Abstract
Clostridium difficile is the bacterial pathogen identified as the cause of pseudomembranous colitis and is principally responsible for nosocomial antibiotic-associated diarrhea and colitis. The pathologic findings associated with this infection are believed to be caused by two large (∼300-kDa) exotoxins, toxins A and B. Because of the mucosal nature of this infection, vaccination strategies aimed at providing prophylactic or therapeutic immune protection have included immunization by mucosal routes. Using the hamster model of C. difficile infection, we examined the protective efficacy of inactivated toxin (toxoid) vaccine formulations prepared as either culture filtrate or partially purified toxoid. We compared combination parenteral and mucosal vaccination regimens involving intranasal, intragastric, or rectal routes of immunization and found that rectal immunization in conjunction with intramuscular (i.m.) vaccination provided full protection of hamsters from death and diarrhea while the other mucosal routes did not. Protection was associated with high levels of toxin-neutralizing antibodies in serum. The requirement for adjuvants for protection was assessed by using sequential i.m. and rectal or i.m. vaccination regimens. Unexpectedly, i.m. immunization without adjuvant conferred the highest protection from death and diarrhea; this regimen elicited the highest serum anti-toxin B titers as well as toxin B neutralizing titers. Passive transfer of mouse antitoxin antibodies protected hamsters in a dose-dependent manner, demonstrating the principal role of circulating antitoxin antibodies in immunity from this toxin-mediated mucosal disease. These results suggest that prophylactic parenteral vaccination or intravenous immunotherapy could provide protection from C. difficile disease in humans.
Clostridium difficile is the bacterial pathogen identified as the cause of pseudomembranous colitis and is principally responsible for nosocomial antibiotic-associated diarrhea (AAD) and colitis. AAD results from antibiotic-induced alteration of the normal flora of the intestine, allowing C. difficile to proliferate. Old age, hospitalization, antibiotic usage, and underlying illness are all risk factors for C. difficile disease (31). Approximately 20% of patients uncolonized at admission to hospital became colonized during hospitalization and more than one-third developed diarrhea in one study (29). The economic impact of this disease is significant. There are an estimated 300,000 cases annually in the United States alone. A recent study estimated the C. difficile disease added, on average, more than 2 weeks to the length of hospitalization at an additional cost of $10,000 per patient (36). No vaccine to prevent or treat symptoms of C. difficile disease is currently available.
The manifestations of this infection are believed to be caused by two exotoxins, toxins A and B. The toxins are large (∼300-kDa) proteins, each containing a ∼100-kDa carboxy terminus consisting of repeating carbohydrate recognition domains responsible for binding to host cell surface oligosaccharides. The N-terminal domain comprises an enzymatic region with glucosyltransferase activity which catalyzes the modification of small GTP-binding proteins. Toxin A is both a cytotoxin and an enterotoxin capable of inducing fluid accumulation in ligated intestinal loops. Toxin B is a more potent cytotoxin, but both toxins are lethal when administered systemically to animals. The pathway of toxic activity begins in the gut lumen, where, following secretion from C. difficile, toxin A attaches to defined epithelial cell surface oligosaccharide receptors (34) and is internalized. Subsequently, toxin A modified small GTP-binding proteins (Rho, Rac, and cdc42), leading to the loss of actin cytoskeletal integrity, which comprises the barrier function of the epithelium through the loss of tight junctions (42).
In certain animal models, the disruption of the epithelium by toxin A probably facilities the entrance of toxin B. When given intragastrically (i.g.) to hamsters, toxin B is lethal only when small amounts of toxin A are given in advance or when the intestine has been surgically modified (26). Recently, cell surface glycoconjugate receptors for toxin B have been demonstrated in the human colon (6) suggesting that in humans, prior insult to the epithelial barrier by toxin A is not required. Subsequently, the toxins generate local inflammation of the mucosa by inducing chemotaxis of neutrophils and inducing the release of inflammatory mediators from macrophages, neutrophils, and mast cells (24, 30, 31, 33, 38, 46).
Animal studies have clearly shown that the toxins are responsible for both diarrhea and inflammation of the intestinal mucosa. Most toxigenic strains synthesize both toxins, whereas nontoxigenic strains are not associated with disease. Immune protection from C. difficile disease has been induced following vaccination of hamsters with culture filtrates containing inactivated toxins A and B (9, 17, 23, 39, 40), whereas filtrates of nontoxigenic strains have no capacity to protect (17, 23). Immunization with inactivated toxin A alone conferred protection to hamsters, but immunization with inactivated toxin B alone did not (17). Passive administration of a monoclonal antibody directed toward the binding domain of toxin A also protected against disease in gnotobiotic mice (7). Recently, passive-immunization experiments with hamsters demonstrated a therapeutic role for immunity to both toxins while only anti-toxin A was required for prophylactic protection (19). Taken together, these findings suggest that both toxins contribute to C. difficile disease in animal models.
Clinical studies have also implicated the toxins as the primary mediators of AAD. Antitoxin levels in serum have been found in some studies to correlate with both decreased severity of disease and the absence of relapse (3, 44). Sera from convalescent patients were found to contain immunoglobulin A (IgA) antibodies which neutralized both the cytotoxic and enterotoxic activities of toxin A (13). When exposed to human colonic explants, toxin B exhibited enterotoxic activity which was 10 times more potent than that of toxin A (35). Human cells other than the colonic epithelium appear to be stimulated by toxin B also; monocytes release inflammatory mediators in the presence of toxin B, probably contributing to the local inflammation characteristic of C. difficile colitis in humans (10).
To develop a vaccine to protect humans from C. difficile disease, immunity to both toxins will probably be required. Previously, we have shown that vaccination of hamsters with toxoid, using a combination of parenteral and mucosal immunization, conferred fill protection in this highly sensitive model of C. difficile disease (39, 40). In this paper, we present data demonstrating that protective immunity can be induced through parenteral vaccination and that toxin-specific circulating antibodies alone have the capacity to protect from both systemic and mucosal disease.
MATERIALS AND METHODS
Animals.
Female Syrian hamsters (Mesocricetus auratus; Charles River Laboratories, Wilmington, Mass.), 4 to 5 weeks old, were used for immunization and challenge studies. The hamsters were caged in groups of five except for the period following C. difficile challenge, when they were caged individually. Female Swiss Webster mice (Taconic, Germantown, N.Y.), 6 to 8 weeks old, were used for generation of antitoxin ascitic fluid. The animals were fed a standard laboratory diet ad libitum. All procedures involving animals were conducted under protocols approved by the Institutional Animal Care and Use Committee.
C. difficile toxoid vaccine.
Toxins A and B of C. difficile can be fractionated by ion-exchange chromatography to high purity, but toxin B, in particular, is sensitive to precipitation following formalin inactivation (23). All attempts to preserve toxin solubility by addition of stabilizing agents have failed (data not shown). For this reason, all immunizations with inactivated C. difficile toxins A and B published to date have been performed with crude or fractionated culture filtrates. In this study, initial experiments were performed with inactivated culture filtrates and subsequent immunizations were performed with an enriched toxin-containing preparation fractionated by gel filtration chromatography.
Culture filtrate C. difficile toxoid was prepared essentially as described previously (23, 39). Dialysis flasks were inoculated with C. difficile VPI 10463, which was grown anaerobically for 72 h, centrifuged, and sterile filtered. The culture filtrate had a toxin A concentration of 56 μg/ml as determined by enzyme-linked immunosorbent assay (ELISA) (25). Electron microscopy-grade paraformaldehyde (Electron Microscopy Sciences, Fort Washington, Pa.) was added to the filtrate to 1% (vol/vol) with stirring for 20 min at room temperature, and the mixture was held at 4°C for 10 days to fully inactivate the toxins. Inactivation of toxins was determined by the absence of cell culture cytotoxicity (8). The toxoid was washed with 4 volumes of phosphate-buffered saline (PBS; pH 7.4) by ultrafiltration through a 100-kDa membrane in a 500-ml cell concentrator (Amicon, Beverly, Mass.). Toxoid was concentrated 16-fold, filter sterilized, and stored at 4°C until used. Given the roughly equivalent molecular masses of the toxins (308 kDa for toxin A; 269 kDa for toxin B) and the similar levels of expression and assuming no losses during concentration, we estimated a concentration of 912 μg of each inactivated toxin per ml of concentrated toxoid.
