Abstract
Isomeric peptide analyses are an analytical challenge of great importance to therapeutic monoclonal antibody and other biotherapeutic product development workflows. Aspartic acid (Asp, D) to isoaspartic acid (isoAsp, isoD) isomerization is a critical quality attribute (CQA) that requires careful control, monitoring, and quantitation during the drug discovery and production processes. While the formation of isoAsp has been implicated in a variety of disease states such as autoimmune diseases and several types of cancer, it is also understood that the formation of isoAsp results in a structural change impacting efficacy, potency, and immunogenic properties, all of which are undesirable. Currently, lengthy ultrahigh-performance liquid chromatography (UPLC) separations are coupled with MS for CQA analyses; however, these measurements often take over an hour and drastically limit analysis throughput. In this manuscript, drift tube ion mobility spectrometry–mass spectrometry (DTIMS–MS) and both a standard and high-resolution demultiplexing approach were utilized to study eight isomeric Asp and isoAsp peptide pairs. While the limited resolving power associated with the standard DTIMS analysis only separated three of the eight pairs, the application of HRdm distinguished seven of the eight and was only unable to separate DL and isoDL. The rapid high-throughput HRdm DTIMS–MS method was also interfaced with both flow injection and an automated solid phase extraction system to present the first application of HRdm for isoAsp and Asp assessment and demonstrate screening capabilities for isomeric peptides in complex samples, resulting in a workflow highly suitable for biopharmaceutical research needs.
Graphical Abstract

Proteins and peptides are capable of undergoing several spontaneous chemical reactions even under standard physiological conditions, including deamidation,1,2 isomerization, 1,3–5 and racemization.6 These post-translational modifications occur readily in proteins and peptides and are extremely challenging to detect and study with mass spectrometry (MS), as several do not have mass shifts.1,7 Of specific interest is the formation of isoaspartic acid (isoAsp, isoD), which can be created either through the deamidation of asparagine (Asn, N) or the isomerization of aspartic acid (Asp, D) (Scheme 1).1 The conversion of Asp and Asn to isoAsp has been implicated in several aging-related disorders, particularly those of the eyes and brain, as well as cancer and decreased autoimmune response due to protein structural changes.7–9 During the development of biotherapeutics,10–12 including but not limited to mAbs and bispecific antibodies, it is also critical to unequivocally identify and quantify critical quality attributes (CQAs)13–15 that could negatively impact the stability and activity of the candidate molecules. Biotherapeutic candidates are therefore subjected to a series of forced degradation studies to determine their stability, appropriate shelf life, and suitable storage and transport conditions, a process known as Molecular Assessment.16 The chemical liabilities are also monitored and quantified during late stage product development within a current good manufacturing process (cGMP) environment using the multiattribute method (MAM).17 IsoAsp is one of the degradation products routinely screened by biopharmaceutical companies, since the spontaneous, nonenzymatic conversion of Asp and Asn to isoAsp can produce a structural change in the protein structure, resulting in protein aggregation and potential decreases in drug potency.3,5,18,19 Conversion of Asp and Asn residues located on the complement determining region (CDR) of antibodies has also led to a substantial decrease in potency of therapeutics, even for the conversion of a single Asp residue.3 Thus, having an effective, high-throughput method to screen potential new drug products for this isomerization at each of the crucial steps in the research and production pipeline would be greatly advantageous for the development of these novel biotherapeutics.
Scheme 1. Isomerization of l-Asp and d-Asp to l-isoAsp or d-isoAsp Proceeds through the Formation of a Succinimide Intermediatea.
al-Asn can also be converted to l-Asp or l-isoAsp through succinimide intermediate formation.
One challenging aspect of performing biotherapeutic characterizations is the development of analytical techniques that can rapidly and accurately detect and quantify these isomeric peptides. This is in part because they share the same m/z values and similar physicochemical properties, making analyses with MS and chromatographic methods nontrivial. Commonly, these spontaneous degradation products are detected using a combination of targeted peptide mapping followed by lengthy chromatographic separations and MS/MS detection.3 While these standard practices are robust, they are extremely low-throughput and preclude screening capabilities. To address this limitation, advances have been made in the use of immunoassay and enzymatic-based screening tests, as well as bioluminescence and fluorescence assays,19,20 which exclude chromatographic separation and are not as time-consuming as other commonly applied methods such as peptide mapping. However, while these screening tools are capable of rapidly detecting isoAsp, they lack specificity and fail to provide the location of the isomerization on the protein or peptide sequence.19,20 MS detection of isoAsp is therefore preferable for this reason, particularly in pharmaceutical preparations where the location of the isomerization can significantly decrease the potency of the mAb-based therapeutics.3
The majority of MS methods used in the detection of isoAsp rely on fragmentation-based dissociation techniques, such as electron capture dissociation (ECD) and electron transfer dissociation (ETD), where the presence of isoAsp is verified by certain characteristic ions.3,21–23 Recently MALDI-ToF/ToF was demonstrated as an effective alternative to the aforementioned electron-based fragmentation techniques for Asp and isoAsp differentiation.24 While these techniques yielded reproducible results, they are reliant on the assumption that the fragment ions will be abundant enough for detection, which may not be the case if isoAsp quantities are low. Attempts at modifying isoAsp-containing peptides prior to fragmentation have also been utilized. In particular, enzymatic labeling of isoAsp with 18O and gas-phase ion chemistry with carbidiimide have shown promise in detecting isoAsp peptides important to mAb-based pharmaceuticals.25,26 However, while the analytical workflows for the detection and quantification of isoAsp exist, the procedures often require peptide fractionation and lengthy (>1 h) chromatographic separation of the Asp-and isoAsp-containing peptides prior to tandem-MS analyses.3 Thus, the development of a robust, high-throughput method for isoAsp content determination in biotherapeutics would benefit both product development and quality control for mAb-based therapeutics.
