Abstract
Atypical lymphoproliferative disorders (LPDs) related with autoimmune disease (AID) show marked clinicopathological diversity, which are defined as three distinct clinicopathological subtypes such as those resembling Castleman disease (CD), atypical paracortical hyperplasia with lymphoid follicles (APHLF), and atypical lymphoplasmacytic and immunoblastic proliferation (ALPIB). We studied excisional biopsy specimens from 31 patients with atypical LPDs associated with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren syndrome (SjS). The lesions in these 31 cases were classified into 6 (19.4%) cases resembling CD, 14 (45.2%) cases of APHLF, and 11 (35.5%) cases of ALPIB. Five cases (83.3%) resembling CD were in the active stage with systemic symptoms and multicentric lymphadenopathy. Thirteen cases (92.9%) of APHLF showed systemic symptoms, multicentric lymphadenopathy and abnormal laboratory findings. Histologic findings for cases resembling CD were rare in patients with RA and SjS. In AID patients, histologic findings for cases resembling CD or APHLF findings correlated with disease activity and multicentric lymphadenopathy. Six cases (54.5%) of ALPIB were in the active phase with systemic symptoms and multicentric lymphadenopathy. ALPIB tended to be unrelated to AID activity, especially in the majority of patients with no abnormal laboratory findings. Atypical LPDs associated with AID is a group of diseases that may be overdiagnosed and overtreated. The diagnosis of atypical LPDs associated with AID requires an understanding of the histological findings as well as a comprehensive assessment of the presence of systemic symptoms, the distribution of lymphadenopathy, and abnormal laboratory findings.
Keywords: autoimmune disease, lymphadenopathy, atypical lymphoproliferative disorders, Castleman disease, atypical lymphoplasmacytic and immunoblastic proliferation
INTRODUCTION
Reactive lymph node lesions in patients with autoimmune disease (AID) and related disorders represent marked histologic diversity and occasionally exhibit atypical lymphoproliferative disorders (LPDs).1,2 In 1984, Koo et al. reported atypical lymphoplasmacytic and immunoblastic proliferation (ALPIB) associated with various AIDs including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren syndrome (SjS).3 Frizzera et al. defined ALPIB and multicentric Castleman disease (CD) as a finding of atypical LPD associated with AID.4 Several reports have also mentioned ALPIB and multicentric CD in the lymph nodes of patients with AID as included in atypical LPD associated with AID.5-10 Atypical LPDs associated with AID are rare and are characterized by prominent lymphoplasmacytic infiltration with various numbers of immunoblasts.4,5 Kojima et al. reported that patients with atypical LPDs associated with AID exhibit multicentric lymphadenopathy, constitutional symptoms and abnormal laboratory findings.11,12 Atypical LPD associated with AID must be differentiated from malignant lymphoma to determine a treatment strategy.11-14 To avoid overdiagnosis and overtreatment, the diagnosis of LPDs associated with AID requires confirmation of these clinical and laboratory findings as well as histological findings.
Atypical LPDs associated with AID are defined by Kojima et al. as three distinct clinicopathological subtypes such as those resembling CD, atypical paracortical hyperplasia with lymphoid follicles (APHLF), and ALPIB.13,15 This classification has made it possible to define atypical LPDs associated with AID that have clinicopathological findings similar to malignancy but cannot be confirmed as malignant. We performed a comprehensive cross-sectional analysis of atypical LPDs associated with AID using a total of 31 cases, including 23 cases previously reported by Kojima et al.10-12 and 8 cases studied at our institution. This review article first presents the clinical characteristics of atypical LPDs associated with AID and then discuss differential diagnosis for a resembling CD, APHLF, and ALPIB, respectively.
CLINICOPATHOLOGICAL FINDINGS OF ATYPICAL LYMPHOPROLIFERATIVE DISORDERS (LPDs) ASSOCIATED WITH AUTOIMMUNE DISEASE (AID)
In Table 1, we summarize 31 cases of atypical LPDs associated with AID diagnosed by performing an excisional lymph node biopsy. The patients were predominantly female (83.9%), with a median age of 48 years (24-71), and 15 (48.4%), 10 (32.3%) and 6 (19.4%) patients had SLE, RA and SjS, respectively, as a clinically diagnosed AID. At the time of the lymph node biopsy, 24 patients (77.4%) had systemic symptoms of AIDs. Multicentric lymphadenopathy was diagnosed from clinical or imaging findings in 24 patients (77.4%). Abnormal laboratory findings, including positivity for various autoantibodies, leukocytopenia, and a reduced serum complement level, were recognized in 19 patients (61.3%).
