Abstract
Vibrio cholerae 638 (El Tor, Ogawa), a new CTXΦ-negative hemagglutinin/protease-defective strain that is a cholera vaccine candidate, was examined for safety and immunogenicity in healthy adult volunteers. In a double-blind placebo-controlled study, no significant adverse reactions were observed in volunteers ingesting strain 638. Four volunteers of 42 who ingested strain 638 and 1 of 14 who received placebo experienced loose stools. The strain strongly colonized the human small bowel, as evidenced by its isolation from the stools of 37 of 42 volunteers. V. cholerae 638, at doses ranging from 4 × 107 to 2 × 109 vibrios, elicited significant serum vibriocidal antibody and anti-Ogawa immunoglobulin A antibody secreting cell responses.
Cholera is an acute diarrheal disease consisting of the passage of voluminous rice-water stools. Vibrio cholerae of serogroups O1 and O139, the causative agents of cholera, secrete a potent enterotoxin, cholera toxin (CT), which causes the clinical symptoms of the disease (17). CT is composed of one A subunit (CTA) which catalyzes NAD-dependent ADP-ribosylation and five B subunits (CTB) that carry the ganglioside GM1 receptor binding site (8). The genes encoding CTA (ctxA) and CTB (ctxB) are encoded by the genome of filamentous phage CTXΦ (40). The toxigenic V. cholerae is a CTXΦ lysogen (40). The ctxA and ctxB genes are located adjacent to other phage genes encoding the core-encoded pilus (cep), the accessory cholera enterotoxin (Ace), and the zonula occludens toxin (Zot) in a core region (40). The core region is flanked by repeat sequences of RS1 and RS2 types that encode proteins required for CTXΦ integration and replication (41). The CTXΦ receptor is the toxin coregulated pilus, a type IV pilus essential for intestinal colonization in humans (13). In addition to CT, V. cholerae produces other putative toxic factors such as Ace, Zot, hemolysin, hemagglutinin/protease (HA/P), and others (17). The roles of these factors in disease have not been determined.
To cause infection, cholera-causing vibrios must overcome the gastric acid barrier, attach to and penetrate the mucous coat, and reach the underlying epithelial cells (17). Intestinal colonization in humans is evidenced by the detection of cholera vibrios in stools; their presence reflects their multiplication in the small bowel and subsequent detachment. Vibrios are also taken up by M cells in which they could interact with macrophages and lymphocytes to elicit immune response (31).
The spread of the seventh pandemic, characterized by the predominance of El Tor biotype vibrios, to the Western hemisphere reemphasizes the need for a safe and effective vaccine against cholera caused by V. cholerae El Tor. The recognition that protection against V. cholerae infection is highly dependent on stimulation of a mucosal immune response has favored the concept of an oral vaccine (36). An inactivated whole-cell oral vaccine has been extensively tested in field trials (14). Although this vaccine is safe and well tolerated, it is costly, requires multiple doses, and provides less than optimal protection, particularly for children. Volunteer studies have shown that orally administered virulent or genetically attenuated V. cholerae strains can be highly immunogenic (23). These studies have demonstrated that solid protection can be achieved in the absence of antitoxin immunity (23–25). CVD103HgR, a CTA− CTB+ derivative of the classical biotype Inaba strain 569B, has been extensively tested in volunteers (26). This vaccine is safe and well tolerated and was protective in volunteers.
Unfortunately, most El Tor biotype vaccine strain candidates, even those lacking the whole core region of the CTX element (ΔAce, ΔZot, ΔCT) have been considered too reactogenic for wide-scale usage (37, 38). Reactogenicity is manifested as mild to moderate diarrhea, abdominal cramps, malaise, vomiting, or low-grade fever (37, 38). The cellular basis of these adverse reactions is not clear. Reactogenicity could be due to colonization of the intestinal mucosa per se and/or synthesis by the vaccine strain candidate of unrecognized virulence factors.
Recently, the nonmotile El Tor Inaba candidate vaccine strain Peru-15 was reported to be well tolerated and immunogenic in volunteers (18). The efficacy of this experimental vaccine remains to be confirmed by field trials in areas where cholera is endemic, including epidemiological settings in which the Ogawa serotype is predominant. Experiments with Peru-15 have provided evidence that the capacity of motile vaccine strains to penetrate the mucous coat contributes to reactogenicity (18, 28). In summary, despite cholera being an old scourge, we are still challenged to develop a vaccine that is affordable for afflicted nations and that integrates long-lasting protection with clinical and environmental safety.
In the present study, we describe a preliminary assessment of the safety and immunogenicity in adult volunteers of a new El Tor vaccine strain candidate that was constructed by deletion of CTXΦ prophage and inactivation of the HA/P gene by insertion of celA encoding Clostridium thermocellum endoglucanase A.
MATERIALS AND METHODS
Media.
V. cholerae and Escherichia coli strains were routinely grown in LB medium and stored in Trypticase soy broth (Difco Laboratories) supplemented with 15% glycerol and 10% skim milk (Oxoid Ltd.) at −70°C. Ampicillin (100 μg/ml) and polymyxin B (100 U/ml) were added when necessary. For determination of CT production, cholera vibrios were grown in AKI cultures (16).
Construction of vaccine strains.
