Abstract
France faced an unusual situation of dengue transmission in 2022, with 65 autochthonous cases spread over nine transmission events by 21 October. This exceeded the number of cases observed during the entire period 2010 to 2021. Six of these events occurred in departments that had never experienced autochthonous dengue transmission. We provide an update of dengue surveillance data in mainland France in 2022. The multiplication of transmission events calls for continuous adaption of preparedness and response to arbovirus-related risks.
Keywords: Dengue, mainland France, autochthonous, vector-borne disease
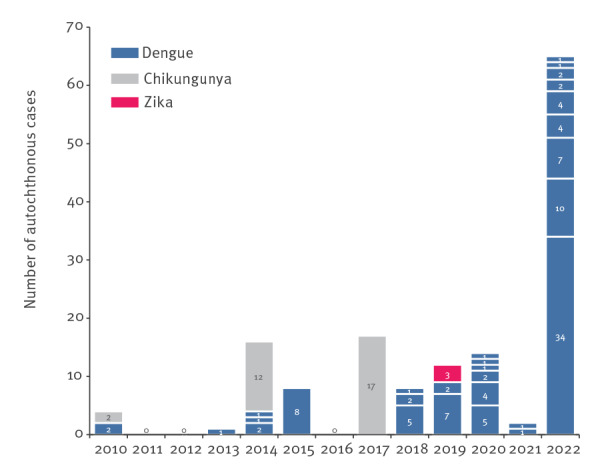
Because Aedes albopictus mosquito is expanding globally, increasing areas worldwide and in Europe have become at risk for Aedes-borne viruses. The French arbovirus surveillance system – deployed in mainland France since 2006 – has detected an increasing number of autochthonous transmissions of dengue virus (DENV), chikungunya virus (CHIKV) and Zika virus (ZIKV) since 2010 [1–5]. In 2022, the situation regarding DENV transmission in mainland France appears to be exceptional both in terms of number of transmission events and number of autochthonous cases (Figure 1).
Figure 1.
Distribution of arbovirus autochthonous events in mainland France, 2010–2022
Data for 2022 are not yet consolidated.
By 21 October 2022, the surveillance had identified 65 autochthonous cases of dengue fever, across nine transmission events. We describe here these events and their main characteristics.
Arboviruses surveillance in France
Dengue is a notifiable disease year-round in France. The surveillance is enhanced from May to November, during the Ae. albopictus activity period [6], for timely implementation of vector control measures to prevent local virus transmission. Each year, regional health authorities launch the enhanced surveillance period with awareness campaigns targeting health professionals on diagnosis and reporting of dengue, but also chikungunya and Zika cases. In addition, Santé publique France Regional Offices review daily arbovirus diagnostic tests conducted in a nationwide network of laboratories to identify non-notified cases. Epidemiological investigations are carried out for each case, whether imported or autochthonous. An autochthonous case is defined as a case with no history of travel during the 2 weeks before symptom onset. Adapted vector control measures are then implemented in the different locations visited by the cases during their viraemic period (from 2 days before to 7 days after symptom onset).
The National Reference Centre for arboviruses (NRC) is in charge of laboratory confirmation of the first autochthonous cases in a local transmission event. According to the time between onset of symptoms and date of sampling (DPSO), the NRC performs RT-qPCR (DPSO < 7 days), ELISA using whole inactivated antigens (DPSO > 5 days), or both. When a RT-qPCR is positive, whole genome sequencing is attempted regardless of the quantification cycle (Cq) value, and virus isolation when Cq < 30. The NRC systematically tests for several flaviviruses (DENV, ZIKV and West Nile virus) and CHIKV. When an autochthonous case is identified, active case finding is immediately implemented in the area to determine the extent of local transmission: door-to-door survey in a 150 m to 250 m radius area, with fingertip blood sampling for suspected cases [7], health professional outreach and a press release for general population awareness. Any situation of autochthonous transmission is risk-assessed with regard to the safety of substances of human origin.
Dengue transmission in mainland France in 2022
From 1 May to 21 October 2022, 217 imported cases of dengue were identified in mainland France. The majority of cases were imported from Cuba (n = 71), Ivory Coast (n = 16) and Mexico (n = 14). The number of imported cases in 2022 was similar to that observed in 2021 (164 cases), but considerably lower than in 2019 (n = 657) and 2020 (n = 834). In 2022, however, nine events of autochthonous dengue transmission were identified in mainland France, resulting in 65 total cases since the beginning of the surveillance period (Table).
