostrum and milk, which protect piglets against infections during the suckling period (10, 21). After being weaned, however, the pigs are deprived of this passive protection and become susceptible to ETEC infections (13). At that moment, an active mucosal immunity is required for protection. An efficient activation of the protective intestinal mucosal immune mechanisms can occur following oral infection but is not obtained by parenteral immunization (28). Therefore, competent oral veterinary vaccines for inducing mucosal protection are not yet available. It has recently been demonstrated that oral administration of solubilized purified F4 fimbriae induces an intestinal mucosal immune response in F4R-positive (F4R+) piglets (27). The absence or presence of these F4R is based on genetic inheritance and can be determined in vitro (12).
In the present study, we analyzed whether F4R, present on brush borders of villous enterocytes, play a key role in the induction of a mucosal immune response. Furthermore, it was evaluated if oral F4 vaccination can elicit mucosal protection against a subsequent challenge.
MATERIALS AND METHODS
Pigs.
Fifteen pigs (Belgian Landrace × Piétrain), which were seronegative for antibodies against F4, were weaned at the age of 5 weeks and immediately housed in groups of five in isolation units, where they obtained water and food ad libitum. All animals were orally treated with colistine (150,000 U/kg of body weight/day; Colivet; Prodivet Pharmaceuticals, Eynatten, Belgium) from 7 days before until 3 days after weaning to prevent ETEC infections. At the end of the experiment, all animals were killed to determine the presence of F4R on their villous enterocytes and/or the number of F4-specific-antibody-secreting cells (ASC) in different tissues. Euthanasia was performed by intravenous injection of pentobarbital (24 mg/kg; Nembutal; Sanofi Sante Animale, Brussels, Belgium) followed by exsanguination.
Bacterial inoculum.
The hemolytic E. coli strain GIS 26, serotype O149:K91:F4ac, producing the heat-labile enterotoxin (LT+) and heat-stable enterotoxin types a and b (STa+, STb+), was cultured for 24 h on brain heart infusion agar (Oxoid, Unipath, Drongen, Belgium), and bacteria were collected by washing the agar with phosphate-buffered saline (PBS; 150 mM, pH 7.4). Subsequently, the bacteria were washed once in PBS and suspended in PBS and the concentration of bacteria in the suspension was determined by measuring the optical density at 660 nm (OD660). An OD of 1 equals 109 bacteria/ml, as determined by counting CFU. The concentration of the suspension was adjusted to 109 bacteria per ml.
Purification of F4 fimbriae.
The F4 fimbriae of the bacteria were isolated as previously described (14) with slight modifications. Briefly, the bacteria were cultured in tryptone soy broth (Difco Laboratories, Biotrading, Bierbeek, Belgium) at 37°C for 18 h, collected by centrifugation, and washed in PBS. Subsequently, the F4 fimbriae were isolated by homogenization of the bacterial suspension, followed by centrifugation to remove larger fragments. The fimbriae, solubilized in the supernatant, were precipitated with 40% ammonium sulfate, and the pellet was dissolved and dialyzed overnight against ultrapure H2O. The protein concentration of the isolated fimbrial solution was determined by the bicinchoninic acid reaction (Sigma-Aldrich, Bornem, Belgium) with bovine serum albumin as a standard. The purity was assessed by electrophoresis on a sodium dodecyl sulfate–12% polyacrylamide slab gel.
In vitro villous adhesion assay for F4R.
In order to determine the presence of F4R on the small intestinal villous enterocytes, an in vitro villous-adhesion assay was performed.
(i) Collection of small intestinal villi.
Immediately after euthanasia, the abdomens of the pigs were opened and a 15-cm-long intestinal segment was excised from the mid jejunum of each pig, after which the intestinal contents were removed by washing the segments three times with PBS at 4°C (7). Subsequently, the segments were opened and washed in Krebs-Henseleit buffer (120 mM NaCl, 14 mM KCl, 25 mM NaHCO3, 1 mM KH2PO4 [pH 7.4]) containing 1% formaldehyde at 4°C. The villi were gently scraped from the mucosae with a glass slide and suspended and washed four times in the same buffer until the supernatant was clear. The villi were stored in this buffer until the adhesion assay was performed.
(ii) Villous-adhesion assay.
The in vitro villous-adhesion assay was based on the technique described by Girardeau (12). Prior to the assay, villi were washed four times in Krebs-Henseleit buffer without formaldehyde and finally suspended in PBS supplemented with 1% (wt/vol) d-mannose (Fluka, Sigma-Aldrich, Bornem, Belgium). d-Mannose was added to prevent adhesion of E. coli by type 1 pili (F1). Subsequently, 4 × 108 F4+ E. coli organisms were added to an average of 50 villi in 0.5 ml of PBS with 1% d-mannose and incubated at room temperature for 1 h while being gently shaken. After the incubation, the villi were examined by phase-contrast microscopy at a magnification of 600 and the adhesion of the bacteria was quantified by counting the number of bacteria adhering along a 50-μm length of villous brush border at 20 different places, after which the bacterial adhesion per 250 μm length of villous brush border was calculated.