Here, we developed a rapid solid phase extraction method coupled with DTIMS–MS (SPE-DTIMS–MS) to evaluate isoAsp isomerization and facilitate high-throughput screening of peptide samples for these degradation products. Since DTIMS–MS is a gas-phase technique capable of separating analytes by mass, size, and shape,27,28 it has great utility for separating isomeric molecule analyses.29,30 By evaluating eight isomeric peptide pairs containing either Asp or isoAsp modifications found within the antistreptavidin IgG1 mAb, we assessed the feasibility of SPE-DTIMS–MS for this type of screening. The application of both standard DTIMS acquisition and the use of a novel high-resolution demultiplexing (HRdm) strategy31 provided an increase in the DTIMS resolving power from ~60 to over 200 while still allowing millisecond IMS separation and not requiring instrument modifications. This work presents the first application of HRdm for the assessment of peptides containing isoAsp and Asp and demonstrates its strong potential for facilitating the detection and screening of these important protein degradation products. These findings also serve as a strong starting point for more in-depth characterization of protein-based therapeutics by demonstrating the utility and versatility of HRdm within different analytical pipelines, ranging from flow analysis to rapid SPE.
EXPERIMENTAL SECTION
Chemicals.
Optima grade methanol, acetonitrile, formic acid, and ammonium acetate were purchased from Fisher Scientific (Pittsburgh, PA) and used as received. Millipore deionized water (18.2 MΩ) was purified using an in-house water filtration system (ELGA PureLab Flex purification system, High Wycombe, UK). All synthetic peptides were obtained from Anaspec Inc. (Freemont, CA) and prepared in water to a concentration of 10 μM prior to analysis.
Flow Injection Analysis (FIA)-DTIMS–MS Experiments.
All FIA experiments were performed with electrospray ionization (ESI, Agilent Jetstream) using an Agilent 1290 HPLC (Santa Clara, CA) coupled to an Agilent 6560 IM-QTOF MS (Santa Clara, CA). This instrument has previously been characterized and consists of a ~78 cm drift cell with ~4 Torr of nitrogen drift gas.32,33 Prior to all experiments, mass calibration of the DTIMS–MS platform was performed. Experiments to determine CCS values and evaluate separation efficiency were performed with FIA using the HPLC to inject 2 μL of each peptide into a stream consisting of 50% mobile phase A (MPA) and 50% mobile phase B (MPB) at a flow rate of 0.2 mL/min. Here, two different MPAs and MPBs were evaluated for the best ionization of the peptides. Our first MP set consisted of MPA1: 5 mM ammonium acetate in deionized water and MPB1: 95:5 methanol:5 mM ammonium acetate in deionized water. Our second MP set was MPA2: 0.1% formic acid in deionized water and MPB2: 0.1% formic acid in acetonitrile. Each peptide standard or mixture was analyzed in triplicate using both positive and negative ionization mode over a range of 100 to 1700 m/z. Standard DTIMS–MS measurements were conducted using a single IMS trap and release cycle, with a trap fill time of 40 ms and a release of 100 μs. For HRdm measurements,31 IMS-MS data was acquired using a 4 bit multiplexing method, with a trap fill time of 3.9 ms and a release time of 100 μs. The uniform field strength used for DTIMS separations was between 12 and 17 V/cm. Additional parameters used for DTIMS–MS data acquisition are detailed in the Supporting Information (Tables S1–S3 for standard IMS acquisition and Tables S4–S6 for 4 bit multiplexed acquisition).
SPE-IMS-MS Experiments.
High-throughput screening experiments were performed for both individual peptide standards and peptide mixtures using the Agilent RapidFire 365 (Santa Clara, CA) SPE system interfaced with the Agilent 6560 IM-QTOF MS. Two types of SPE cartridges (C4 and C18) and the two mobile phase compositions from the FIA experiments were used with a 20:80 MPA:MPB composition for the elution step. The four main stages for the SPE analysis are aspiration, load, elution, and re-equilibration, which had times of 0.6, 3, 6, and 1 s. The solvent and mobile phase compositions utilized in the three stages following aspiration consisted of (MPA:MPB): load – 100:0, elution – 20:80, and re-equilibration – 100:0. To minimize sample carry-over, the sipper and injection valve loops were washed between samples using both water and acetonitrile. All SPE-DTIMS–MS measurements were also conducted using the 4-bit multiplexing approach described in the previous section.
CCS Determination.
Prior to CCS analyses, multiplexed data files were demultiplexed using the PNNL PreProcessor34 (v. 3.0 build 2020.11.24, currently in beta, https://omics.pnl.gov/software/pnnl-preprocessor) and a newly introduced data interpolation feature where three points were substituted for one data point. Additional parameters are described in the Supporting Information (Figure S1). CCS value determination was performed using the Agilent MassHunter Workstation Software IM-MS Browser Version B.10.00 and the single-field CCS method, which has been described in detail previously.33 In this study, the CCS value obtained for each isomeric peptide ion and its m/z were then imported as a transition list into Skyline35,36 (MacCoss Lab Software, v. 21.1), which was subsequently used to determine peak areas for both the FIA and SPE analyses.