Table 1. Clinicopathological findings of atypical lymphoproliferative disorders associated with autoimmune disease (n=31).
| Subtype | Cases | M:F | Age (median) |
SLE (%) | RA (%) | SjS (%) | Systemic symptoms (%) | Multicentric lymphadenopathy (%) | Abnormal laboratory findings (%) |
|---|---|---|---|---|---|---|---|---|---|
| Resembling Castleman’s disease (CD) | 6 | 0:6 | 24-42 (33) |
6 (100) | 0 (0) | 0 (0) | 5 (83.3) | 5 (83.3) | 4 (66.7) |
| Atypical paracortical hyperplasia with lymphoid follicles (APHLF) | 14 | 5:9 | 31-68 (51) |
5 (35.7) | 5 (35.7) | 4 (28.6) | 13 (92.3) | 13 (92.3) | 13 (92.3) |
| Atypical lymphoplasmacytic immunoblastic proliferation (ALPIB) | 11 | 0:11 | 25-71 (49) |
4 (36.4) | 5 (45.5) | 2 (18.2) | 6 (54.5) | 6 (54.5) | 5 (45.5) |
| All cases | 31 | 5:26 | 24-71 (48) |
15 (48.4) | 10 (32.3) | 6 (19.4) | 24 (77.4) | 24 (77.4) | 22 (71.0) |
Abbreviations: M:F, male: female ratio; SLE, systemic lupus erythematosus; RA, rheumatoid arthritis; SjS, Sjögren’s syndrome.
Atypical LPDs associated with AID were classified into 6 (19.4%) cases resembling CD, 14 (45.2%) cases of APHLF, and 11 (35.5%) cases of ALPIB. All cases resembling CD were in SLE, not RA or SjS. Five cases (83.3%) resembling CD were in the active stage with systemic symptoms and multicentric lymphadenopathy. Four cases (66.7%) resembling CD had abnormal laboratory findings, with an elevated erythrocyte sedimentation rate, proteinuria, and autoimmune hemolytic anemia in all 4 cases, thrombocytopenia in one case, and with none exhibiting polyclonal hypergammaglobulinemia. In group APHLF, SLE, RA and SjS were seen in 5 (35.7%), 5 (35.7%) and 4 (28.6%) patients, respectively. Thirteen cases (92.9%) of APHLF showed systemic symptoms, multicentric lymphadenopathy and abnormal laboratory findings, including polyclonal hypergammaglobulinemia in 10 patients (71.4%) and thrombocytopenia in 2 patients (14.3%). EBV-positive cases retrieved by in-situ hybridization for EBV-encoded RNA (EBER) were found in 3 of 14 cases (21.4%) only in the APHLF group (not listed in Table 1). In the ALPIB group, SLE, RA and SjS were seen in 4 (36.4%), 5 (45.5%) and 2 (18.2%) patients, respectively. Six cases (54.5%) were in the active phase with systemic symptoms and multicentric lymphadenopathy. Abnormal laboratory findings were seen in 5 (45.5%) cases of ALPIB, with polyclonal hypergammaglobulinemia in all 5 cases and thrombocytopenia in none.
In summary, atypical LPDs associated with ALD in patients with SLE, RA, and SjS were classified into three groups, each with its own clinical characteristics. The group with a syndrome resembling CD was younger and it was found only in patients with SLE. The syndrome resembling CD and APHLF groups had more patients with active AID, whereas the ALPIB group tended to have fewer patients in the active phase of AIDs.
