Abstract
Research has observed evidence for both hypo-(supposedly due to a broken mirror neuron system) and hyper-(thought to be the result of deficits in adaptive control) imitation in autism spectrum disorder (ASD). This work sought to adjudicate between these findings using an automatic imitation (AI) paradigm with the novel manipulation of the need to engage adaptive control of imitation. Results demonstrated that ASD participants do not display a specific deficit in AI capability, are able to engage in proactive control of AI, and that relative to a well-matched effector condition, AI is not selectively associated with ASD symptom severity. These data cast doubt upon the notion of impairments in imitation or its control in ASD.
Keywords: Automatic imitation, Imitation, Cognitive control, Proactive control
One of the most defining symptoms of autism spectrum disorder (ASD) is a deficit in social functioning (American Psychiatric Association 2013), the set of abilities integral for interacting and behaving in accordance with other members of the social world (Merhoum et al. 2015; Smeekens et al. 2015). Problems in this area are demonstrably present across the entire lifespan, and are therefore considered to be one of the most significant challenges for individuals with ASD (Gantman et al. 2012). Although attributing the social deficits present in ASD to a single aetiological factor is difficult due to the considerable degree of heterogeneity in both the ASD phenotype and social functioning, one avenue of work has specifically focused on the role of imitative capability. Imitation, the process by which an individual observes and replicates the behavior of another, is considered to be key to the development of a multitude of abilities inherent to social functioning including language, theory of mind, and empathy (Ingersoll 2008; Rogers and Pennington 1991; Sowden et al. 2016). Given that research has highlighted that those with ASD display specific deficits in these areas, a burgeoning literature has suggested that difficulties in imitation may in fact underlie the social deficits observed in ASD.
Although debate exists (cf., Ferrari and Rizzolatti 2014), the mirror neuron system (MNS), a set of brain regions that respond to both the execution and observation of action (di Pellegrino et al. 1992), has been posited to comprise the neural mechanism underlying a number of social-cognitive processes, including imitative capability (Catmur et al. 2007; Cook and Bird 2012; Iacoboni et al. 1999). Given that a number of the purported functions of the MNS (e.g., language acquisition and empathy) display considerable overlap with areas of impairment in ASD (see Fishman et al. 2014), one theory posits that the disorder may be, at least partially, characterized by a “broken” MNS (Oberman and Ramachandran 2007; Williams et al. 2001). However, despite the seemingly intuitive nature of this hypothesis, neurological investigations of MNS structure and function in those with ASD have provided mixed results (for an extensive review see Hamilton 2013). Behavioral investigations of voluntary imitation have, on the whole, provided evidence for an imitative deficit (i.e., hypo-imitation) in ASD (Williams et al. 2004). However, voluntary imitation paradigms require participants to consciously imitate an observed action, a process that is likely to rely on multiple cognitive mechanisms including, but not limited to, executive function, attention, and an understanding of communication (Press et al. 2010; Sowden et al. 2016). Given that such higher-level cognitive processes have been shown to be impaired in ASD (Bird et al. 2006), attributing the results of these paradigms to a specific imitation deficit is specious at best.
To minimize these confounding factors and isolate a measure of imitation that does not rely on multiple cognitive mechanisms, researchers have instead employed stimulus–response compatibility (SRC) paradigms (Kornblum et al. 1990) in order to achieve a measure of so-called ‘automatic imitation’ (AI)—the degree to which observed actions modulate action execution. In such paradigms, participants are typically required to execute a response based on the identity of a presented stimulus, while simultaneously being exposed to a task-irrelevant stimulus that either depicts the same (congruent) or a different (incongruent) movement (for example participants may be required to perform a manual movement in response to a numeric cue, while a task-irrelevant manual movement is also presented to them). These paradigms therefore require a degree of ‘self-other’ control, i.e., an inhibition of an ‘other’ activated movement representation in favor of a ‘self’ activated movement representation. It is important to note that, in line with previous research on the topic (see Cook and Bird 2012; Hamilton et al. 2007; Schunke et al. 2016; Sowden et al. 2016), the remainder of this manuscript periodically uses the term imitation to refer to automatic imitation, however use of this term should not be taken to imply anything about voluntary or controlled imitative response tendencies.