In order to certify the F4 specificity of this bioassay, blocking experiments were performed with F4ac-specific monoclonal antibodies (MAb) (clone CVI F4ac-5; ID-DLO, Lelystad, The Netherlands) (30) and with an irrelevant MAb of similar isotype, i.e., an anti-swine immunoglobulin G (IgG) MAb (29). Furthermore, E. coli organisms of the same strain grown at 18°C for 2 days and consequently not expressing F4 fimbriae (11) were used as negative-control bacteria to corroborate the specificity of the F4-mediated adhesion.
Experimental procedures. (i) Oral vaccination with purified F4 fimbriae.
At the age of 6 weeks, 10 animals were orally given the F4 antigen (V-animals [vaccinated]) on three successive days (2 mg/day). Therefore, the antigen was solubilized in 10 ml of PBS and administered orally after the animals had been deprived of food and water for 3 h. Subsequently, they were deprived for an additional two hours. Five animals were placebo vaccinated with PBS (C-animals [control]). All animals received one second homologous oral vaccination on day 16 post-primary vaccination (ppv). Antigen-specific antibodies in serum were determined at days 0, 16, 23, 30, and 36 ppv.
(ii) Oral challenge with virulent F4+ ETEC.
On day 36 ppv, all animals were orally infected with the virulent F4+ ETEC strain as described previously (8). In short, pigs were pretreated for 3 days with thiamphenicol (Urfamycine; Inpharzam NV, Brussels, Belgium), solubilized in milk (2,000 mg/animal/day). Subsequently, they were orally infected with 1010 F4+ ETEC, after the acidic gastric pH was neutralized with 62 ml of NaHCO3 (1.4% [wt/vol] in distilled water), 15 to 30 min earlier. F4-specific antibodies in serum were measured 8, 15, and 22 days postchallenge (pc) (44, 51, and 58 days ppv, respectively). Fecal excretion of F4+ ETEC was examined daily until 13 days pc, and F4-specific antibodies in feces were measured −1, 2, 4, 6, 8, 13, and 20 days pc.
(iii) Oral boost with purified F4 fimbriae.
To demonstrate the presence or absence of antigen-specific memory cells, five V-animals and four C-animals were immunized orally once again with purified F4, 37 to 42 days pc (73 to 78 days ppv). F4-specific ASC were determined in jejunal and ileal mesenteric lymph nodes (MLN) and in peripheral blood (PB) 5 days after the last oral administration of F4.
Test samples. (i) Serum.
Blood was taken from the jugular vein. After 18 h of incubation at room temperature, serum was collected, inactivated at 56°C for 30 min, and subsequently treated with kaolin (Sigma-Aldrich) to decrease the background reading in enzyme-linked immunosorbent assays (ELISA) (2). Therefore, 4 volumes of a kaolin suspension (25% [wt/vol] in PBS) were added to 1 volume of serum and incubated at room temperature for 30 min. The suspension was centrifuged at 5,500 × g for 10 min, and the supernatant was diluted in ELISA dilution buffer (PBS plus 0.2% [vol/vol] Tween 20 plus 5% [wt/vol] bovine serum albumin), yielding a final serum dilution of 1/10.
(ii) Feces.
Fecal samples were collected daily from 1 day before till 13 days pc and once on day 20 pc. Samples were stored at −80°C until they were examined for the presence of hemolytic F4+ ETEC and F4-specific IgA antibodies. Prior to the testing, 1% (wt/vol) and 50% (wt/vol) suspensions were prepared. The 1% fecal suspension was made in PBS and used for quantifying hemolytic F4+ ETEC excretion. The 50% suspension was made in PBS supplemented with fetal calf serum (20% [vol/vol]), kanamycin (100 μg/ml), penicillin (100 IU/ml), streptomycin (100 μg/ml), and Tween 20 (0.2% [vol/vol]) and subsequently heat inactivated for 30 min at 56°C. Following centrifugation at 5,500 × g for 30 min, the supernatant was collected and used in the F4-specific IgA ELISA.
(iii) PB MC.
Peripheral blood (PB) was collected from the jugular vein and immediately mixed with an equal volume of Alsever’s solution. The monomorphonuclear cells (MC) were isolated by density gradient centrifugation (500 × g at 18°C for 45 min) on Lymphoprep (NYCOMED Pharma AS, Life Technologies, Merelbeke, Belgium). After lysis of erythrocytes in ammonium chloride (74.7% [wt/vol]) and subsequent centrifugation (380 × g at 4°C for 10 min), the pelleted cells were washed and suspended at 107 cells/ml in leukocyte medium (RPMI 1640 [GIBCO BRL, Life Technologies, Merelbeke, Belgium] containing fetal calf serum [10% vol/vol], 2-mercaptoethanol [5 × 10−5 M], nonessential amino acids, Na pyruvate [100 μg/ml], l-glutamine [292 μg/ml], penicillin [100 IU/ml], streptomycin [100 μg/ml], and kanamycin [100 μg/ml]).
(iv) MLN MC.
At the moment of slaughter, jejunal and ileal MLN were aseptically dissected. After the surrounding fat was removed from the specimens, the MC were isolated by teasing the tissues apart, followed by lysis of erythrocytes with ammonium chloride. After centrifugation (380 × g at 4°C for 10 min), the pelleted cells were washed and suspended in leukocyte medium at 107 cells/ml.
ELISA for F4-specific IgG, IgA, and IgM.