RESEMBLING CASTLEMAN DISEASE (resembling CD)
Histological findings
A syndrome resembling CD must to be differentiated from idiopathic CD, which is divided into hyaline-vascular and plasma cell types based on histological characteristics, and especially from idiopathic multicentric CD histologically exhibiting a plasma cell type. Histologic findings of a syndrome resembling CD are hyaline-vascular lymphoid follicles, plasmacytosis, and vascular proliferation of the interfollicular areas. Flendrig et al. described the histological characteristics of a syndrome resembling CD as “mixed type”, whereas idiopathic CD is divided into two histological types.16,17
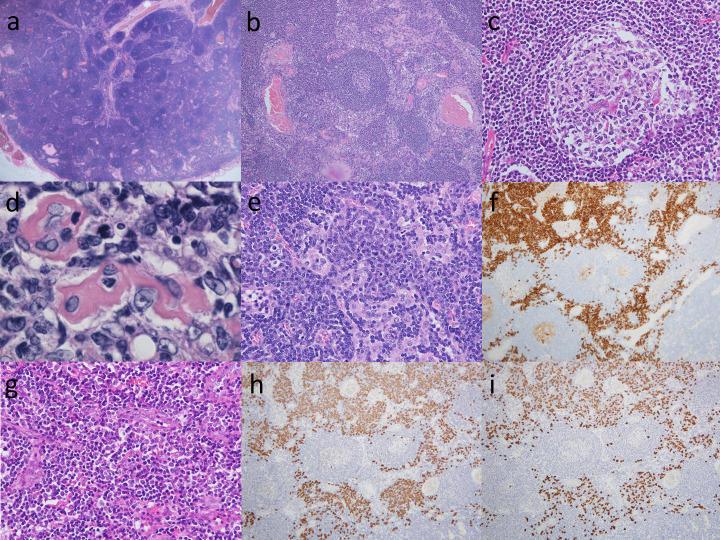
Representative histological findings of a syndrome resembling CD are shown in Fig. 1. A syndrome resembling CD is often limited to a lymph node enlargement of less than 2.0 cm in diameter. The number of lymphoid follicles with germinal centers varies (Fig. 1a). The lymphoid follicles have a wide morphological spectrum that ranges from cases with predominantly hyperplastic lymphoid follicles (hyperplastic lymphoid type) to those with abundant small hyalinized vessels (hyaline-vascular type).4 In the hyperplastic lymphoid type (Fig. 1b), numerous plasma cells are intermingled. In the hyaline-vascular type, the capillaries that entered from the tissue surrounding the lymphoid follicles often penetrate the germinal center (Fig. 1c), and these capillaries often have thickened and hyalinized walls (Fig. 1d). The mantle zone of the hyperplastic lymphoid type is thin and consists of hyaline-vascular type vessels concentrically arranged in small hyalinized vessels (Fig. 1b). It should be noted that syndromes resembling CD often produce intermediate findings between the hyperplastic lymphoid type and the hyaline-vascular type, and both types of histological findings can be mixed. In the interfollicular area and medullary cords, numerous mature plasma cells may infiltrate a sheet (Fig. 1e), with CD38-positive (Fig. 1f), and immature plasma cells and immunoblasts may be observed (Fig. 1g). The in situ hybridization of immunoglobulin light chain determinants demonstrated a mixture of Kappa-positive (Fig. 1h) and Lambda-positive (Fig. 1i) cells, indicating that mature plasma cells and their precursors are polytypic. Intracytoplasmic immunoglobulins of plasma cells and their precursors are reportedly usually polytypic in nature.8,10 Lymphocytes also infiltrate to various degrees, and a small number of neutrophils and eosinophils may be present.
Fig. 1.
Representative histological findings of cases resembling Castleman disease.
(a) In a low power view of the lymph node, numerous lymphoid follicles were noted including an atrophic germinal center surrounded by a thick mantle zone and a capillary that penetrated the follicle. HE, x20.
(b) Lymphoid follicles including an atrophic germinal center surrounded by a thick mantle zone and a capillary that penetrated the follicle were noted. HE, x100.
(c) A large hyperplastic follicle with active germinal center was present. HE, x400.
(d) A germinal center to small hyaline-vascular was present. HE, x1000.
(e, f) Aggregates of mature plasma cells and plasmacytoid cells immunoreactive to CD38 were observed in the interfollicular area. (e) HE, x400; (f) CD38, x100.
(g) Interfollicular area containing aggregates of numerous arborizing capillaries were also present. HE, x400.
(h, i) In situ hybridization of immunoglobulin light chain determinant demonstrated the polytypic condition of mature plasma cells and their precursors. (h) Kappa, x100; (i) Lambda, x100.