Representative findings from the use of AI paradigms in typically developing (TD) participants show that people are faster and more accurate to execute a response when that response is paired with the presentation of a similar movement (i.e., when the task-irrelevant stimulus performs the same manual movement as required by the numeric cue; congruent trials), suggesting that people display a propensity to imitate others even when it is detrimental to overall task performance (e.g., reducing accuracy and increasing response latency—Brass et al. 2001; Brass et al. 2000). Although relatively few studies have used SRC paradigms to probe AI in ASD, the results from those that have almost exclusively support the notion that AI is not impaired in ASD (Hamilton et al. 2007; Press et al. 2010; Schunke et al. 2016; Sowden et al. 2016).
In contrast, some research using SRC paradigms has demonstrated evidence for increased AI in ASD (Bird et al. 2007; Deschrijver et al. 2017; Spengler et al. 2010). Although these data speak against the view that imitative capacity is equivalent between those with ASD and TD, they are somewhat consistent with clinical observations of echolalia (the repetition of spoken words) and echopraxia (the repetition of movements). These findings suggest that rather than the generalized imitation deficit predicted by the broken MNS hypothesis, it is the ability to exert top-down control of social-imitative behaviors that may be impaired in ASD, leading to deficits in social functioning (Spengler et al. 2010). Although it is clear that the general tendency to imitate others results in a higher quality of social interaction (Lakin and Chartrand 2003), evidence also suggests that effective social imitation is reliant upon context-dependent modulation (Chartrand and Lakin 2013). This suggests that the ability to engage adaptive control of imitation is critical to normative social functioning. Consequently, the deficits in social functioning observed in ASD may be driven by an inability to modulate imitative processes to levels that are appropriate to the specific context. In support of this notion, Cook and Bird (2012) found that TD participants showed increased imitation following pro-social priming, whereas ASD participants did not, suggesting atypical social modulation of imitation. Proponents of this ‘aberrant-control’ theory argue that the observed hyper-imitation in ASD is driven by impairments in dissociating self from other representations. This results in a maladaptive upregulation of representation of another’s action at the expense of one’s own action representation and a reduced capacity to inhibit AI when the observed action is incongruent. In favor of this notion, Spengler et al. (2010) demonstrated that imitative behavior negatively correlated with theory of mind measures in ASD, suggesting that a reduced ability to represent the distinct mental states of self and others led to an increase in AI. Likewise, despite demonstrating intact AI in individuals with ASD in their first experiment, Sowden et al. (2016) demonstrated that imitative behavior positively correlated with autism general symptom severity measured by the Autism Diagnostic Observation Schedule (ADOS) in their second.
However, evidence for the aberrant-control theory is also mixed, with a number of studies (although not all using AI) failing to find evidence for hyper-imitation in ASD (Forbes et al. 2017; Gowen et al. 2008; Grecucci et al. 2013; Press et al. 2010), as well as a recent meta-analysis finding no evidence for deficits in imitative control using a large sample (N = 220) of ASD participants (Cracco et al. 2018). One possible reason for these discrepant results is that previous research is considerably heterogeneous with respect to sample sizes and the methodologies used to assess the control of imitation, often relying on correlational analyses with ostensibly control-relevant variables. One clear omission is that no previous research has directly manipulated the requirement for top-down control of imitation within an AI paradigm with ASD participants.
One robust method for inducing the need for top-down control is a manipulation of the ratio of congruent-to-incongruent trials (for a comprehensive review see Bugg and Crump 2012). AI research has routinely demonstrated that incongruent trials (trials in which the relevant and irrelevant stimulus dimensions do not prompt the same response) result in slower and less accurate responding than do congruent trials, often termed an ‘interference’ effect. However, research primarily using the Stroop and Flanker tasks has demonstrated that the magnitude of this interference is attenuated when the ratio of incongruent to congruent trials is increased (e.g., Kane and Engle 2003; Lowe and Mitterer 1982; Lindsay and Jacoby 1994; West and Baylis 1998). Attenuated interference effects observed may represent a switch to a more proactive form of control in which participants’ expectations of incongruence bias attention and response preparation towards the task-relevant dimension (or away from the task-irrelevant dimension) facilitating faster and more accurate responding (Braver et al. 2007; Braver et al. 2009). This proportion congruency effect is therefore thought to be a clear index of a person’s ability to implement top-down control over their responses (Bugg and Crump 2012). Such a manipulation in the context of an AI paradigm therefore provides a principled and novel approach to investigating the control of AI in ASD as it directly influences the requirement for top-down control of imitation, thus permitting a stronger test of the aberrant-control theory than previous work.