High-Resolution Demultiplexing (HRdm).
The methods utilized for HRdm have been previously described by May et al. and herein were applied to the assessment of isomeric Asp- and isoAsp-containing peptides.31 Feature finding was performed on each data file using the internal feature finding tool in Agilent IM-MS Browser v 10.00 (Figure S2), and the resulting feature list was exported along with the raw data file, demultiplexed data file, and resulting feature list into the Agilent High Resolution Demultiplexing tool 1.0 (prerelease build 52) or Agilent High Resolution Demultiplexing tool 2.0 (prerelease build 2.0.116 E). Both HRdm 1.0 and 2.0 were used in processing the data due to the release of 2.0 following initial manuscript submission. This software allows for postprocessing resolving power enhancement at three separate processing levels (low, medium, and high). For the analyses presented herein, all three processing levels were assessed for both resolving power enhancement and preservation of peak area under the curve (AUC).
RESULTS AND DISCUSSION
Investigating the CCS Values of Isomeric Peptide Ions.
To assess standard acquisition and HRdm applied to the DTIMS separation of Asp and isoAsp peptide pairs, eight isomeric pairs were evaluated as individual peptide standards and isomeric mixtures. The eight pairs were: (D/isoD)L, L(D/isoD)A, AL(D/isoD)GK, A(D/isoD)GLK, AL(D/isoD)GE, AL(D/isoD)EK, G(D/isoD)LLLK, and GL(D/isoD)LLK. While the isoAsp/Asp isomerizations were of primary interest, two pairs of sequence isomers were also included. Both pairs contained a sequence isomerization in which the location of the Asp residue within the sequence was switched with Leu (L) from the second amino acid from the N-terminus to the third residue from the N-terminus (A(D/isoD)LGK vs AL(D/isoD)GK and G(D/isoD)LLLK vs GL(D/isoD)LLK). While the vast majority of proteomics work is commonly carried out using only positive mode ESI,37 here peptides were assessed using both positive and negative modes to compare the separation efficiency of the different ion types. Thus, CCS values were obtained for all 16 peptide standards in both ion modes and for each observed ion type. In positive ion mode, the peptide ions were observed as both [M + H]+ and [M + Na]+ ions (32 total peptide ions), while in negative ion mode [M − H]− and [M − 2H + Na]− ions were detected (32 total peptide ions). The single-field CCS values for each peptide ion investigated are listed in the Supporting Information (Tables S7 and S8).
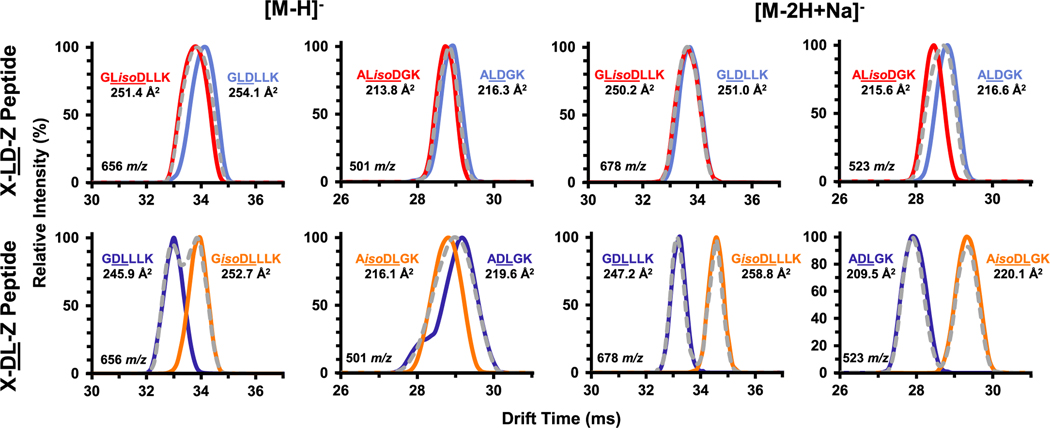
As adduction to metal ions has previously been demonstrated to facilitate IMS separations,30,38,39 we also evaluated sodium adducts to understand their potential for adding confidence to the measurements. Differences in isomeric pair separations were noted in the different ion modes and for different ion types in the same ion modes. In several cases, adduction to sodium was demonstrated to improve peptide isomer separations compared to protonation or deprotonation. Specifically, in the case of peptides having DL and LD sequence isomers, investigations into both the deprotonated and [M − 2H + Na]− adducts revealed that the location of the Asp residue within the peptide chain was influential in differentiating the peptides (Figure 1). In their deprotonated forms, both peptide pairs containing the generic sequences X–L(D/isoD)–Z and X–(D/isoD)L–Z (where X represents the N-terminus amino acid and Z represents the remaining amino acid sequence) displayed either minimal or no separation (Figure 1). However, upon sodium adduction, the isomeric peptide pair containing the X–(D/isoD)L–Z sequence was baseline-resolved, while the peptides containing X–L(D/isoD)–Z remained unresolved. Adduction of sodium in ESI is often associated with the availability of oxygen atoms within a particular molecule,40,41 and sequence dependence has been specifically noted for spontaneous isoAsp formation in peptides and proteins.42,43 This is particularly exacerbated by the localization of amino acids with smaller, more hydrophilic functional groups, such as glycine (Gly, G), serine (Ser, S), and histidine (His, H) on the C-terminus side of Asp and Asn residues.42 Previous work has demonstrated that the presence of these less bulky amino acids allows for a certain degree of peptide flexibility, making them amenable to succinimide formation.44 In particular, protein regions containing the sequence motif Asp–Gly are particularly prone to succinimide formation,42,45 particularly in the mildly acidic storage environments most commonly employed for antibodies.4,46,47 While future studies characterizing peptides containing these sequence isomers are necessary, these findings serve as a promising starting point for computational investigations into the structure–mobility relationship of these isomeric peptides.