Table. Main characteristics of dengue virus transmission events in mainland France, 2022 (n = 9).
| Place of transmission | Number of autochthonous cases | Identification of the primary case | Serotype | Date of symptoms onset | |||
|---|---|---|---|---|---|---|---|
| City | Department | Region | Earliest | Latest | |||
| Perpignan | Pyrénées-Orientalesa | Occitanie | 1 | No | DENV-3 | 12 June | NA |
| Fayence | Var | Paca | 7 | No | DENV-1 | 20 June | 22 July |
| Andrest/Rabastens-de-Bigorre | Hautes-Pyrénéesa | Occitanie | 4 | Reunion Island, France | DENV-1 | 11 July | 28 August |
| Saint-Jeannet/Gattières | Alpes-Maritimes | Paca | 34 | No | DENV-3 | 25 July | 22 September |
| La Salvetat-Saint-Gilles | Haute-Garonnea | Occitanie | 4 | Democratic Republic of the Congo | DENV-3 | 14 August | 20 August |
| Saint-Laurent-du-Var | Alpes-Maritimes | Paca | 10 | No | DENV-1 | 15 August | 16 September |
| Montauban | Tarn-et-Garonnea | Occitanie | 1 | No | ND | 30 August | NA |
| Toulouse | Haute-Garonne | Occitanie | 2 | No | DENV-3 | 15 September | 21 September |
| Porto Vecchio region | Corse-du-Suda | Corsica | 2 | No | DENV-3 | 20 September | 20 September |
DENV: dengue virus; NA: not applicable; ND: not determined; Paca: Provence-Alpes-Côte d’Azur.
a First historic autochthonous circulation of Aedes-borne virus in the department.
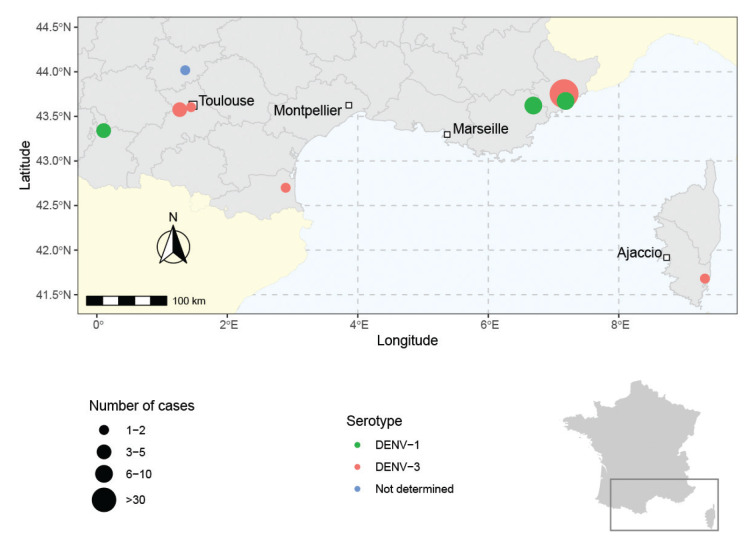
The index case was notified by health professionals for six events. In addition, laboratory surveillance identified three transmission events. The size of the different autochthonous events ranged from 1 to 34 cases. The largest event occurred in the municipalities of Saint-Jeannet and Gattières, where respectively 23 and 11 cases were identified, belonging to a single transmission chain. All events occurred in suburban residential areas.
For seven of the nine events, the primary imported case was not identified. One local transmission chain was initiated by an imported case returning from Reunion Island (identified by laboratory-based surveillance), the other by a person arriving from the Democratic Republic of the Congo who did not seek medical attention during the symptomatic period (identified during the door-to-door survey). Neither of these two primary cases were notified by health professionals. The dengue serotype could be determined for eight events: five were serotype 3 (DENV-3), identified for the first time in mainland France, and three were serotype 1 (DENV-1). Whole genomes sequences were obtained for seven of the nine clusters. The DENV-3 sequence from the Corsica cluster could not be determined due to low viral load. The near complete sequences of the four remaining DENV-3 clusters were distinct from each other, suggesting distinct introductions in agreement with the available epidemiological data. Additional virological and phylogenetic analyses are still ongoing.
Six of these events occurred in departments that had never previously experienced autochthonous transmission of an Aedes-borne virus, five in south-western France (Occitanie region) and one in Corsica (Figure 2). None of the cases presented with a severe form of dengue fever. The clinical manifestations of the 65 cases were mainly non-specific and included fever (98%), asthenia (78%), headache (75%), myalgia/arthralgia (62%), rash (43%) and retro-orbital pain (15%). Cases occurred throughout the surveillance period, from mid-June to the end of September.
Figure 2.
Dengue transmission events reported in mainland France, October 2022
Map created using GADM database of Global Administrative Areas, Version 4.1 available at www.gadm.org.
Discussion
Given the history of colonisation by Ae. albopictus, events of autochthonous transmission of Aedes-borne viruses are expected in southern France [8] and several events have already been observed [2,4,5], as well as in other Mediterranean countries [9-11]. However, the evolving epidemiological situation of dengue in France in 2022 is exceptional, not only because of a large number of events, but also because the number of reported autochthonous cases (n = 65) exceeded that observed during the entire period 2010 to 2021 (n = 48) [2,12]. Among the nine events reported this year, one is the largest ever documented in Europe, with 34 cases of dengue identified on 21 October 2022 (Figure 2). Moreover, six events took place in departments where such transmission had never been described (south-western France, Corsica).