Differential diagnosis
In our data, a syndrome resembling CD was found in all SLE patients and multicentric lymphadenopathy was found in 5 out of 6 patients. A syndrome resembling CD in SLE patients must be differentiated from idiopathic multicentric CD in particular to determine the treatment strategy. Clinical findings are helpful in making this distinction. Idiopathic multicentric CD occasionally presents with AID-like clinical symptoms, is positive for various autoantibodies, and exhibits some of the clinical diagnostic criteria for SLE,4,17,18 although they do not fully meet the diagnostic criteria for SLE. On the other hand, our experience with cases resembling CD showed constitutional symptoms such as fever, arthralgia, skin rash, as well as various laboratory abnormalities such as an elevated erythrocyte sedimentation rate, proteinuria, and autoimmune hemolytic anemia. These clinical manifestations are also similar to those of idiopathic multicentric CD.10,19,21-25 Kojima et al. described the clinicopathological characteristics of idiopathic multicentric CD in Japan.26 Based on their clinicopathological findings, two types were delineated, including a group that did not exhibit marked polyclonal hypergammaglobulinemia. This group was predominantly female, higher in age, with a high incidence of pleural effusion or ascites, and frequent association with thrombocytopenia and autoantibody positivity during the course of disease. All the cases resembling CD that we experienced were similar to this group in that there was an absence of polyclonal hypergammaglobulinemia. Histologically, the majority of idiopathic multicentric CD cases have been reported to be a plasmacytoid type, while resembling CD cases presented as a mixed type.10,19,21-25 The diagnosis must be made based on a combination of clinicopathological findings.
The concept of a case resembling CD associated with RA and SjS is controversial. None of the cases resembling CD that we reviewed met the diagnostic criteria for RA and SjS. The histological findings in patients with RA and SjS all varied in terms of the size and shape of the germinal center, were distinguished from the surrounding mantle zone, showed a proliferation of T cells with mild atypia in the interfollicular area, and lacked small hyalinized vessels in germinal center. Cases resembling CD associated with RA and SjS are rare.9,22 SjS that can be treated with lymphadenopathy may resolve, and the disease may not have been biopsied at the time of progression. However, resembling associated with RA may indeed be rare.
ATYPICAL PARACORTICAL HYPERPLASIA WITH LYMPHOID FOLLICLES (APHLF)
Histological findings
Kojima et al. reported an unusual APHLF lymph node lesion of associated with SLE or RA.13,15 APHLF is referred to as “resembling T-zone dysplasia” and is defined histologically by marked hyperplasia of the germinal center and proliferation of T cells with mild atypia in the interfollicular area.11,20
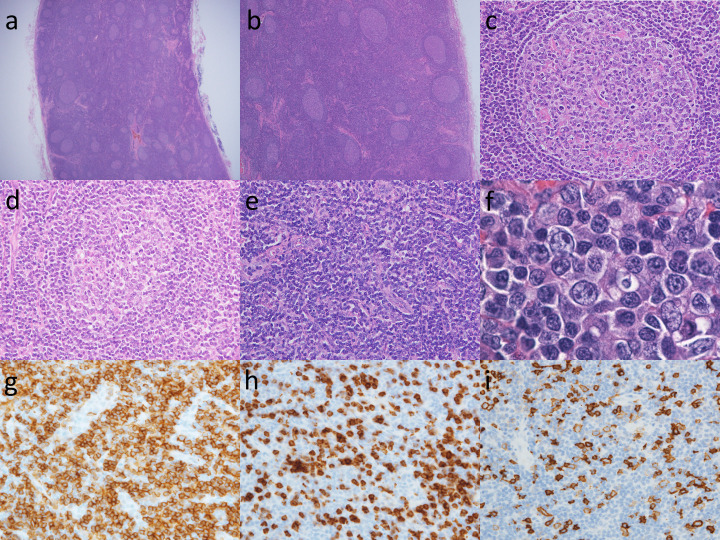
Figure 2 shows representative histological findings of APHLF. Lymph node enlargement is often limited to less than 2.0 cm in diameter in APHLF. Histological findings of APHLF showed paracortical hyperplasia and numerous lymphoid follicles (Fig. 2a). Many of the germinal centers show hyperplasia (Fig. 2b). Several small vessels with flattened endothelial cell nuclei may penetrate the germinal center, which shows hyperplasia (Fig. 2c). The germinal center is destroyed by lymphocytic infiltration into the mantle zone (follicular lysis) (Fig. 2d). In the interfollicular area, there are numerous small vessels with enlarged endothelial cell nuclei and a thickened basement membrane (Fig. 2e). The cells proliferating around the small vessels are numerous small lymphocytes that have rounded nuclei with mild cytologic atypia (Fig. 2f). Different numbers of plasma cells, immunoblasts, and histiocytes are intermingled. Interfollicular T-cells are a mixture of CD4-positive cells (Fig. 2g) and CD8-positive cells (Fig. 2h), with CD4-positive cells being slightly more numerous than CD8-positive cells. The majority of the immunoblasts in the interfollicular area express CD20 (Fig. 2i).
Fig. 2.