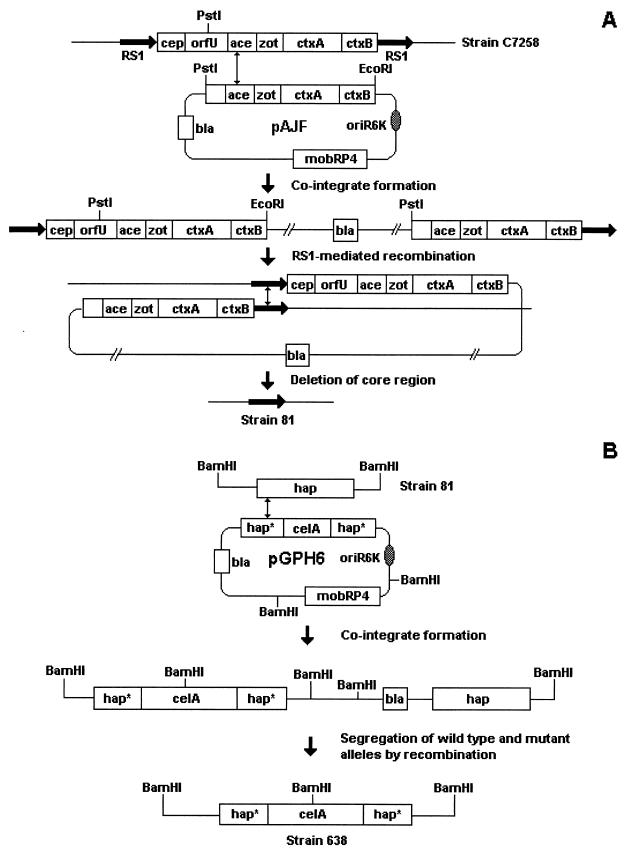
The construction of strains 81 and 638 is illustrated in Fig. 1. The suicide plasmid pAJF containing part of the core region cloned in vector pGP704 (3) was transferred by conjugation from the permissive host E. coli SM10λpir (33) to V. cholerae C7258 (El Tor, Ogawa, Perú, 1991) to produce an ampicillin-resistant cointegrate (Fig. 1A). Southern blot hybridization analysis confirmed that the cointegrate was a partial duplication of core genes separated by vector DNA and flanked by RS sequences (Fig. 1A) (3, 4). The cointegrate was allowed to segregate in antibiotic-free medium, and antibiotic-sensitive colonies were selected. The vast majority of ampicillin-sensitive colonies lacked the core genes and contained a single RS element in their chromosome (Fig. 1A) (3, 4). A segregant designated V. cholerae 81 was confirmed to contain a single RS element of type RS1 in the chromosomal site previously occupied by the CTXΦ prophage (Fig. 1A) (4). For construction of strain 638 (Fig. 1B), suicide vector pGPH6 containing the HA/P gene (hap) inactivated by insertion of reporter gene celA (30) was transferred from E. coli SM10λpir to strain 81 to produce an ampicillin-resistant cointegrate. Southern hybridization demonstrated that the cointegrate contains the insertionally inactivated hap gene (hap::celA) and its wild-type allele separated by vector DNA (30). The ampicillin-resistant cointegrate described above was allowed to segregate in antibiotic-free medium, and ampicillin-sensitive colonies were selected. An ampicillin-sensitive colony designated 638 was characterized by Southern analysis and shown to contain the hap::celA mutant allele (30).
FIG. 1.
Construction of candidate vaccine strain 638. Construction of strain 81 (A) and strain 638 (B). Gene designations: cep, core-encoded pilus; orfU, open reading frame U; ace, accessory cholera enterotoxin; zot, zonula occludens toxin; ctxA, cholera toxin A subunit; ctxB, cholera toxin B subunit; RS1, repeat sequence; hap, hemagglutinin/protease; hap*, 5′ or 3′ half of hap; bla, β-lactamase; celA, C. thermocellum endoglucanase A; oriR6K, R6K origin of replication; mobRP4, RP4 mob (mobilization) region.
Measurement of CT.
CT was measured in culture supernatants by GM1 enzyme-linked immunosorbent assay (ELISA) by using monoclonal antibody 1G10G5 against CTA and peroxidase-conjugated anti-mouse immunoglobulin G (IgG) as the primary and secondary antibodies, respectively (7).
Animal studies.
Ileal loop experiments were performed as described previously (10). Briefly, New Zealand adult rabbits were not allowed food for 24 h, and their small intestines were withdrawn and ligated approximately 10 cm from the appendix. The intestine was divided into 5- to 6-cm segments by ligature, and 108 CFU vibrios in 0.5 ml of phosphate-buffered saline were injected. After 16 to 18 h, the animals were sacrificed and loop length and fluid volume were determined. Results are expressed as fluid accumulation (FA) = fluid volume (in milliliters)/length (in centimeters). The 50% lethal doses (LD50s) was determined by using the infant mouse model. Dilutions containing from 103 to 107 vibrios in 20 μl of phosphate-buffered saline were orally inoculated to groups of 6 to 10 2- or 3-day-old newborn mice. Mortality was determined after 72 h, and the LD50 was calculated as described elsewhere (29).
Volunteers.
Volunteers were recruited from 18- to 40-year-old male students or workers from the municipality La Lisa of Havana. All volunteers enjoyed good health, did not have recent history of diarrheal disease or cholera vaccination, and were not taking any medication at the time of recruitment. Before admittance, volunteers were medically and psychologically screened and passed a written exam to assure their understanding of the study. Finally, each volunteer signed a witnessed informed consent.
Study design.