In order to investigate the possibility of specific deficits in imitative capability, and more importantly top-down control of imitation in ASD, the present study investigated AI effects in a large, well-defined cohort of ASD and TD participants. In a first step we sought to provide robust evidence for or against the notion that ASD is characterized by a specific impairment in imitative tendencies by examining whether ASD participants exhibited hypo-imitation. On the basis of prior research we expected to find no difference between ASD and TD. Second, and most importantly, we sought to investigate possible differences in the ability to exert top-down control of AI between ASD and TD by using a well-validated proportion congruency manipulation. To examine this we used an experimental manipulation of proportion congruence across two blocks of an AI paradigm. According to the aberrant-control theory, ASD participants should demonstrate reduced modulation of interference (relative to TD) when faced with a higher ratio of incongruent-to-congruent trials, which would suggest that they have a reduced ability to exert control over their level of imitation dependent upon context.
Methods
Participants
Participants in this study consisted of 42 individuals with confirmed autism spectrum disorder (ASD; mean age: 18.05, range 13–24, 5 female) and 50 individuals with typical development (TD; mean age: 18.04, range 14–24, 8 female) who were enrolled in the Cognitive Control in Autism (CoCoA) study examining the development of cognitive functioning in ASD from adolescence into early adulthood, at the University of California, Davis MIND Institute (see Table 1). To address the fact that this sample demonstrated significant differences in IQ between ASD and TD diagnosis groups, ensure that our results were not due to general deficits in cognitive function routinely observed in ASD samples, and to be more consistent with prior literature, we reanalyzed our data on an IQ-matched subsample of participants (N = 66). For pragmatic reasons we report the comprehensive details of these analyses in the supplementary materials. Importantly, matching in this manner did not meaningfully change the pattern of results reported in the main body of this manuscript. Participants were recruited from the greater Sacramento area into the CoCoA study through advertisements, advocacy groups, and the MIND Institute’s subject tracking system and research volunteer registry. All procedures were approved by the UC Davis Institutional Review Board, and all participants received financial remuneration for their participation.
Table 1.
Participant characteristics
| ASD (N = 42) | TH (N = 50) | Group comparison | |
|---|---|---|---|
|
| |||
| Age in years (SD) | 18.05 (2.93) | 18.04 (2.52) | t = 0.013, p = .989 |
| Sex (M, F) | 37, 5 | 42, 8 | X2(1) = 0.315, p = .574 |
| WASI-II FSIQ | 99.71 (14.49) | 112.72 (12.65) | t = 4.597, p < .001 |
| WASI-II VCI | 94.98 (12.81) | 109.16 (12.24) | t = 5.420, p < .001 |
| WASI-II PRI | 105.14 (18.64) | 113.54 (14.43) | t = 2.435, p = .017 |
| SCQ | 21.31 (5.82) | 3.18 (3.55) | t = 17.630, p < .001 |
| ADOS—severity (SD) | 7.76 (1.69) | – | – |
NB: VCI verbal comprehension, PRI perceptual reasoning, SCQ social communication questionnaire, ADOS Autism Diagnostic Observation Schedule
Inclusion Criteria
In order to be eligible for the study, all ASD participants were required to have a previous community diagnosis of ASD and needed to meet the diagnostic criteria for ASD on a Diagnostic and Statistical Manual (DSM)—5 Criteria Checklist and the Autism Diagnostic Observation Schedule 2 (ADOS-2; Lord et al. 2000). ASD participants were also required to have a total score greater than or equal to 15 on the Social Communication Questionnaire (SCQ), which measures social and communication problems (Rutter et al. 2003). Two ASD participants did not meet criteria on the SCQ ( SCQtotal = 7 and 11, respectively). However, in both cases the decision was made to retain these participants by a licensed clinical psychologist with extensive ASD expertise using the balance of available evidence (i.e., community diagnosis, ADOS-2, and DSM-5 checklist). TD participants needed a score of less than or equal to 11 on the SCQ, no social communication disorders as assessed by a DSM-5 symptoms checklist, and no first degree relative with a history of ASD nor any AXIS 1 psychopathology or neurodevelopmental disorders. Finally, all participants were required to have a Wechsler Abbreviated Scale of Intelligence (WASI)-II FSIQ of at least 70 (Wechsler 2011).