A partially purified toxoid preparation was also manufactured. Briefly, C. difficile-inoculated dialysis flasks were cultured for 3 days, and the contents were centrifuged and sterile filtered. The toxin concentrations in the filtrate were determined by ELISA, and the filtrate was concentrated by addition of (NH4)2SO4 to 60% and incubation at 4°C for 24 h. After precipitation, the toxin-containing pellet was resuspended in PBS and dialyzed in PBS with a 12,000- to 14,000-molecular-weight-cutoff dialysis sac (Spectrum, Houston, Tex.) at 4°C for 24 h. Material was harvested, clarified by centrifugation, and filtered through a 0.8-μm-pore-size filter. The toxin A- and B-containing preparation was separated by gel filtration chromatography with Sephacryl S-200 resin (Pharmacia, Uppsala). The toxin A and B concentrations were determined by ELISA (25), and the total protein concentration of the preparation was calculated by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.). From these values, we determined that both toxins in total represented 32% of the protein content. Electron microscopy-grade paraformaldehyde was added to the toxin preparation to 0.25% and the mixture was stirred for 20 min at room temperature and held at 4°C for 12 days to fully inactivate toxins. The toxoid was dialyzed against 0.02% paraformaldehyde in PBS and stored at 4°C until use. Prior to use, the toxoid was further concentrated in a Macrosep centrifugal concentrator (30-kDa membrane; Filtron Technology, Northborough, Mass.) to final concentrations of 953 and 340 μg/ml for toxins A and B, respectively, as measured by ELISA prior to formaldehyde inactivation.
Active-immunization regimens.
Various immunization regimens involving parenteral or combination parenteral and mucosal vaccination protocols are outlined in Tables 1 and 2. For intranasal (i.n.) administration, unanesthetized hamsters were each given toxoid diluted in PBS in a 50-μl total volume administered into both external nares with a micropipettor. For coadministration with Escherichia coli heat-labile toxin (LT; Swiss Serum and Vaccine Institute, Berne, Switzerland), 5 μg of LT was added to the vaccine formulation and administered in the 50-μl volume. For i.g. administration, hamsters were anesthetized with isoflurane (Ohmeda PPD Inc., Liberty Corner, N.J.) and then toxoid plus 5 μg of LT in a 200-μl volume was delivered with a feeding needle (Popper and Sons, New Hyde Park, N.Y.) following pretreatment with 0.3 ml of 0.1 M NaHCO3 (pH 8.3). For rectal (r.) administration, anesthetized hamsters were given toxoid plus 5 μg of LT in a 200-μl volume with a feeding needle. For intramuscular (i.m.) immunization, toxoid was adsorbed to Al2O3 (Rehydragel LV; Reheis Chemical, Berkeley Heights, N.J.), hereafter referred to as alum. Prior to each experiment, the appropriate ratio of toxoid to alum which resulted in complete adsorption of vaccine was determined. Alum was titrated against a quantity of toxoid with PBS as diluent, and after a 30-min incubation at room temperature, preparations were centrifuged and supernatants were analyzed for free toxin A by capture ELISA. After determining the minimum quantity of alum required for full adsorption of toxoid, a safety margin of excess alum was included in the final vaccine formulations. The formulation was administered to anesthetized hamsters in a 100-μl volume via a 25-gauge needle in the right caudal thigh.
TABLE 1.
Combination parenteral-mucosal immunization regimens with formalin-inactivated C. difficile culture filtrate toxoid
| Regimen | Group | Route (days) | Dosea (μg) | Adjuvant | No. of animals |
|---|---|---|---|---|---|
| Mucosal prime, i.m. boost | A | i.n. (0) | 25 | None | 5 |
| i.m. (14, 28) | 10 | Alum | |||
| B | i.n. (0) | 25 | LT | 5 | |
| i.m. (14, 28) | 10 | Alum | |||
| C | i.g. (0) | 100 | LT | 4 | |
| i.m. (14,28) | 10 | Alum | |||
| D | r. (0) | 100 | LT | 5 | |
| i.m. (14, 28) | 10 | Alum | |||
| E | i.m. (0, 14, 28) | 10 | Alum | 5 | |
| F | No treatment | ||||
| i.m. prime, mucosal boost | A | i.m. (0) | 10 | Alum | 5 |
| i.n. (14, 21, 28) | 25 | None | |||
| B | i.m. (0) | 10 | Alum | 4 | |
| i.n. (14, 21, 28) | 25 | LT | |||
| C | i.m. (0) | 10 | Alum | 5 | |
| i.g. (14, 21, 28) | 100 | LT | |||
| D | i.m. (0) | 10 | Alum | 5 | |
| r. (14, 21, 28) | 100 | LT | |||
| E | No treatment | 5 |
Based on the toxin A concentration as determined by ELISA prior to formaldehyde inactivation.
TABLE 2.
Sequential i.m. and r. or i.m. immunization with partially purified C. difficile toxoid
| Group | Route (days) | Dosea (μg) of:
|
Adjuvant | No. of animals | |
|---|---|---|---|---|---|
| Toxin A | Toxin B | ||||
| A | i.m. (0) | 25 | 9 | Alum | 20 |
| r. (14, 21) | 25 | 9 | LT | ||
| B | i.m. (0) | 25 | 9 | Alum | 20 |
| r. (14, 21) | 25 | 9 | None | ||
| C | i.m. (0, 14, 21) | 25 | 9 | Alum | 15 |
| D | i.m. (0, 14, 21) | 25 | 9 | none | 15 |
| E | i.m. (0) | 0 | 0 | Alumb | 10 |
| r. (14, 21) | 0 | 0 | LTb | ||
| F | Weight control (unchallenged) | None | None | None | 5 |
Toxin A or B concentration determined by ELISA prior to formaldehyde inactivation.
Adjuvant administered in PBS.
Passive-immunization regimens.
Hyperimmune hamster serum was prepared following i.m. immunization of 10 hamsters with partially purified toxoid (25 μg of toxin A; 9 μg of toxin B) without adjuvant on days 0, 7, 14, 21, and 35. The hamsters were bled on day 54, and serum samples were pooled. The pooled serum exhibited neutralizing titers of 3,200 for both toxins (see “Cytotoxicity inhibition assay” below). Naive animals (two per group) each received 2 ml of immune or normal sera on three consecutive days (days −3, −2, and −1) by the intraperitoneal (i.p.) route. Blood and fecal samples were taken 1 day later (day 0), and the animals were challenged, as described below.
Mouse polyclonal antitoxin ascites fluid was generated as follows. Groups of mice were immunized parenterally with partially purified toxoid and challenged with purified active C. difficile toxin A (200 ng) or B (100 ng) by the i.p. or intravenous route, respectively, as part of another study. Animals surviving the challenge were given booster immunizations with toxoid. Mice were inoculated with s-180/TG sarcoma cells i.p., and 10 days later ascitic fluid was drained, pooled, clarified by centrifugation, and held at −20°C. This polyclonal ascites preparation exhibited toxin-neutralizing titers of 16,000 for both toxins. Various doses (6, 2, 0.6, and 0.2 ml) of immune ascites or 2 ml of nonimmune ascites was administered to groups of five hamsters each 2 days prior to challenge. Passive antitoxin titers were determined from prechallenge bleeds, and the challenges were performed as described below.
Goat anti-toxin A and B (Techlab Inc., Blacksburg, Va.) serum demonstrated high toxin-neutralizing titers in vitro (toxin A, 25,600; toxin B, 12,800). Immune or normal serum (2 ml) was administered on days −2, −1, 0, and 1, with challenge on day 0 to groups of two hamsters each. The hamsters were challenged as described below. Blood and fecal samples were taken at 2-day intervals postchallenge from unchallenged controls receiving immune serum to assess passive antitoxin titers in sera and fecal extracts.
Sampling of blood and secretions.