Atypical paracortical hyperplasia with lymphoid follicles (APHLF)
(a) In a low power view of the lymph node, there are numerous lymphoid follicles. HE, x20.
(b) Numerous lymphoid follicles including an atrophic germinal center and paracortical hyperplasia. HE, x40.
(c) The germinal centers were usually hyperplastic, although a few were somewhat atrophic. HE, x40.
(d) The germinal center is destroyed by lymphocytic infiltration into the mantle zone (follicular lysis). HE, x400.
(e) High power field of the interfollicular area. Proliferation of arborizing vessels with high endothelium was also noted. HE, x400.
(f) There were intermingled with various numbers of plasma cells, immunoblasts and histiocytes in the paracortical area. Numerous small lymphocytes with minimal cytological atypia. HE, x1000.
(g, h) Sixty percent of interfollicular T-cells expressed CD4 and the remaining 40% were CD8-positive. (g) CD4, x400; (h) CD8, x400.
(i) The majority of the immunoblasts in the interfollicular area expressed CD20. x400.
Differential diagnosis
APHLF was termed T-zone dysplasia because of the possibility of a tumor due to the presence of atypical T cells in the interfollicular area. T-zone dysplasia with follicular hyperplasia was also reported by Suchi et al.27 However, Kojima et al. reported no complications of malignant lymphoma in patients with APHLH during a follow-up period of 44 to 225 months (median 103 months).11 In addition, polymerase chain reaction analysis demonstrated no clonal rearrangement of the T-cell receptor γ-chain gene.11 APHLF should be treated as a nonneoplastic entity and should be distinguished from T-cell lymphoma with lymphoid follicles or angioimmunoblastic lymphoma with hyperplastic germinal centers.
In our data, we found constitutional symptoms and multicentric lymphadenopathy in all but one case. Seven cases presented with lymphadenopathy as the onset of disease. Hepatomegaly was noted in four cases and splenomegaly was evident in two cases. APHLF is often associated with clinical symptoms and also with multicentric lymphadenopathy during active AID, so it can be relatively easily distinguished from the clinical course. To avoid overdiagnosis and overtreatment, we emphasize the need to pay close attention to the clinical and laboratory findings as well as the histologic findings.
ATYPICAL LYMPHOPLASMACYTIC AND IMMUNOBLASTIC PROLIFERATION (ALPIB)
Histological findings
Koo et al. reported an unusual lymph node lesion associated with various AIDs including SLE, RA, SjS and autoimmune hemolytic anemia.3 They also suggest that a proliferative finding of a mixture of immunoblasts and plasma cells is ALPIB. ALPIB is a rare LPD associated with AIDs.12
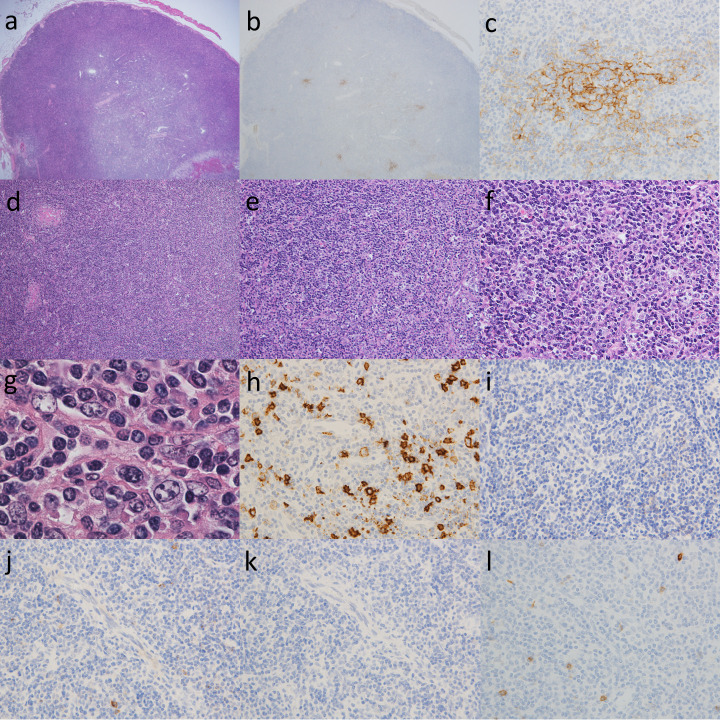
Representative histological findings of ALPIB are presented and explained in Fig. 3. The enlarged lymph nodes that indicate ALPIB are generally up to 3.0 cm in size. Basically, the number of lymphoid follicles decreases and individual lymphoid follicles atrophy (Fig. 3a). CD21 staining shows that the follicular dendritic cell meshwork is atrophied or destroyed, with only a little remaining (Figs. 3b and 3c). Hyperplasia and atrophy are mixed in some germinal centers. In some cases, the lymph sinuses seem to have disappeared (Fig. 3d). A polymorphic cell population consisting of numerous mature plasma cells, plasmacytoid cells, large basophilic transformed lymphocytes (immunoblasts), and various sized lymphocytes is observed in the paracortex (Figs. 3e and 3f). Although some of the immunoblasts have large vesicular nuclei with prominent nucleoli resembling Hodgkin cells, typical Reed-Sternberg cells are not detected (Fig. 3g). Small to medium lymphocytes exhibit minimal cytological atypia. Medium to large lymphocytes with clear cytoplasm, called