Figure 1.
Separation of sequence isomers revealed a potential influence of the isoAsp location and the ability of DTIMS to separate the pairs. For example, the four isomeric pairs on the left illustrate that peptides with X–LD–Z sequences (top) had minimal to no DTIMS separation, while those with a X–DL–Z sequence (bottom) had more distinct CCS values and baseline separation upon sodium adduction (right). Mixtures are denoted with gray dashed lines, and all drift spectra are normalized to maximum peak height to illustrate potential separation efficiency of this method.
Characterization of Isomer Separations by ΔCCS%.
Previous work by Dodds and co-workers investigated the utility of percent difference in CCS (ΔCCS%) as a means of assessing difficulty of separation for analytes by IMS and predicting the separability of compounds at a stated Rp.48 The Agilent 6560 has an average Rp of 60 (CCS/ΔCCS), and as such, two analytes need to possess a ΔCCS% of ~2.3% or greater for half-height separation, while those with ΔCCS% above 1.7% attain moderate separation (10% valley), and any analytes with less than 1.4% ΔCCS% are not separable or have minimal separation in a mixture at this given resolving power (Figure 2).48 Representative drift spectra from each classification are shown in the bottom panel of Figure 2. Upon examination of the drift spectra and CCS values for the isomeric peptide pairs, varying degrees of separation were observed. The resulting ΔCCS% for seven of the eight peptide pairs are illustrated in Figure S3. The eighth pair, DL and isoDL, was not assessed due to “smearing” in their IMS drift spectra, which is commonly attributed to multimerization in some small molecules during ESI or ion trapping, followed by the subsequent dissociation within the drift cell.49 This phenomenon was particularly pronounced in negative ionization mode (Figure S4), preventing assessment of DTIMS separation for this particular dipeptide pair. Close examination of the other peptide pairs however revealed that only two pairs, G(D/isoD)LLLK and A(D/isoD)LGK, meet the ΔCCS% threshold necessary for near baseline resolution in negative ionization mode at the average Rp of 60, while only GL(D/isoD)LLK was half-height differentiable when using positive ionization. While a majority of the peptide pairs had at least one adduct that exceeded the 1.4% ΔCCS% threshold necessary for 10% separation, the ability to distinguish isomer pairs at 10% separation in complex mixtures becomes challenging and unreliable.
Figure 2.
Three distinct categories were used to assign the seven isomeric pairs in this study based on the Agilent 6560 standard Rp of 60. The three separate categories were based on their DTIMS CCS differences: <1.4% CCS difference (Minimal Separation), between 1.4 and 2.3% difference (Moderate Separation), and >2.3% (Baseline Separation). All drift spectra are presented as normalized to maximum peak height.
To address the limited Rp of the standard DTIMS separation used, HRdm was evaluated. HRdm has been described in detail previously by May et al. and provides a significant increase in effective DTIMS Rp when applied postacquisition to data acquired in 4 bit multiplexing mode.31 There are currently three separate modes of HRdm processing termed low, medium, and high. Each mode performs a different amount of data fitting with low having the least effect and high having the most.31 An example of each of these processing modes applied to one of our isomeric peptide pairs is displayed in Figure 3A. The lowest available HRdm method provides the least improvement in Rp (average of ~ 80 from previous work), and the highest HRdm improves the average Rp to ~210, although values of over 300 have previously been reported.31 However, this heightened mode often reduces the number of data points observed within each drift spectra and may contribute to poor quantitation (Figure S5). The medium-processing mode available yields an average Rp of ~120 and allows for half-height separation of isomers who differ in CCS by ~1.2% (Figure 3B), but it does not reduce the number of points across the drift spectra peak as severely as high mode. To demonstrate the utility of HRdm for the assessment of Asp/isoAsp pairs, the three processing modes were applied to each peptide pair. An example of this process for the isomeric peptides A(D/isoD)LGK is illustrated in Figure 3A. In their deprotonated forms, these analytes possess CCS values of 217.0 Å2 and 220.3 Å2, with a percent difference of 1.5% ΔCCS%. Drift spectra for the individual peptide standards using standard multiplexing revealed an Rp of 55 for ADLGK and 54 for AisoDLGK (Figure 3A). When assessed as an equimolar mixture, DTIMS–MS analysis revealed a single peak for the associated drift spectra, and thus, the associated isomers could not be readily differentiated. Postacquisition application of HRdm to the data in its low-processing mode increased the observed Rp of the peptide standards to 143 and 151. This enhanced resolving power readily facilitated the separation of these two isomeric peptide standards when in an equimolar mixture, resulting in a 60% separation of the peptide pairs. The medium-processing mode led to further enhancement of the Rp of each drift spectrum, with the observed Rp for ADLGK and AisoDLGK increasing to 210 and 220 resulting in a 90% separation of the peptide pairs. Interestingly, further enhancement using the high-processing mode maintained the 90% separation of the peptide pairs in the equimolar mixture. Examination of each set of peptides revealed that the use of the low processing could separate three of out of the seven pairs examined to half-height separations (Figure 3B). Both the medium- and high-processing levels were capable of half-height separation for all seven peptide pairs given the average Rp achieved by each processing mode as denoted by the vertical dashed lines (Figure 3B). It was also noted that all seven peptide pairs could be separated in negative mode just by their deprotonated ions, and five of the seven could be separated in positive mode with their protonated ions. Therefore, emphasis for the rapid screening of these peptides was put on separation of their deprotonated ions.