To evaluate antibody responses in sera and secretions, preimmunization and prechallenge samples were taken on day −1 and 7 days after the last immunizing dose, respectively. Blood (200 to 400 μl) was withdrawn from the retroorbital sinus of anesthetized animals and left to clot overnight at 4°C, and serum was obtained by centrifugation. Following intraperitoneal administration of pilocarpine (0.4 mg in PBS), saliva was collected with a micropipette and two fecal pellets were placed in preweighed microcentrifuge tubes. For passive-immunization studies, pilocarpine was omitted and freshly voided fecal pellets were collected. The net weights of fecal samples were determined and samples were suspended in PBS plus protease inhibitors (0.2 μM aminoethylbenzenesulfonyl fluoride [Calbiochem, La Jolla, Calif.]; 1 μg of aprotinin [Sigma, St. Louis, Mo.], per ml; 10 μM leupeptin [Sigma]), adding 5 μl per mg of feces. The samples were vortexed vigorously, and insoluble material was removed by centrifugation.
C. difficile challenge.
Hamsters were challenged by i.g. administration of 0.5 mg of clindamycin-HCl (Sigma) followed 3 h later with 105 cells of C. difficile (VPI 10463) washed with PBS to eliminate free toxins. Hamster weights, extent of diarrhea, and general health status were recorded at 2-day intervals postchallenge for 14 days, and weight loss was determined by comparison to the prechallenge weight.
For active-immunization studies, hamsters were challenged 14 days after the last immunizing dose. For passive-immunization experiments, challenges were performed according to the schedules detailed above.
ELISA for antibodies to toxins A and B.
Toxin-specific antibody titers in sera, feces, and saliva were measured by indirect ELISA. Microtiter plates (Corning-Costar, Cambridge, Mass.) were coated with 100 μl of purified toxin A or B (Techlab) at 1 μg/ml in 0.1 M carbonate-bicarbonate buffer (pH 9.8) overnight at 4°C. After washing wells four times with PBS-Tween (PBS, 0.05% Tween 20) and blocking with 2.5% nonfat dry milk in PBS-Tween for 1 h at 37°C, twofold serial dilutions of serum samples or 1:10 dilutions of secretions were prepared in duplicate and incubated for 1 h at 37°C. Various starting dilutions, ranging from 1:1,000 to 1:10,000, were used for sera. Following four washes with PBS-Tween, bound antibody was detected after a 1-h incubation of either goat anti-hamster IgG-alkaline phosphatase conjugate (Southern Biotech Associates, Birmingham, Ala.) or rabbit anti-hamster IgA (prepared at OraVax, Inc., with hamster colostral IgA as the immunogen) followed by goat anti-rabbit IgG-alkaline phosphatase (Southern Biotech). Negative controls were toxin-coated wells reacted with detection antibodies and substrate. After 5 washes, p-nitrophenyl phosphate substrate (1 mg/ml [Sigma]) in 0.1 M diethanolamine buffer (pH 9.6) containing 0.5 mM MgCl2 was incubated on plates at room temperature for 20 min and optical densities at 405 nm were recorded on a Vmax plate reader (Molecular Devices, Menlo Park, Calif).
Cytotoxicity inhibition assay.
IMR-90 fibroblast cells were grown to confluence in 96-well plates in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, N.Y.) containing 10% fetal calf serum. Twofold dilutions of hamster sera (starting at 1:50 and 1:25 for toxin A and B, respectively) were incubated for 1 h at 37°C with 4 CTU100 of toxin A (25 ng/ml) or toxin B (125 pg/ml), where 1 CTU100 is the minimum dose of toxin required to induce rounding of 100% of cells. Goat anti-toxin A or B (Techlab) served as positive controls. The mixtures were added to cells and incubated for 24 h, and the proportion of rounded cells was determined. The cytotoxicity inhibition titer was defined as the reciprocal of the highest serum dilution that inhibited >50% cell rounding.
Immunohistochemical assessment of toxoid binding.
Hamster intestinal tissue was prepared as previously described (11). Reactive aldehydes in extracted epoxy tissue sections were quenched with 50 mM NH4Cl in PBS for 10 min, and nonspecific protein binding was blocked with 0.2% gelatin (type A porcine skin) in PBS for 30 min in a humid chamber. Purified toxin A (Techlab) or partially purified toxoid was diluted to 10 μg/ml in gelatin-PBS and overlaid on the sections for 60 min at room temperature. After sections were washed three times (5 min each) with gelatin-PBS, anti-toxin A monoclonal antibody (PCG-4; Techlab) or goat anti-toxin B was incubated at a 1:500 dilution for 60 min. Following washes, either goat anti-mouse IgG–tetramethylrhodamine-5-isocyanate (TRITC) (American Qualex, La Mirada, Calif.) or rabbit anti-goat IgG-TRITC (American Qualex) was incubated at 1:500 for 30 min. The slides were briefly washed in PBS and then distilled H2O, and coverslips were mounted with Moviol (Calbiochem) containing 2.5% DABCO (1,4-diazabicyclo[2.2.2]octane [Sigma]). Photography was performed using a Zeiss Axioskop microscope equipped for epifluorescence with Kodak Elite 400 slide film. Control slides were prepared by using gelatin-PBS instead of either toxin A or toxoid during the labeling procedure.
Statistical analysis.
The difference in immune responses between groups was tested for significance by the Wilcoxon/Kruskal-Wallis test (rank sums) with JMP software (SAS Instutite, Cary, NC).
RESULTS
Protective efficacy of combination parenteral/mucosal vaccination regimens.
Previous experiments demonstrated that administration of vaccine preparations containing inactivated C. difficile toxins A and B (toxoid) to hamsters in regimens incorporating both parenteral and mucosal routes yielded high levels of protection from diarrhea and death following challenge (39, 49). To build on these findings by developing regimens which could be applied in the clinic, we hoped to define a mucosal route in hamsters which could confer protection in combination with the clinically acceptable i.m. route of administration. We compared the effectiveness of a culture filtrate toxoid formulation administered by the i.n., i.g., or rectal r. route as either a priming dose followed by i.m. boosting or for booster doses following i.m. priming. Because immune responses following mucosal vaccination are substantially enhanced when antigen is coadministered with a mucosal adjuvant, we included E. coli LT in i.g. and r. immunization regimens, while i.n. vaccination was performed with and without LT as outlined in Table 1. Intramuscular immunization with toxoid included alum as the adjuvant. Also, to assess the relative contribution of i.m. dosing to protection in the combined parenteral-mucosal regimen, i.m. vaccination alone (three doses) was tested in parallel. Hamsters were challenged 2 weeks after the last immunizing dose as described in Materials and Methods and evaluated for protection from diarrhea and death.
The clindamycin-C. difficile challenge is a stringent test of immunity. Following challenge, all sham-immunized control animals were dead or required euthanasia within 4 days. Gross pathological examination of control animals showed severe hemorrhagic necrosis of the cecum and colon typical of clindamycin-induced cecitis and colitis (4). Of the immunized groups, only those which were either primed or boosted by the r. route in conjunction with i.m. vaccination were fully protected from death and diarrhea (Fig. 1). The weight loss observed following challenge correlated well with protection from diarrhea (data not shown). We found that the i.g. route was least effective in protecting hamsters from diarrhea when used as the route to prime or boost animals in combination with i.m. vaccination. The i.n. route generally afforded intermediate levels of protection relative to the other mucosal routes, and no clear enhancement of protection was observed following LT coadministration. Parenteral vaccination with toxoid by the i.m. route with alum yielded moderate protection; 40 and 60% of hamsters were protected from diarrhea and death, respectively.
FIG. 1.
Protection against C. difficile disease (diarrhea [solid bars] or death [hatched bars]) in hamsters vaccinated with combination parenteral-mucosal dosing regimens. Hamsters were either primed or boosted with inactivated C. difficile culture filtrate toxoid given by various mucosal routes in conjunction with i.m. administration. The outcomes for intestinal and systemic disease after clindamycin-C. difficile challenge are shown. Protection is defined as either the number of animals free of detectable diarrhea or the number of survivors, both as a percentage of the total number of animals per group. See Table 1 for a description of the immunization regimens used.
Protective efficacy of parenteral vaccination regimens.
The above studies used a culture filtrate containing inactivated C. difficile toxins A and B. To minimize the potential that other C. difficile antigens may contribute to protection and to develop a vaccine formulation appropriate for clinical use, a partially purified preparation containing both toxins and using a milder inactivation protocol was manufactured. We tested this toxoid formulation by using the sequential i.m. and r. combination regimen in parallel with parenteral vaccination alone and assessed the requirements for parenteral (alum) or mucosal (LT) adjuvants. i.m. priming with toxoid and alum was followed by r. boosting with or without LT. Pilot studies revealed that inclusion of alum in the priming dose in this regimen resulted in a modest increase in protection over that for comparable groups without alum (data not shown). These combination regimens were compared to i.m. vaccination alone with and without alum (Table 2).
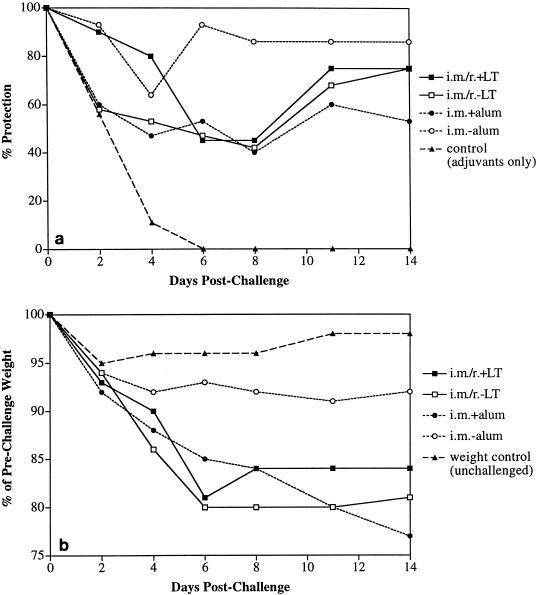
We found that, like the previously tested crude filtrate toxoid, immunization with the partially purified toxoid induced protective immunity against clindamycin and C. difficile challenge. All vaccinated groups showed a high level of protection from death (>90%) and various levels of diarrhea. Surprisingly, we found that i.m. vaccination without alum conferred the highest level of protection from diarrhea (Fig. 2a). While 64% protection was seen on day 4 postchallenge, approximately 90% of hamsters were completely protected from days 6 through 14 postchallenge (the end of the observation period). Protection from diarrhea correlated well with the reduced levels of weight loss observed in the i.m./no-alum group (Fig. 2b). The sequential i.m.-r. and i.m.-plus-alum regimens yielded reduced levels of protection from diarrhea and weight loss. Confirming our earlier observations with crude filtrate toxoid given i.n., there was no clear enhancement of protection from diarrhea and weight loss when LT was coadministered.
FIG. 2.
Protection against C. difficile disease in hamsters vaccinated with partially purified C. difficile toxoid by sequential i.m. and r. regimens or i.m. regimens with or without adjuvant. Protection against diarrhea (a) and weight loss (b) at various times points postchallenge is shown. See Table for a description of the immunization regimens used. In panel b, each point represents the group mean weight as a percentage of the prechallenge weight. The weight loss observed for unchallenged controls reflects the response of animals to individual housing following separation at the time of challenge.
Assessment of antibody responses following vaccination with toxoid.
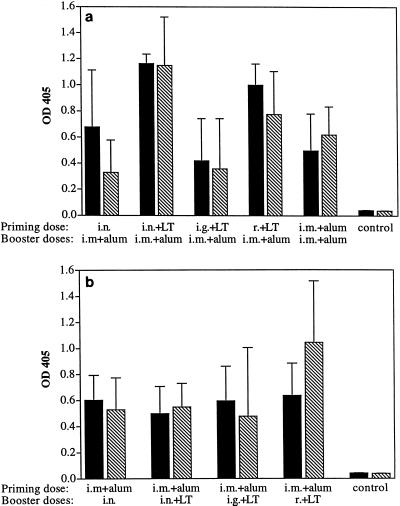
Since previous studies have demonstrate that antibody responses to inactivated C. difficile toxins correlated with protection in the hamster model (18, 39, 40), we quantitated by ELISA toxin-specific IgG and IgA levels elicited in prechallenge sera and secretions by the various vaccination regimens and measured the capacity of serum antibodies to neutralize cytotoxic activity in vitro. In the initial experiment, which involved crude filtrate toxoid and compared different mucosal routes in combination with parenteral vaccination (Table 1), we found that all regimens elicited substantial toxin-specific IgG titers in prechallenge serum samples. IgG titers were more variable in groups which were primed by different mucosal routes (Fig. 3a) than in those boosted by mucosal routes (Fig. 3b). Animals primed i.n. and given LT had significantly higher IgG titers against both toxins (P = 0.012) than did hamsters primed i.g. and given LT. The group primed i.n. and given LT also had significantly higher anti-toxin A IgG levels than did the group primed i.m. and not given LT (P = 0.012) and higher anti-toxin B IgG titers than did animals primed i.n. and not given LT (P = 0.012). Animals boosted by mucosal routes did not have significantly different IgG responses to either toxin (Fig. 3b). Antitoxin IgA responses in serum overall were relatively modest (data not shown). Analysis of toxin-specific antibodies in secretions revealed that levels of local antitoxin responses sometimes depended on the mucosal route used for priming or booster doses. For example, i.n. immunization with LT typically induced higher salivary IgA titers than did other mucosal routes (results not shown). However, all regimens incorporating mucosal immunization stimulated only modest levels of toxin-specific fecal antibody. No detectable antitoxin IgA or IgG was found in feces after i.m. immunization alone. Groups immunized by the i.g. or r. routes, which stimulate intestinal antibody-inductive sites, did not show elevated IgA or IgG titers in feces (data not shown). We assessed the relationship of antitoxin levels elicited in serum or mucosa by different regimens to the protection conferred to hamsters. We found that antitoxin levels did not appear to correlate directly with protection, since rectally immunized hamsters did not consistently demonstrate higher titers of any particular antibody isotype in sera or secretions.
FIG. 3.
Serum IgG response to toxin A or B in prechallenge samples. Sera from hamsters either primed (a) or boosted (b) by various mucosal routes with C. difficile culture filtrate toxoid were analyzed by indirect ELISA. Samples in panel a were diluted to 1:50,000 or 1:10,000 for anti-toxin A (solid bars) or B (hatched bars) responses, respectively. Samples in panel b were diluted to 1:12,500 or 1:8,000 for anti-toxin A (solid-bars) or B (hatched bars) responses, respectively. Each bar represents the mean and standard deviation for five animals. OD 405, optical density at 405 nm. See Table 1 for a description of the immunization regimens used.
Anti-LT IgG responses in serum were also examined to assess the immunogenicity of LT as an indicator of adjuvant activity. In general, anti-LT responses were variable within groups and were route dependent. For example, when one dose of LT was administered with antigen, the highest IgG titers were elicited by the rectal route (data not shown). However, three LT doses plus antigen by the i.n. route induced high circulating IgG titers whereas the r. route induced relatively modest titers. i.g. administration of LT induced nonuniform responses; only one in five animals yielded detectable anti-LT responses following two doses of vaccine.
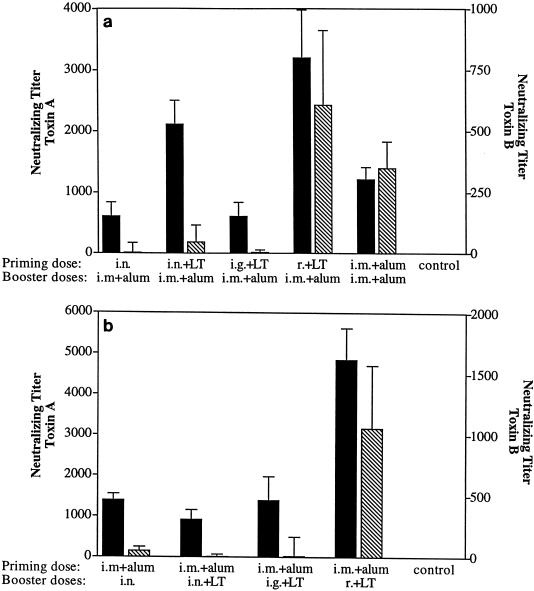
The ability of serum and secretory antibodies to inhibit (neutralize) toxin A or B cytotoxicity in an in vitro cell culture assay was tested. Immune sera were clearly capable of neutralizing the toxins in this assay. By contrast, immune saliva or fecal extracts revealed no detectable inhibition of toxin A or B cytotoxicity (data not shown). Compared to antitoxin levels in serum as measured by ELISA, toxin neutralization titers correlated more closely with protection. Regimens which incorporated priming or boosting by the r. route yielded toxin neutralization titers which were higher than those in most other groups, correlating with the full protection from death and diarrhea observed (Fig. 4a). Priming by the r. route yielded significantly higher toxin A inhibition titers (P < 0.05) than in other groups except the group primed i.n. and given LT, which was the only other group to exhibit full protection from death. Toxin B inhibition titers in this group were also significantly higher than in all groups except the i.m.-only group, which yielded the highest protection from diarrhea (40%) among the suboptimally protected groups. r. boosting following i.m. priming induced significantly higher toxin A and B neutralizing titers than did the other regimens tested, again correlating with the full protection observed (Fig. 4b). These results demonstrated that the production of elevated toxin neutralizing-antibody titers in serum was associated with protection from C. difficile disease in this model.
FIG. 4.
In vitro C. difficile toxin-neutralizing activity in prechallenge sera of vaccinated hamsters. Sera from hamsters either primed (a) or boosted (b) by various mucosal routes with C. difficile culture filtrate toxoid were analyzed for neutralizing activity against toxin A (solid bars) or toxin B (hatched bars) as determined by inhibition of toxicity in the cell culture assay described in Materials and Methods. Each bar represents the geometric mean titer and standard error of samples from five animals.
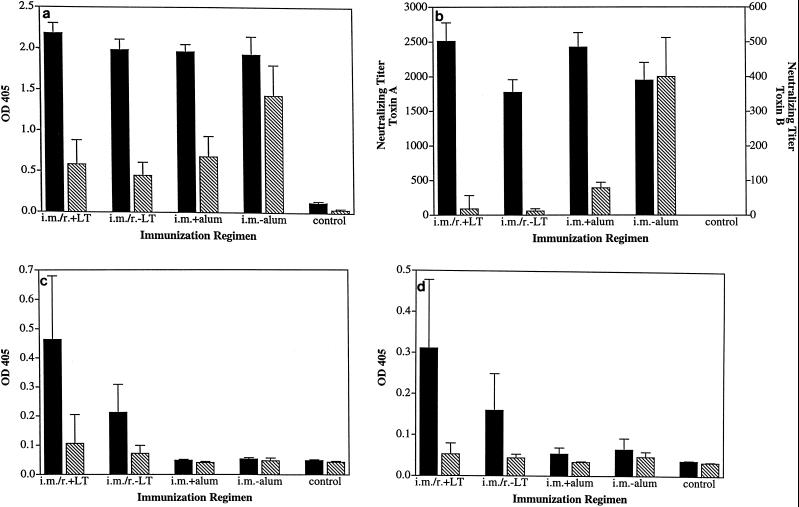
Analysis of antibody responses in animals immunized with the partially purified toxoid vaccine (Table 2) revealed the immunogenicity of this preparation in the different regimens. However, there were clear differences in the magnitude of antibody titers between the groups. Notably, prechallenge serum samples from animals receiving vaccine i.m. without alum contained significantly higher anti-toxin B IgG titers (Fig. 5a) as well as toxin B neutralizing titers (Fig. 5b) than did all other groups (P < 0.05). Fecal IgA and IgG antibodies directed against the toxins were found only in animals given the combination regimen (Fig. 5c and d). IgA titers in saliva were also highest in mucosally vaccinated animals, indicating the induction of panmucosal antibody responses. IgG levels in saliva appeared to correlate with IgG titers in serum, since the group primed i.m. and given no alum exhibited the highest anti-toxin B titers (data not shown). The addition of LT to the r. booster doses did not result in significantly higher antitoxin or toxin neutralizing titers in serum or secretions, correlating with the similar levels of protection elicited in the presence or absence of LT. In summary, i.m. immunization with toxoid without adjuvant induced the highest level of protection from diarrhea and weight loss in the absence of a distinct intestinal antibody response. The presence of high circulating anti-toxin and toxin neutralizing antibody titers in these animals correlated with the enhanced protection observed, further implicating serum antitoxin antibodies as effector molecules in this disease model.
FIG. 5.
Antitoxin antibody responses and toxin-neutralizing activity in prechallenge samples from vaccinated hamsters. Hamsters were immunized with partially purified C. difficile toxoid by sequential i.m. and r. regimens or i.m. regimens with or without adjuvant. (a) Antitoxin A (solid bars) or B (hatched bars) levels in serum were assessed at 1:50,000 dilution by ELISA. (b) Toxin-neutralizing activity against toxin A (solid bars) or B (hatched bars). (c and d) Toxin A-specific (solid bars) or B-specific (hatched bars) IgA or IgG levels in fecal extracts were analyzed at a 1:10 dilution. See Table 2 for a description of the immunization regimens used.
Histopathology of hamster cecal tissue following immunization and challenge.
To assess the ability of the vaccination regimens to minimize the proinflammatory action of the toxins, pathological examination of the cecal tissue was performed for selected surviving hamsters following sacrifice 3 weeks after challenge. As opposed to the severe hemorrhagic necrosis of the cecum seen within 48 h of challenge of control hamsters, surviving hamsters typically demonstrated minimal to moderate epithelial hyperplasia, minimal to mild goblet cell hyperplasia, and mild chronic/active inflammation of the mucosa (data not shown). The histopathological outcome did not vary among vaccination regimens, probably because the survivors typically exhibited mild or no diarrhea. The absence of substantial tissue damage in parenterally immunized hamsters (i.m. with no alum) correlate with the absence of diarrhea in these animals postchallenge. These findings demonstrated that immune protection of the cecal mucosa from C. difficile disease was induced through systemic vaccination.
Mucosal binding of toxoid vaccine.
The immunogenicity of toxoid following mucosal vaccination may be related to properties of the toxoid preparation. The lectin-like binding of toxin A to intestinal cell surface receptors is essential for enterotoxic activity. In the hamster model, gut epithelial cells express abundant toxin A-binding sites in both the small and large intestines (21) whereas sites for toxin B have not been demonstrated in animal tissue. Immunohistochemical analysis of toxoid binding to hamster rectal mucosa showed clear binding to cell membrane receptors, with a pattern and intensity similar to those of native toxin A (Fig. 6). These results were observed when anti-toxin A antibody was used to detect bound toxoid, demonstrating that the binding property of toxin A is largely retained following formalin inactivation. Anti-toxin B antibodies did not recognize bound toxoid, suggesting that toxin B did not gain the ability to bind the mucosa by virtue of aldehyde cross-linking to toxin A. The omission of toxin A or toxoid from the staining protocol resulted in no detectable signal (data not shown). The adherent property of the toxoid demonstrated in situ may contribute to the immunogenicity of this inactivated toxin preparation.
FIG. 6.
Binding of partially purified C. difficile toxoid to hamster rectal mucosa. Sections of rectal mucosa from naive hamsters were overlaid with purified toxin A (A) or toxoid (B), and binding was assessed by antibody detection, as described in Materials and Methods. The distribution of binding sites and intensity of signal is similar for purified toxin A and toxoid, as determined by fluorescence microscopy. Omission of either toxin A or toxoid resulted in no detectable signal (data not shown). Bar, 50 μm.
Passive immunization with immune sera and ascites.
The high level of protection conferred by parenteral (i.m.) vaccination suggested that circulating antibodies may be effector molecules in this model. To separate the protective activity in serum from the possibility that local (mucosal) immune mechanisms were induced following vaccination by the i.m. route, passive immunization of naive hamsters with different antitoxin preparations was performed. We first transferred pooled sera (6 ml total) from hyperimmunized hamsters vaccinated with partially purified toxoid (i.m. with no alum) to naive animals along with control sera in parallel and challenged them (see Materials and Methods). The animals which received immune sera developed mild diarrhea lasting 1 to 3 days and then remained free of disease, while control animals developed severe diarrhea and were sacrificed at 2 days postchallenge (data not shown). To generate sufficient antitoxin antibody to repeat the study with larger groups of animals, we prepared mouse polyclonal ascites fluid, as described in Materials and Methods. Hamsters were given either various doses of immune ascites or control ascites by the i.p. route 2 days before challenge, as outlined in Materials and Methods.
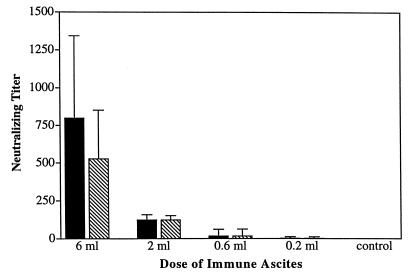
Animals receiving 6 ml of immune ascites were highly protected from challenge; all the animals survived, and one animal developed diarrhea lasting for 1 day (Fig. 7). Reducing the dose to 2 ml resulted in 0 and 60% protection form diarrhea and death, respectively. Lowering the passive dose further (to 0.6 or 0.2 ml) resulted in little or no protection from death. Control animals were all dead within 2 days of challenge.
FIG. 7.
Passive protection of hamsters from C. difficile disease with antitoxoid immune ascites. Naive hamsters received various doses of immune ascites by the i.p. route and were challenged 2 days later with clindamycin-C. difficile, as detailed in Materials and Methods. Protection against diarrhea (solid bars) and death (hatched bars) is shown.
We also assessed the ability of hyperimmune goat antiserum raised against C. difficile toxoid to protect hamsters from challenge. We administered multiple 2-ml volumes of this antiserum by the i.p. route prior to and following challenge, as described in Materials and Methods. In contrast to the protection conferred by transfer or hamster or mouse antitoxin antibodies, we found that recipient hamsters were poorly protected from challenge (data not shown). Hamsters given control nonimmune goat serum died by 4 days postchallenge, while recipients of immune serum developed severe diarrhea and were euthanized by 6 days postchallenge.
Appearance of serum neutralizing antibodies in passively immunized hamsters.
Because toxin neutralizing titers in serum following active immunization correlated best with protection from challenge, we assessed the passive titers in serum from the recipient hamsters. We found that passive administration of 6 ml of mouse ascites resulted in toxin neutralizing titers in sera which were comparable to that induced by active immunization in hamsters vaccinated by the i.m. route without adjuvant (Fig. 8). This passive dose conferred high-level protection from challenge, while lower doses yielded proportionately reduced toxin neutralizing titers and less protection. To determine the antibody isotype responsible for toxin-neutralizing activity in this preparation, separation of antitoxin ascites by protein G-affinity chromatography into IgG-containing and IgG-depleted fractions (which contained IgA and IgM) was performed. We found that all of the toxin-neutralizing activity was associated with the IgG-containing fraction (data not shown).
FIG. 8.
Levels of in vitro toxin-neutralizing activity in sera from hamsters given various doses of immune ascites. The geometric mean titer of neutralizing activity against toxin A (sold bars) or B (hatched bars) in prechallenge hamster sera is shown, along with the standard error.
Quantitative analysis of goat antitoxin antibodies in sera of unchallenged recipient hamsters revealed substantial anti-toxin A and B titers by ELISA as well as toxin neutralizing titers approaching the levels elicited in hamsters by active immunization (Fig. 9). No toxin-specific IgA or IgG antibodies were detected by ELISA in feces following passive administration of any of the antitoxin preparations (data not shown). Despite the presence of neutralizing antibodies at levels associated with protection following active immunization or passive transfer of hamster or mouse antitoxin, hamsters which received goat antitoxin serum were poorly protected from challenge. These results suggest a functional requirement for homologous antibodies in this disease model. In summary, immune protection from C. difficile challenge could be transferred to naive hamsters by using immune hamster sera or mouse ascites containing neutralizing IgG antibodies, thus confirming the role of circulating antibodies in mucosal and systemic protection from C. difficile disease.
FIG. 9.
Levels of in vitro toxin-neutralizing activity and antitoxin antibodies in recipient hamster sera following passive administration of goat antitoxin serum. The geometric mean titer of neutralizing activity (solid bars) against toxin A or B and the goat anti-toxin A or B levels (hatched bars) in sera or unchallenged hamsters are shown, along with the standard error. Anti-toxin A and B levels in serum were assessed by ELISA at a 1:1,000 dilution. OD 405, optical density at 405 nm.
DISCUSSION
The role of the exotoxins of C. difficile as principal effectors of disease manifestations in both animal models and humans is now well established. The toxins act by stimulating fluid secretion in the gut, compromising the integrity of the intestinal epithelium, and inducing the release of proinflammatory mediators by various cells. Furthermore, in animal models, systemic administration of the toxins results in death apparently by neutrotoxicity (2). Accordingly, complete immune protection requires that antibodies neutralize the distinct activities of the toxins, which have diverse pathological consequences. In this study, we have shown that antitoxin antibodies which appear in serum following vaccination of hamsters with an inactivated toxoid preparation can prevent or minimize the symptoms of disease caused by active toxins. The high level of mucosal and systemic protection elicited through parenteral immunization and the transfer of immunity to naive hamsters by using serum or ascites demonstrated that circulating antitoxin antibodies mediate the effector functions in this model of C. difficile infection.
Previous studies have shown that passively administered circulating antitoxin antibodies could protect animals from this disease. Antibodies directed against the related Clostridium sordellii toxin, which neutralize C. difficile toxins in vitro, provided immune protection to hamsters when administered parenterally at high doses (1). In a gnotobiotic-mouse model of C. difficile infection, various monoclonal antibodies directed toward the carbohydrate recognition domains of toxin A were found to confer immunity to C. difficile challenge following intravenous administration (7). These studies provided evidence for a protective role of circulating antibodies in the prevention of mucosal and systemic C. difficile disease in animal models.
A protective role for antitoxin antibodies in human disease has been suggested on the basis of various studies. As a consequence of natural exposure to C. difficile, humans often develop antitoxin antibodies in both serum and secretions. One survey revealed that >60% of adults were seropositive for both toxins and found that the antitoxin titers were significantly higher in convalescent-phase sera than in control samples (41). Patients found to have low anti-toxin A titers in serum and feces were more likely to have C. difficile disease of longer duration and to have relapses than were individuals with higher anti-toxin titers (44). Antibodies that neutralize cytotoxicity in vitro also appear in sera or secretions of exposed individuals. The toxin-neutralizing activity in serum was found to reside in the IgA fraction, despite the presence of anti-toxin IgG molecules (13). Analysis of human colostrum or milk showed the presence of toxin-neutralizing antibodies (probably secretory IgA [sIgA]), suggesting a mechanism for passive protection of infants (16, 43).
Secretory antibodies also appear in the intestinal tract in response to C. difficile exposure. In patients undergoing colonoscopy, colonic aspirates were found to contain toxin A-specific sIgA levels higher than those in duodenal samples, correlating with the site of C. difficile infection (14). Toxin A-specific sIgA present in the colonic aspirates inhibited the binding of toxin A to brush border glycoconjugate receptors, perhaps modeling early events in immune protection in the gut.
Because C. difficile colonization and early toxin production occurs in the intestinal tract, investigators have examined the protective efficacy of orally administered antitoxin antibodies. Female hamsters immunized with inactivated toxins provided neonates with immunity to C. difficile disease through maternal milk containing toxin-neutralizing antibodies (17). Furthermore, hyperimmune bovine colostral IgG, which neutralizes both the cytotoxic and enterotoxic activities of the toxins (15), protected naive hamsters from disease when given orally (27). More recently, toxin-neutralizing antibodies purified from egg yolks of hens immunized with fragments of both C. difficile toxins were found to protect hamsters prophylactically and therapeutically from clindamycin and C. difficile challenge when administered orally (19). In addition, only anti-toxin A antibodies were necessary to prevent disease, while immunity to both toxins was required for therapeutic activity. Interestingly, the successful treatment of hamsters by passive immunotherapy was not followed by relapse and rendered many animals refractory to subsequent challenge, contrasting markedly with results following antibiotic therapy (19) and treatment with bovine colostral IgG (27).
The essential role of antitoxin immunity in protection from C. difficile disease in the hamster model is clear. Toxin-neutralizing antibodies in serum appear responsible for the protective activity observed, since the neutralization titers induced through active immunization best correlated with protection, irrespective of the regimen used. Fractionation of antibody isotypes in immune ascites revealed that the in vitro toxin-neutralizing activity resided in the IgG component. Nonetheless, this observation does not identify toxin-neutralizing IgG as the effector molecule in vivo. It was previously suggested, however, that toxin-specific serum IgG associated with cecal tissue of immunized hamsters was responsible for protection (18). Administration of fractionated immune serum or ascites will be necessary to conclusively determine which isotype(s) confers protection from C. difficile disease following passive administration in vivo.
Because serum toxin-neutralizing antibodies were implicated in protection of actively immunized hamsters, we routinely quantitated these levels in recipient hamsters following passive immunization. Despite achieving serum neutralizing titers at or near the levels seen following active immunization (Fig. 9), the goat antiserum offered only limited protection. Conversely, hamsters which demonstrated similar neutralizing titers following administration of hamster serum or mouse ascites were highly protected. Analysis of the kinetics of clearance of toxin-specific antibodies of hamster, mouse, or goat origin from sera of recipient hamsters did not reveal differences which were likely to account for the different protective capacities observed (data not shown). These results suggest that the mechanism(s) involved in protection requires homologous or highly related antibodies for efficacy.
The precise mechanism by which circulating antitoxin antibodies mediate protection from diarrhea in this model is unknown. The potent enterotoxic activity of toxin A in hamsters rapidly induces diarrhea following challenge of unprotected animals with clindamycin and C. difficile. Parenteral immunization with toxoid or passive immunization with sera or ascites induced partial or full protection from diarrhea, suggesting that anti-toxin A antibodies can minimize or prevent enterotoxic activity at the earliest stages of the disease process. This mode of immune protection would probably require that antibodies react with toxin at the level of the epithelium, be it in the intestinal lumen or within the epithelium itself. Histopathological analysis of cecal tissue from hamsters surviving challenge revealed only modest epithelial changes and mild inflammation of the mucosa, which was consistent with the extent of diarrhea seen in these animals. It is possible that circulating toxin-neutralizing antibodies (IgG) gain access to the gut lumen as a consequence of the mild inflammation of the mucosa observed in protected animals, but this would have to occur in the absence of observable fluid loss, since most animals did not develop diarrhea. However, irrespective of the source of antitoxin, we did not detect toxin-specific antibodies in feces following passive administration. Furthermore, the inability of goat antitoxin antibodies to protect hamsters argues against a mechanism of passive antibody diffusion in this disease model.
The absence of appreciable amounts of toxin-specific antibodies in feces following parenteral immunization supports the paradigm that locally synthesized polymeric IgA and IgM which are actively transported into intestinal secretions can be effectively stimulated only through mucosal immunization. Hamsters immunized by combined parenteral and mucosal regimens also showed modest but distinct fecal IgG responses against toxin A, suggesting that r. administration of toxoid was responsible for the appearance of IgG in feces. We did not determine whether this was a consequence of systemic priming with antigen or whether r. immunization alone can elicit toxin-specific fecal IgG antibodies. IgG antibodies, although not the predominant isotype, can be measured in intestinal secretions following vaccination. For example, r. immunization of humans with V. cholerae whole-cell or recombinant cholera toxin B subunit elicited IgA and IgG directed against the B subunit in rectal secretions (20).
Despite the inactivation of the toxic activity of both toxins with formaldehyde, the toxoid preparation exhibited the same mucosal binding property as native toxin A. Similarly, the vacuolating cytotoxin of Helicobacter pylori retains its cell-binding capacity following formaldehyde inactivation, and this inactivated form induces high-titer neutralizing antibodies (28). The retention of the lectin-like property of toxin A in the toxoid preparation may be immunologically relevant, since adherent antigens are generally more immunogenic than nonadherent antigens when administered by mucosal routes (12, 47).
When administered by the i.m. route, the toxoid vaccine exhibited the unexpected property of being more immunogenic in the absence of alum adjuvant. The adsorption of toxoid to alum suppressed both the IgG response to toxin B and the toxin B neutralizing titers following i.m. vaccination. The protective capacity of the vaccine given i.m. without alum correlated with the enhanced immune response to toxin B. The mechanism for the reduced immunogenicity of the alum-adjuvanted vaccine is unknown. The lectin-like property of inactivated toxin A may also contribute to greater immunogenicity by the i.m. route. Immunohistochemical examination of muscle tissue from the hamster caudal thigh revealed no detectable toxin A or B binding sites (data not shown). The ability of toxoid to adhere to immune cells located at or recruited to injection sites is unknown.
In certain systems, mucosal protection from enteric pathogens has been induced through parenteral immunization. Clearance of Pseudomonas aeruginosa from the mouse gastrointestinal tract was found to be mediated by circulating antibodies against lipopolysaccharide O antigen elicited by i.p. immunization (32). Protection of mice from intestinal rotavirus infection by circulating oligomeric IgA monoclonal antibodies through a possible mechanism of intraepithelial immune complex formation has also been reported (5). An essential role for serum antibodies in immunity from both systemic and mucosal diseases has been proposed (37).
The demonstration of mucosal and systemic protection of hamsters from C. difficile disease by circulating antitoxin antibodies suggests that immune protection of humans could be elicited following systemic immunization with a vaccine formulation containing inactivated C. difficile toxins. Furthermore, administration of intravenous Ig (IVIG) preparations containing antibodies directed against both toxins of C. difficile may provide a means of conferring more rapid immune therapy which might be required for acute or relapsing disease symptoms. IVIG preparations with low titers of antitoxin antibodies have already been shown to be effective in isolated cases of chronic relapsing disease (22, 45). Further clinical studies will be necessary to assess the effectiveness of a toxoid-based vaccine for at-risk individuals and to define the utility of vaccine-induced antibodies administered as IVIG for the prevention and/or treatment of C. difficile disease.
ACKNOWLEDGMENTS
We thank Cynthia Lee and Robert Gerety for the critical evaluation of the manuscript.
This work was supported in part by a SBIR grant R43 AI40397 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Allo M, Silva J, Jr, Fekety R, Rifkin G D, Waskin H. Prevention of clindamycin-associated colitis in hamsters by Clostridium sordellii antitoxin. Gastroenterology. 1979;76:351–355. [PubMed] [Google Scholar]
- 2.Arnon S S, Mills D C, Day P A, Henrickson R V, Sullivan N M, Wilkins T D. Rapid death of infant rhesus monkeys injected with Clostridium difficile toxins A and B: physiology and pathologic basis. J Pediatr. 1984;101:34–40. doi: 10.1016/s0022-3476(84)80585-5. [DOI] [PubMed] [Google Scholar]
- 3.Aronsson B, Granstrom M, Mollby R, Nord C E. Serum antibody response to Clostridium difficile toxins in patients with Clostridium difficile diarrhoea. Infection. 1985;13:97–101. doi: 10.1007/BF01642866. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett J G, Onderdonk A B, Cisneros R L, Kasper D L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977;136:701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- 5.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 6.Catagliuolo I, Valenick L, Riegler M, LaMont J T, Pothoulakis C. Oligosaccharides containing sialic acid and N-acetyl-glucosamine mediate Clostridium difficile toxin B binding and biological effects in human colonic mucosa. Gastroenterology. 1998;114:A949. . (Abstract). [Google Scholar]
- 7.Corthier G, Muller M C, Wilkins T D, Lyerly D, L’Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991;59:1192–1195. doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrich M, Van Tassell R, Libby J, Wilkins T D. Production of Clostridium difficile antitoxin. Infect Immun. 1980;28:1041–1043. doi: 10.1128/iai.28.3.1041-1043.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernie D S, Thompson R O, Batty I, Walker P D. Active and passive immunization to protect against antibiotic associated caecitis in hamsters. Dev Biol Stand. 1983;53:325–332. [PubMed] [Google Scholar]
- 10.Flegel W A, Muller F, Daubener W, Fischer H-G, Hadding U, Northoff H. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Immun. 1991;59:3659–3666. doi: 10.1128/iai.59.10.3659-3666.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannasca P J, Giannasca K T, Falk P, Gordon J I, Neutra M R. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol. 1994;267(Gastrointest. Liver Physiol. 30):1108–G1121. doi: 10.1152/ajpgi.1994.267.6.G1108. [DOI] [PubMed] [Google Scholar]
- 12.Giannasca P J, Boden J A, Monath T P. Targeted delivery of antigen to hamster nasal lymphoid tissue with M-cell-directed lectins. Infect Immun. 1997;65:4288–4298. doi: 10.1128/iai.65.10.4288-4298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson S, Sypura W D, Gerding D N, Ewing SL, Janoff E N. Selective neutralization of a bacterial enterotoxin by serum immunoglobulin A in response to mucosal disease. Infect Immun. 1995;63:3166–3173. doi: 10.1128/iai.63.8.3166-3173.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly C P, Pothoulakis C, Orellana J, LaMont J T. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology. 1992;102:35–40. doi: 10.1016/0016-5085(92)91781-x. [DOI] [PubMed] [Google Scholar]
- 15.Kelly C P, Pothoulakis C, Vavva F, Castagliuolo I, Bostwick E F, O’Keane J C, Keats S, LaMont J T. Anti-Clostridium difficile bovine immunoglobulin concentrate inhibits cytoxicity and enterotoxicity of C. difficile toxins. Antimicrob Agents Chemother. 1996;40:373–379. doi: 10.1128/aac.40.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Pickering K, DuPont H L, Sullivan N, Wilkins T. In vitro and in vivo neutralizing activity of human colostrum and milk against purified toxins A and B of Clostridium difficile. J Infect Dis. 1984;150:57–62. doi: 10.1093/infdis/150.1.57. [DOI] [PubMed] [Google Scholar]
- 17.Kim P-H, Iaconis J P, Rolfe R D. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55:2984–2992. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim P-H, Rolfe R D. Characterization of protective antibodies in hamsters immunized against Clostridium difficile toxins A and B. Microb Ecol Health Dis. 1989;2:47–59. [Google Scholar]
- 19.Kink J A, Williams J A. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998;66:2018–2025. doi: 10.1128/iai.66.5.2018-2025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozlowski P A, Cu-uvin S, Neutra M R, Flanagan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krivan H C, Clark G F, Smith D F, Wilkins T D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect Immun. 1986;53:573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung D Y, Kelly C P, Boguniewicz M, Pothoulakis C, LaMont J T, Flores A. Treatment with intravenously administered gamma globulin of chronic colitis induced by Clostridium difficile toxin. J Pediat. 1991;118:633–637. doi: 10.1016/s0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 23.Libby J M, Jortner B S, Wilkins T D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982;36:822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linevsky J K, Pothoulakis C, Keats S, Warny M, Keats A C, LaMont J T, Kelly C P. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am J Physiol. 1997;273(Gastrointest Liver Physiol. 6):G1333–G1340. doi: 10.1152/ajpgi.1997.273.6.G1333. [DOI] [PubMed] [Google Scholar]
- 25.Lyerly D M, Sullivan N M, Wilkins T D. Enzyme-linked immunosorbent assay for Clostridium difficile. J Clin Microbiol. 1983;17:72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyerly D M, Saum K E, MacDonald D K, Wilkins T D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyerly D M, Bostwick E F, Binion S B, Wilkins T D. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991;59:2215–2218. doi: 10.1128/iai.59.6.2215-2218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manetti R, Massari P, Marchetti M, Magagnoli C, Nuti S, Lupetti P, Ghiara P, Rappuoli R, Telford J L. Detoxification of the Helicobacter pylori cytotoxin. Infect Immun. 1997;65:4615–4619. doi: 10.1128/iai.65.11.4615-4619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McFarland L V, Mulligan M E, Kwok R Y, Stamm W E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 30.Miller P D, Pothoulakis C, Baeker T R, LaMont J T, Rothstein T L. Macrophage-dependent stimulation of T cell depleted spleen cells by Clostridium difficile toxin A and calcium ionophore. Cell Immunol. 1990;126:155–163. doi: 10.1016/0008-8749(90)90308-e. [DOI] [PubMed] [Google Scholar]
- 31.Mitty R D, LaMont J T. Clostridium difficile diarrhea: pathogenesis, epidemiology and treatment. Gastroenterologist. 1994;2:61–69. [PubMed] [Google Scholar]
- 32.Pier G P, Meluleni G, Goldberg J B. Clearance of Pseudomonas aeruginosa from the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect Immun. 1995;63:2818–2825. doi: 10.1128/iai.63.8.2818-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pothoulakis C, Sullivan R, Melnick D A, Triadafilopoulos G, Gadenne A-S, Meshulam T, LaMont J T. Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granuolocytes. J Clin Invest. 1988;81:1741–1745. doi: 10.1172/JCI113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price S B, Phelps C J, Wilkins T D, Johnson J L. Cloning of the carbohydrate-binding portion of the toxin A gene of Clostridium difficile. Curr Microbiol. 1987;16:55–60. [Google Scholar]
- 35.Reigler M, Sedivy R, Pothoulakis C, Hamilton G, Zacheri J, Bishof G, Consentini E, Feil W, Schlessel R, LaMont J T, Wenzel E. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Investig. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley T V, Codde J P, Rouse I L. Increased length of hospital stay due to Clostridium difficile associated diarrhoea. Lancet. 1995;345:455–456. doi: 10.1016/s0140-6736(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 37.Robbins J B, Schneerson R, Szu S C. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 38.Siffert J-C, Baldacini O, Kuhry J G, Wachsmann D, Benabdelmoumene S, Faradji A, Monteil H, Poindron P. Effects of Clostridium difficile toxin B on human monocytes and macrophages: possible relationship with cytoskeletal rearrangement. Infect Immun. 1993;61:1082–1090. doi: 10.1128/iai.61.3.1082-1090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres J F, Lyerly D M, Hill J E, Monath T P. Evaluation of formalin-inactivated Clostridium difficile vaccine administered by parenteral and mucosal routes of immunization in hamsters. Infect Immun. 1995;63:4619–4627. doi: 10.1128/iai.63.12.4619-4627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres J F, Thomas W D, Lyerly D M, Giel M A, Hill J E, Monath T P. Clostridium difficile vaccine: influence of different adjuvants and routes of immunization on protective immunity in hamsters. Vaccine Res. 1996;5:149–162. [Google Scholar]
- 41.Viscidi R, Laughon B E, Yolken R, Bo-Linn P, Moench T, Ryder P W, Bartlett J G. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]
- 42.von Eichel-Streiber C, Boquet P, Sauerborn M, Thelestam M. Large clostridial cytotoxins—a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol. 1996;4:375–382. doi: 10.1016/0966-842X(96)10061-5. [DOI] [PubMed] [Google Scholar]
- 43.Wada N, Nishida N, Iwaki S, Ohi H, Mikawaki T, Taniguchi N, Migita S. Neutralizing activity against Clostridium difficile toxin in the supernatants of cultured colostral cells. Infect Immun. 1980;29:545–550. doi: 10.1128/iai.29.2.545-550.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warny M, Vaerman J P, Avesani V, Delmee M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1994;62:384–389. doi: 10.1128/iai.62.2.384-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warny M, Denie C, Delmee M, Lefebvre C. Gamma globulin administration in relapsing Clostridium difficile-induced pseudomembranous colitis with a defective antibody response to toxin A. Acta Clin Belg. 1995;50:36–39. doi: 10.1080/17843286.1995.11718419. [DOI] [PubMed] [Google Scholar]
- 46.Wershil B K, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 1998;114:956–964. doi: 10.1016/s0016-5085(98)70315-4. [DOI] [PubMed] [Google Scholar]
- 47.Wu H-Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]