The study was performed in a isolation ward at the Institute for Tropical Medicine “Pedro Kouri.” The clinical trial protocol was revised and approved by the Ethics Committees of the Institute for Tropical Medicine “Pedro Kouri” and the Instituto Finlay. Finally, the study was authorized by the State Center for Drug Control and the National Biosecurity Laboratory. In the present study, 42 volunteers received strain 638 and 14 received placebo. In the first group, 16 volunteers received 2 × 109 CFU (high dose) and 6 received placebo. In the second group, 7 volunteers received 109 CFU (high dose), 7 received 4 × 107 CFU (low dose), and 4 received placebo. In the third group, 6 volunteers received 2 × 109 CFU (high dose), 6 received 2 × 108 CFU (medium dose), and 4 received placebo. In the fourth group, eight volunteers received 109 CFU of strain JBK70 (25) and four received placebo.
Preparation of inoculum and administration of vaccine strain and placebo.
The vaccine strain was grown on brain heart infusion (BHI) agar and suspended in saline, and the resulting solutions were standardized spectrophotometrically. The suspensions were diluted appropriately and plated to determine the viable count. Volunteers ingested 120 ml of 2% bicarbonate buffer followed by the inoculum in 30 ml of the same buffer. The placebo consisted of bicarbonate buffer alone and was indistinguishable from the vaccine preparation. To ensure double-blinding, identical flasks, containing either inoculum or placebo, were coded by an outside monitor. The clinical investigator assigned a letter to each volunteer. The code was kept by the monitor till the end of the experiment and analysis of all samples. Food and drinks were withheld from volunteers for 90 min before and after dosing.
Clinical surveillance.
During the first 2 days, volunteers were allowed to acclimate to the isolation ward, the medical screening was completed, and baseline samples were taken. Volunteers were monitored by a clinical investigator for adverse reactions for 120 h after inoculation. All stools were collected in disposable plastic bedpans and weighed, and their consistency was graded on a five-point scale (grade 1, formed stool; grade 2, soft but formed stool; grade 3, thick liquid stool; grade 4, opaque watery stool; and grade 5, rice-water stool). Diarrhea was defined as the passage of two or more loose stools (grades 3 to 5) within 48 h and at least 200 g or a single loose stool of 300 g or greater. Other symptoms (fever, abdominal cramps, vomiting, malaise, gurgling, and headache) were recorded. On the fifth day (120 h) after inoculation, volunteers received a single dose of doxycycline (300 mg) and were discharged from quarantine after the third negative coproculture.
Bacteriology.
The determination of the number of vibrios excreted per gram of stool was achieved by dispersion of stool specimens in saline, serial dilution, and plating in thiosulfate-citrate-bile salt-sucrose (TCBS) agar. The identity of the colonies was determined by agglutination with specific antisera, resistance to polymyxin B, and expression of the celA reporter gene (30).
Vibriocidal antibody assay.
Sera were collected on days 0, 7, 14, 21, and 28, and vibriocidal antibody titers were determined in a microassay (1). Briefly, 25 μl of twofold dilutions of serum in saline were placed in 96-well tissue culture plates. Next, 25 μl of a 107-CFU/ml suspension of V. cholerae VC12 (classical, Ogawa) or VC13 (classical, Inaba), containing fivefold-diluted human complement without anticholera activity, was added to each well and incubated for 1 h at 37°C. Finally, BHI broth containing 2% dextrose and 2% bromocresol purple was added and the plates were incubated 3 h at the same temperature. The vibriocidal antibody titer was defined as the highest dilution of serum causing complete inhibition of bacterial growth as judged by visual color comparison of the culture medium with a control without serum.
Anti-LPS IgG and IgA.
Antibacterial antibodies were determined in an ELISA with V. cholerae Ogawa LPS as solid-phase antigen and peroxidase-conjugated anti-human IgG and IgA (Sigma Chemical Co., St. Louis, Mo.). The plates were developed with o-phenylenediamine and the absorbance was read at 492 nm. The antibody titer was defined as the dilution of serum, calculated by interpolation, giving an absorbance value 0.4 unit above the background value (27, 35).
Antibody-secreting cells.
Venous blood was obtained before and 7 days after inoculation to enumerate LPS-specific IgA antibody-secreting cells (ASC) by a modification of the original enzyme-linked immunospot assay (5). Briefly, peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation on Histopaque 1077 (Sigma Chemical Co.). Individual wells of MultiScreen-HA plates (Millipore, Bedford, Mass.) were coated with V. cholerae Ogawa LPS (25 μg/ml), 0.5% bovine serum albumin (BSA) (negative control) or anti-human IgA (positive control) in phosphate-buffered saline overnight at 4°C. Plates were washed, blocked with RPMI 1640 complete medium supplemented with 10% fetal calf serum (Sigma Chemical Co.) and gentamicin (50 μg/ml) and incubated for 30 min at 37°C in a CO2 incubator. A volume of 90 μl was withdrawn from each well and replaced with 50 μl of PBMC suspension (106 to 105 cells), and incubation continued for 4 h at 37°C in a 7.5% CO2 atmosphere. The imprints of specific ASC were revealed by addition of peroxidase-conjugated anti-human IgA and overnight incubation at 4°C. Spots were counted under low magnification, and results were expressed as ASC per 106 PBMC. Volunteers who experienced a twofold increase in the number of ASC after inoculation and had a final count higher than two spots per 106 PBMC were considered positive.
Statistical analysis.
Statistical analysis of experimental data was performed with software Epi Info 6, version 6.04a, July 1996 (Centers for Disease Control and Prevention, Atlanta, Ga.). Means and proportions were compared by the paired t and exact Fisher’s test, respectively.
RESULTS
Properties of vaccine strain.