The wells of a 96-well microtiter plate (NUNC, Polysorp Immuno Plates; Life Technologies) were coated with an F4ac-specific MAb (30) at a concentration of 1 μg/ml of coating buffer (carbonate-bicarbonate buffer, 50 mM, pH 9.4). After 2 h of incubation at 37°C, the remaining binding sites were blocked for 30 min at room temperature with PBS supplemented with 0.2% (vol/vol) Tween 20. Subsequently, the F4 antigen was added to the wells at a concentration of 50 μg/ml of ELISA dilution buffer and incubated for 1 h at 37°C. Then, treated sera were added in series of twofold dilutions in ELISA dilution buffer, starting from the dilution 1/10, and plates were incubated for 1 h at 37°C. Thereafter, the wells were treated for 1 h at 37°C with optimal dilutions of anti-swine IgG, IgM, and IgA conjugates. Conjugates had been prepared by coupling anti-swine IgG-, IgM-, and IgA-specific MAb (29) to peroxidase with a peroxidase labeling kit (Boehringer Mannheim, Brussels, Belgium). The substrate, ABTS, was added, and the OD405 was spectrophotometrically measured after 15 min of incubation at 37°C. Between each incubation step, plates were washed three times with PBS-T (PBS plus 0.2% [vol/vol] Tween 20). The obtained ODs of all the sera (dilution, 1/10) at day 0 were averaged, and the standard deviation was calculated. The mean, increased by 2 times the standard deviation, was considered the cutoff value. The obtained cutoff values were 0.176, 0.139, and 0.292 for F4-specific IgG, IgA, and IgM, respectively. The antibody titer was the inverse of the highest dilution which still had an OD higher than the calculated cutoff values.
For detection of F4-specific IgA antibodies in feces, supernatants of the prepared 50% fecal suspensions were added to the F4-coated wells and series of twofold dilutions in ELISA dilution buffer were made, after which an optimal dilution of the anti-swine IgA-peroxidase conjugate and subsequently ABTS were added. Incubation times and conditions were similar to those in the F4 serum antibody ELISA. The obtained ODs of the undiluted 50% fecal suspensions of pigs that were not immunized were averaged, and the cutoff value was determined as described above (obtained cutoff value = 0.140).
Elispot assay for F4-specific IgG-, IgA-, and IgM-secreting cells.
F4-coated plates were prepared as described above. Subsequently, 100 μl of MC suspensions were added to the wells at a concentration of 107 cells/ml of leukocyte medium and the plates were incubated for 3 h at 37°C in a humidified 5% CO2 atmosphere. After the cells were removed by three subsequent washes with PBS-T, the plates were treated with anti-swine IgG-, IgM-, and IgA-conjugates (see the ELISA procedure) for 1 h at 37°C. Unbound conjugates were removed by three PBS-T washes, and the substrate solution, consisting of 4 volumes of 3-amino-9-ethylcarbazole (AEC) working solution (0.67 ml of AEC stock solution [0.4%, wt/vol, in dimethylformamide] in 10 ml of Na acetate [0.1 M, pH 5.2] plus 10 μl of 30% H2O2) and 1 volume of 3% (wt/vol) low-melting-point agarose (BIOzym, Landgraaf, The Netherlands), was added. Developed brown-red spots were counted with an inverted microscope after plates had been incubated at least overnight in the dark at room temperature. For each MC suspension, spots in five wells (106 MC/well) were counted, so that finally the amount of ASC per 5 × 106 MC was obtained. Results are presented as mean numbers ± standard errors of the means (SEM) of F4-specific ASC.
Fecal excretion of F4+ ETEC.
Excretion of ETEC in feces was demonstrated by inoculating fecal samples onto blood agar plates (Difco Laboratories) at 37°C for 24 h. Hemolytic E. coli colonies were examined for the production of F4 fimbriae by agglutination with F4ac-specific MAb. When excretion of ETEC was demonstrated, bacteria were quantified by inoculating 0.5 ml of the 1% fecal suspension onto blood agar plates. After 24 h of incubation at 37°C, the colonies were blotted onto polyvinylidene fluoride membranes (Gelman Sciences, Leuven, Belgium) during 2 h at room temperature. Subsequently, the remaining binding sites were blocked overnight with blocking solution (5% [wt/vol] nonfat dry milk in PBS). After the membranes were rinsed in PBS, F4ac-specific MAb were added at a concentration of 0.4 μg/ml of blocking solution and the membranes were incubated for 1 h at room temperature. Subsequently, the membranes were washed three times in PBS and peroxidase-conjugated rabbit-anti-mouse Ig antibodies (DAKO, Merelbeke, Belgium), diluted 1/1,000 in blocking solution, were added. After 1 h of incubation at room temperature and three washes in PBS, the substrate solution (0.67 ml of AEC stock solution [0.4%, wt/vol, in dimethylformamide] in 10 ml of sodium acetate [0.1 M, pH 5.2] plus 10 μl of 30% H2O2) was added. The enzymatic reaction was stopped after 30 min by rinsing the membranes in PBS. Developed brown-red dots were counted, and the average within each group was calculated. Results are presented as the mean numbers ± SEM of excreted F4+ ETEC per 5 mg of feces.
RESULTS
Specificity of the in vitro villous-adhesion assay.
