Abstract
Background
Unlike the numerous studies concerning the role of dietary inflammatory potential in chronic diseases, limited studies focused on the association of dietary inflammatory potential with handgrip strength (HGS) and probable sarcopenia (PS). This study tends to explore the association between dietary inflammatory potential and PS among older adults in Tehran.
Methods
The cross-sectional study was conducted between May and October 2019 on 201 randomly selected older adults in Tehran, Iran. A validated food frequency questionnaire was utilized for recording dietary intake. Dietary habits were evaluated through Dietary Inflammatory Index (DII) and Empirical Dietary Inflammatory Index (EDII). PS assessment was done by HGS estimation. Statistical evaluation included descriptive analyses, logistic, and linear regression.
Results
Those probably suffering from sarcopenia were older than healthy ones (p < 0.0001) and had significantly higher DII scores (p = 0.05) but not EDII (p = 0.85). Besides, PS subjects had a lower intake of anti-inflammatory nutrients. The odds of PS were doubled in people on the top tertile of DII (OR = 2.49, 95% (CI) = 1.11–5.58) and second tertile of EDII (OR = 2.29, 95% (CI) = 1.03–5.07) relative to bottom tertiles after adjusting for confounders. The relationships between index scores and HGS were simply significant in the adjusted model of EDII and HGS (B = -0.49, p = 0.04).
Conclusion
Conclusively, participants adhering to a pro-inflammatory diet had more likelihood of PS. Findings are in line with current recommendations to reduce unhealthy foods with more inflammatory potential. These findings warrant confirmation in high-quality interventional studies.
Keywords: Aging, Dietary inflammatory index, Empirical dietary inflammatory index, Handgrip strength, Probable sarcopenia
Introduction
Physical changes happen gradually as a consequence of aging. Loss of muscle strength and muscle mass are the most prevalent modifications after age 50 [1]. The reduction of 3% muscle strength and 1% muscle mass happens annually in adulthood which is the pathologic form of the decline called sarcopenia [2, 3]. Sarcopenia is a multifactorial, age-dependent disorder associated with a sedentary lifestyle and malnutrition [1]. Sarcopenia has different causes, which include age-related factors like decreased physical activity, anorexia of aging, low vitamin D, weight loss, and elevated pro-inflammatory cytokines [4]. In 2018, the European Working Group on Sarcopenia in Older People (EWGSOP) defined probable sarcopenia (PS) by low muscle strength as a powerful predictor of sarcopenia [5]. Muscle weakness increases the odds of falling and causes serious injuries in different parts of the body [6]. It could also predict poor patient outcomes e.g. prolonged hospitalization, poor health-related quality of life, and death, and is a frailty marker that increases the possibility of mobility limitation [5, 7, 8]. Sarcopenia progression could be prevented by the assessment of PS to provide applicable information about sarcopenia. In the current situation of global aging, the future increase in sarcopenia health costs is evident and some interventions have been required to decrease the loss of muscle mass or restore it in older adults [9]. Although the loss of muscle mass and decrease of muscle strength could occur due to aging, different grades of this reduction have been observed in the population. It shows that changeable habits like diet may have a role in the progression of sarcopenia [10, 11].
Assessing diet quality is one of the ways to demonstrate a person’s diet status. The Dietary Inflammatory Index (DII) and very recently Empirical Dietary Inflammatory Index (EDII) was developed by Shivappa (a priori) and Tabung et al. (a posteriori) to assess the inflammatory potential of a dietary pattern. A high score of these indexes has a significant association with increased serum and blood inflammatory markers [12, 13]. Furthermore, they evaluate the association between diet quality and chronic inflammatory outcomes like metabolic and pulmonary diseases, cancer, and fractures [14–17].
Several studies have considered the association of DII and EDII with a risk of different morbidities. Post-menopausal women with a high risk of osteoporosis tend to have a higher score of DII which indicates a pro-inflammatory diet [18]. The further risk of cardiovascular disorder, metabolic syndrome, hyperglycemia, and abdominal obesity were associated with a pro-inflammatory diet [16, 19]. The high risk of frailty was associated with a high score of DII in older adults [20]. On the other hand, few studies have focused on the association between dietary inflammatory potential and muscle weakness or sarcopenia. The pro-inflammatory diet, evaluated by DII, leads to high odds of sarcopenia and osteosarcopenic obesity [21, 22]. Low gait speed and increased risk of fractures were associated with the inflammatory potential of diet [17]. Cervo et al. suggested that a pro-inflammatory diet might be harmful to musculoskeletal health in men relative to women [23].
A rapid growth in the Iranian elderly population from 6.4% to 20.2% within 2019–2050 [24] turns the age-related complications (i.e. PS and sarcopenia) into a nationally important issue that needs particular emphasis. Despite the investigation of the association between dietary inflammatory potential and sarcopenia in various studies, none has assessed the association with PS. Hence, this cross-sectional study aimed to consider the association of dietary inflammatory potential, evaluated by DII and EDII, with PS in older residents of Tehran, Iran.