Strain 638 was constructed by deletion of the CTXΦ prophage from the chromosome of C7258 to yield strain 81 and subsequent inactivation of the hap gene by insertion of celA (Fig. 1) (3, 4). Strains 81 and 638 lack DNA sequences homologous to the core region of the CTX element but retain a RS1 in their chromosome (3, 4). Strain 638 does not produce soluble HA/P activity or immunoreactive material (30). The parent strain C7258, a clinical isolate from Perú, produced CT and was highly virulent in the infant mouse cholera model (Table 1). Strains 81 and 638 were markedly attenuated when tested for enterotoxicity in rabbit ileal loops and virulence in infant mice (Table 1). Disruption of hap in strain 638 slightly increased the LD50 over that of strain 81 (Table 1). Aside from the above genetic manipulations, V. cholerae 638 was indistinguishable in morphology, motility, biochemical tests, nutritional requirements, and growth rate from its parental strain (C7258). During characterization of recombinant strains, we noticed that strain 81 lost its capacity to assemble cell surface mannose-sensitive hemagglutinin (6). This phenotype did not affect intestinal colonization of its 638 derivative in mice (30) and humans (present study).
TABLE 1.
Attenuation of genetically modified strains
| Strain | Amt (ng/ml) of CT | Fluid accumulation (ml/cm)a | LD50 in infant mouse |
|---|---|---|---|
| C7258 | 64.0 ± 3.0 | 1.44 ± 0.2 | (1.5 ± 0.02) × 103 |
| 81 | NDb | 0.45 ± 0.4 | (2.7 ± 0.05) × 106 |
| 638 | ND | 0.29 ± 0.3 | (1.0 ± 0.05) × 107 |
Mean ± standard deviation of five independent determinations.
ND, not detectable.
Reactogenicity.
A summary of clinical findings after ingestion of strain 638 and placebo is shown in Table 2. All clinical manifestations observed were mild and of short duration. No statistical significance could be demonstrated between the inoculated and placebo groups with the present data. Gurgling and abdominal cramps were the adverse reactions most frequently reported by volunteers irrespective of dose. Four of 42 volunteers inoculated developed mild diarrhea (grade 3). Among these volunteers, two from the first group (n = 16) and one from the second group (n = 7) received the high dose. The fourth volunteer with diarrhea ingested the medium dose. One volunteer who received the high dose had five loose stools (72 h after inoculation) and a total output of 680 g. Two volunteers had two loose stools 28 and 72 h after inoculation with total outputs of 220 and 500 g, respectively. Another volunteer had a single diarrheal output of 300 g 73 h after inoculation.
TABLE 2.
Frequency of occurrence of adverse reactions after ingestion of El Tor Ogawa candidate vaccine strain 638
| Symptom | No. of volunteersa
|
Relative risk | 95% Confidence interval | Probability (Fisher) | |||
|---|---|---|---|---|---|---|---|
| Inoculated (n = 42)
|
Given placebo (n = 14)
|
||||||
| + | − | + | − | ||||
| Diarrheab | 4 | 38 | 1 | 13 | 1.33 | 0.16–10.96 | 0.6329 |
| Abdominal cramps | 13 | 29 | 2 | 12 | 2.17 | 0.56–8.44 | 0.1944 |
| Gurgling | 14 | 28 | 3 | 11 | 1.56 | 0.42–4.63 | 0.3143 |
| Heartburn | 6 | 36 | 2 | 12 | 1.00 | 0.23–4.40 | 0.6850 |
| Headache | 7 | 35 | 0 | 14 | 0.1163 | ||
| Vomiting | 1 | 41 | 0 | 14 | 0.7500 | ||
| Fever | 0 | 0 | 0 | 0 | |||
| Malaise | 0 | 0 | 0 | 0 | |||
Number of volunteers in treatment group with (+) or without (−) the symptom shown.
The volunteers with diarrhea had a mean diarrheal stool weight of 425 g (range, 220 to 680 g). The mean number of diarrheal stools per ill volunteer was 2 (range, 1 to 5).
In order to evaluate if the scarcity of adverse reactions observed in Cuban volunteers was due to a lower susceptibility, we carried out a control experiment with strain JBK70. This is a first-generation (ΔctxA ΔctxB) vaccine strain discarded because of its elevated reactogenicity in North American volunteers (25). This strain colonized the human small bowel well, as evidenced by its isolation from the stools of seven of eight volunteers. Five of eight volunteers (63%) who ingested JBK70 experienced diarrhea with total stool weight peaking at 1.2 kg (three volunteers, grade 3; 2 volunteers, grade 4; mean diarrheal stool weight per ill volunteer, 588 g; mean number of diarrheal stools per ill volunteer, 2.6). Other symptoms associated with ingestion of JBK70 were vomiting (one volunteer), abdominal cramps (two volunteers), headache (three volunteers); gurgling (two volunteers), and heartburn (one volunteer). No symptoms were observed in volunteers who received placebo in this control experiment.
Bacteriological isolation of vaccine strain.
As indicated in Table 3, strain 638 was recovered in 37 of 42 volunteers inoculated (88%). For the higher dose, excretion of the vaccine strain tends to peak at 72 h after inoculation. Three of the four cases of diarrhea occurred at this time. Of the 37 volunteers who excreted vibrios, 12 excreted on at least 4 days, 19 excreted on at least 3 days, and 28 excreted on 2 days. The number of volunteers excreting strain 638 and the mean number of vibrios per gram of stool declined in volunteers given the lower dose. Vibrios isolated from the stools of volunteers produced endoglucanase A, indicating that the celA reporter gene was stably maintained during growth in the human intestine. No vaccine strain was detected in the stools of volunteers receiving placebo.
TABLE 3.