Figure 3.
Low-, medium-, and high-HRdm processing improved IMS resolving power. (A) The separation of A(D/isoD)LGK illustrates improvements in resolving power based on the increasing rigor of the resolving power enhancement applied. (B) The half-height separation was determined in terms of percent difference in CCS (ΔCCS%) at the given average resolving power (Rp) of each mode of HRdm enhancement. Comparison of the low-, medium-, and high-processing modes showed low separated five out of the seven isomer pairs, while both the medium- and highest-processing levels provide half-height separations for all the investigated isomer pairs. Drift spectra are presented as normalized to maximum peak height.
Since deprotonation illustrates such potential for rapid screening of the peptides from this study, HRdm enhancement was also applied to the equimolar mixture of all isomer pairs investigated in this study. Figure 4A shows the nested spectra for the m/z window in which the [M − H]− ions for three peptide pairs (A(D/isoD)LGK, AL(D/isoD)GK, and AL(D/isoD)GE) were observed after routine demultiplexing. To further complicate this m/z area, two of three pairs (A(D/soD)LGK, AL(D/isoD)GK) are isomeric, and the third pair (AL(D/isoD)GE) has a nominal m/z value matching the [M + 1] peak for the others. In this complex mixture, a single peak was observed for both A(D/isoD)LGK and AL(D/isoD)GK as well as for ALDGE and ALisoDGE (Figure 4B). Upon application of the medium-processing level of HRdm to the same data file, three peaks for the [M − H]− for A(D/isoD)LGK and AL(D/isoD)GK could be observed (Figure 4C). This was due not to pair overlap but overlap of AisoDLGK and ALDGK, which share the same CCS of 217 Å2. However, these two peaks are readily differentiated using their [M − 2H + Na]− adduct (Figure S6), which demonstrates the utility of the sodiated adducts for screening candidate peptides for this isomerization. The [M − H]− peak for AL(D/isoD)GE was baseline-differentiated, with relative peak abundances maintained between routine DTIMS and HRdm DTIMS (Figure 4D). This was also observed for the [M − 2H+Na]− adduct of this pair, as illustrated in Figure S6. These initial results are promising, as no chromatographic separations were employed for the isomeric peptides. However, while sample throughput would be sacrificed, it should be noted the use of a fast LC gradient could potentially facilitate further resolution of the peptide isomers.
Figure 4.
HRdm DTIMS–MS analyses for complex mixtures of Asp-and isoAsp-containing peptides. (A) Three peptide pairs (A(D/isoD)LGK, AL(D/isoD)GK, and AL(D/isoD)GE) are shown in the nested spectra as [M − H]− ions. (B) Before HRdm processing, two separable isomers (AL(D/isoD)GE) are observed at m/z 502.2163, while four isomers occur at m/z 501.2684 in only a single IMS peak. (C) Following medium-HRdm processing, the IMS separations improved such that two baseline differentiated peaks were observed for AL(D/isoD)GE, and three peaks were observed for A(D/isoD)LGK and AL(D/isoD)GK. (D) Upon examination, AisoDLGK and ALDGK share the same CCS value, causing the overlap at m/z 501.2684. All drift spectra are normalized to maximum peak height.
While the significant improvements in DTIMS separations upon application of HRdm enhancement are extremely exciting, consistent with previous work utilizing HRdm,38 a reduction in the number of points across the drift spectra was observed upon increasing enhancement due to further reduction in the low-intensity tails (Figure S5). As there are strict guidelines for monitoring the formation of isoAsp in biopharmaceuticals, it was necessary to determine how severe the impact of this application would be on relative peak area. To compensate for the reduction of data points used in this process, the PNNL PreProcessor’s34 data interpolation feature was utilized to increase the number of data points across the curve from one to three. Additionally, lower field strengths (~12 V/cm) for DTIMS separations were employed in order to increase the sampling across drift spectra. The ratio of the peak area under the curve (AUC) for each IMS drift spectrum of isoAsp to Asp peptides was then determined before and after each level of HRdm enhancement. For comparison, the percent difference between the routine 4-bit multiplexed drift spectra and those obtained using each HRdm enhancement level were found to only differ on average by 2.2% and not exceed 11% for any of the ions assessed (Table S9). Thus, the reduction of data points across the IMS peak does not appear to have significant impacts on the AUC and would not preclude quantitation capabilities.
SPE-IMS-MS for Isomeric Peptide Screening.