Methods and materials
This cross-sectional study was carried out on 201 randomly-selected older residents (60 years old ≤) of Tehran, Iran between May and October 2019. The sample size was defined according to type I error of α = 0.05 and type II error of β = 80%, thus, 191 overall subjects were needed for this study. Finally, 201 participants were included to further increase statistical power. Those with energy intake between 800–4200, no changes in their dietary habits over the last year, walking without any helping equipment, prosthetic or artificial limbs, and without an acute form of any disease were entered in the present study. For sampling, Tehran was divided into 5 regions: east, west, north, south, and city center. Details of the sampling process were described elsewhere [25]. After taking written consent from participants, their demographic and socioeconomic information, physical activity, and medical history were questioned by a standardized questionnaire. The physical activity was the amount of daily average time used to exercise, jog or do other sports which were estimated by participants. Socioeconomic status was defined by collecting data about education and economic state. Considering the possibility of refusals for declaring monthly income, a 9-item questionnaire (possession of house, car, side-by-side refrigerator, washing machine, dishwasher, laptop/personal computer, sofa, microwave, and handmade carpet) [26] was used in addition to querying about the house and car ownership for quantifying the economic status. The subjects’ economic status was classified as: Very bad: ≤ 3 items without any personal home and car. Bad: ≤ 3 items with personal home or car. 4–6 items without personal home and car. Average: 4–6 items with personal home or car. 7 items ≤ without a personal home and car. Good: 7 ≤ items with personal home or car. Very good: 7 items ≤ with personal home and car.
This study was approved by the ethics committee of Tehran University of Medical Sciences. The protocol number of the local ethics committee was IR.TUMS.VCR.REC.1398.476.
Anthropometric measurements
Waist, hip, and arm circumferences, weight, and height were measured in the current study. Body mass index (BMI), waist-to-hip ratio (WHR), and waist-to-height ratio (WHtR) were calculated in regard to the aforementioned measures. Weight (kg) and height (cm) were measured with light clothes and without shoes on with a Camry EB9011 scale (Camry Co, Zhongshan, China) and a Fiber-Glass tape measure, respectively. The measurement of a midpoint between the lower edge of the chest and the upper edge of the iliac crest and the last rib formed waist circumference (WC), and hip circumference was the maximum circumference of the hip when a participant stood firmly. The mid-upper arm circumference (MUAC) was the measured circumference of the scapular and olecranon midway of the non-dominant hand with the elbow flexed 90°. Circumferences were measured by a Fiber-Glass tape measure as well.
Participants with BMI lower than 23.5 and higher than 30.9 kg/m2 were underweight and obese, respectively [27]. Abdominal obesity was specified as waist circumference higher than 88 cm and 102 cm in women and men, respectively. Moreover, people with WHtR ≥ 0.6 and women with a WHR higher than 0.85 were classified as abdominally obese as well [28, 29]. Notably, WHR was not used for men due to cultural and religious matters.
Dietary data collection
A previously validated semi-quantitative food frequency questionnaire (FFQ) was utilized with 147 items for estimation of the usual dietary intake throughout last year [30]. Major items contained intake of bread and grains, legumes, meat, and meat-derived products, poultry, fish, eggs, dairies, kinds of butter, vegetables, pickles, fruits and fruit juices, oils, seeds, and nuts, added sugar, drinks, spices, and salt. The frequencies and portion sizes of each item were asked. Finally, the dietary intake quality was assessed by DII and EDII. Questionnaires were completed by trained dietitians.
Calculation of Dietary Inflammatory Index
DII was determined according to the approach suggested by Shivappa et al [12]. Considering the usage of the 147-item FFQ, 29 out of 45 components of DII were scored in this study which includes 24 nutrients, onion, garlic, turmeric, pepper, and tea. The DII scoring procedure is as follows: 1) Each component’s Z-score has been calculated based on the global mean and standard deviation which has been reported elsewhere [12], 2) The Z-score was converted to the percentile to minimize the effect of the right skewing, 3) The percentile value doubled and subtracted by 1 for computing the centered percentile, 4) Multiplying the centered percentile by the overall inflammatory effect score made each parameter’s DII score. Finally, the sum of all derived values forms the overall DII score.
Calculation of Empirical Dietary Inflammatory Index
Eighteen food groups form EDII following the Tabung et al. system [13] which wine and beer were not used to make EDII in the present study due to religious reasons. High- and low-energy beverages were considered as one food item in the FFQ, thus, 15 food parameters included in this study as inflammatory (processed meat, red meat, organ meat, other fish, other vegetables, refined grains, high-energy beverages, and tomatoes) and anti-inflammatory (dark yellow vegetables, leafy green vegetables, snacks, fruit juice, pizza, tea, and coffee) categories with more positive and negative scores, respectively. The mean daily intake of each food group was identified by defined serving sizes and weighted by the proposed regression coefficients. The weighted food group intakes were summed to constitute EDII and rescaled by dividing by 1000 to reduce the magnitude of the score for facilitating the interpretation.
Probable sarcopenia
As stated by EWGSOP2, handgrip strength (HGS) was evaluated as a surrogate measurement of muscle strength to determine PS [5]. A squeeze dynamometer (Saehan SH5008, Co, Seoul, Korea) was used as the HGS determinant. Participants sat on a chair with the arm bent at 90°; were asked to squeeze the dynamometer 3 times with the extreme force of each hand and held it for 10 s with 30 s rest between every attempt. Eventually, the average maximum power of each hand was ascertained as the participant’s HGS. Since the aforementioned dynamometer has not been used in former studies, the accuracy of the dynamometer was checked against a Jamar dynamometer, the gold standard for testing HGS [3]. The results of the squeeze dynamometer would comparable with the Jamar dynamometer if the amounts are multiplied by 1.6. Thereby, participants were defined to have a high probability of sarcopenia when the HGS was < 10 kg (women) and < 16.8 kg (men) in the present study.