Measures
ADOS-2: Autism Diagnostic Observation Schedule 2 (Lord et al. 2000)
The ADOS-2 is a semi-structured interview administered by a licensed clinical psychologist (trained to reliability) to participants with ASD to assess symptoms of ASD. Participants are assessed on social affect, including communication and reciprocal social interaction, and restricted and repetitive behaviors. In order to be eligible for the study, participants with ASD had to have a total score ≥ 7. The ADOS-2 also provides a measure of relative severity to compare across modules (calibrated severity score, CSS) as well as specific severity scores for social affect (ADOS-SA) and restricted and repetitive behaviors (ADOS-RRB).
WASI-II: Wechsler Abbreviated Scale of Intelligence Second Edition (Wechsler 2011)
The WASI-II provides a brief, reliable measure of cognitive ability. Verbal Comprehension and Perceptual Reasoning indices were calculated based on performances of various tasks in the battery, including block design, vocabulary, matrix reasoning, and similarities. These scales are combined to form a measure of general intelligence, or FSIQ. In order to be eligible for the study, participants were required to have an FSIQ ≥ 70.
SCQ: Social Communication Questionnaire (Rutter et al. 2003)
The SCQ is a parent-report autism screening questionnaire that assesses the core behavioral domains of ASD. A total score ≥ 15 is indicative of ASD. The SCQ was primarily used to screen TD participants for the presence of ASD symptoms, and all TD participants were required to have a total SCQ < 11, indicating that they do not have ASD (Berument et al. 1999; Rutter et al. 2003).
Social Responsiveness Scale, Second Edition (Constantino and Gruber 2012)
The Social Responsiveness Scale, Second Edition (SRS-2) is a parent-report questionnaire that identifies the presence and severity of social communication impairments and restrictive and repetitive behaviors in ASD. The SRS-2 generates a total score that serves as an objective index of symptoms associated with autism, as well as two subscales: the Social Communication and Interaction (SRS-SCI) subscale and the Restricted Interests and Repetitive Behavior (SRS-RRB) subscale.
Stimuli
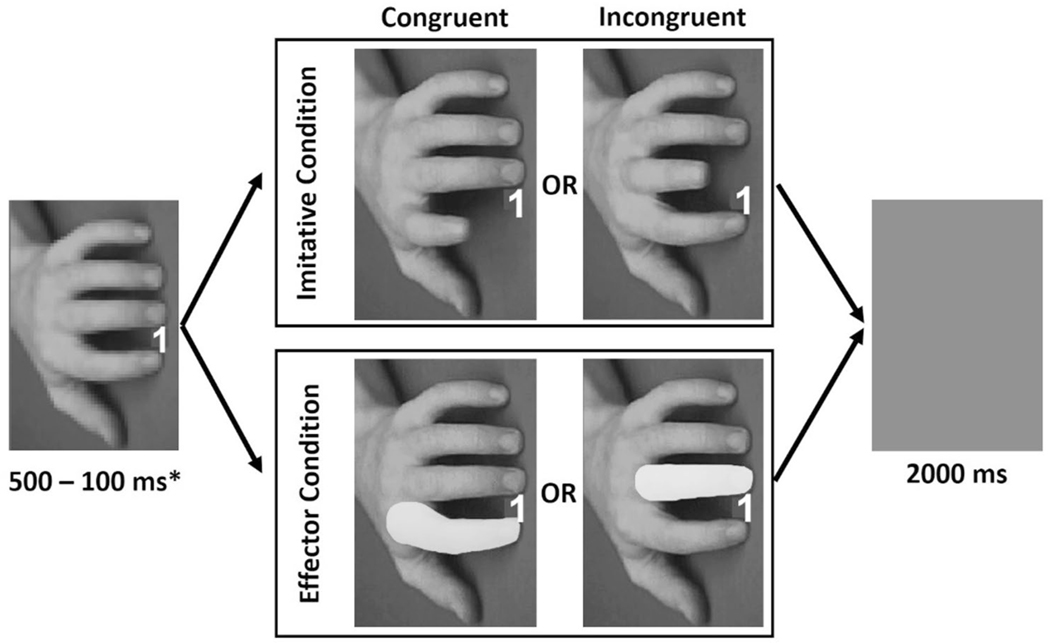
The experiment was programmed in Experiment (Python 3.5.2; Krause and Lindemann 2014) and administered using an HP laptop with a 14-inch LCD monitor. Stimuli were adapted from prior research on automatic imitation (e.g., Hogeveen and Obhi 2013; Sowden et al. 2016) and consisted of a set of 3 images that displayed a hand either executing a finger lift (imitation condition) or having a finger masked (effector condition). Effector trials are an ideal non-imitative reference condition as they draw attention to the effector (i.e., the highlighted finger) and create a resultant impact on response times without the ‘imitative’ action expressed in the counterpart condition. The first image consisting of a hand in a neutral position was displayed for 500–1000 ms.1 Subsequently, a numeric cue (either a ‘1’ or a ‘2’) was presented which cued a response from participants (see procedures below) while at the same time the hand was manipulated in one of two ways: (i) the hand performed either a congruent or incongruent index or middle finger lift action (imitative condition; congruent: the displayed finger lift matched that which the participant was required to perform; incongruent: the displayed finger lift was opposite that which the participant was required to perform), or (ii) a congruent or incongruent finger was highlighted in yellow (effector condition). Regardless of condition the hand manipulation lasted for 568 ms; this was done in order to be consistent with prior research using the same stimuli (e.g., Hogeveen and Obhi 2013; Obhi et al. 2014). Following the manipulation, the hand image disappeared from the screen and was replaced by a blank image for a fixed inter-trial interval (ITI) of 2000 ms. In order to ensure that any behavioral differences between congruent and incongruent trials were the result of motoric, as opposed to spatial, stimulus–response compatibility (cf., Cho and Proctor 2004), each image was rotated orthogonally (90°) relative to the participant (see Fig. 1).