clear cells, are absent (Fig. 3g). Staining with CD20 reveals that medium to large lymphocytes show B cell phenotypes, which indicate B-immunoblastic infiltration (Fig. 3h). The majority of medium- and large-sized lymphocytes were negative for programmed cell death protein 1 (PD-1), C-X-C motif chemokine ligand 13 (CXCL13), and inducible co-stimulator (ICOS), CD10, which are T follicular helper cell-associated markers (Fig. 3i-l). Small vessels in the paracortex usually have plump endothelial cell nuclei. High endothelial venules showing arborization were not prominent. Monocyte-like B cells may be seen in the paracortex.
Fig. 3.
Atypical lymphoplasmacytic and immunoblastic proliferation (ALPIB)
(a) In a low power view of the lymph node, there are a few lymphoid follicles including usually atrophic germinal center. HE, x20.
(b, c) Staining with CD21 highlighted the atrophic follicular dendritic cell meshwork. (b) x20; (c) x400.
(d) Obvious paracortical expansion with diffuse effaced lymph node architecture. HE, x100.
(e) The paracortical area was diffusely infiltrated by a polymorphous population of small to medium-sized lymphocytes. Each case contained small mild to moderate vessels in the interfollicular area. HE, x200.
(f) The paracortical area was diffusely infiltrated by a polymorphous population consisting of numerous mature plasma cells, plasmacytoid cells, large basophilic transformed lymphocytes (immunoblasts), small to medium-sized lymphocytes. Scattered histiocytes were also present. HE, x400.
(g) A portion of immunoblasts had large vesicular nuclei and prominent nucleoli resembling Hodgkin cells, but typical Reed-Sternberg cells were not detected. There were no medium-to-large lymphoid cells with clear cytoplasms (clear cells). HE, x1000.
(h) Staining with CD20 revealed that medium to large lymphocytes show B cell phenotypes, which indicate B-immunoblastic infiltration. x 400
(i-l) The majority of medium- and large-sized lymphocytes were negative for T follicular helper cell-associated markers. (i) PD-1, x400; (j) CXCL13, x400; (k) ICOS, x400; (l) CD10, x400.
Differential diagnosis
ALPIB is characterized histologically by a prominent infiltration of large B-immunoblasts in the paracortex. These immunoblasts look similar to Reed-Sternberg cells or pale/clear cells and should be differentiated from Hodgkin lymphoma and angioimmunoblastic T-cell lymphoma (AITL). Therefore, the differential diagnosis of ALPIB is particularly problematic with AILT.13 The concept of AITL has recently changed, and in the WHO classification, AITL is now included under “Angioimmunoblastic T-cell lymphoma and other T-follicular helper (TFH) cell-origin nodal lymphomas”.28 AITL is a proliferation of tumor cells with a TFH cell trait that is positive for at least two, ideally three of the following, CD10, BCL6, PD1, CXCL13, ICOS, and SAP, with a marked proliferation of high endothelial venules (HEVs) and follicular dendritic cells.28 By contrast, the important histological findings related to ALPIB are the absence of pronounced arborizing vascular proliferation and the absence of CD10-positive “clear cells”.12 ALPIB can be histologically distinguished from AITL by the immunophenotype of TFH and the findings of HEV. In addition, ALPIB consisted of a genetically undetected T cell receptor rearrangement, and the overall 5-year survival rate was 83%, suggesting the disease was a benign reactive process.12 Clinical manifestations are also useful in differentiation. AITL often presents with a variety of symptoms, and as the disease progresses, systemic lymphadenopathy, hepatosplenomegaly, rash, fever, weight loss, polyarthritis, pleural effusion and ascites may occur. ALPIB, on the other hand, was asymptomatic with single lymphadenopathy in half of the cases in our study.