Statistical analysis
DII and EDII were divided into tertiles to assess dietary quality. Normality distribution was checked using Kolmogorov–Smirnov’s test. Independent Student’s t-test and test was applied respectively to determine the significant differences of quantitative (Mean ± standard deviation (SD)) and qualitative variables (frequencies (%)) between the two groups (probably sarcopenic and non-sarcopenic). Age, gender, CVD medication, BMI, family number, and physical activity were adjusted to compare the mean-dependent variables by analysis of covariance (ANCOVA). A multiple linear regression model was performed to adjust for confounders of HGS to assess the actual relationship of DII and EDII with HGS. Finally, binary logistic regression was utilized for evaluating the association of adherence to DII and EDII with PS by adjusting the above-mentioned covariates. Statistical significance α was accepted at 0.05. Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA, version16) was used for all statistical analyses.
Results
Participant Characteristics
A total of 46 men (23%) and 155 women (77%) with a mean age of 66 years (ranging from 60 to 85) were included in this study. They had a daily physical activity of 32 min and a BMI of 29 kg/m2. The most common diseases among participants were cardiovascular diseases and skeletal disorders. PS subjects were older than healthy ones (67 vs 64 years, p < 0.0001) and constitute 61% of the total study population. Additionally, they had low MUAC (p = 0.02) and a worse economic state in relation to subjects with normal HGS (p = 0.002). The summary of the main characteristics of these participants was demonstrated in Table 1.
Table 1.
Participant characteristic
| Variables | Non-sarcopenic (N=78) |
Probable Sarcopenia (N=123) |
P-value* | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (year) | 63.9 | 3.66 | 67.54 | 5.94 | <0.0001 |
| Postmenopausal age (year) | 47.35 | 4.86 | 47.91 | 5.64 | 0.53 |
| Physical activity (min) | 37.20 | 42.3 | 29.64 | 42.3 | 0.07 |
| Weight (kg) | 74.16 | 9.96 | 72.2 | 11.56 | 0.22 |
| Height (m) | 1.59 | 0.08 | 1.58 | 0.09 | 0.17 |
| Waist circumference (cm) | 97.67 | 8.92 | 97.28 | 10.38 | 0.79 |
| MUAC (cm) | 32.5 | 2.09 | 31.56 | 3.01 | 0.02 |
| Body mass index (kg/m2) | 29.26 | 4.07 | 28.82 | 4.09 | 0.45 |
| WHtR | 0.61 | 0.07 | 0.61 | 0.08 | 0.7 |
| WHR a | 0.88 | 0.06 | 0.86 | 0.1 | 0.25 |
| HGS (Kg) | 13.29 | 3.52 | 9.16 | 3.03 | <0.0001 |
| N (%) | N (%) | ||||
| Gender | 0.12 | ||||
| Male | 14 (30.4) | 32 (69.6) | |||
| Female | 64 (41.3) | 91 (58.7) | |||
| Marital status | 0.16 | ||||
| Married | 61 (41.8) | 85 (58.2) | |||
| Other | 17 (27.33) | 38 (72.67) | |||
| Head of the family | 0.18 | ||||
| Father | 15 (32.6) | 31 (67.4) | |||
| Mother | 62 (41.9) | 86 (58.1) | |||
| Education | 0.14 | ||||
| High school or lower | 52 (36.1) | 92 (63.9) | |||
| University | 26 (45.6) | 31 (54.4) | |||
| Economic status | 0.002 | ||||
| Very bad | 7 (21.2) | 26 (78.8) | |||
| Bad | 20 (48.8) | 21 (51.2) | |||
| Average | 9 (25) | 27 (75) | |||
| Good | 9 (26.5) | 25 (73.5) | |||
| Very good | 32 (57.1) | 24 (42.9) | |||
| Supplements | |||||
| Vitamin D | 56 (40.9) | 81 (59.1) | 0.24 | ||
| Multivitamins | 32 (41) | 46 (59) | 0.36 | ||
| Minerals | 39 (39) | 61 (61) | 0.52 | ||
| Disorders | |||||
| Diabetes | 19 (38) | 31 (62) | 0.52 | ||
| Cardiovascular | 33 (29.5) | 79 (70.5) | 0.004 | ||
| Pulmonary | 7 (35) | 13 (65) | 0.46 | ||
| Renal | 4 (21.1) | 15 (78.9) | 0.07 | ||
| Skeletal | 46 (34.8) | 86 (65.2) | 0.08 | ||
| Psychological | 19 (31.7) | 41 (68.3) | 0.12 | ||
| Medication | |||||