A villous-adhesion assay was optimized and controlled for its F4 specificity. For each pig of the experiment, the adhesion assay was repeated twice with similar results, indicating that the quantification of adhesion was reproducible. By the use of this test, the experimental animals could be divided into F4R+ animals, with between 17 ± 3 and 65 ± 7 (means ± SEM) bacteria adhering to a 250-μm length of villous brush border, and F4R− pigs, with less than 5 bacteria adhering to a 250-μm length of villous brush border (Fig. 1B).
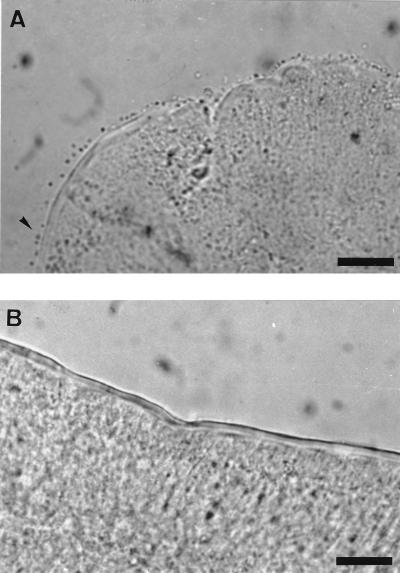
FIG. 1.
Small intestinal villous brush borders after the in vitro adhesion assay with F4ac+ E. coli. (A) F4R+ brush border with strong adhesion; (B) F4R− brush border without adhesion. Bars, 10 μm.
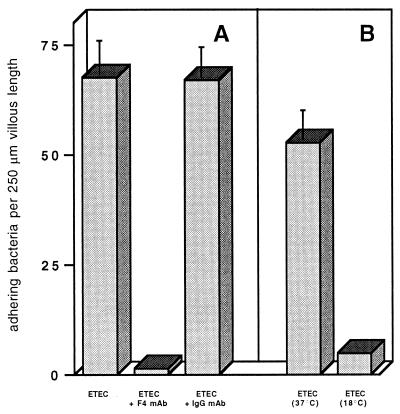
To ascertain the F4 specificity of the adhesion test, an F4ac-specific MAb and an irrelevant MAb of the same isotype were tested for their capacity to block the binding of the bacteria to the villi. Anti-F4ac blocked adhesion almost completely, while the irrelevant antibodies had a negligible effect on adhesion (Fig. 2A). Moreover, bacteria which had been cultured at 18°C and consequently did not express their F4 fimbriae had almost completely lost their ability to adhere to the fimbriae (Fig. 2B).
FIG. 2.
Adhesion of F4+ ETEC to F4R+ intestinal villi (of two pigs) with and without addition of 10 μg of F4ac- and swine IgG-specific MAb per ml (A) and adhesion of ETEC grown at 37 or 18°C to F4R+ intestinal villi (of two pigs) (B). Bars and T bars represent mean numbers of adhering bacteria per 250-μm length of villous brush border ± SEM (n = 2).
F4R characterization of the experimental animals.
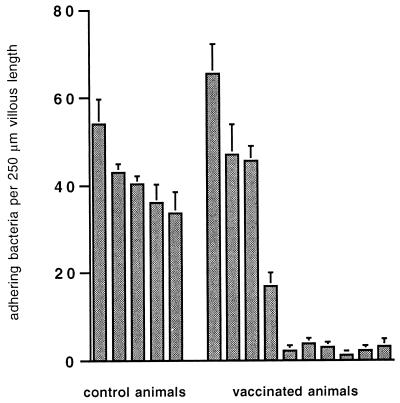
All C-animals and four V-animals expressed the F4R, whereas six V-animals lacked the receptor (Fig. 3). Based on these results, three groups were obtained: a placebo-vaccinated receptor-positive (CF4R+, n = 5) group, an F4-vaccinated receptor-positive (VF4R+, n = 4) group, and an F4-vaccinated receptor-negative (VF4R−, n = 6) group (Table 1). The mean number of adhering bacteria per 250-μm length of villus in the F4R+ groups was about 40 (Fig. 4).
FIG. 3.
Adhesion of F4+ ETEC to intestinal villi of control pigs (n = 5) and vaccinated pigs (n = 10). The adhesion assay was repeated twice for each pig. Bars and T bars represent mean numbers of adhering bacteria per 250-μm length of villous brush border for individual pigs ± SEM (n = 3).
TABLE 1.
Numbers of F4R+ and F4R− pigs in V-animal and C-animal groups at the moment of immunization and infection
| Treatment (days ppv) | No. of:
|
||
|---|---|---|---|
| V-animals
|
C-animals
|
||
| F4R+ | F4R− | F4R+ | |
| Vaccination (0–3, 16) | 4 | 6 | 5 |
| ETEC infection (36) | 4 | 6 | 5 |
| F4 oral boost (73 or 78) | 3 | 2 | 4 |
FIG. 4.
Adhesion of F4+ ETEC to intestinal villi of pigs in the CF4R+ group (n = 5), the VF4R+ group (n = 4), and the VF4R− group (n = 6). Bars and T bars represent mean numbers of adhering bacteria per 250-μm length of villous brush border ± SEM.
Oral vaccination of F4R+ and F4R− pigs with purified F4.