Fig. 1.
4 of 8 possible trials in the experiment, representing all possible index finger trials (for the sake of brevity we do not present middle finger trials, cued by a ‘2’). Trials can either be imitative or effector trials, congruent or incongruent, and require the participant to lift either their index or middle finger (cued by ‘1’ or ‘2’ respectively). *Except the first trial of each block - see footnote above
Procedure
Participants completed two blocks of the experiment on two separate days as part of their participation in the CoCoA Study. Block order was counterbalanced across participants. Before beginning each block of the experiment, participants were asked to sit centrally aligned with the laptop and place their index and middle fingers of their right hand on the ‘v’ and ‘b’ keys respectively on the laptop keyboard. Participants were instructed that during the experiment they would be required to make index and middle finger lifts “as quickly and accurately as possible” in response to the presentation of a numeric cue (‘1’ and ‘2’, respectively), and to ignore all other stimuli on the screen. The experimenter sat directly behind the participant during an initial practice block to ensure that the participant was performing the task properly and remained present in the room, but unable to directly observe participants’ responses, throughout the remainder of the task. Task instruction was the same for both blocks of the experiment.
Each experimental block consisted of 144 trials. One block consisted of 75% congruent and 25% incongruent trials (mostly congruent; MC) and one consisted of 75% incongruent and 25% congruent trials (mostly incongruent; MI). Trials were split equally between imitative and effector trials (72 trials each) in both blocks. Each block was preceded by a practice block consisting of 16 trials and was followed by a main block split into 3 sections, between which participants were allowed to take short breaks. Each section of the block consisted of 48 pseudorandomized trials.
Data Analysis
Behavioral analyses focused on participants responses to the cue and analyzed them in terms of accuracy rates (%) and median reaction times (RT)2 on correct trials (in ms). Additionally, in order to account for a potential speed-accuracy trade-off in participants’ responses (see Schouten and Bekker 1967; Wickelgren 1977), a single measure combining accuracy and RT was computed. Specifically, we used the ‘EZ’ diffusion model (Wagenmakers et al. 2007). This model assumes that, when presented with a binary choice, participants accumulate evidence in favor of one or the other response until they reach a certain threshold, allowing them to settle on a single response. The rate at which this threshold is reached from a given starting point measures the mean rate of evidence accumulation in the decision-making process (hereafter referred to as drift rate); as such, lower values of this parameter indicate a lower signal-to-noise ratio in the evidence accumulation process. Behavioral scores for all three variables (accuracy, median reaction time, drift rate) were transformed and submitted to independent ANOVAs to examine both imitative tendencies in general as well as top-down control over imitation.3 We were also interested in examining previous findings that increases in autism severity were related to increases in AI effects (Sowden et al. 2016, experiment 2), and extending them by investigating whether the same relationship could be observed for control of AI effects. To do so we correlated behavioral scores for our three variables of interest with scores on two ASD symptom-severity measures. Specifically, we used subscale scores from both the clinician-administered ADOS-2 [social affect severity (ADOS-SA) and restricted and repetitive behaviors severity (ADOS-RRB)] and the informant-reported Social Responsiveness Scale [social communication severity (SRS-SCI) and restricted and repetitive behaviors severity (SRS-RRB)].