| Diabetes | 14 (33.3) | 28 (66.7) | 0.28 | ||
| Cardiovascular | 25 (26) | 71 (74) | <0.0001 | ||
| Skeletal | 17 (32.7) | 35 (67.3) | 0.2 | ||
| Psychological | 11 (42.3) | 15 (57.7) | 0.41 | ||
| BMI Status | 0.49 | ||||
| Underweight | 4 (36.4) | 7 (63.6) | |||
| Normal | 50 (38.8) | 79 (61.2) | |||
| Overweight | 24 (39.3) | 37 (60.7) | |||
| WC Status. | 0.11 | ||||
| Normal | 17 (30.9) | 38 (69.1) | |||
| Abdominal obesity | 61 (41.8) | 85 (58.2) | |||
| WHtR status | 0.19 | ||||
| Normal | 31 (36.9) | 53 (63.1) | |||
| Abdominal obesity | 47 (40.2) | 70 (59.8) | |||
| WHR status a | 0.38 | ||||
| Normal | 23 (39) | 36 (61) | |||
| Abdominal obesity | 41 (42.7) | 55 (57.3) | |||
HGS Handgrip strength, SD standard deviation, BMI body mass index, WC waist circumference, MUAC Mid-upper arm circumference, WHtR Waist- to-height ratio, WHR Waist-to-hip ratio
*P ≤ 0.05; Student’s t-test was used for comparing the means difference of quantitative variables, X2 test was used for qualitative variables
a Calculated in women
Dietary inflammatory potential and hand grip strength
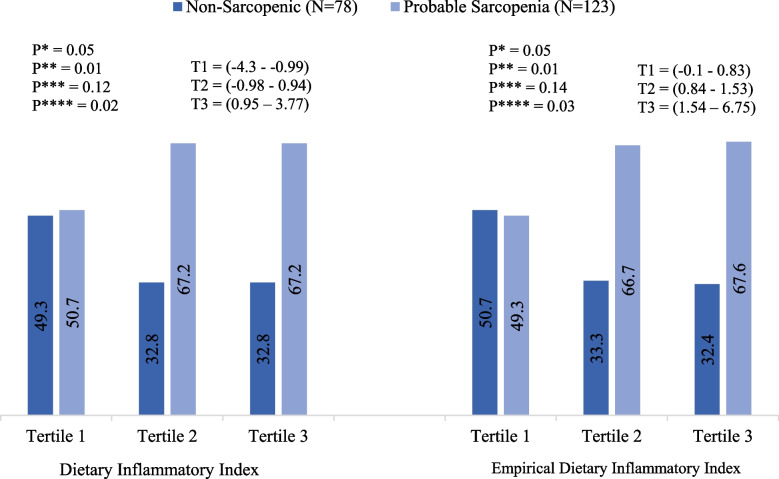
While mean DII scores varied significantly between non-sarcopenic (-0.3 (± 1.91)) and PS subjects (0.15 (± 1.85)) (p = 0.05), the association between EDII and PS remained insignificant (p = 0.85). Table 2 presents associations between components of the indexes across healthy and PS subjects. The probable-sarcopenic subjects had positive DII scores for saturated fats and thiamin, and a negative score for iron compared to healthy ones. None of the EDII components showed a significant association with PS. The prevalence of PS subjects was significantly reduced from 67.2% and 67.6% in the highest tertile of DII and EDII to 49.3% and 50.7% in the lowest tertile, respectively (Fig. 1).
Table 2.
DII, EDII, and the components scores across probable and non-sarcopenic subjects
| Variables (mean ± SD) |
HGS | P-value* | P-value** | |||
|---|---|---|---|---|---|---|
| Non-sarcopenic (N = 78) |
Probable Sarcopenia (N = 123) |
|||||
| Mean | SD | Mean | SD | |||
| Dietary Inflammatory Index | -0.3 | 1.91 | 0.15 | 1.85 | 0.1 | 0.05 |
| Total Energy | 0.003 | 0.11 | -0.0007 | 0.1 | 0.79 | 0.73 |
| Total Protein | 0.002 | 0.01 | -0.001 | 0.01 | 0.1 | 0.07 |
| Total Carbohydrate | 0.0003 | 0.06 | 0.0006 | 0.06 | 0.97 | 0.67 |
| Total Fat | -0.02 | 0.17 | 0.01 | 0.2 | 0.22 | 0.59 |
| Total Cholesterol | 0.006 | 0.07 | -0.003 | 0.06 | 0.3 | 0.13 |
| Total SFA | -0.04 | 0.2 | 0.03 | 0.2 | 0.02 | 0.06 |
| Total Iron | 0.004 | 0.02 | -0.002 | 0.02 | 0.02 | 0.02 |
| Total B12 | -0.001 | 0.06 | 0.002 | 0.06 | 0.72 | 0.72 |
| Total MUFA | 0.0001 | 0.005 | -0.0001 | 0.005 | 0.81 | 0.79 |
| Total PUFA | -0.006 | 0.2 | 0.0008 | 0.2 | 0.82 | 0.39 |
| Total Fiber | -0.04 | 0.39 | 0.02 | 0.38 | 0.25 | 0.39 |
| Total Magnesium | -0.04 | 0.3 | 0.02 | 0.27 | 0.13 | 0.18 |
| Total Zinc | -0.02 | 0.18 | 0.01 | 0.18 | 0.24 | 0.4 |
| Total Folate | -0.01 | 0.11 | 0.01 | 0.11 | 0.2 | 0.17 |
| Total Niacin | -0.02 | 0.15 | 0.01 | 0.14 | 0.07 | 0.07 |
| Total Riboflavin | -0.004 | 0.04 | 0.002 | 0.04 | 0.35 | 0.24 |
| Total Thiamin | -0.01 | 0.06 | 0.006 | 0.05 | 0.04 | 0.05 |
| Total Vitamin A | -0.01 | 0.24 | 0.005 | 0.23 | 0.59 | 0.44 |
| Total Vitamin C | -0.009 | 0.24 | 0.002 | 0.25 | 0.77 | 0.59 |
| Total Vitamin E | -0.01 | 0.25 | 0.004 | 0.24 | 0.65 | 0.27 |
| Total Vitamin D | -0.03 | 0.26 | 0.01 | 0.26 | 0.28 | 0.28 |
| Total Pyridoxine | -0.009 | 0.21 | 0.003 | 0.21 | 0.7 | 0.62 |
| Total Selenium | -0.01 | 0.11 | 0.008 | 0.11 | 0.16 | 0.4 |
| Garlic | 0.01 | 0.23 | -0.01 | 0.24 | 0.48 | 0.71 |