We assume that the increase in cases is not a surveillance artefact because surveillance was maintained during the COVID-19 outbreak. The drivers of arbovirus transmission are undoubtedly multiple but are mainly influenced by the interactions between vector populations, virus strains and the global environment [13]. Environmental conditions thus have a major impact on the efficiency of the vector system as well as on vector density and host–vector contacts [14,15]. Spring and summer have been especially warm in 2022 [16], which promoted vector activity and transmission efficacy of dengue virus [17]. Adaptation between viral and vector genotypes is also a major factor in transmission, and the occurrence of numerous episodes involving DENV-3 raises questions. Further research is needed to better characterise determinants (climatic, socio-economic, environmental) for local transmission events and their extension. Such analysis needs to take into account all autochthonous transmission events observed in France since 2010.
Conclusion
France is the only European country to have declared autochthonous dengue cases this year [18]. The French surveillance system appears to be sufficiently sensitive to detect autochthonous transmission of arboviruses and efficient to limit their spread. However, the multiplication and the geographical extent of transmission events are challenging its sustainability. Ensured sustainability of this surveillance requires promotion of main stakeholder involvement through (i) consolidation of the network of reporting laboratories, (ii) raising awareness among patients to seek medical consultation for influenza-like illness without respiratory symptoms, especially in the context of negative COVID-19 results and (iii) orientation of health professionals to the diagnosis and reporting of arboviral diseases.
Ethical statement
Ethical approval was not needed for this study as it was performed as part of routine surveillance activities.
Acknowledgements
The authors wish to acknowledge the agents from regional health authorities, Santé publique France, mosquito control operators, laboratories and health professionals who helpfully contributed to manage these local transmission events.
The members of the Investigation team were;
Michèle Auzet-Caillaud, Catherine Aventini, Alice Borel, Christiane Bruel, Anne Decoppet, Maurin Gossa, Mounira Krouk, Chloé Laboureyras, Alexia Mazza, Marie Mihoubi, Alexandra Muriel, Françoise Peloux-Petiot, Clément Piétin, Jérôme Raibaut, Laurent Saintillan, Camille Schmitt, Monique Travanut, Camille Vassal (Agence régionale de santé Provence-Alpes-Côte d’Azur); Patricia Albert, Léa Bulmanski-Then, Cédric Cahuzac, Laura Catala, Aline Cot, Pierre-Marie Creton, Angélique Dubois, Jean Sébastien Dehecq, Isabelle Estève-Moussion, Fanny Gaillard, Christine Giraud, Olivier Glass, Vincent Lagarde, Aurélie Larrose, Catherine Lecerf, Radia Ould Larabi, Blandine Maes, Guylaine Peiffer, Christine Rico, Isabelle Rouvié-Laurie, Nicolas Roux, Giselle Santana, Nicolas Sauthier, Sarah Sellami, Stéphane Wagner, Betty Zumbo (Agence régionale de santé Occitanie) ; Estelle Balle, Lauranne Coiplet, Stéphanie Gaucher, Gabriel Leccia (Agence régionale de santé Corse) ; Delphine Morel (Agence régionale de santé Grand-Est) ; Fabienne Jouanthoua (Agence régionale de santé Nouvelle-Aquitaine) ; Lorène Belkadi, Oriane Broustal, Cécile Durand, Damien Mouly, Gabriel Yubero, Adeline Riondel, Leila Bekheira, David Kelly, Isabelle Mertens-Rondelart, Miguel-Angel Sanchez-Ruiz, Yvan Souares, Nathan Yanwou (Santé publique France); Alexia Barbry, Thibaut Durand, Anne Ovize, Anaïs Soares (Biomnis) ; Bénédicte Roquebert, Laura Verdurme (Cerba); Jean-Michel Mansuy (CHU Toulouse), Hugues Aumaître (CH Perpignan); Lionel Chanaud, Grégory L’Ambert, Yves-Marie Kervella (EID-Méditerranée) ; Guillaume Lacour, Antoine Mignotte, Charles Tizon (Altopictus); Anthony Biancarelli, Jean-Luc Maestracci, Barthélémy Pompa, Jean-Baptiste Santoni (Collectivité de Corse).
Conflict of interest: None declared.
Authors’ contributions: AC, CC, FJ, GG, GAD, AG, HN, MCP, FF and all members of the investigation team participated to the investigation, data collection and/or to the biological analyses. AC, CC, FJ, GG, GAD, AG, HN, MCP and FF participated in the writing of the original draft. All authors reviewed and edited the article.