Rapid solid-phase extraction (SPE) systems have previously been utilized in conjunction with DTIMS–MS to develop high-throughput screening methods for isomeric compounds, such as glycans and xenobiotics.39,50 Rapid SPE-MS has also used for assessing levels of acrylamide covalent modification to a target sulphydryl residue51 and multispecific antibody clone screening.52 In the SPE-DTIMS–MS workflow, sample cleanup is performed by loading the sample onto a SPE cartridge, which is selected based on the analyte’s compatibility with the cartridge stationary phase. After loading, the sample is washed and subsequently eluted for direct DTIMS–MS analysis. This rapid SPE-DTIMS–MS platform performs sample cleanup and subsequent DTIMS–MS analysis with a ~10 s sample-to-sample throughput, making it amenable for screening pipelines. Since C4 and C18 materials are commonly employed for the analysis of small molecules, proteins, and peptides (Figure 5), both SPE cartridges were analyzed in this study.50,52,53 Additionally, two mobile phase systems were investigated to determine which solvent conditions resulted in optimal sample cleanup, elution, and ion abundance for each peptide. Peak areas were log2-transformed for the protonated ions in each condition, and the results are highlighted in Figure 5 (for deprotonated adducts, see Figure S7). For both positive and negative ionization, a majority of peptides retained best on the C18 cartridge and then subsequently eluted with 80:20 0.1% FA in water:0.1% FA in ACN. However, it should be noted that all peptide standards were above the limit of detection, except for D/isoDL due to the smearing mentioned previously. Furthermore, adduction to sodium was decreased when using SPE-DTIMS–MS as opposed to FIA-DTIMS–MS (Figure S8). While assessment of the protonated to sodiated species ratio varied slightly, an approximate 1.3:1 ratio was observed for FIA-DTIMS–MS and 120.0:1 was observed for the comparable SPE-IMS-MS analysis (Table S10). These findings suggest that SPE significantly reduced sodium (Figure S8B), benefiting researchers who require quantitative assessment of unadducted proteins and peptides. This may also be desirable for those seeking more informative MS/MS obtained from protonated as opposed to sodiated ions, as the latter may result in the loss of the metal cation and lead to less informative MS/MS spectra. Thus, the use of SPE may offer a better starting point for exploring the role of protonated or deprotonated ions in IMS–MS separations of isomeric peptide pairs.
Figure 5.
Two different SPE cartridges, C4 and C18, and two mobile phase systems were investigated for the peptide standards using SPE-DTIMS–MS. Peak areas for the protonated peptides were determined in Skyline using the anticipated m/z and CCS for each peptide standard and then log2-transformed to facilitate comparisons between peptides of differing abundances. C18 with 0.1% formic acid (FA) and acetonitrile (ACN) provides the most comparable ion signals for all peptides studied. In triplicate analyses, all peak areas illustrated differences less than 5%.
CONCLUSION
In this work, we utilized a novel HRdm data processing capability with DTIMS to maintain millisecond evaluations and greatly improve the IMS resolving power for isomeric peptide pairs containing either Asp or isoAsp residues. For our specific study, prior to HRdm processing, we were only able to separate three of eight isomeric pairs; however, HRdm enabled differentiation of seven of the eight peptide pairs and facilitated the use of SPE-DTIMS–MS for rapid screening of isoAsp presence. Furthermore, the pair that did not separate was due to smearing in the IMS separation from multimer formation, which has been observed previously in small amino acids and dipeptides. Therefore, this SPE-DTIMS–MS platform provides a promising starting point for the screening of isomeric molecules such as peptides containing either Asp or the degradation product isoAsp. From a biopharmaceutical perspective, the application of DTIMS for Asp and isoAsp characterization is also attractive, as it compliments analytical techniques already being applied within biopharmaceutical research and development24,54 and increases the peak capacities possible within traditional LC–MS workflows.55 One can therefore envisage the combination of rapid chromatographic56 or electrophoretic separation being combined with both IMS and an electron-based fragmentation method3,22 for unequivocal determination of Asp to isoAsp isomerization.57 DTIMS–MS therefore shows promise for both LC and SPE coupling in biotherapeutic evaluations to provide additional identification confidence and enable not just early screening campaigns58 but also application in later stage biomolecule development analytics, such as MAM.14
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank John Fjeldsted from Agilent Technologies for his advice and discussions. Andrew Dykstra, John O. Hui, and Qingchun Zhang from Amgen are thanked for their initial discussions regarding aspartatic- and isoaspartic-acid-containing peptides. This work was funded, in part, by grants from the National Institutes of Health (P30 ES025128, P42 ES027704 and P42 ES031009) and a cooperative agreement with the United States Environmental Protection Agency (STAR RD 84003201). The DTIMS–MS measurements were carried out in the Molecular Education, Technology and Research Innovation Center (METRIC) at North Carolina State University.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.1c05533.
Supplemental figures and tables detailing additional instrumentation parameters, collision cross section values for isomeric peptides, nested spectra for isomeric peptide pairs, and assessments of drift spectra area under the curve with HRdm (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.analchem.1c05533
The authors declare no competing financial interest.
Contributor Information
Karen E. Butler, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States
James N. Dodds, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States.
Tawnya Flick, Pivotal Attribute Sciences, Amgen Process Development, Thousand Oaks, California 91320, United States.
Iain D. G. Campuzano, Discovery Attribute Sciences, Amgen Research, Thousand Oaks, California 91320, United States.
Erin S. Baker, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States; Center for Human Health and the Environment and Comparative Medicine Institute, North Carolina State University, Raleigh, North Carolina 27695, United States.