| Onion | 0.001 | 0.16 | -0.003 | 0.18 | 0.87 | 0.86 |
| Turmeric | -0.02 | 0.46 | 0.005 | 0.43 | 0.73 | 0.18 |
| Pepper | -0.003 | 0.08 | 0.0008 | 0.07 | 0.73 | 0.18 |
| Tea | 0.009 | 0.32 | -0.01 | 0.3 | 0.67 | 0.83 |
| Empirical Dietary Inflammatory Index | 1.36 | 1.17 | 1.42 | 0.91 | 0.69 | 0.85 |
| Processed Meat | 0.01 | 0.03 | 0.1 | 0.02 | 0.87 | 0.75 |
| Other Fish | 0.01 | 0.008 | 0.007 | 0.01 | 0.37 | 0.22 |
| Red Meat | 0.03 | 0.02 | 0.03 | 0.03 | 0.19 | 0.67 |
| Organ Meat | 0.0003 | 0.0008 | 0.0001 | 0.0003 | 0.12 | 0.08 |
| Grains | 1.1 | 1.12 | 1.14 | 0.9 | 0.78 | 0.94 |
| Other Vegetables | -0.04 | 0.35 | -0.38 | 0.26 | 0.42 | 0.11 |
| Tomatoes | 0.02 | 0.01 | 0.01 | 0.01 | 0.38 | 0.17 |
| High Energy Beverages | 0.01 | 0.03 | 0.02 | 0.07 | 0.11 | 0.13 |
| Leafy Green Vegetables | -0.11 | 0.2 | -0.09 | 0.07 | 0.24 | 0.32 |
| Dark Yellow Vegetables | -0.02 | 0.01 | -0.02 | 0.03 | 0.69 | 0.86 |
| Fruit Juice | -0.003 | 0.01 | -0.004 | 0.01 | 0.54 | 0.75 |
| Snacks | 0.42 | 0.007 | -0.009 | 0.04 | 0.29 | 0.28 |
| Tea | -0.05 | 0.05 | -0.04 | 0.04 | 0.75 | 0.62 |
| Pizza | -0.004 | 0.04 | -0.003 | 0.007 | 0.46 | 0.48 |
| Coffee | -0.05 | 0.04 | -0.03 | 0.12 | 0.27 | 0.32 |
DII Dietary Inflammatory Index, EDII Empirical Dietary Inflammatory Index, HGS Handgrip strength, SD standard deviation, SFA Saturated Fatty Acid, MUFA Mono-Unsaturated Fatty Acid, PUFA Poly- Unsaturated Fatty Acid
*P ≤ 0.05, Student’s t-test; **P ≤ 0.05, Analysis of covariance (ANCOVA), adjusted for age, family number, gender, CVD medication, BMI, and physical activity
Fig. 1.
Association between PS and tertiles of DII and EDII. The bars indicate the percentages DII: Dietary Inflammatory Index. EDII: Empirical Dietary Inflammatory Index. T: Tertile. P*: Differences between tertiles. P**: Differences between T1 and other tertiles. P***: Differences between T3 and other tertiles. P****: Differences between T1 and T3. P ≤ 0.05; X2 test.
Dietary inflammatory potential, hand grip strength, and confounders
Regarding the findings of multiple linear regression analyses (Table 3), there was a negative significant association between the DII score and HGS in the unadjusted model (adjusted R2 = 0.03, B = -0.37, p = 0.009), plus EDII score and HGS after adjusting for confounders (adjusted R2 = 0.52, B = -0.49, p = 0.04). Considering the adherence to indexes, those in the top tertile of DII (indicating a more pro-inflammatory diet) had a higher likelihood of PS in comparison with normal ones in both unadjusted (OR = 2.11, 95% (CI) = 1.05–4.24) and adjusted models (OR = 2.7, 95% (CI) = 1.25–5.8; OR = 2.49, 95% (CI) = 1.11–5.58). Besides, subjects in the second tertile of EDII were 2.29 times (95% (CI) = 1.03–5.07) more likely to have PS than those in the lower tertile in the adjusted model (Table 4).
Table 3.
Multiple linear regression for the association of handgrip strength with DII and EDII
| Variables | Adjusted R2 | Unstandardized Coefficients | 95% (CI) | P-value* | |
|---|---|---|---|---|---|
| B (SE) | |||||
| Dietary Inflammatory Index | Crude model | 0.03 | -0.37 (0.14) | (-0.65—-0.09) | 0.009 |
| Model Ia | 0.46 | -0.16 (0.11) | (-0.37 – 0.05) | 0.14 | |
| Model IIb | 0.51 | -0.29 (0.24) | (-0.77 – 0.18) | 0.22 | |
| Empirical Dietary Inflammatory Index | Crude model | -0.005 | -0.07 (0.26) | (-0.59 – 0.44) | 0.78 |
| Model Ia | 0.46 | -0.23 (0.19) | (-0.61 – 0.15) | 0.23 | |
| Model IIb | 0.52 | -0.49 (0.24) | (-0.95—-0.02) | 0.04 | |
*P ≤ 0.05
a Adjusted for age and gender
b Adjusted for age, family number, gender, CVD medication, Body Mass Index and physical activity
Table 4.