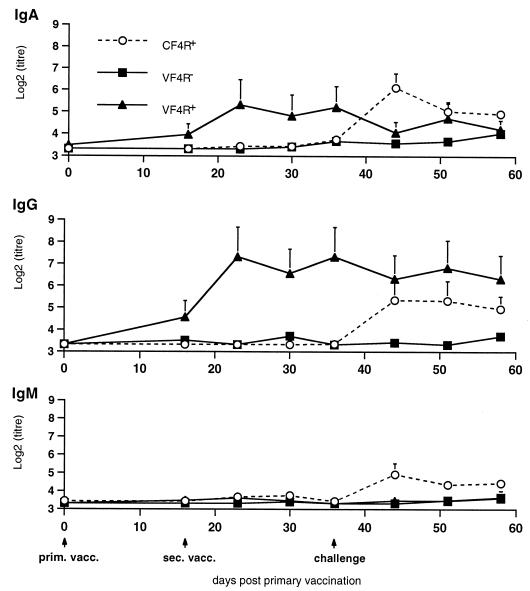
Four F4R+ and six F4R− pigs were orally vaccinated with purified F4 fimbriae and received a booster immunization 16 days later (VF4R+ and VF4R− pigs, respectively), and five control F4R+ animals were vaccinated with a placebo (CF4R+ pigs). The humoral immune response was analyzed till day 36. Following primary vaccination, a weak antibody response occurred in VF4R+ pigs (Fig. 5), with IgG and IgA antibodies increasing slightly but with IgM remaining under the detection limit. After the second vaccination on day 16 ppv, the antigen-specific IgG and IgA titers increased rapidly and reached a maximum on day 23, after which they both declined slightly. In contrast, oral vaccination of F4R− animals did not induce an F4-specific immune response. The placebo-vaccinated animals remained seronegative.
FIG. 5.
Evolution of the F4-specific IgG, IgM, and IgA antibody titers in serum (±SEM) in CF4R+ animals (n = 5), in VF4R+ animals (n = 4), and in VF4R− animals (n = 6) following vaccination on days 0 and 16; all animals were challenged on day 36, and F4-specific IgG, IgM, and IgA antibodies in serum were quantified. prim. vacc., primary vaccination; sec. vacc., secondary vaccination.
Examination of fecal samples 1 day before the challenge infection (19 days post-secondary vaccination, 35 days ppv) revealed F4-specific IgA antibodies in the feces of two of the VF4R+ pigs. These antigen-specific IgA antibodies were not detected in feces of VF4R− and CF4R+ pigs (Fig. 6).
FIG. 6.
Mean F4-specific IgA titers in feces of VF4R+ pigs (n = 4), VF4R− pigs (n = 6), and CF4R+ pigs (n = 5) 35 days ppv (1 day before oral ETEC challenge). Bars and T bars represent mean titers ± SEM.
Oral challenge of CF4R+, VF4R+, and VF4R− animals with F4+ ETEC.
Thirty-six days ppv (20 days post-secondary vaccination), all animals were infected with the virulent F4+ ETEC strain and the F4-specific antibody response was determined until day 58 (Fig. 5). Only the CF4R+ animals displayed an immune reaction, with an increase of all three Ig isotypes. Both IgM and IgA peaked 8 days pc (44 days ppv), after which they subsided, whereas IgG antibodies remained high and declined only slightly 22 days pc (58 days ppv). Infection of the VF4R+ piglets evoked no serum antibody booster response. At the moment of the infection, F4-specific IgG and IgA antibodies were still present and IgG remained at the same level during the period of analysis whereas IgA decreased slightly 8 days pc (44 days ppv), to increase afterwards to its level prior to the infection. The IgM antibody titer remained at background level. Infection of VF4R− pigs induced no immune reaction, i.e., all three isotype responses remained low.
F4-specific fecal IgA antibodies could be detected in only two of the four VF4R+ pigs the day before challenge (−1 day pc), with titers of 8 and 128, respectively (Fig. 7). After challenge those two animals showed a booster response 6 days pc, which subsequently gradually decreased. In none of the other pigs were F4-specific fecal IgA antibodies detected.
FIG. 7.
Evolution of the F4-specific IgA titers (±SEM) in feces of VF4R+ pigs (n = 4), VF4R− pigs (n = 6), and CF4R+ pigs (n = 5) following oral ETEC challenge.
An examination of fecal samples after the infection revealed that only the CF4R+ pigs excreted hemolytic F4+ ETEC on days 3, 4, and 5 pc (Table 2). At day 3, the excretion was maximal, amounting to approximately 1.22 × 104 F4+ ETEC per g of feces. In the feces of VF4R+ and VF4R− pigs, hemolytic F4+ ETEC could not be detected.
TABLE 2.
Fecal excretion of hemolytic F4+ ETEC after oral challenge of CF4R+, VF4R+, and VF4R− pigs
| Vaccination group (n) | Mean no. ± SEM of hemolytic F4+ ETEC per 5 mg of feces on day pc:
|
||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |
| CF4R+ (5) | —a | 61 ± 43 | 8 ± 4 | 2 ± 1 | — |
| VF4R+ (4) | — | — | — | — | — |
| VF4R− (6) | — | — | — | — | — |
—, no excretion detected.
F4-specific ASC in MLN and PB 5 days after an oral boost with purified F4.