Recovery of V. cholerae 638 from the stools of volunteers
| Dose | No. of volunteers excreting vaccine strain/total no.
|
Mean CFU/g of stoola | |||||
|---|---|---|---|---|---|---|---|
| Time after inoculation (h)
|
Total | ||||||
| 24 | 48 | 72 | 96 | 120 | |||
| High (1 × 109 to 2 × 109) | 7/29 | 10/29 | 16/29 | 15/29 | 10/29 | 28/29 | 4.4 × 106 |
| Medium (2 × 108) | 6/6 | 5/6 | 4/6 | 4/6 | 3/6 | 6/6 | 5.5 × 106 |
| Low (4 × 107) | 1/7 | 2/7 | 2/7 | 2/7 | 2/7 | 3/7 | 2.7 × 105 |
Overall mean.
Immune response to vaccine strain.
Strain 638 elicited a significant and consistent immune response in terms of serum Ogawa vibriocidal antibodies, serum anti-Ogawa LPS IgG or IgA (Table 4), and Ogawa LPS-specific IgA ASC (Table 5). Although reciprocal geometric mean titer (GMT) peaked 14 days after inoculation, seroconversion was attained on day 7 and titers remained high till day 28. Seroconversion rates, peak reciprocal GMT, and ELISA titers were dose dependent. However, even at the lowest dose, strain 638 elicited a significant vibriocidal antibody response compared to that of the placebo group. A significant proportion of the volunteers who experienced seroconversion developed relatively high (≥1,024) vibriocidal titers (Table 4). All volunteers who seroconverted for Ogawa vibriocidal antibodies seroconverted for the Inaba serotype (data not shown). The high percentage of responders in the ASC evaluation (Table 5) reflects an effective stimulation of mucosal immunity, mainly secretory IgA (sIgA), by strain 638 which corresponds with the elevated anti-LPS IgA titers encountered 14 days after inoculation. One volunteer who ingested placebo seroconverted for anti-LPS IgG. This volunteer had very low preinoculation anti-LPS serum IgG that increased to the cutoff value at day 7 and remained constant thereafter. Another volunteer who ingested placebo reached the cutoff value of ASC. Similarly, this volunteer had a very low preinoculation number of LPS-specific ASC.
TABLE 4.
Serum antibody response in volunteers given V. cholerae El Tor Ogawa strain 638 orally
| Immune response | Volunteers given:
|
|||
|---|---|---|---|---|
| High dose | Medium dose | Low dose | Placebo | |
| Ogawa vibriocidal antibodiesa | ||||
| No. seroconverted (fourfold) (%) | 24/29 (82) | 5/6 (83) | 5/7 (71) | 0/14 (0) |
| GMTb (range) | ||||
| Preinoculation | 47 (0–160) | 32 (0–40) | 33 (0–40) | 37 (0–320) |
| Postinoculation peak (14 days) | 873 (0–20,480) | 639 (0–2,560) | 389 (40–2,560) | 46 (0–320) |
| No. of responders with titers ≥ 1,024/total no. | 10/24 | 4/5 | 4/5 | 0 |
| Anti-Ogawa LPS IgGc | ||||
| No. seroconverted (twofold) (%) | 23/29 (79) | 4/6 (67) | 3/7 (43) | 1/14 (7) |
| No. seroconverted (fourfold) (%) | 21/29 (71) | 4/6 (67) | 3/7 (43) | 1/14 (7) |
| Log reciprocal titerd ± SDe | ||||
| Preinoculation | 0.12 ± 0.25 | 0.12 ± 0.29 | 0 | 0.03 ± 0.12 |
| Postinoculation peak (14 days) | 1.86 ± 1.12 | 1.56 ± 1.49 | 1.07 ± 1.36 | 0.19 ± 0.57 |
| Anti-Ogawa LPS IgAc | ||||
| No. seroconverted (twofold) (%) | 26/29 (90) | 6/6 (100) | 5/7 (71) | 0/14 (0) |
| No. seroconverted (fourfold) (%) | 24/29 (83) | 6/6 (100) | 4/7 (57) | 0/14 (0) |
| Log reciprocal titer ± SD | ||||
| Preinoculation | 0.1 ± 0.37 | 0 | 0.27 ± 0.37 | 0.13 ± 0.39 |
| Postinoculation peak (14 days) | 2.43 ± 1.0 | 2.68 ± 0.48 | 1.96 ± 1.25 | 0.19 ± 0.53 |
Number of volunteers with fourfold increase in titer/total number of volunteers. The seroconversion rate is shown as a percentage in the parentheses.
GMT, geometric mean titer.
Number of volunteers with two- or fourfold increase in titer/total number of volunteers.
Logarithm of the reciprocal arithmetic mean titer.
SD, standard deviation of the mean.
TABLE 5.
Anti-LPS IgA ASC response in peripheral blood samples from volunteers following ingestion of V. cholerae 638
| Dose | No. positivea (%) | Mean no. of ASC per 106 PBMC (range) |
|---|---|---|
| High | 27/29 (93.1) | 48.5 (0–475.0) |
| Medium | 6/6 (100) | 37.7 (4.0–128.5) |
| Low | 6/7 (85.7) | 37.1 (0–204.0) |
| None | 1/14 (7.1) | 0.5 (0–6.5) |
No. of volunteers with anti-LPS IgA ASC response/total no. of volunteers.
DISCUSSION
V. cholerae C7258 is a toxigenic vibrio isolated from a patient during the 1991 cholera outbreak in Perú. This strain produced CT in AKI cultures and was very virulent in the infant mouse cholera model (Table 1). The genetic modifications shown in Fig. 1 resulted in the marked attenuation of strains 81 and 638 (Table 1).