Logistic regression: probable sarcopenia
| Variables | Crude model | Model I a | Model II b |
|---|---|---|---|
| OR 95% (CI) | OR 95% (CI) | OR 95% (CI) | |
| Dietary Inflammatory Index model | |||
| Tertile 1 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 2.11 (1.05–4.24)† | 2.31 (1.09–4.9)† | 2.3 (1.05–5.12)† |
| Tertile 3 | 2.11 (1.05–4.24)† | 2.7 (1.25–5.8)† | 2.49 (1.11–5.58)† |
| P-trend | 0.04 | 0.01 | 0.02 |
| Empirical Dietary Inflammatory Index model | |||
| Tertile 1 | 1.00 | 1.00 | 1.00 |
| Tertile 2 | 2.06 (1.02–4.15)† | 2.67 (1.24–5.71)† | 2.29 (1.03–5.07)† |
| Tertile 3 | 2.15 (1.07–4.33)† | 2.35 (1.1–5.00)† | 1.91 (0.85–4.25) |
| P-trend | 0.03 | 0.03 | 0.10 |
† P ≤ 0.05
aAdjusted for age and gender
bAdjusted for age, family number, gender, CVD medication, Body Mass Index and physical activity
Discussion
For all we know, this is the first study attempt to investigate the association between the inflammatory potential of the diet and PS among older adults by both DII and EDII regardless of comparing the results of both indexes with each other. The findings of this study represented that a more pro-inflammatory diet doubled the odds of PS in older adults even after adjusting the association for confounders. The cutoff values of EWGSOP2 were used in the current study which has been reported to be good indicators in Iranian populations [31].
The number of studies on the association between dietary inflammatory potential and muscle weakness is limited and findings are a point of contention. Similar to our study, an increased odds of low grip hand was found by Kim et al. in older individuals adhering to a pro-inflammatory diet [32]. As reported by Laclaustra et al., there was a link between a pro-inflammatory diet and frailty in older adults [17]. The possibility of osteosarcopenic obesity increased in postmenopausal Korean women with high DII scores through the findings of Park et al [21]. Unlike the association between dietary inflammatory potential, evaluated by DII, with greater risk of sarcopenia, Bagheri et al. failed to show a significant difference between abnormal HGS and tertiles of DII [22]. Besides, the suggested linkage between energy-adjusted DII and abnormal HGS by Cervo et al. was in significant as well [23]. These conflicts might be explained by the dissimilarity of dynamometers and populations among studies. It appears that additional data is required to give insight into the association between dietary inflammatory indexes and muscle strength.
In the present study, PS subjects consumed more saturated fats and had a less dietary intake of anti-inflammatory nutrients compared to healthy people. Based on the findings, it seems that people with a high possibility of sarcopenia consumed less fruit and vegetable as the main sources of these anti-inflammatory nutrients concerning subjects with normal HGS. Consistent with our study, Hashemi et al. showed that older adults with high adherence to the Mediterranean diet had low odds of sarcopenia [33]. Participants with a high probability of sarcopenia consumed less fruit and vegetable with less adherence to Healthy Eating Index, Dietary Quality Index, and Mediterranean Diet in several studies [25, 34–36]. Although the levels of inflammatory markers have not been assessed in the current study, it has been remarked that higher hs-CRP is directly associated with oxidative stress which has been introduced as a major underlying mechanism of sarcopenia pathogenesis in previous studies [37, 38]. On the one hand, rising pro-inflammatory cytokine levels like TNF-α, IL-6, and hs-CRP happen through aging which exacerbates the inflammatory process, and consequently, accelerates muscle weakness [39]. Moreover, saturated fats provoke inflammatory responses through the NF-kB pathway. Thus, contrary to mono- or polyunsaturated fatty acids (MUFAs or PUFAs) as anti-inflammatory nutrients, high consumption of saturated fats might play role in impaired muscle strength [40, 41]. On the other hand, inflammatory mediators downregulate insulin and insulin-like growth factor-1 (IGF-1) which decrease muscle protein synthesis [42]. In this case, muscle atrophy tends to occur. A decrease in muscle mass might impair muscle strength as well, unnecessarily in a linear relationship. Notably, muscle weakness could occur rapidly compare to muscle mass decline [5, 43–46]. Nevertheless, the findings of the different studies aroused much controversy on the association between muscle mass and muscle strength and more investigations are required to clarify this association.
Though these findings were novel in this concept, PS was distinguished by using the recent definition of EWGSOP, and subjects were randomly selected from Tehran’s all regions which provides a good portrayal of Tehran’s older adults, this study has some limitations. Primarily, the squeeze dynamometer used here has a lower accuracy relative to digital ones. This is a cross-sectional study in that the serum concentration of inflammatory markers wasn’t measured and unable to verify any causality as well as it cannot specify the role of diet in PS precisely. Since the FFQ was used for dietary intake assessment, we can’t ignore the recall bias and over-report or under-report of participants. Finally, some of the DII components were not included in the calculation of total DII in this study which may cause underestimation of the relationship, although, Shivappa et al. reported that including at least 28 dietary parameters for its calculation did not drop DII’s predictive ability [12].