References
- 1. Calba C, Guerbois-Galla M, Franke F, Jeannin C, Auzet-Caillaud M, Grard G, et al. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Euro Surveill. 2017;22(39):17-00647. 10.2807/1560-7917.ES.2017.22.39.17-00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Franke F, Giron S, Cochet A, Jeannin C, Leparc-Goffart I, de Valk H, et al. Autochthonous chikungunya and dengue fever outbreak in Mainland France, 2010-2018. Eur J Public Health. 2019;29(Supplement_4):ckz186-628. 10.1093/eurpub/ckz186.628 [DOI] [Google Scholar]
- 3. Giron S, Franke F, Decoppet A, Cadiou B, Travaglini T, Thirion L, et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Euro Surveill. 2019;24(45):1900655. 10.2807/1560-7917.ES.2019.24.45.1900655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. La Ruche G, Souarès Y, Armengaud A, Peloux-Petiot F, Delaunay P, Desprès P, et al. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15(39):19676. 10.2807/ese.15.39.19676-en [DOI] [PubMed] [Google Scholar]
- 5. Succo T, Leparc-Goffart I, Ferré JB, Roiz D, Broche B, Maquart M, et al. Autochthonous dengue outbreak in Nîmes, South of France, July to September 2015. Euro Surveill. 2016;21(21):30240. 10.2807/1560-7917.ES.2016.21.21.30240 [DOI] [PubMed] [Google Scholar]
- 6. Lacour G, Chanaud L, L’Ambert G, Hance T. Seasonal synchronization of diapause phases in Aedes albopictus (Diptera: Culicidae). PLoS One. 2015;10(12):e0145311. 10.1371/journal.pone.0145311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matheus S, Huc P, Labeau B, Bremand L, Enfissi A, Merle O, et al. The use of serum spotted onto filter paper for diagnosing and monitoring chikungunya virus infection. J Clin Virol. 2015;71:89-92. 10.1016/j.jcv.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 8. Rodhain F. Aedes albopictus: a potential problem in France. Parassitologia. 1995;37(2-3):115-9. [PubMed] [Google Scholar]
- 9. Aranda C, Martínez MJ, Montalvo T, Eritja R, Navero-Castillejos J, Herreros E, et al. Arbovirus surveillance: first dengue virus detection in local Aedes albopictus mosquitoes in Europe, Catalonia, Spain, 2015. Euro Surveill. 2018;23(47):1700837. 10.2807/1560-7917.ES.2018.23.47.1700837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lazzarini L, Barzon L, Foglia F, Manfrin V, Pacenti M, Pavan G, et al. First autochthonous dengue outbreak in Italy, August 2020. Euro Surveill. 2020;25(36):2001606. 10.2807/1560-7917.ES.2020.25.36.2001606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt-Chanasit J, Haditsch M, Schoneberg I, Gunther S, Stark K, Frank C. Dengue virus infection in a traveller returning from Croatia to Germany. Euro Surveill. 2010;15(40):19677. 10.2807/ese.15.40.19677-en [DOI] [PubMed] [Google Scholar]
- 12. Jourdain F, Roiz D, de Valk H, Noël H, L’Ambert G, Franke F, et al. From importation to autochthonous transmission: Drivers of chikungunya and dengue emergence in a temperate area. PLoS Negl Trop Dis. 2020;14(5):e0008320. 10.1371/journal.pntd.0008320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zouache K, Fontaine A, Vega-Rua A, Mousson L, Thiberge JM, Lourenco-De-Oliveira R, et al. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc Biol Sci. 2014;281(1792):201141078. 10.1098/rspb.2014.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roche B, Léger L, L’Ambert G, Lacour G, Foussadier R, Besnard G, et al. The spread of Aedes albopictus in Metropolitan France: contribution of environmental drivers and human activities and predictions for a near future. PLoS One. 2015;10(5):e0125600. 10.1371/journal.pone.0125600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Semenza JC, Rocklöv J, Ebi KL. Climate change and cascading risks from infectious disease. Infect Dis Ther. 2022;11(4):1371-90. 10.1007/s40121-022-00647-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Météo-France. 2022: les bilans climatiques. [2022: climate assessments]. Saint Mande Cedex: Météo-France. [Accessed: 7 Oct 2022]. Available from: https://meteofrance.fr/actualite/publications/2022-les-bilans-climatiques
- 17. Mercier A, Obadia T, Carraretto D, Velo E, Gabiane G, Bino S, et al. Impact of temperature on dengue and chikungunya transmission by the mosquito Aedes albopictus. Sci Rep. 2022;12(1):6973. 10.1038/s41598-022-10977-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control (ECDC). Autochthonous transmission of dengue virus in mainland EU/EEA, 2010-present. Stockholm: ECDC. [Accessed: 12 Oct 2022]. Available from: https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea