Abstract
Purpose
Acute respiratory distress syndrome (ARDS) is an acute and critical disease among children and adults, and previous studies have shown that the administration of corticosteroids remains controversial. Therefore, a meta-analysis of randomized controlled trials (RCTs) was performed to evaluate the safety and efficacy of corticosteroids.
Methods
The RCTs investigating the safety and efficacy of corticosteroids in ARDS were searched from electronic databases (Embase, Medline, and the Cochrane Central Register of Controlled Trials). The primary outcome was 28-day mortality. Heterogeneity was assessed using the Chi square test and I2 with the inspection level of 0.1 and 50%, respectively.
Results
Fourteen RCTs (n = 1607) were included for analysis. Corticosteroids were found to reduce the risk of death in patients with ARDS (relative risk (RR) = 0.78, 95% confidence interval (CI): 0.70–0.87; P < 0.01). Moreover, no significant adverse events were observed, compared to placebo or standard support therapy. Further subgroup analysis showed that variables, such as adults (RR = 0.78; 95% CI: 0.70–0.88; P < 0.01), non-COVID-19 (RR = 0.71; 95% CI: 0.62–0.83; P < 0.01), methylprednisolone (RR = 0.70; 95% CI: 0.56–0.88; P < 0.01), and hydrocortisone (RR = 0.79; 95% CI: 0.63–0.98; P = 0.03) were associated with 28-day mortality among patients who used corticosteroids. However, no association was found, regarding children (RR = 0.21; 95% CI: 0.01–4.10; P = 0.30).
Conclusion
The use of corticosteroids is an effective approach to reduce the risk of death in ARDS patients. However, this effect is associated with age, non-COVID-19 diseases, and methylprednisolone and hydrocortisone use. Therefore, evidence suggests patients with age ≥ 18 years and non-COVID-19 should be encouraged during the corticosteroid treatment. However, due to substantial differences in the use of corticosteroids among these studies, questions still remain regarding the dosage, optimal corticosteroid agent, and treatment duration in patients with ARDS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-022-02186-4.
Keywords: Acute respiratory distress syndrome, Corticosteroids, Children, Adults, Meta-analysis
Introduction
Acute respiratory distress syndrome (ARDS) is a serious condition, which is characterized as refractory hypoxemia caused by various factors and is usually secondary to a variety of lung diseases or extrapulmonary conditions, such as pneumonia, drowning and non-pulmonary sepsis [1]. It could lead to respiratory failure, critical illness, and even death, particularly in children [2,3]. A previous study showed that ARDS is estimated to be prevalent in 10.4% of patients requiring intensive care unit (ICU) care [4]. Although mechanical ventilation and fluid management have proved their efficacy for ARDS treatment in recent history, effective treatment remains a challenge, such as tidal volume, high positive end-expiratory pressure, advanced infection, and fewer drugs [5–7].
The concept of ARDS was first proposed in 1967 by Ashbaugh et al. [8]. ARDS is known as an excessive inflammatory response which is caused by damage to the alveolar capillary endothelial cells and alveolar epithelial cells, resulting in pulmonary interstitial edema [4], alveolar edema, airway occlusion, and alveolar atrophy [1,9–11]. Corticosteroids were considered as one of the potential therapeutic drugs[8] as early use of corticosteroids were shown to reduce systemic inflammatory response and accelerate the recovery of pulmonary infection [4]. This may be related to the role of glucocorticoids, which can inhibit the synthesis of cytokines and reduce the proliferation and regulation of T cells, macrophages and others [12].
Currently, as few as 30–35% of children diagnosed with ARDS are administered steroids in clinical practice [13]. However, in adults, many randomized controlled trials (RCTs) using corticosteroids have been performed [14,15]. Unfortunately, there is still a lack of conclusive evidence and guidelines for the use of corticosteroids in ARDS patients [16]. This may be due to the conflicting data between recent and previous studies [17–22], and a significant heterogeneity being found among the results of the RCTs [18,23–27]. In addition, the efficacy of corticosteroids has been found to be associated with several clinical variables, and in some cases, corticosteroids are not recommended routinely [1,28].
Therefore, the safety and efficacy of corticosteroids are still unclear. Hence, in this study, we performed a systematic review and meta-analysis to assess the safety and efficacy of corticosteroid administration in patients with ARDS, compared to those without corticosteroids. In addition, subgroup analysis of glucocorticoid regarding age, etiology, type of corticosteroids, and treatment duration was also performed.
Material and methods
Databases and search strategy
The study was registered on PROSPERO (CRD42022314505) and performed according to the PRISMA statement [29]. We systematically searched Embase, Medline, and the Cochrane Central Register of Controlled Trials (from 1963 to March 15th, 2022). The terms, including corticosteroids, hydrocortisone, methylprednisolone, dexamethasone, acute respiratory distress syndrome, ARDS, and randomized controlled trial, were searched alone or in combination without language restriction. Only human studies were included and the search strategy is detailed in the Supplementary Materials.
Eligibility criteria
Eligible RCT studies were searched and selected for analysis according to the criteria for participants, interventions, comparators, outcomes, and study design. Patients were eligible if they were diagnosed with ARDS (based on manifestations [8], the American-European Consensus Conference and the Berlin Definitions [3,7]) and aged > 28 days (onset). The intervention included any corticosteroid treatment and the comparator included standard supportive care (such as mechanical ventilation, antibiotics, and fluid replacement) and placebo administration [1].
Two independent investigators (X.C. and S.L.) screened the titles and abstracts of the included articles. Any discrepancies between the two investigators were resolved through discussion, or decided by the third investigator (C.L.). When the data of the included articles was missing, the corresponding authors were contacted to obtain the data. The risk of bias and methodological quality were assessed using the Cochrane Risk of Bias Tool 2.0 [30].
Data collection process
The primary outcome was 28-day mortality. In-hospital or ICU mortality was used to calculate the pooled 28-day mortality, unless actual 28-day mortality rates were reported or obtained from primary authors [31]. Secondary outcomes included ICU mortality, in-hospital mortality, 60-day mortality, length of ICU stay, length of in-hospital stay, and ventilation-free days to day 28. Adverse events included hyperglycemia and gastroduodenal bleeding. Patients with chronic diseases requiring long-term corticosteroid and immunosuppressive therapy were excluded. Data was collected by the two investigators (X.C. and S.L.) separately using a standardized table and any discrepancy was resolved through discussion, or decided by the third investigator (C.L.).
Subgroup analysis
Subgroup analysis was performed on the following variables: children/adults (children, < 18 years, and > 28 days; adults, ≥ 18 years); etiology (Coronavirus disease 2019 (COVID-19) or non-COVID-19); corticosteroid type (hydrocortisone, dexamethasone, methylprednisolone, etc.); treatment duration (≤ 7 days, 8–14 days, and ≥ 15 days); and methylprednisolone dose (high, > 2 mg/kg/d; low, ≤ 2 mg/kg/d).
Data synthesis
The statistical analysis was performed using Review Manager, version 5.4.1 (Cochrane Collaboration), and Stata, version 16.0 (College Station, Texas, USA). Heterogeneity was assessed using the Chi square (Chi2) test and the I2 test with an inspection level of 0.1 and 50%, respectively. If the I2 was < 50% and Chi2 > 0.1, fixed-effects models were employed for the analysis. Otherwise, random-effects models were used. When there was a difference between the two, the Chi2 test results follow the I2. Dichotomous data was analyzed using the Mantel–Haenszel method, and the pooled risk ratios (RR) and corresponding 95% confidence intervals (CI) were then calculated. Continuous data was analyzed using the Inverse Variance method and expressed as mean difference (MD) and 95% CIs. If only median and interquartile range were available, mean and standard deviation were estimated using the Mean–Variance Estimation [32]. Potential publication bias was assessed by funnel plot, Egger's test, and Begg's test [33].
Results
Study selection and study characteristics
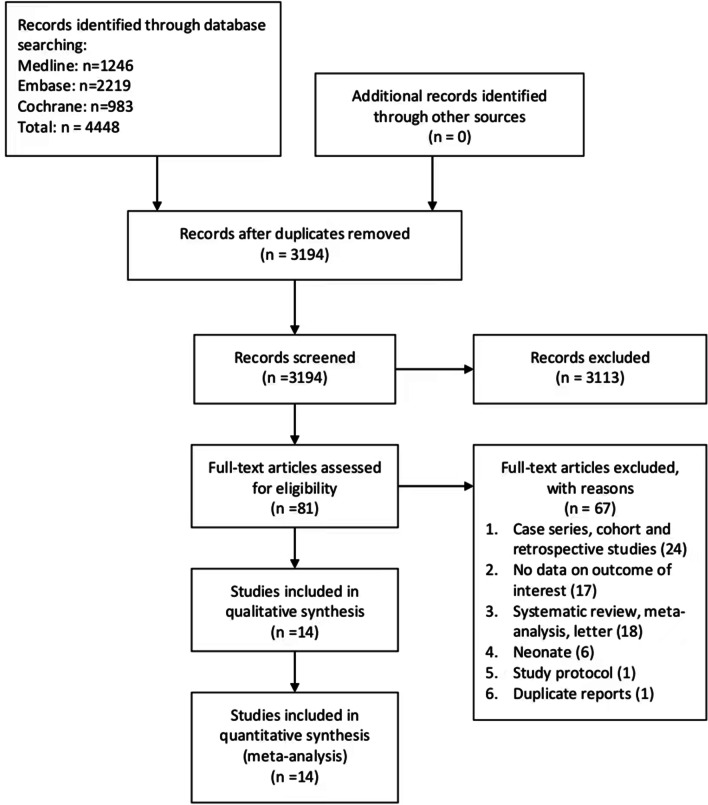
A total of 4448 potentially eligible records were found through a comprehensive literature search (Additional file 1: Table S1). The duplication was performed using MedRef (Jinyetiancheng, Beijing, China) [34]. After excluding duplicates and reviewing the titles and abstracts, 81 studies remained in the study. After reading the full text, 14 studies [14,15,17,18,23–26,35–40] met the eligibility criteria, were included for the final analysis and had a sample size ranging from 24 to 393 (Fig. 1).
Fig. 1.
Selection process for RCTs included for the analysis
The 14 studies were published between 1963 and 2022, including 1607 participants. Of these, nine studies were multicenter [14,17,18,23,25,26,37–39], ten studies used placebo as controls and another four studies used standard therapy [15,17,18,35] Seven studies [14,23,25,26,35,39,40] used methylprednisolone, four [24,36–38] used hydrocortisone, and three [15,17,18] used dexamethasone. Treatment duration ranged from 1 to 32 days. The corticosteroid dose varied widely among the included studies, ranging from 0.125 to 120 mg/kg/d of methylprednisolone (or equivalent; Additional file 2: Table S2).
Risk of bias and quality of evidence
First, seven studies were considered as having a low risk of bias [14,18,24–26,38,39]. Second, another seven studies were assessed as having low risk, regarding “deviations from intended interventions”, “missing outcome data”, “measurement of the outcome”, and “selection of the reported results category”. Among them, allocation concealments were not clearly described in six studies, which were assessed as unclear risk [15,23,35–37,40]. In addition, there was an open RCT without allocation concealment, which was considered to have a high risk of bias [17] (Additional file 1: Fig. S1).
Primary outcomes: 28-day mortality
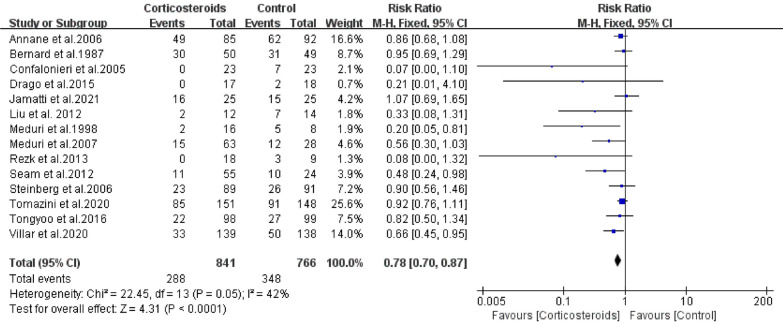
Twenty-eight-day mortality was reported in fourteen studies (n = 1607). Overall, 28-day mortality was reported in 34.2% of those taking corticosteroids and 45.4% of those without corticosteroid use. Hence, the RR is estimated at 0.78 (95% CI: 0.70–0.87; P < 0.01), which revealed an association between corticosteroid therapy and 28-day mortality (Fig. 2). Therefore, it was demonstrated that corticosteroid use could decrease the risk of 28-day mortality. No heterogeneity between the studies was found according to Chi2 following to I2 (22.45 and 42%, respectively).
Fig. 2.
The effect of corticosteroids on Mortality at 28 days among patients with ARDS
Secondary outcomes
Four studies [14,18,37,40] reported ICU mortality, five studies [18,23,25,37,38] reported in-hospital mortality, and four studies [14,18,24,38] reported 60-day mortality. Corticosteroids were associated with in-hospital mortality (RR = 0.68, 95% CI: 0.47–0.98; P = 0.04) and ICU mortality (RR = 0.67, 95% CI: 0.45–0.98; P = 0.04), but not 60-day mortality (RR = 0.73; 95% CI: 0.50–1.06; P = 0.1). Therefore, we found that corticosteroid use could decrease the risk of in-hospital and ICU mortality. However, heterogeneity existed between the studies reporting on in-hospital mortality (Chi2 = 8.33, I2 = 52%), ICU mortality (Chi2 = 6.96, I2 = 57%), and 60-day mortality (Chi2 = 6.3, I2 = 52%) (Additional file 1: Fig. S2).
Six studies [[[[17,18,23–25,36]]]] (n = 925) provided data on ventilation-free days at 28 days. Pooled analysis suggested a significant increase of ventilation-free days at day 28 (MD = 3.53 days; 95% CI: 2.32–4.74; P < 0.01), and no heterogeneity among studies (Chi2 = 7.26, I2 = 31%) was found (Additional file 1: Fig. S3). This means that the corticosteroid used could reduce the dependence of ventilation. There was no association between corticosteroid use and duration of hospital stay (MD = − 4.19 days; 95% CI: − 13.49–5.12; P = 0.38) and ICU stay (MD = -2.90 days; 95% CI: − 10.42–4.62; P = 0.45). However, high heterogeneity among studies was found in in-hospital stay (Chi2 = 84.26, I2 = 96%) and ICU stay (Chi2 = 219.62, I2 = 99%) (Additional file 1: Fig. S4).
Adverse events
The incidence of hyperglycemia (RR = 1.11; 95% CI: 1.0–1.23, P = 0.05) and gastroduodenal bleeding (RR = 1.34; 95% CI: 0.51–3.51; P = 0.56) was not statistically different between the groups. There was no heterogeneity found among the studies in hyperglycemia (Chi2 = 2.41, I2 = 0%) and gastroduodenal bleeding (Chi2 = 1.35, I2 = 0%) (Additional file 1: Fig. S5).
Sensitivity analysis and publication bias
Subgroup analysis
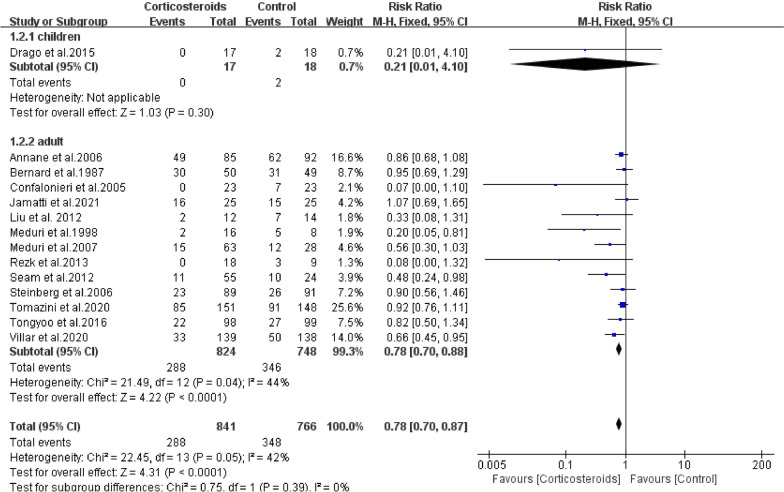
Adults/children: Ten studies included adult patients (n = 841, glucocorticoid group; n = 766, control group) and one included children (n = 17, glucocorticoid group; n = 18, control group). A fixed-effect model was used and the pooled data from the meta-analysis showed a significant association between mortality and glucocorticoid use in the adult subgroup (RR = 0.78; 95% CI: 0.70–0.88; P < 0.01), and there was no heterogeneity among included studies (Chi2 = 21.49, I2 = 44%). However, no significant association was found in the children subgroup (RR = 0.21; 95% CI: 0.01–4.10; P = 0.30) (Fig. 3).
Fig. 3.
The effect of corticosteroids on Mortality at 28 days. Studies subdivided by adults and children
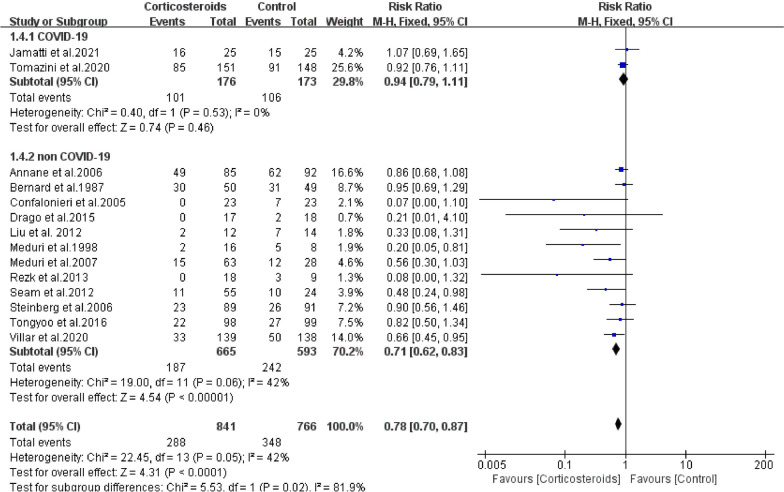
Etiology: Two studies (n = 176, glucocorticoid group; n = 173, control group) included ARDS patients with COVID-19, and 12 studies (n = 665, glucocorticoid group; n = 593, control group) included ARDS patients without COVID-19. No beneficial effect of glucocorticoid treatment was found in the COVID-19 subgroup (RR = 0.94; 95% CI: 0.79–1.11; P = 0.46). Whereas, in the non-COVID-19 subgroup, corticosteroids were found to significantly reduce patient’s risk of mortality (RR = 0.71; 95% CI: 0.62–0.83; P < 0.01). Heterogeneity was not found among the studies, including in the COVID-19 subgroup (Chi2 = 0.4, I2 = 0%) and the non-COVID-19 subgroup (Chi2 = 19.0, I2 = 42%) (Fig. 4).
Fig. 4.
The effect of corticosteroids on Mortality at 28 days. Studies subdivided by COVID-19 status
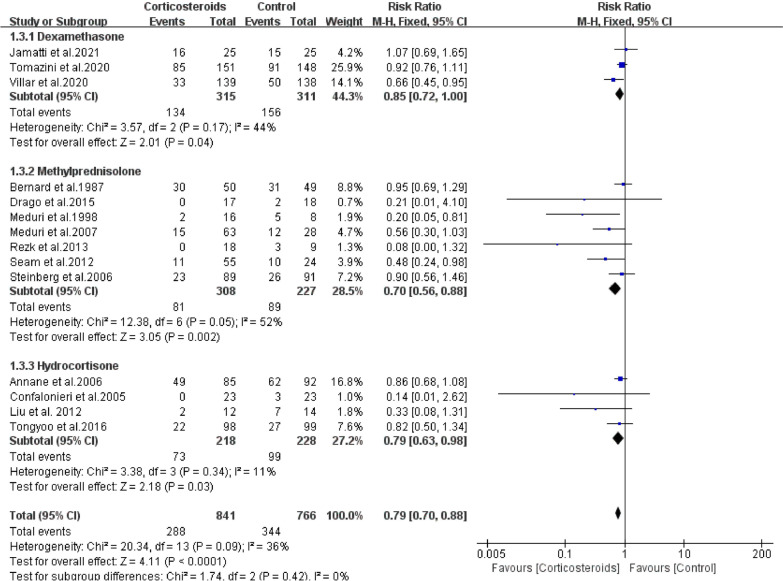
Corticosteroid type: Methylprednisolone (RR = 0.70; 95% CI: 0.56–0.88; P = P < 0.01) was used in seven RCTs (n = 535) and hydrocortisone (RR = 0.79; 95% CI: 0.63–0.98; P = 0.03) in four RCTs (n = 446), both of which were found to significantly improve outcomes. However, three RCTs (n = 626) of dexamethasone (RR = 0.85; 95% CI: 0.72–1.00; P = 0.04) demonstrated a negative result. There was no heterogeneity among the studies, including hydrocortisone (Chi2 = 3.38, I2 = 11%) and dexamethasone (Chi2 = 3.57, I2 = 44%). However, heterogeneity exists among the studies in methylprednisolone (Chi2 = 12.38, I2 = 52%) (Fig. 5).
Fig. 5.
The effect of corticosteroids on Mortality at 28 days. Studies subdivided by corticosteroids types
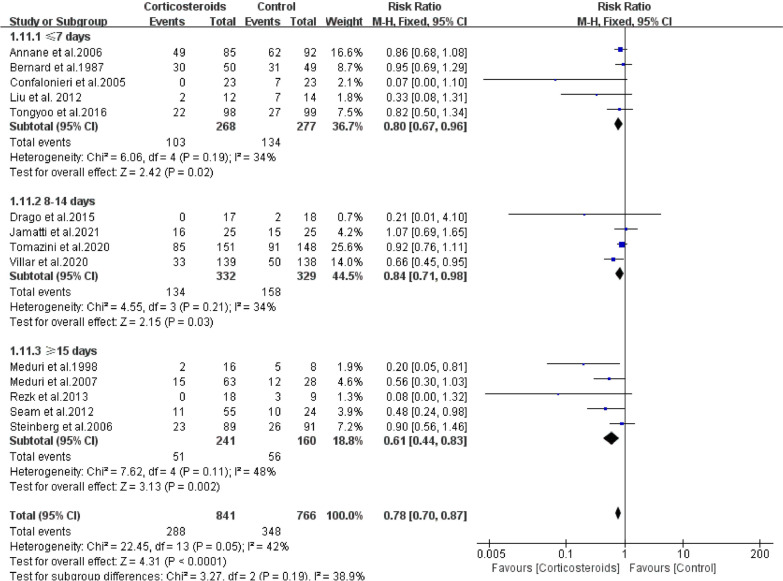
Treatment duration: Our data suggested that glucocorticoids use ≤ 7 days (RR = 0.80; 95% CI: 0.67–0.96; P = 0.02), 8–14 days (RR = 0.84; 95% CI: 0.71–0.98; P = 0.03) and ≥ 15 days (RR = 0.61; 95% CI: 0.44–0.83; P < 0.01) could significantly reduce risk of mortality among patients using corticosteroid. No heterogeneity among studies was found in ≤ 7 days (Chi2 = 6.06, I2 = 34%), 8–14 days (Chi2 = 4.55, I2 = 34%) and ≥ 15 days (Chi2 = 7.62, I2 = 48%) (Fig. 6).
Fig. 6.
The effect of corticosteroids on Mortality at 28 days. Studies subdivided by treatment duration of corticosteroids
Dose: Since methylprednisolone is known to have a very high efficacy, a secondary analysis of methylprednisolone dose was performed. We found that a high-dose of methylprednisolone (> 2 mg/kg/d) did not affect patient mortality (RR = 0.95; 95% CI: 0.69–1.29; P = 0.74), whereas a low-dose of methylprednisolone (≤ 2 mg/kg/d) could significantly reduce patient’s risk of mortality (RR = 0.53; 95% CI: 0.33–0.86; P = 0.01). No significant heterogeneity was found among studies, including low-dose (Chi2 = 8.15, I2 = 39%) (Additional file 1: Fig. S6).
Treatment duration: Based on the methylprednisolone data, compared to short treatment duration (≤ 14 days) (RR = 0.90, 95% CI: 0.65–1.23; P = 0.49), long treatment duration (≥ 15 days) was more effective to reduce the risk of mortality (RR = 0.61, 95% CI: 0.44–0.83; P = P < 0.01). No significant heterogeneity among studies was found in either the short treatment duration (Chi2 = 1.04, I2 = 4%) and long treatment duration (Chi2 = 7.62, I2 = 48%) (Additional file 1: Fig. S7).
Publication bias
Publication bias was assessed using Egger’s test (P = 0.052) and Begg’s test (P = 0.119), and no evidence was found (Additional file 1: Fig. S8).
Discussion
Compared to a previous meta-analysis published in 2021 [21], the present study has several advantages as it has six additional RCTs and contains children and participants with COVID-19. Moreover, the sample size of our study was relatively large and the research was of high quality. In the present study, we found that corticosteroids could significantly reduce the 28-day mortality of ARDS patients, which is inconsistent with several previous meta-analyses [19,26,41]. Corticosteroid use can improve ICU mortality, in-hospital mortality, and ventilation-free days at 28 days, and these beneficial effects have also been confirmed by other studies [21]. In addition, glucocorticoid use could potentially increase the risk of hyperglycemia (but not gastroduodenal bleeding) among ARDS patients, which is consistent with findings by Fang et al. [31]. Therefore, we recommend that blood glucose should be monitored in ARDS patients who receive glucocorticoids. Subgroup analysis showed that the efficacy of corticosteroids in ARDS patients was associated with several variables, such as age, etiologies, corticosteroid type and dosage, and courses of treatment. Therefore, ARDS patients could benefit from steroid therapy.
Various efficacy of glucocorticoid treatment have been observed in children and adults with ARDS. First, there are a limited number of studies investigating the role of glucocorticoid therapy for ARDS in children. Most treatment choices for children with ARDS are made based on the experience of adult ARDS treatment, such as mechanical ventilation, prone position ventilation, drug therapy, and others [42]. Second, the corticosteroid treatment is not recommended in children with ARDS according to the current guidelines[43]. Our findings in this study confirmed that corticosteroid therapy is beneficial to adults with ARDS, but not children. An RCT of children with ARDS (n = 35) was included in our study [23]. The results showed that although glucocorticoids increased the oxygenation index (PaO2/FiO2) on the 8th and 9th days, they did not reduce mortality. The different efficacy of glucocorticoid treatment in children with ARDS compared to adults with ARDS may be explained by the different disease patterns, immune responses, and lung growth/development abilities [23]. Therefore, current evidence does not support corticosteroid treatment for the management of children with ARDS, and further investigation is required to fill this gap in the research. In addition, previous studies have revealed that corticosteroids may affect growth and metabolism [44]. As a result, caution is required for the indication of corticosteroid therapy.
The effect of corticosteroid treatment is different between non-COVID-19 and COVID-19 patients. In non-COVID-19 ARDS patients, corticosteroids can reduce mortality in the experimental group, while no improvements in mortality were observed in patients with COVID-19. Similar results were obtained by Baek et al. [45,46]. However, this finding conflicts with the current recommendation of steroid treatment for ARDS caused by COVID-19 [47]. The heterogeneous results may be caused by the disease severity and the different types of glucocorticoids used in patients with COVID-19.
Each corticosteroid treatment identified had a varying effect on ARDS patients. In the past, when assessing the efficacy of corticosteroids, most studies target the dose and duration of corticosteroid treatment [20,21], and ignore the specific type of corticosteroid. In the subgroup analysis of our study, it was found that methylprednisolone and hydrocortisone could reduce the mortality of ARDS patients, with methylprednisolone performing particularly well. This effect difference may be explained by the fact that methylprednisolone has a longer in vivo residence time and relatively high concentration in the lung when compared to hydrocortisone [48]. However, dexamethasone does not show a significant effect, although it appears to reduce mortality. In general, our data suggests that different types of glucocorticoids could lead to variable effects on the outcome of ARDS patients. Selecting the appropriate glucocorticoid treatment should be dependent on the patient’s condition.
The use of corticosteroids is always a complex problem in practice. Long-term administration could increase the risk of hyperglycemia and make the disease worse [31]. Hence, an appropriate duration of corticosteroid treatment is required. Our data showed that corticosteroid treatment with a duration of ≤ 7 days, 8–14 days, or ≥ 15 days, could significantly reduce the mortality of ARDS patients. To avoid adverse events, a short duration of corticosteroid treatment is therefore recommended based on our findings. However, a low-dose and long-course of methylprednisolone was found to reduce the mortality of the experimental group significantly, and similar results were confirmed in previous studies [22,48].
The recommended duration is inconsistent which could be explained by the heterogeneity due to different corticosteroids used (hydrocortisone and dexamethasone vs methylprednisolone). The metabolic pathway of the two glucocorticoids (hydrocortisone and dexamethasone) is different to that of methylprednisolone [49], resulting in a different conclusion. Meanwhile, the data confirms the importance of selecting the correct corticosteroid. Unfortunately, the choice of corticosteroid for the management of ARDS patients is still uncertain and further assessments are needed to evaluate the indicators (such as clinical phenotype, immunology, and ARDS treatment) for each corticosteroid.
Limitations
Although several interesting findings were found, the study also has some limitations. First, due to the nature of systematic reviews and meta-analyses, heterogeneity is an inherent disadvantage, and several variables (such as treatment protocol, dosage, and types of corticosteroids used) varied widely across the studies. Second, the sample size is small, adverse events are infrequent, and the insufficient data makes the assessment of adverse events between subgroups difficult. In addition, due to the short follow-up period of RCTs, the long-term adverse effects of corticosteroid use were neither evaluated nor recorded. Further observational studies are needed to provide more details regarding the potential long-term effects of corticosteroid use in ARDS patients.
Conclusions
Corticosteroids are an effective approach to reduce the risk of death in ARDS patients. However, this effect is associated with age, non-COVID-19 diseases, and methylprednisolone and hydrocortisone use. Therefore, evidence suggests patients with age ≥ 18 years and non-COVID-19 should be encouraged during the corticosteroid treatment. However, due to substantial differences in the use of corticosteroids among these studies, questions still remain regarding the dosage, optimal corticosteroid agent, and treatment duration in patients with ARDS. Therefore, further investigation is required.
Supplementary Information
Additional file 1: Table S1. Search strategy. Figure S1. Assess risk of bias. A. Risk of bias summary; B. Risk of bias graph. Figure S2. The effect of corticosteroids on mortality in ICU, in hospital and 60-days. A. Mortality in ICU; B. Mortality in hospital; C. Mortality at 60-days. Figure S3. Ventilation-free days at day 28 among patients with ARDS. Figure S4. Duration of hospital stay and ICU stay among patients with ARDS. A. ICU stay; B. Hospital stay. Figure S5. Adverse events among patients with ARDS. A. Hyperglycemia; B. Gastroduodenal bleeding. Figure S6. The effect of corticosteroids on mortality at 28 days. Studies subdivided by different dosage of methylprednisolone. Figure S7. The effect of corticosteroids on mortality at 28 days. Studies subdivided by different treatment duration of methylprednisolone. Figure S8. Assess the potential publication bias. (A) Egger's test; (B) Begg’s test.
Additional file 2: Table S2. Characteristics of the 14 randomized clinical trials of corticosteroids in patients with ARDS.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- Chi2
Chi square
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- ICU
Intensive care unit
- MD
Mean difference
- RCTs
Randomized controlled trials
- RR
Risk ratios
Author contributions
XC contributed to the overall design, data collection, data processing and interpretation of the research project; SL participated in data collection, data processing and interpretation; CL participated in data collection, data processing and interpretation; HD and YF made important contributions to the interpretation of the research results. All authors read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
The data not published within the present study is available in a public repository for any of the participating studies. Requests for information on procedures, statistical analysis and formal data requests can be submitted to investigators (X.C.; S.L.)
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xinyan Chang, Email: xinyan1203@163.com.
Shaojun Li, Email: lishaojun1980@hotmail.com.
Yueqiang Fu, Email: fuyueqiang@sina.com.cn.
Hongxing Dang, Email: dhxdoc@163.com.
Chengjun Liu, Email: liucwd@163.com.
References
- 1.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–637. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 3.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012; 307(23): 2526–33. [DOI] [PubMed]
- 4.Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP. Nuclear factor-kappaB- and glucocorticoid receptor alpha-mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. NeuroImmunoModulation. 2005;12(6):321–338. doi: 10.1159/000091126. [DOI] [PubMed] [Google Scholar]
- 5.Determann RM, Royakkers A, Wolthuis EK, Vlaar AP. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):1–14. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan E, Del Sorbo L, Goligher EC, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 7.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1): 818–24. [DOI] [PubMed]
- 8.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 9.Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med. 2014;189(11):1301–1308. doi: 10.1164/rccm.201403-0535OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay MA, Zemans RL, Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. doi: 10.1146/annurev-physiol-030212-183756. [DOI] [PubMed] [Google Scholar]
- 12.Masjedi M, Esmaeil N, Saffaei A, et al. Cytokine Indexes in Pemphigus Vulgaris: perception of its immunpathogenesis and hopes for non-steroidal treatment. Iran J Pharm Res. 2017;16(3):1223–1229. [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca D, Piastra M, Chidini G, et al. The use of the Berlin definition for acute respiratory distress syndrome during infancy and early childhood: multicenter evaluation and expert consensus. Intensive Care Med. 2013;39(12):2083–2091. doi: 10.1007/s00134-013-3110-x. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 15.Jamaati H, Hashemian SM, Farzanegan B, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947. doi: 10.1016/j.ejphar.2021.173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutts S, Talboys R, Paspula C, Prempeh EM, Fanous R, Ail D. Adult respiratory distress syndrome. Ann R Coll Surg Engl. 2017;99(1):12–16. doi: 10.1308/rcsann.2016.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA J Am Med Assoc. 2020;324(13):1307. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar J, Ferrando C, Martinez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Nath A, Aggarwal AN, Gupta D. Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A meta-analysis. Respirology. 2007;12(4):585–590. doi: 10.1111/j.1440-1843.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 20.Lamontagne F, Briel M, Guyatt GH, Cook DJ, Bhatnagar N, Meade M. Corticosteroid therapy for acute lung injury, acute respiratory distress syndrome, and severe pneumonia: a meta-analysis of randomized controlled trials. J Crit Care. 2010;25(3):420–435. doi: 10.1016/j.jcrc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Lin P, Zhao Y, Li X, Jiang F, Liang Z. Decreased mortality in acute respiratory distress syndrome patients treated with corticosteroids: an updated meta-analysis of randomized clinical trials with trial sequential analysis. Crit Care. 2021;25(1):122. doi: 10.1186/s13054-021-03546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junhai Z, Bangchuan H, Shijin G, Jing Y, Li L. Glucocorticoids for acute respiratory distress syndrome: a systematic review with meta-analysis and trial sequential analysis. Eur J Clin Invest. 2021;51(6):e13496. doi: 10.1111/eci.13496. [DOI] [PubMed] [Google Scholar]
- 23.Drago BB, Kimura D, Rovnaghi CR, et al. Double-blind, placebo-controlled pilot randomized trial of methylprednisolone infusion in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med. 2015;16(3):e74–81. doi: 10.1097/PCC.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 24.Tongyoo S, Permpikul C, Mongkolpun W, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care. 2016;20(1):329. doi: 10.1186/s13054-016-1511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 26.Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317(25):1565. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 27.Mok YH, Lee JH, Rehder KJ, Turner DA. Adjunctive treatments in pediatric acute respiratory distress syndrome. Expert Rev Respir Med. 2014;8(6):703–716. doi: 10.1586/17476348.2014.948854. [DOI] [PubMed] [Google Scholar]
- 28.Pediatric acute respiratory distress syndrome consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang F, Zhang Y, Tang J, et al. Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(2):213–223. doi: 10.1001/jamainternmed.2018.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.SUN Wen-Ying-ge, MA Lu. Comparative analysis of reference management software. Chin J Med Libr Inf Sci. 2016; 25(9).