Conclusion
In conclusion, adherence to a diet with greater inflammatory potential might significantly impact the possibility of sarcopenia in older adults. These results are in line with recent recommendations to substitute healthy foods and emphasize the consideration of dietary choices in elderly health status. These findings warrant confirmation in further well-designed studies.
Acknowledgements
This study was supported by the Tehran University of Medical Science, Tehran, Iran. We would like to express our gratitude to the research deputy of the School of Nutritional Sciences and Dietetics and the dietitians who helped us collect the data, and especially, to the health centers and subjects who participated in this study.
Abbreviations
- HGS
Handgrip strength
- PS
Probable sarcopenia
- DII
Dietary Inflammatory Index
- EDII
Empirical Dietary Inflammatory Index
- EWGSOP
European Working Group on Sarcopenia in Older People
- BMI
Body mass index
- WHR
Waist to hip ratio
- WHtR
Waist to height ratio
- MUAC
Mid-upper arm circumference
- FFQ
Food frequency questionnaire
- MUFAs
Monounsaturated fatty acids
- ANCOVA
Analysis of covariance
- PUFAs
Polyunsaturated fatty acids
- IGF-1
Insulin-like growth factor-1
Authors’ contributions
Z. Esmaeily: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing—Original Draft Z. Tajary, Sh. Daei, M. Rezaei, A. Eyvazkhani: Investigation, Resources. M. Mansouri: Resources A. D. Motlagh: Conceptualization, Methodology, Supervision, Project administration.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Availability of data and materials
Data described in the manuscript, code book, and analytic code will be made available by the corresponding author upon request pending.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Tehran University of Medical Sciences with a protocol number of IR.TUMS.VCR.REC.1398.476. All methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was taken from participants by explaining the purpose of the study.
Consent for publication
Not Applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim JS, Wilson JM, Lee SR. Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutr Biochem. 2010;21:1–13. doi: 10.1016/j.jnutbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of community-dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7:290–298. doi: 10.1002/jcsm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5:253–259. doi: 10.1007/s13539-014-0161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Ancum JM, Pijnappels M, Jonkman NH, et al. Muscle mass and muscle strength are associated with pre- and post-hospitalization falls in older male inpatients: a longitudinal cohort study. BMC Geriatr. 2018;18:116. doi: 10.1186/s12877-018-0812-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syddall H, Cooper C, Martin F, et al. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–656. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 8.Organization WH (2017) Integrated Care for Older People: Guidelines on Community-Level Interventions to Manage Declines in Intrinsic Capacity. [PubMed]
- 9.Waters DL, Baumgartner RN, Garry PJ, et al. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging. 2010;5:259–270. doi: 10.2147/CIA.S6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloom I, Shand C, Cooper C et al. (2018) Diet Quality and Sarcopenia in Older Adults: A Systematic Review. Nutrients. 10. [DOI] [PMC free article] [PubMed]
- 11.Yokoyama Y, Nishi M, Murayama H, et al. Association of Dietary Variety with Body Composition and Physical Function in Community-dwelling Elderly Japanese. J Nutr Health Aging. 2016;20:691–696. doi: 10.1007/s12603-015-0632-7. [DOI] [PubMed] [Google Scholar]
- 12.Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and Validation of an Empirical Dietary Inflammatory Index. J Nutr. 2016;146:1560–1570. doi: 10.3945/jn.115.228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivappa N, Stubbs B, Hebert JR, et al. The Relationship Between the Dietary Inflammatory Index and Incident Frailty: A Longitudinal Cohort Study. J Am Med Dir Assoc. 2018;19:77–82. doi: 10.1016/j.jamda.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ümüş Ö, Aslı U, Nitin S et al. (2019) The Relationship between Dietary Inflammatory Index, Pulmonary Functions and Asthma Control in Asthmatics. Iranian Journal of Allergy, Asthma and Immunol. 18. [DOI] [PubMed]
- 16.Shivappa N, Godos J, Hébert JR et al. (2018) Dietary Inflammatory Index and Cardiovascular Risk and Mortality—A Meta-Analysis. 10:200. [DOI] [PMC free article] [PubMed]
- 17.Laclaustra M, Rodriguez-Artalejo F, Guallar-Castillon P, et al. The inflammatory potential of diet is related to incident frailty and slow walking in older adults. Clin Nutr. 2020;39:185–191. doi: 10.1016/j.clnu.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Sohn C, Kwon M et al. (2018) Positive Association between Dietary Inflammatory Index and the Risk of Osteoporosis: Results from the KoGES_Health Examinee (HEXA) Cohort Study. Nutrients. 10. [DOI] [PMC free article] [PubMed]
- 19.Shakeri Z, Mirmiran P, Khalili-Moghadam S, et al. Empirical dietary inflammatory pattern and risk of metabolic syndrome and its components: Tehran Lipid and Glucose Study. Diabetol Metab Syndr. 2019;11:16. doi: 10.1186/s13098-019-0411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, Park Y (2018) Association between the Dietary Inflammatory Index and Risk of Frailty in Older Individuals with Poor Nutritional Status. Nutrients. 10. [DOI] [PMC free article] [PubMed]
- 21.Park S, Na W, Sohn C. Relationship between osteosarcopenic obesity and dietary inflammatory index in postmenopausal Korean women: 2009 to 2011 Korea National Health and Nutrition Examination Surveys. J Clin Biochem Nutr. 2018;63:211–216. doi: 10.3164/jcbn.18-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagheri A, Soltani S, Hashemi R, et al. Inflammatory potential of the diet and risk of sarcopenia and its components. Nutr J. 2020;19:129. doi: 10.1186/s12937-020-00649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cervo MM, Shivappa N, Hebert JR, et al. Longitudinal associations between dietary inflammatory index and musculoskeletal health in community-dwelling older adults. Clin Nutr. 2020;39:516–523. doi: 10.1016/j.clnu.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Nations U (2020) World Population Ageing 2019.