CONCLUSION
There is no evidence that atypical LPD associated with AID is a neoplasm and it often has a good prognosis. Differentiation is necessary because of the different treatment modalities. A syndrome resembling CD is often associated with the active phase of SLE, and its histology resembles a mixed type of idiopathic CD. APHLF occurs in the active phase of SLE, RA, and SjS and the histological findings are characterized by hyperplasia of the lymphoid follicles with a proliferation of T cells showing mild atypia in the interfollicular area. Cases resembling CD and APHLF should be distinguished from idiopathic multicentric CD and malignant lymphoma by focusing on histological findings and clinical symptoms. ALPIB occurs in SLE, RA, and SjS, and is histologically characterized by prominent lymphoplasmacytic infiltration with various numbers of immunoblasts. ALPIB may be a single lymphadenopathy. With ALPIB it should be confirmed whether or not the immunoblasts in the interfollicular area resemble the clear cells of AITL and that there is no expression of the TFH cell markers. Atypical LPD associated with AID classified as resembling CD, APHLF, and ALPIB should be diagnosed by a comprehensive assessment of clinicopathologic findings.
ACKNOWLEDGMENTS
We are sincerely grateful to the late Professor Masaru Kojima for his excellent guidance and kindness.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Henry K. Connective tissue disorders/autoimmune diseases. In: Henry K, Symmers WStC (eds): Thymus, Lymph Nodes, Spleen and Lymphatics, Systemic Pathology, vol. 7, 3rd ed., Edinburgh, Churchill Livingstone. 1992; pp. 141-325. [Google Scholar]
- 2.O’Malley DP, Gerorge TI, Orazi A, Abbondanzo SL. Atlas of Nontumor Pathology, First Series, Fascicle 7. Benign and Reactive Conditions of Lymph Node and Spleen. Washington DC, Armed Forces Institute of Pathology. 2009; pp. 375-404. [Google Scholar]
- 3.Koo CH, Nathwani BN, Winberg CD, Hill LR, Rappaport H. Atypical lymphoplasmacytic and immunoblastic proliferation in lymph nodes of patients with autoimmune disease (autoimmune-disease-associated lymphadenopathy). Medicine (Baltimore). 1984; 63: 274-290. [DOI] [PubMed] [Google Scholar]
- 4.Frizzera G, Peterson BA, Bayrd ED, Goldman A. A systemic lymphoproliferative disorder with morphologic features of Castleman’s disease: clinical findings and clinicopathologic correlations in 15 patients. J Clin Oncol. 1985; 3: 1202-1216. [DOI] [PubMed] [Google Scholar]
- 5.Frizzera G. Atypical lymphoproliferative disorders, In: D.M. Knowles (Ed.), Neoplastic Hematopathology, Lippincott Williams & Wilkins, Philadelphia. 1992; pp. 459-495. [Google Scholar]
- 6.Ioachim HL, Jeffrey L. Ioachim’s lymph node pathology, 4th ed. Part four Lymphadenopathies. J.B. Lippincott Co., Philadelphia. 2009; pp. 213-222. [Google Scholar]
- 7.Blanco R, Mclaren B, Davis B, Steele P, Smith R. Systemic lupus erythematosus-associated lymphoproliferative disorder: report of a case and discussion in light of the literature. Hum Pathol. 1997; 28: 980-985. [DOI] [PubMed] [Google Scholar]
- 8.Kojima M, Nakamura S, Morishita Y, et al. Reactive follicular hyperplasia in the lymph node lesions from systemic lupus erythematosus patients: A clinicopathological and immunohistological study of 21 cases. Pathol Int. 2000; 50: 304-312. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Chetrit E, Flusser D, Okon E, Ackerman Z, Rubinow A. Multicentric Castleman’s disease associated with rheumatoid arthritis: a possible role of hepatitis B antigen. Ann Rheum Dis. 1989; 48: 326-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima M, Nakamura S, Itoh H, et al. Systemic lupus erythematosus (SLE) lymphadenopathy presenting with histopathologic features of Castleman disease: a clinicopathologic study of five cases. Pathol Res Pract. 1997; 193: 565-571. [DOI] [PubMed] [Google Scholar]