An advantageous property of strain 638 is that the genetic manipulation did not introduce deleterious mutations affecting the growth rate in vitro and in vivo. In fact, inactivation of hap increased colonization in infant mice (30). Another desirable property of strain 638 is that it is marked with a reporter gene encoding an activity not present in other enteric bacteria. The β-(1-4) endoglucanase activity encoded by celA is easily detectable in carboxymethylcellulose indicator agar stained with Congo red (30). In this test, the vaccine strain appears as a red colony surrounded by a transparent halo in a red field. This rapid, simple, and sensitive technique is particularly useful in minimally equipped laboratories or research stations created for field experiments.
Strain 638 was well tolerated in volunteers (Table 2). All the clinical symptoms recorded were mild and of short duration. The rate of diarrhea observed in volunteers ingesting 638 was 9.5%. The preceding El Tor biotype candidate vaccine strains JBK70 and CVD110 induced mild to moderate diarrhea in 50 to 70% of North American volunteers, with total stool volumes peaking 1.8 liters (2 to 13 loose stools per ill volunteer) (25, 37). The observation that Cuban volunteers challenged with strain JBK70 experienced adverse reactions to a similar extent supports the conclusion that strain 638 is a comparatively well-tolerated vaccine candidate. The nonreactogenic classical biotype vaccine strain CVD103-HgR produced diarrhea in 0.5 to 11% volunteers, depending on the target population (21, 34). The rate of diarrhea observed after ingestion of strain 638 fell within the range of strain CVD103-HgR. However, in comparing CVD103-HgR and 638, some important differences should be considered. First, in volunteer studies conducted in Thailand (34) and Peru (11) in which 10% of volunteers developed mild diarrhea, a different definition of diarrhea was used; diarrhea was defined as the passage of three to four loose stools in 24 h. No volunteer ingesting strain 638 had diarrhea by this definition. Second, in the above studies with CVD103-HgR, the heat-inactivated E. coli K-12 placebo itself elicited symptoms. In the present study, we used buffer as the placebo to maximize the likelihood of detecting statistically significant differences with the group ingesting the attenuated strain. A third difference is that our volunteers ingested strain 638 harvested from a plate. A lower rate of diarrhea was observed when volunteers received lyophilized CVD103-HgR (26). The cellular basis of reactogenicity is not clear but the detection of proinflammatory cytokines and lactoferrin in the stools of volunteers administered strain CVD110 suggests a local inflammatory response (32).
More recently, the El Tor biotype candidate vaccine strain Peru-15 was shown to be immunogenic, protective, and well tolerated in volunteers (18). It has been hypothesized that Peru-15 is not reactogenic because it is nonmotile and incapable of penetrating the mucous gel to interact with enterocytes and induce an inflammatory response (28). Strain 638 is motile and does not produce HA/P (30). It is not possible to evaluate with the present data the role of HA/P in reactogenicity. Inactivation of the HA/P gene increased the duration of adherence of cholera-causing vibrios to cultured intestinal epithelial cells (9), augmented adherence and multiplication of choleragenic vibrios on mucus-coated cultured human intestinal cells (2), and increased adherence and colonization in infant mice (30). Infant mice colonization data have been shown to correlate well with colonization in humans (13, 39). An early hypothesis was that reactogenicity is due to the synthesis by candidate vaccine strains of other virulence factors. It is possible that HA/P could be a virulence factor contributing to the reactogenicity of genetically attenuated (CT−) candidate vaccine strains. Several lines of evidence argue in favor of this possibility. First, HA/P belongs to a family of zinc-containing metalloproteases widely distributed in pathogenic bacteria (12). Second, HA/P enhances the enterotoxicity of live vibrios in rabbit ileal loops (15). Third, it has been reported that HA/P causes morphological changes and perturbs the paracellular barrier function in cultured epithelial cells (42). Alternatively, the absence of the mucinase activity associated with HA/P could prevent penetration of mucus as the motility defect in Peru-15.
The recovery of strain 638 from 88% of the volunteers inoculated indicates that this strain colonizes the human small bowel well (Table 3). Early studies suggested that colonization correlates positively with reactogenicity (25, 37). Strain 638 is an example of a V. cholerae candidate vaccine strain that colonizes the small bowel very well without exerting severe side effects (Table 2). CVD103-HgR does not adhere to cultured cells and colonizes animal models and humans poorly (2, 23, 24). Our results suggest that this vaccine could be improved by enhancing its adherence and colonization without increasing its reactogenicity.
Strain 638 elicited significant serum (Table 4) and ASC (Table 5) responses at all doses tested. The vibriocidal antibody response is considered the best immunological correlate with protection (23, 24). The magnitude of the serum vibriocidal antibody response (Table 4) to ingestion of strain 638 was similar to that of the classical biotype vaccine CVD103-HgR (23). Long-lasting protection against V. cholerae infection is due to induction of high levels of sIgA antibodies and immunologic memory for these antibodies in the intestine (36). An alternative approach to determining sIgA antibodies in intestinal fluids is enumeration of the specific ASC circulating in the blood; ASC reflect priming of the mucosal immune system to locally presented antigens (36). The high ASC response rate induced at all doses examined (Table 5) suggests that ingestion of strain 638 efficiently elicits the correct immune response. The slight decline of both serum and ASC responses at the lower dose was paralleled by a decline in the recovery of vibrios from the stools of volunteers (Table 3). Since strain 638 does not produce HA/P, the above results demonstrate that hap is an appropriate locus for insertion of foreign antigens without affecting immunogenicity.