- 25.Esmaeily Z, Tajary Z, Daei Sh, et al. Association between Healthy Eating Index-2015 scores and probable sarcopenia in community-dwelling Iranian older adults: a cross-sectional study. JNS. 2021;10:1–9. doi: 10.1017/jns.2021.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safarpour M, Dorosty Motlagh A, Hosseini SM, et al. Prevalence and outcomes of food insecurity and its relationship with some socioeconomic factors. Knowledge And Health. 2014;8:193–198. [Google Scholar]
- 27.Winter JE, MacInnis RJ, Wattanapenpaiboon N, et al. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99:875–890. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 28.Organization WH (2011) Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008.
- 29.Schneider HJ, Friedrich N, Klotsche J, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010;95:1777–1785. doi: 10.1210/jc.2009-1584. [DOI] [PubMed] [Google Scholar]
- 30.Mirmiran P, Esfahani F, Azizi F. Relative validity and reliability of the food frequency questionnaire used to assess nutrient intakes: Tehran Lipid and Glucose Study. Iran J Diabetes Lipid. 2009;9:185–197. [Google Scholar]
- 31.Shafiee G, Heshmat R, Ostovar A et al. (2020) Comparison of EWGSOP-1and EWGSOP-2 diagnostic criteria on prevalence of and risk factors for sarcopenia among Iranian older people: the Bushehr Elderly Health (BEH) program. Journal of Diabetes & Metabolic Disorders. [DOI] [PMC free article] [PubMed]
- 32.Kim D, Park Y. Association between the Dietary Inflammatory Index and Risk of Frailty in Older Individuals with Poor Nutritional Status. Nutrients. 2018;10:1363. doi: 10.3390/nu10101363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashemi R, Motlagh AD, Heshmat R, et al. Diet and its relationship to sarcopenia in community dwelling Iranian elderly: a cross sectional study. Nutrition. 2015;31:97–104. doi: 10.1016/j.nut.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Chan R, Leung J, Woo J. A Prospective Cohort Study to Examine the Association Between Dietary Patterns and Sarcopenia in Chinese Community-Dwelling Older People in Hong Kong. J Am Med Dir Assoc. 2016;17:336–342. doi: 10.1016/j.jamda.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Koyanagi A, Veronese N, Solmi M, et al. Fruit and Vegetable Consumption and Sarcopenia among Older Adults in Low- and Middle-Income Countries. 2020;12:706. doi: 10.3390/nu12030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo S, Kim D-Y, Lim H. Sarcopenia in relation to nutrition and lifestyle factors among middle-aged and older Korean adults with obesity. Eur J Nutr. 2020;59:3451–3460. doi: 10.1007/s00394-020-02179-3. [DOI] [PubMed] [Google Scholar]
- 37.Semba RD, Lauretani F, Ferrucci L. Carotenoids as protection against sarcopenia in older adults. Arch Biochem Biophys. 2007;458:141–145. doi: 10.1016/j.abb.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cottone S, Mulè G, Nardi E, et al. Relation of C-reactive protein to oxidative stress and to endothelial activation in essential hypertension. Am J Hypertens. 2006;19:313–318. doi: 10.1016/j.amjhyper.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–715. doi: 10.1093/gerona/55.12.M709. [DOI] [PubMed] [Google Scholar]
- 40.Thoma A, Lightfoot AP. NF-kB and Inflammatory Cytokine Signalling: Role in Skeletal Muscle Atrophy. Adv Exp Med Biol. 2018;1088:267–279. doi: 10.1007/978-981-13-1435-3_12. [DOI] [PubMed] [Google Scholar]
- 41.Sears B, Ricordi C. Anti-inflammatory nutrition as a pharmacological approach to treat obesity. J Obes. 2011;2011:431985. doi: 10.1155/2011/431985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granic A, Sayer AA, Robinson SM. Dietary Patterns. Skeletal Muscle Health, and Sarcopenia in Older Adults. 2019;11:745. doi: 10.3390/nu11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choe Y-R, Jeong J-R, Kim Y-P. Grip strength mediates the relationship between muscle mass and frailty. J Cachexia Sarcopenia Muscle. 2020;11:441–451. doi: 10.1002/jcsm.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tak YJ, Lee JG, Yi YH, et al. Association of Handgrip Strength with Dietary Intake in the Korean Population: Findings Based on the Seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1), 2016. Nutrients. 2018;10:1180. doi: 10.3390/nu10091180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles, ligaments and tendons journal. 2013;3:346–350. doi: 10.32098/mltj.04.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100:1460–1466. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available by the corresponding author upon request pending.