- 11.Kojima M, Nakamura S, Oyama T, et al. Autoimmune disease-associated lymphadenopathy with histological appearance of T-zone dysplasia with hyperplastic follicles. A clinicopathological analysis of nine cases. Pathol Res Pract. 2001; 197: 237-244. [DOI] [PubMed] [Google Scholar]
- 12.Kojima M, Nakamura N, Tsukamoto N, et al. Atypical lymphoplasmacytic and immunoblastic proliferation of autoimmune disease: clinicopathologic and immunohistochemical study of 9 cases. J Clin Exp Hematop. 2010; 50: 113-119. [DOI] [PubMed] [Google Scholar]
- 13.Kojima M, Motoori T, Nakamura S. Benign, atypical and malignant lymphoproliferative disorders in rheumatoid arthritis patients. Biomed Pharmacother. 2006; 60: 663-672. [DOI] [PubMed] [Google Scholar]
- 14.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005; 165: 2337-2344. [DOI] [PubMed] [Google Scholar]
- 15.Kojima M, Motoori T, Asano S, Nakamura S. Histological diversity of reactive and atypical proliferative lymph node lesions in systemic lupus erythematosus patients. Pathol Res Pract. 2007; 203: 423-431. [DOI] [PubMed] [Google Scholar]
- 16.Flendrig JA. Benign giant lymphoma: clinicopathologic correlation study, in: R.L. Clark, R.W. Gumley (Eds.), The Year Book of Cancer, Year Book Medical Publishers, Chicago. 1970; pp. 296-299. [Google Scholar]
- 17.Frizzera G. Castleman’s disease and related disorders. Semin Diagn Pathol. 1988; 5: 346-364. [PubMed] [Google Scholar]
- 18.Frizzera G. Atypical lymphoproliferative disorders, In: D.M. Knowles (Ed.), Neoplastic Hematopathology, 2nd ed., Lippincott Williams & Wilkins, Philadelphia. 2000; pp. 569-622. [Google Scholar]
- 19.Weisenburger DD, Nathwani BN, Winberg CD, Rappaport H. Multicentric angiofollicular lymph node hyperplasia: a clinicopathologic study of 16 cases. Hum Pathol. 1985; 16: 162-172. [DOI] [PubMed] [Google Scholar]
- 20.Kojima M, Nakamura S, Morishita Y, et al. Reactive follicular hyperplasia in the lymph node lesions from systemic lupus erythematosus patients: A clinicopathological and immunohistological study of 21 cases. Pathol Int. 2000; 50: 304-312. [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Corton RS. Robbins SL Basic Pathology, 7th ed. The Hematopoietic and Lymphoid Systems. W.B. Saunders, Philadelphia. 2003; pp. 103-164. [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31: 315-324. [DOI] [PubMed] [Google Scholar]
- 23.McCurley TL, Collins RD, Ball E, Collins RD. Nodal and extranodal lymphoproliferative disorders in Sjogren’s syndrome: a clinical and immunopathologic study. Hum Pathol. 1990; 21: 482-492. [DOI] [PubMed] [Google Scholar]
- 24.Kojima M, Shimizu K, Ikota H, et al. “Follicular variant” of hyaline-vascular type of Castleman’s disease: histopathological and immunohistochemical study of 11 cases. J Clin Exp Hematop. 2008; 48: 39-45. [DOI] [PubMed] [Google Scholar]
- 25.Kawabata H, Takai K, Kojima M, et al. Castleman-Kojima disease (TAFRO syndrome): a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly: a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop. 2013; 53: 57-61. [DOI] [PubMed] [Google Scholar]
- 26.Kojima M, Nakamura N, Tsukamoto N, et al. Clinical implications of idiopathic multicentric castleman disease among Japanese: a report of 28 cases. Int J Surg Pathol. 2008; 16: 391-398. [DOI] [PubMed] [Google Scholar]
- 27.Suchi T, Ueda R, Suzuki H, Namikawa R. T-zone dysplasia with hyperplastic follicles-an incipient T-cell lymphoma. In: Hanaoka M, Kadin ME, Mikata A, Watanabe S (Eds). Lymphoid Malignancy, Immunology and Cytogenetics. Field & Wood Medical Publishers Inc, New York. 1990; pp. 155-163. [Google Scholar]
- 28.Dogan A, Gaulard P, Jaffe ES, Muller-Hermelink HK, de Leval L. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Angioimmunoblastic T-cell lymphoma and other nodal lymphomas of T follicular helper cell origin. Lyon: IARC Press. 2017; pp. 407-412. [Google Scholar]