REFERENCES
- (1).Zheng X; Deng L; Baker ES; Ibrahim YM; Petyuk VA; Smith RD Chem. Commun 2017, 53, 7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Liu DT-Y Trends Biotechnol. 1992, 10, 364–369. [DOI] [PubMed] [Google Scholar]
- (3).Eakin CM; Miller A; Kerr J; Kung J; Wallace A. Frontiers in Pharmacology 2014, 5, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wakankar AA; Borchardt RT J. Pharm. Sci 2006, 95 (11), 2321–2336. [DOI] [PubMed] [Google Scholar]
- (5).Cacia J; Keck R; Presta LG; Frenz J. Biochemistry 1996, 35 (6), 1897–1903. [DOI] [PubMed] [Google Scholar]
- (6).Radkiewicz JL; Zipse H; Clarke S; Houk KN J. Am. Chem. Soc 1996, 118 (38), 9148–9155. [DOI] [PubMed] [Google Scholar]
- (7).Truscott RJW; Schey KL; Friedrich MG Trends Biochem. Sci 2016, 41, 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Roher AE; Lowenson JD; Clarke S; Woods AS; Cotter RJ; Gowing E; Ball MJ Proc. Natl. Acad. Sci. U.S.A 1993, 90 (22), 10836–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Tao Y; Julian RR Anal. Chem 2014, 86 (19), 9733–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Eakin CM; Miller A; Kerr J; Kung J; Wallace A. Frontiers in pharmacology 2014, 5, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zhong X; D’Antona AM Antibodies 2021, 10 (2), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Deshaies RJ Nature 2020, 580 (7803), 329–338. [DOI] [PubMed] [Google Scholar]
- (13).Yu LX; Amidon G; Khan MA; Hoag SW; Polli J; Raju GK; Woodcock J. AAPS Journal 2014, 16 (4), 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Rogers RS; Nightlinger NS; Livingston B; Campbell P; Bailey R; Balland A. mAbs 2015, 7 (5), 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rogers RS; Abernathy M; Richardson DD; Rouse JC; Sperry JB; Swann P; Wypych J; Yu C; Zang L; Deshpande R. AAPS Journal 2018, 20 (1), 7. [DOI] [PubMed] [Google Scholar]
- (16).Nowak C; Cheung JK; Dellatore SM; Katiyar A; Bhat R; Sun J; Ponniah G; Neill A; Mason B; Beck A; Liu H. mAbs 2017, 9 (8), 1217–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ren D. Trends Biotechnol. 2020, 38 (10), 1051–1053. [DOI] [PubMed] [Google Scholar]
- (18).Harris RJ; Kabakoff B; Macchi FD; Shen FJ; Kwong M; Andya JD; Shire SJ; Bjork N; Totpal K; Chen AB Journal of Chromatography B: Biomedical Sciences and Applications 2001, 752 (2), 233–245. [DOI] [PubMed] [Google Scholar]
- (19).Hsiao K; Alves J; Patel R; Adams M; Nashine V; Goueli SJ Pharm. Sci 2017, 106 (6), 1528–1537. [DOI] [PubMed] [Google Scholar]
- (20).Puri A; Quan Y; Narang AS; Adams M; Gandhi R; Nashine VC AAPS PharmSciTech 2017, 18 (3), 803–808. [DOI] [PubMed] [Google Scholar]
- (21).DeGraan-Weber N; Zhang J; Reilly JP Journal of The American Society for Mass Spectrometry 2016, 27 (12), 2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Ni W; Dai S; Karger BL; Zhou ZS Anal. Chem 2010, 82 (17), 7485–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Cournoyer JJ; Lin C; Bowman MJ; O’connor PB J. Am. Soc. Mass Spectrom 2007, 18 (1), 48–56. [DOI] [PubMed] [Google Scholar]
- (24).Hui JO; Flick T; Loo JA; Campuzano IDG J. Am. Soc. Mass Spectrom 2021, 32 (8), 1901–1909. [DOI] [PubMed] [Google Scholar]
- (25).Liu M; Cheetham J; Cauchon N; Ostovic J; Ni W; Ren D; Zhou ZS Anal. Chem 2012, 84 (2), 1056–1062. [DOI] [PubMed] [Google Scholar]
- (26).Ayrton ST; Chen X; Bain RM; Pulliam CJ; Achmatowicz M; Flick TG; Ren D; Cooks RG Journal of The American Society for Mass Spectrometry 2018, 29 (7), 1339–1344. [DOI] [PubMed] [Google Scholar]
- (27).May JC; McLean JA Anal. Chem 2015, 87 (3), 1422–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Dodds JN; Baker ES Journal of The American Society for Mass Spectrometry 2019, 30 (11), 2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wu Q; Wang J-Y; Han D-Q; Yao Z-P TrAC Trends in Analytical Chemistry 2020, 124, 115801. [Google Scholar]
- (30).Xie C; Wu Q; Zhang S; Wang C; Gao W; Yu J; Tang K. Talanta 2020, 211, 120719. [DOI] [PubMed] [Google Scholar]
- (31).May JC; Knochenmuss R; Fjeldsted JC; McLean JA Anal. Chem 2020, 92 (14), 9482–9492. [DOI] [PubMed] [Google Scholar]