The above results justify further improvement of strain 638. The ability to induce antitoxin immunity would be desirable. Enhancement of biosafety is a second major concern. Strain 638 contains an RS element of type RS1 in the position previously occupied by CTXΦ (4). On one side, the RS1 can provide an attachment site for reinsertion of CTXΦ and be considered a negative factor. On the other hand, the product of rstR confers immunity to reinfection by El Tor CTXΦ (20) and its presence in strain 638 could be a biosafety-enhancing feature. PCR cloning and DNA sequencing of the RS1 present in strain 638 confirmed the presence of open reading frames rstR, rstA1, rstB1, and rstC (4). A desirable property for a live-organism vaccine candidate is that it be deficient in genetic recombination (e.g., recA). However, recA mutations could have negative effects. It can render a vaccine strain costly by affecting colonization and immunogenicity (19, 22) or making it more sensitive to lyophilization. Thus, since no live cholera vaccine has yet proved to be effective in the field, caution should be taken not to overemphasize all the properties that could be anticipated for an ideal vaccine.
ACKNOWLEDGMENTS
We thank Richard A. Finkelstein (University of Missouri, Columbia) for encouragement and fruitful discussions. We are grateful to Roberto Fernández for biosecurity assurance; Teresa Serrano, Jorge Menéndez, Daniel González, and Sara Palma for their skillful assistance in volunteer recruitment, medical screening, and clinical surveillance; and Arlenis Moreno for technical assistance in bacteriology.
REFERENCES
- 1.Benenson A, Saad S, Mosley A. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull W H O. 1968;38:277–285. [PMC free article] [PubMed] [Google Scholar]
- 2.Benitez J A, Spelbrink R G, Silva A, Phillips T E, Stanley C M, Boesman-Finkelstein M, Finkelstein R A. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect Immun. 1997;65:3474–3477. doi: 10.1128/iai.65.8.3474-3477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benitez J A, Silva A, Rodríguez B L, Fando R, Campos J, Robert A, Garcia H, Garcia L, Perez J L, Oliva R, Torres C A, Ledon T. Genetic manipulation of Vibrio cholerae for vaccine development: construction of live attenuated El Tor candidate vaccine strains. Arch Med Res. 1996;27:275–283. [PubMed] [Google Scholar]
- 4.Campos J, Fando R, Silva A, Rodriguez B L, Benitez J A. Replicating function of the RS1 element associated with Vibrio cholerae CTXΦ prophage. FEMS Microbiol Lett. 1998;164:141–147. doi: 10.1111/j.1574-6968.1998.tb13079.x. [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky C, Nillson L-A, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for the enumeration of specific antibody secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 6.Falero G, Rodriguez B L, Valmaseda T, Perez M E, Benitez J A, Sierra G. Production and characterization of a new monoclonal antibody against mannose-sensitive hemagglutinin of Vibrio cholerae. Hybridoma. 1998;17:63–67. doi: 10.1089/hyb.1998.17.63. [DOI] [PubMed] [Google Scholar]
- 7.Fando R, Pérez J L, Rodríguez B L, Campos J, Robert A, García L, Silva A, Benitez J A. Promoter activities in Vibrio cholerae ctxΦ prophage. Infect Immun. 1997;65:1561–1565. doi: 10.1128/iai.65.4.1561-1565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein R A. Cholera enterotoxin (choleragen): a historical perspective. In: Barua D, Greenough W B, editors. Cholera. New York, N.Y: Plenum Medical Book Company; 1992. pp. 155–187. [Google Scholar]
- 9.Finkelstein R A, Boesman-Finkelstein M, Chang Y, Häse C. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Formal S B, Kundel D, Schneider H, Kunev N, Sprinz H. Studies with Vibrio cholerae in ligated loops of the rabbit intestine. Br J Exp Pathol. 1961;42:504–510. [PMC free article] [PubMed] [Google Scholar]
- 11.Gotuzzo E, Butron B, Seas C, Penny M, Ruiz R, Losonsky G, Lanata C F, Wasserman S S, Salazar E, Kaper J B, Cryz S, Levine M M. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun. 1993;61:3994–3997. doi: 10.1128/iai.61.9.3994-3997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häse C, Finkelstein R A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993;57:823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrington D A, Hall R H, Losonsky G A, Mekalanos J J, Taylor R K, Levine M M. Toxin, the toxin co-regulated pili and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmgren J, Osek J, Svennerholm A-M. Protective oral cholera vaccine based on a combination of cholera toxin B subunit and inactivated cholera vibrios. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: American Society for Microbiology; 1994. pp. 415–424. [Google Scholar]
- 15.Ichinose Y, Ehara M, Honda T, Miwatani T. The effect on enterotoxicity of protease purified from Vibrio cholerae. FEMS Microbiol Lett. 1994;115:265–272. doi: 10.1111/j.1574-6968.1994.tb06649.x. [DOI] [PubMed] [Google Scholar]
- 16.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol. 1986;30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaper J B, Morris G, Jr, Levine M M. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenner J R, Coster T S, Taylor D N, Trofa A F, Barrera-Oro M, Hyman T, Adams J M, Beattie D T, Killeen K P, Spriggs D R, Mekalanos J J, Sadoff J C. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995;172:1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 19.Ketley J M, Kaper J B, Herrington J B, Losonsky D A, Levine M M. Diminished immunogenicity of a recombination-deficient derivative of Vibrio cholerae vaccine strain CVD103. Infect Immun. 1990;58:1481–1484. doi: 10.1128/iai.58.5.1481-1484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimsey H, Waldor M. Abstracts of the 33rd Joint Conference on Cholera and Related Diarrheal Disease, Clearwater Beach, Fla. 1997. Heteroimmunity amongst CTXΦ; pp. 42–46. [Google Scholar]