Five V-animals and four C-animals were reimmunized orally with purified F4, 37 to 42 days pc. Analysis of F4-specific IgG, IgM, and IgA ASC in lymphoid tissues 5 days after the oral boost revealed no or only a few cells in jejunal and ileal MLN of infected VF4R+ and VF4R− pigs (Table 3). In contrast, larger numbers of F4-specific ASC were present in the jejunal and ileal MLN (total of 69 and 17 ASC per 5.106 MC, respectively) of the infected CF4R+ animals (Table 3). These cells were mainly of the IgM and IgA isotypes. F4-specific ASC were almost undetectable in PB of pigs of all three groups.
TABLE 3.
Number of F4-specific ASC in jejunal and ileal MLN and in PB 5 days after oral F4 immunization of challenged CF4R+ pigs (n = 4), challenged VF4R+ pigs (n = 3), and challenged VF4R− pigs (n = 2)
| Isotype | Mean no. ± SEM of F4-specific ASC per 5 × 106 MC in challenged:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| CF4R+ animals
|
VF4R+ animals
|
VF4R− animals
|
|||||||
| Jejunal MLN | Ileal MLN | PB | Jejunal MLN | Ileal MLN | PB | Jejunal MLN | Ileal MLN | PB | |
| IgG | 4 ± 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| IgM | 40 ± 27 | 10 ± 6 | 0 | 1 ± 1 | 1 ± 1 | 0 | 0 | 0 | 3 ± 3 |
| IgA | 25 ± 12 | 7 ± 2 | 0 | 1 ± 0 | 0 | 1 ± 1 | 0 | 0 | 0 |
| Total | 69 | 17 | 0 | 2 | 1 | 1 | 0 | 0 | 3 |
DISCUSSION
The ability of purified F4 fimbriae to induce an immune response upon oral administration in pigs has been demonstrated before (27). However, a clear explanation for the immunogenic capacity of this antigen was still missing. The importance of an F4R interaction for the immune response is postulated in this study. The presence or absence of F4R was demonstrated and quantified by an in vitro villous-adhesion assay. The specificity of this test was shown by completely blocking bacterial adhesion with F4ac-specific MAb. These MAb bind to the fimbrial c epitope (30) that partially constitutes the receptor-binding site of the fimbriae (3). Furthermore, bacteria cultured at 18°C and consequently not expressing F4 (11) do not adhere to F4R+ villi. A third indication of the specificity of the in vitro adhesion assay is that purified F4 fimbriae block the subsequent adhesion of F4+ ETEC (28). All these data prove that the adhesion assay is a reliable test to demonstrate the presence of the F4R.
In the present study, it was clearly demonstrated, by vaccinating pigs with and without the F4R, that the presence of the antigen-specific receptors on brush borders of villous enterocytes is a prerequisite for the induction of an immune response following oral immunization with purified F4 fimbriae. Indeed, oral F4 vaccination of F4R+ pigs induced an immune reaction with a clear increase of F4-specific antibodies in serum. Moreover, both IgG and IgA responses were enhanced by a second vaccination 16 days later, resulting in maxima at day 23. Furthermore, examination of fecal samples 35 days ppv demonstrated that upon oral F4 vaccination of F4R+ animals, F4-specific IgA antibodies could be detected, indicating that the mucosal immune system had been activated. In contrast, F4-specific antibodies were not detected in serum or feces of F4R− animals following the two vaccinations. All these findings indicate that the presence of the antigen-specific receptor plays an important role in the induction of an immune response following oral immunization. This possibility was confirmed by a subsequent oral challenge of the CF4R+ and VF4R− animals with the virulent F4+ ETEC strain. The VF4R− pigs remained F4 seronegative after infection, whereas the CF4R+ animals showed clear serum IgG, IgA, and IgM responses following challenge. Even a subsequent oral administration of F4 to the VF4R− pigs, 37 to 42 days after the infection could not induce F4-specific ASC in the jejunal and ileal MLN. All these data confirm our hypothesis that the immunostimulating capacity of the F4 antigen following oral feeding is due to the presence of an antigen-specific receptor. Moreover, as purified F4 fimbriae are able to bind to villous brush borders, as demonstrated in an ELISA and in an F4+ ETEC adhesion-inhibition assay (28), an antigen-receptor interaction is likely to have been crucial in the present experiments in stimulating the intestinal mucosal immune system. This finding suggests that, as an alternative to the use of mucosal adjuvants, liposomes, and biodegradable microspheres, a receptor-mediated mechanism should be considered as a way to activate mucosal immune responses and to prevent the induction of oral tolerance. Indeed, it has been demonstrated for pigs that an oral administration of ovalbumin (OVA), a protein antigen that does not bind to an epithelial receptor, suppresses the serum OVA-specific IgG response upon a subsequent systemic OVA immunization, which suggests that a state of oral tolerance is induced (28). In mice, it has been shown that OVA feeding evokes intestinal mucosal suppression as well (23). The fact that the mucosal immune system can be suppressed by administration of an oral antigen is still one of the important obstacles in the development of oral vaccines. This suppression can be prevented or even reversed by simultaneously administered mucosal adjuvants such as the E. coli heat-labile enterotoxin and the cholera toxin (5, 23). A possible adjuvant effect of F4 on coadministered antigens such as OVA could not be demonstrated, as a combined oral administration of purified fimbriae and OVA did induce an F4-specific but not an OVA-specific immune response (28). Whether conjugation of tolerogens to F4 fimbriae, resulting in an antigen complex which binds to F4R, can stimulate the mucosal immune system against the conjugated tolerogens is under study and may contribute to the development of oral porcine vaccines.