- (32).May JC; Goodwin CR; Lareau NM; Leaptrot KL; Morris CB; Kurulugama RT; Mordehai A; Klein C; Barry W; Darland E; Overney G; Imatani K; Stafford GC; Fjeldsted JC; McLean JA Anal. Chem 2014, 86 (4), 2107–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Stow SM; Causon TJ; Zheng X; Kurulugama RT; Mairinger T; May JC; Rennie EE; Baker ES; Smith RD; McLean JA; Hann S; Fjeldsted JC Anal. Chem 2017, 89 (17), 9048–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bilbao A; Gibbons BC; Stow SM; Kyle JE; Bloodsworth KJ; Payne SH; Smith RD; Ibrahim YM; Baker ES; Fjeldsted JC J. Proteome Res 2022, 21, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).MacLean B; Tomazela DM; Shulman N; Chambers M; Finney GL; Frewen B; Kern R; Tabb DL; Liebler DC; MacCoss MJ Bioinformatics (Oxford, England) 2010, 26 (7), 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Adams KJ; Pratt B; Bose N; Dubois LG; St. John-Williams L; Perrott KM; Ky K; Kapahi P; Sharma V; MacCoss MJ; Moseley MA; Colton CA; MacLean BX; Schilling B; Thompson JW J. Proteome Res 2020, 19 (4), 1447–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Riley NM; Rush MJP; Rose CM; Richards AL; Kwiecien NW; Bailey DJ; Hebert AS; Westphall MS; Coon JJ Molecular & cellular proteomics: MCP 2015, 14 (10), 2644–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Chouinard CD; Cruzeiro VWD; Roitberg AE; Yost RA J. Am. Soc. Mass Spectrom 2017, 28 (2), 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Zheng X; Zhang X; Schocker NS; Renslow RS; Orton DJ; Khamsi J; Ashmus RA; Almeida IC; Tang K; Costello CE; Smith RD; Michael K; Baker ES Anal. Bioanal. Chem 2017, 409 (2), 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kuberan B; Lech M; Zhang L; Wu ZL; Beeler DL; Rosenberg RD J. Am. Chem. Soc 2002, 124 (29), 8707–8718. [DOI] [PubMed] [Google Scholar]
- (41).Morisaki N; Kobayashi H; Yamamura Y; Morisaki M; Nagasawa K; Hashimoto Y. Chem. Pharm. Bull 2002, 50 (7), 935–940. [DOI] [PubMed] [Google Scholar]
- (42).Aswad DW; Paranandi MV; Schurter BT J. Pharm. Biomed. Anal 2000, 21 (6), 1129–1136. [DOI] [PubMed] [Google Scholar]
- (43).Yokoyama H; Mizutani R; Noguchi S; Hayashida N. Scientific Reports (Nature Publisher Group) 2019, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Xie M; Schowen RL J. Pharm. Sci 1999, 88 (1), 8–13. [DOI] [PubMed] [Google Scholar]
- (45).Sydow JF; Lipsmeier F; Larraillet V; Hilger M; Mautz B; Mølhøj M; Kuentzer J; Klostermann S; Schoch J; Voelger HR; Regula JT; Cramer P; Papadimitriou A; Kettenberger H. PLoS One 2014, 9 (6), e100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Stephenson RC; Clarke SJ Biol. Chem 1989, 264 (11), 6164–6170. [PubMed] [Google Scholar]
- (47).Shire SJ; Shahrokh Z; Liu JJ Pharm. Sci 2004, 93 (6), 1390–1402. [DOI] [PubMed] [Google Scholar]
- (48).Dodds JN; May JC; McLean JA Anal. Chem 2017, 89 (22), 12176–12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Dodds JN; Hopkins ZR; Knappe DRU; Baker ES Anal. Chem 2020, 92 (6), 4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zhang X; Romm M; Zheng X; Zink EM; Kim Y-M; Burnum-Johnson KE; Orton DJ; Apffel A; Ibrahim YM; Monroe ME; Moore RJ; Smith JN; Ma J; Renslow RS; Thomas DG; Blackwell AE; Swinford G; Sausen J; Kurulugama RT; Eno N; Darland E; Stafford G; Fjeldsted J; Metz TO; Teeguarden JG; Smith RD; Baker ES Clinical mass spectrometry (Del Mar, Calif.) 2016, 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Campuzano IDG; San Miguel T; Rowe T; Onea D; Cee VJ; Arvedson T; McCarter JD Journal of Biomolecular Screening 2016, 21 (2), 136–144. [DOI] [PubMed] [Google Scholar]
- (52).Sawyer WS; Srikumar N; Carver J; Chu PY; Shen A; Xu A; Williams AJ; Spiess C; Wu C; Liu Y; Tran JC Proc. Natl. Acad. Sci. U. S. A 2020, 117 (18), 9851–9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Leveridge M; Buxton R; Argyrou A; Francis P; Leavens B; West A; Rees M; Hardwicke P; Bridges A; Ratcliffe S; Chung C. Journal of Biomolecular Screening 2014, 19 (2), 278–286. [DOI] [PubMed] [Google Scholar]
- (54).Dykstra AB; Flick TG; Lee B; Blue LE; Angell NJ Am. Soc. Mass Spectrom 2021, 32 (8), 1952–1963. [DOI] [PubMed] [Google Scholar]
- (55).Valentine SJ; Kulchania M; Barnes CAS; Clemmer DE Int. J. Mass Spectrom 2001, 212 (1), 97–109. [Google Scholar]
- (56).Fekete S; Schappler J; Veuthey J-L; Guillarme D. TrAC Trends in Analytical Chemistry 2014, 63, 2–13. [Google Scholar]
- (57).Campuzano IDG; Lippens JL Curr. Opin. Chem. Biol 2018, 42, 147–159. [DOI] [PubMed] [Google Scholar]
- (58).Bailly M; Mieczkowski C; Juan V; Metwally E; Tomazela D; Baker J; Uchida M; Kofman E; Raoufi F; Motlagh S; Yu Y; Park J; Raghava S; Welsh J; Rauscher M; Raghunathan G; Hsieh M; Chen Y-L; Nguyen HT; Nguyen N; Cipriano D; Fayadat-Dilman L. mAbs 2020, 12 (1), 1743053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.