- 35.Rezk NA, Ibrahim AM. Effects of methyl prednisolone in early ARDS. Egypt J Chest Dis Tuberc. 2013 doi: 10.1016/j.ejcdt.2013.02.013. [DOI] [Google Scholar]
- 36.Liu L, Li J, Huang YZ, et al. The effect of stress dose glucocorticoid on patients with acute respiratory distress syndrome combined with critical illness-related corticosteroid insufficiency. Zhonghua Nei Ke Za Zhi. 2012;51(8):599–603. [PubMed] [Google Scholar]
- 37.Annane D, Sebille V, Bellissant E. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med. 2006;34(1):22. doi: 10.1097/01.ccm.0000194723.78632.62. [DOI] [PubMed] [Google Scholar]
- 38.Confalonieri M, Urbino R, Potena A, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171(3):242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 39.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA J Am Med Med Assoc. 1998;280(2):159. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 40.Seam N, Meduri GU, Wang H, et al. Effects of methylprednisolone infusion on markers of inflammation, coagulation, and angiogenesis in early acute respiratory distress syndrome. Crit Care Med. 2012;40(2):495. doi: 10.1097/CCM.0b013e318232da5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshihiro S, Hongo T, Ohki S, et al. Steroid treatment in patients with acute respiratory distress syndrome: a systematic review and network meta-analysis. J Anesth. 2022;36(1):107–121. doi: 10.1007/s00540-021-03016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu GP, Wang YX. Drug management of pediatric acute respiratory distress syndrome. Zhongguo Dang Dai Er Ke Za Zhi. 2018;20(9):697–700. doi: 10.7499/j.issn.1008-8830.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamburro RF, Kneyber MC. Pulmonary specific ancillary treatment for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5 Suppl 1):S61–72. doi: 10.1097/PCC.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 44.De Luca D, Piastra M, Tosi F, et al. Pharmacological therapies for pediatric and neonatal ALI/ARDS: an evidence-based review. Curr Drug Targets. 2012;13(7):906–916. doi: 10.2174/138945012800675687. [DOI] [PubMed] [Google Scholar]
- 45.Baek MS, Lee Y, Hong S, Lim C, Koh Y, Huh JW. Effect of corticosteroid therapy in the early phase of acute respiratory distress syndrome: a propensity-matched cohort study. Korean J Intern Med. 2021;36(1):145. doi: 10.3904/kjim.2019.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, Zhang S, Dong X, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130(12):6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA. 2020;324(13):1292–1295. doi: 10.1001/jama.2020.16747. [DOI] [PubMed] [Google Scholar]
- 48.Greos LS, Vichyanond P, Bloedow DC, Irvin CG, Hill MR. Methylprednisolone achieves greater concentrations in the lung than prednisolone. A pharmacokinetic analysis. Am Rev Respir Dis. 1991;144(3 Pt 1):586–592. doi: 10.1164/ajrccm/144.3_Pt_1.586. [DOI] [PubMed] [Google Scholar]
- 49.Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44(1):61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategy. Figure S1. Assess risk of bias. A. Risk of bias summary; B. Risk of bias graph. Figure S2. The effect of corticosteroids on mortality in ICU, in hospital and 60-days. A. Mortality in ICU; B. Mortality in hospital; C. Mortality at 60-days. Figure S3. Ventilation-free days at day 28 among patients with ARDS. Figure S4. Duration of hospital stay and ICU stay among patients with ARDS. A. ICU stay; B. Hospital stay. Figure S5. Adverse events among patients with ARDS. A. Hyperglycemia; B. Gastroduodenal bleeding. Figure S6. The effect of corticosteroids on mortality at 28 days. Studies subdivided by different dosage of methylprednisolone. Figure S7. The effect of corticosteroids on mortality at 28 days. Studies subdivided by different treatment duration of methylprednisolone. Figure S8. Assess the potential publication bias. (A) Egger's test; (B) Begg’s test.
Additional file 2: Table S2. Characteristics of the 14 randomized clinical trials of corticosteroids in patients with ARDS.
Data Availability Statement
The data not published within the present study is available in a public repository for any of the participating studies. Requests for information on procedures, statistical analysis and formal data requests can be submitted to investigators (X.C.; S.L.)