The levels of IgA in the F4 orally immunized (VF4R+) and the ETEC-infected animals (CF4R+) reached approximately the same titers [log 2 (titer) = 5.3 ± 1.2 and 6.1 ± 0.7, respectively], indicating that the mucosal immune system was equally well stimulated. However, the IgG response was slightly higher after oral F4 immunization than after infection [log 2 (titer) = 7.3 ± 1.4 and 5.3 ± 0.9, respectively]. A plausible explanation for the higher IgG (systemic) response after oral immunization with F4 is that solubilized purified F4, after receptor binding, stimulates the mucosal immune system but also enters rapidly into the circulation and elicits a systemic response. On the other hand, F4 fimbriae which are attached to the outer membranes of living bacteria are less likely to enter the circulation after infection, so that stimulation remains more restricted to the mucosal immune system. Alternatively, the adjuvant effect of the heat-labile enterotoxin, produced by the colonized ETEC, may have altered the F4-specific immune response (5).
Examination of fecal samples revealed that only the CF4R+ pigs excreted hemolytic F4+ ETEC 3, 4, and 5 days following infection, indicating their colonization of the small intestine. Subsequently, the immune system was stimulated, resulting in serum IgM, IgG, and IgA antibody responses. In contrast, no excretion was observed in the VF4R− animals, indicating an absence of colonization due to a lack of the F4R. As colonization could not occur, the immune system was not stimulated and these piglets remained F4 seronegative (17). Remarkably, in the VF4R+ piglets F4+ ETEC excretion was not found either. Despite the presence of the receptor, which enables F4+ ETEC to colonize the small intestine, no colonization took place and a systemic booster response remained absent. Nevertheless, a mucosal booster response was observed in two of the four VF4R+ pigs. In these vaccinated animals, F4-specific IgA antibodies, secreted at the mucosal surfaces (18), appear to be responsible for the prevention of colonization and thus protection. However, at this moment it is unclear if the induced protection can prevent diarrhea, as the experimental infection did not induce clinical symptoms in the control placebo-vaccinated animals, probably due to the weak colonization of the small intestine with the F4+ ETEC (maximal excretion, 1.22 × 104 colonies/g of feces). Indeed, it has been demonstrated that hemolytic ETEC strains proliferate in the intestinal tracts of healthy pigs to lower numbers than in littermates that develop diarrhea (15, 24) and that colonization of F107+ E. coli, resulting in a minimal fecal excretion of 106 to 107 colonies/g of feces, is needed to evoke clinical symptoms in recently weaned 4-week-old pigs (4). Possible explanations for the weak colonization are development of some age resistance, due to the release of larger amounts of F4R in the intestinal mucus layers with increasing age (6), and the absence of a number of predisposing factors involved in the multifactorial diarrhea complex in weaned pigs (13). The absence of clinical symptoms can also be explained by an age-related increase in the fluid absorption capacity of the colon (1).
In conclusion, these results demonstrated that F4R have to be present on the brush borders of villous enterocytes in order to induce an immune response in pigs upon oral F4 immunization. Moreover, oral vaccination with purified F4 prevented colonization of the intestine by virulent F4+ ETEC. Consequently, oral vaccination against F4+ ETEC infections with purified F4 fimbriae appears possible and oral immunization with antigens conjugated to F4 carriers to avoid induction of oral tolerance must be considered.
ACKNOWLEDGMENTS
This work was supported by the Research Fund of the Universiteit Gent and the Fund for Scientific Research of Flanders (FWO, Brussels, Belgium). The author has a grant from the Flemish Institute for the Promotion of Scientific-Technological Research in the Industry (IWT, Brussels, Belgium).
We gratefully acknowledge A. Bianchi for providing the pig-specific IgG-, IgM-, and IgA-specific MAb. We are also indebted to Denise Slos for her technical assistance.
REFERENCES
- 1.Argenzio R A, Moon H W, Kemeny L J, Whipp S C. Colonic compensation in transmissible gastroenteritis of swine. Gastroenterology. 1984;86:1501–1509. doi: 10.1016/S0016-5085(84)80165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnouts S. Ph.D. thesis. Leuven, Belgium: Catholic University Leuven; 1994. Somatostatin immunoneutralization in the chicken: immunogen development and effect on growth; p. 42. [Google Scholar]
- 3.Bakker D, Willemsen P T, Simons L H, van Zijderveld F G, de Graaf F K. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol Microbiol. 1992;6:247–255. doi: 10.1111/j.1365-2958.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- 4.Bertschinger H U, Stamm M, Vögeli P. Inheritance of resistance to oedema disease in the pig: experiments with an Escherichia coli strain expressing fimbriae 107. Vet Microbiol. 1993;35:79–89. doi: 10.1016/0378-1135(93)90117-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 6.Conway P L, Welin A, Cohen P S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990;58:3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox E, Houvenaghel A. Comparison of the in vitro adhesion of K88, K99, F41 and P987 positive Escherichia coli to intestinal villi of 4- to 5-week-old pigs. Vet Microbiol. 1993;34:7–18. doi: 10.1016/0378-1135(93)90003-p. [DOI] [PubMed] [Google Scholar]
- 8.Cox E, Schrauwen E, Cools V, Houvenaghel A. Experimental induction of diarrhoea in newly-weaned piglets. J Vet Med Ser A. 1991;38:418–426. doi: 10.1111/j.1439-0442.1991.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 9.De Aizpurua H J, Russell-Jones G J. Oral vaccination—identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med. 1988;167:440–451. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deprez P, Van Den Hende C, Muylle E, Oyaert W. The influence of the administration of sow’s milk on the postweaning excretion of haemolytic Escherichia coli in the pig. Vet Res Commun. 1986;10:469–478. doi: 10.1007/BF02214010. [DOI] [PubMed] [Google Scholar]
- 11.Gaastra W, de Graaf F K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982;46:129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girardeau J P. A new in vitro technique for attachment to intestinal villi using enteropathogenic Escherichia coli. Ann Microbiol (Paris) 1980;131B:31–37. [PubMed] [Google Scholar]
- 13.Hampson D J. Postweaning Escherichia coli diarrhoea in pigs. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, Oxon, United Kingdom: CAB International; 1994. p. 177. [Google Scholar]
- 14.Jacobs A A C, de Graaf F K. Production of K88, K99 and F41 fibrillae in relation to growth phase, and a rapid procedure for adhesin purification. FEMS Microbiol Lett. 1985;26:15–19. [Google Scholar]
- 15.Kenworthy R, Crabb W E. The intestinal flora of young pigs, with reference to early weaning, Escherichia coli and scours. J Comp Pathol. 1963;73:215–228. doi: 10.1016/s0368-1742(63)80025-9. [DOI] [PubMed] [Google Scholar]
- 16.Mowat A M. Oral tolerance and regulation of immunity to dietary antigens. In: Ogra P L, Lamm M E, McGhee J R, Mestecky J, Strober W, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1995. p. 185. [Google Scholar]
- 17.Newby T J, Stokes C R, Evans P A, Bourne F J. The immune response following oral vaccination with E. coli. Curr Top Vet Med Anim Sci. 1981;12:377–388. [Google Scholar]
- 18.Porter P, Kenworthy R, Noakes D E, Allen W D. Intestinal antibody secretion in the young pig in response to oral immunization with Escherichia coli. Immunology. 1974;27:841–853. [PMC free article] [PubMed] [Google Scholar]
- 19.Richards W P C, Fraser C M. Coliform enteritis of weaned pigs. A description of the disease and its association with haemolytic Escherichia coli. Cornell Vet. 1961;51:245–257. [PubMed] [Google Scholar]
- 20.Russell, M. W., and J. Mestecky. 1988. Induction of the mucosal immune response. Rev. Infect. Dis. 10(Suppl. 2):S440–S446. [DOI] [PubMed]
- 21.Rutter J M, Jones G W. Protection against enteric disease caused by Escherichia coli—a model for vaccination with a virulence determinant? Nature. 1973;242:531–532. doi: 10.1038/242531a0. [DOI] [PubMed] [Google Scholar]
- 22.Sojka W J, Lloyd M K, Sweeney E J. Escherichia coli serotypes associated with certain pig diseases. Res Vet Sci. 1960;1:17–27. [Google Scholar]
- 23.Stok W, van der Heijden P J, Bianchi A T J. Conversion of orally induced suppression of the mucosal immune response to ovalbumin into stimulation by conjugating ovalbumin to cholera toxin or its B subunit. Vaccine. 1994;6:521–526. doi: 10.1016/0264-410x(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 24.Svendsen J, Riising H J, Christensen S. Studies on the pathogenesis of enteric Escherichia coli in weaned pigs: bacteriological and immunofluorescence studies. Nord Vetmed. 1977;29:212–220. [PubMed] [Google Scholar]
- 25.Thomas H, Parrot D. The induction of tolerance to a soluble protein antigen by oral administration. Immunology. 1974;27:631–639. [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasi T B., Jr Oral tolerance—a review. Transplantation. 1980;29:353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Van den Broeck W, Cox E, Goddeeris B. Stimulation of the mucosal immune system in pigs after immunization with F4 fimbriae. Immunol Lett. 1997;56:177. [Google Scholar]
- 28.Van den Broeck, W., E. Cox, and B. M. Goddeeris. Unpublished data.
- 29.Van Zaane D, Hulst M M. Monoclonal antibodies against porcine immunoglobulin isotypes. Vet Immunol Immunopathol. 1987;16:23–36. doi: 10.1016/0165-2427(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 30.Van Zijderveld F G, Anakotta J, Brouwers R A M, van Zijderveld A M, Bakker D, de Graaf F K. Epitope analysis of the F4 (K88) fimbrial antigen complex of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect Immun. 1990;58:1870–1878. doi: 10.1128/iai.58.6.1870-1878.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wold A E, Dahlgren U I H, Hanson L Å, Mattsby-Baltzer I, Midvetdt T. Difference between bacterial and food antigens in mucosal immunogenicity. Infect Immun. 1989;57:2666–2673. doi: 10.1128/iai.57.9.2666-2673.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]