- 21.Kotloff K L, Wasserman S S, O’Donnell S, Losonsky G A, Cryz S J, Levine M M. Safety and immunogenicity in North Americans of a single dose of live oral cholera vaccine CVD 103-HgR: results of a randomized, placebo-controlled, double-blind crossover trial. Infect Immun. 1992;60:4430–4432. doi: 10.1128/iai.60.10.4430-4432.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar K K, Srivastava R, Sinha V B, Michalski J, Kaper J B, Srivastava B S. recA mutations reduce adherence and colonization by classical and El Tor strains of Vibrio cholerae. Microbiology. 1994;140:1217–1222. doi: 10.1099/13500872-140-5-1217. [DOI] [PubMed] [Google Scholar]
- 23.Levine M M, Tacket C O. Recombinant live cholera vaccines. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: American Society for Microbiology; 1994. pp. 395–414. [Google Scholar]
- 24.Levine M M, Kaper J B. Live oral vaccines against cholera: an update. Vaccine. 1993;11:107–212. doi: 10.1016/0264-410x(93)90019-t. [DOI] [PubMed] [Google Scholar]
- 25.Levine M M, Kaper J B, Herrington D, Losonsky G, Glenn Morris J, Clements M I, Black R E, Tall B, Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine M M, Kaper J B, Herrington D, Ketley J, Losonsky G, Tacket C O, Tall B, Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD103 and CVD103-HgR. Lancet. 1998;ii:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 27.Lycke N. Cholera toxin and the intestinal immune response. Experimental study at the single cell level. Ph.D. thesis. Göteborg, Sweden: University of Göteborg; 1986. [Google Scholar]
- 28.Mekalanos J J, Waldor M K, Gardel C L, Coster T S, Kenner J, Killeen K P, Beattie D T, Trofa A, Taylor D N, Sadoff J C. Live cholera vaccines: perspectives on their construction and safety. Bull Inst Pasteur. 1995;93:255–262. [Google Scholar]
- 29.Reed L J, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 30.Robert A, Silva A, Benitez J A, Rodriguez B L, Fando R, Campos J, Sengupta D K, Boesman-Finkelstein M, Finkelstein R A. Tagging a Vibrio cholerae El Tor candidate vaccine strain by disruption of its hemagglutinin/protease gene using a novel reporter enzyme, Clostridium thermocellum endoglucanase A. Vaccine. 1996;14:1517–1522. doi: 10.1016/s0264-410x(96)00105-3. [DOI] [PubMed] [Google Scholar]
- 31.Siebers A, Finlay B B. M cells and the pathogenesis of mucosal and systemic infection. Trends Microbiol. 1996;4:22–29. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 32.Silva T M J, Schleupner M A, Tacket C O, Steiner T S, Kaper J B, Edelman R, Guerrant R L. New evidence for an inflammatory component in diarrhea by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect Immun. 1996;64:2362–2364. doi: 10.1128/iai.64.6.2362-2364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon R, Priefer U, Puhler A. A broad host-range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 34.Su-Arehawaratana P, Singharaj P, Taylor D N, Hoge C, Trofa A, Kuvanont K, Migasena S, Pitisuttitham P, Lim Y L, Losonsky G, Kaper J B, Wasserman S S, Cryz S, Echeverria P, Levine M M. Safety and immunogenicity of different immunization regimens of CVD103-HgR live oral cholera vaccine in soldiers and civilians in Thailand. J Infect Dis. 1992;165:1042–1048. doi: 10.1093/infdis/165.6.1042. [DOI] [PubMed] [Google Scholar]
- 35.Svennerholm A M, Sack D A, Holmgren J, Bardhan P K. Intestinal antibody response after immunization with cholera B subunit. Lancet. 1982;i:305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- 36.Svennerholm A M, Jonson G, Holmgren J. Immunity to Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: American Society for Microbiology; 1994. pp. 257–272. [Google Scholar]
- 37.Tacket C O, Losonsky G, Nataro J P, Cryz S J, Edelman R, Fasano A, Michalski J, Kaper J B, Levine M M. Safety and immunogenicity of live oral cholera vaccine candidate CVD110, a ΔctxA Δzot Δace derivative of El Tor Ogawa Vibrio cholerae. J Infect Dis. 1993;168:1536–1540. doi: 10.1093/infdis/168.6.1536. [DOI] [PubMed] [Google Scholar]
- 38.Taylor D N, Killeen K P, Hack D C, Kenner J R, Coster T S, Beattie D T, Ezzell J, Hyman T, Trofa A, Sjogren M J, Friedlander A, Mekalanos J J, Sadoff J C. Development of a live, oral, attenuated vaccine against El Tor cholera. J Infect Dis. 1994;170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 39.Thelin K H, Taylor R K. Toxin coregulated pilus, but not mannose-sensitive hemagglutinin is required for colonization by Vibrio cholerae O1 El Tor and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 41.Waldor M K, Rubin E J, Pearson G D N, Kimsey H, Mekalanos J J. Regulation, replication, and integration functions of the Vibrio cholerae CTXΦ are encoded by region RS2. Mol Microbiol. 1997;24:917–926. doi: 10.1046/j.1365-2958.1997.3911758.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Milton D, Nybom P, Sjö A, Magnusson K-E. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog. 1996;21:111–123. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]