All statistical analyses were conducted using both frequentist and Bayesian techniques, using RStudio (version 1.1.456) and JASP (version 0.9.2.0) respectively and are therefore reported with an associated p value and Bayes Factor (BF). As a brief guide to interpretation of BF’s, a BF10 value represents the likelihood of the observed data under the alternative hypothesis (i.e., the hypothesis of effect) relative to the likelihood of the data under the null hypothesis (i.e., the assumption of no effect). Therefore, the value represents the strength of evidence in favor of one model over another and, unlike p values, is represented on an unbounded continuous scale. Held and Ott (2018) provide guidelines for interpretation of BF10; a BF10 of 1 demonstrates equivocal evidence for either model, values between 1 and 3 are considered as weak evidence for the alternative, 3–10 as intermediate, 10–100 as strong, and values greater than 100 are considered as conclusive evidence in favor of one model over another (in the case of a B F10 this would be conclusive support for the alternative hypothesis, see also Kass and Raftery 1995; Lakens et al. 2018). Any B F10 less than 1 is considered to be evidence in favor of the null hypothesis. Importantly, a BF10 < 1 constitutes evidence with strength equal to its reciprocal. In the current work we report any BF10 < 1 as its reciprocal BF01, thereby quantifying the support for the null hypothesis with respect to the same benchmark values provided above.
Results
Exclusions
Trials where participants responded more quickly than 150 ms: (ASD: 1.14% of trials, TD: 0.11% of trials) or more slowly than 2000 ms (ASD: 0.03% of trials, TD: 0.01% of trials) were excluded from further analyses. Additionally, any incorrect responses (ASD: 6.17% of congruent trials, 14.45% of incongruent trials; 9.23% of MC trials, 11.39% of MI trials; 9.18% of effector trials, 11.44% of imitation trials; TD: 2.86% of congruent trials, 6.71% of incongruent trials; 4.42% of MC trials, 5.15% of MI trials; 4.06% of effector trials, 5.51% of imitation trials) or trials that received no response (ASD: 0.71% of congruent trials, 1.31% of incongruent trials; 0.39% of MC trials, 1.62% of MI trials; 1.16% of effector trials, 0.86% of imitation trials; TD: 0.59% of congruent trials, 1.13% of incongruent trials; 0.56% of MC trials, 1.17% of MI trials; 0.93% of effector trials, 0.79% of imitation trials) were removed for calculation of the reaction time metric. Finally, for each participant, any trial that showed an RT more than three standard deviations above or below the mean for each condition (0.83% of remaining trials) was removed from further analyses. In total, 8.84% of trials were removed across all conditions and diagnosis groups. Within diagnosis groups, 5.77% of trials were removed for TD, 12.49% of trials were removed for ASD.
Automatic Imitation in ASD
To examine whether there exists a selective imitation deficit in participants with ASD, participants’ scores were averaged across block to create a single composite measure for each variable. Interference scores (calculated as scores on congruent—incongruent trials) were then computed for each dependent variable (accuracy, RT, drift rate; see Table 2). It is important to note that in order to provide equivalent (positive) data between measures, interference scores for RT were calculated as incongruent-congruent. For all three dependent variables there was a significant (Holm–Bonferroni corrected—Holm 1979) effect of stimulus congruence for both imitation and effector trials for both diagnosis groups. These interference scores were submitted to three independent 2 (stimulus type: imitation vs. effector) x 2 (diagnosis: ASD vs. TD) ANOVAs (see Fig. 2).
Table 2.
Means and standard deviations of mean scores across the factors diagnosis, stimulus type, and congruence
| Diagnosis | Stimulus type | Measure | Congruent mean (SD) | Incongruent mean (SD) | Comparison t* (p) |
|---|---|---|---|---|---|
|
| |||||
| ASD | Imitation | Accuracy | 92.39 (10.87) | 80.94 (14.66) | 6.214 (< .001) |
| RT | 422.39 (59.41) | 465.76 (62.62) | 9.319 (< .001) | ||
| Drift rate | 0.012 (0.005) | 0.008 (0.005) | 7.777 (< .001) | ||
| Effector | Accuracy | 93.06 (11.77) | 84.92 (13.79) | 4.817 (< .001) | |
| RT | 432.74 (50.07) | 476.80 (58.23) | 9.560 (< .001) | ||
| Drift rate | 0.013 (0.005) | 0.009 (0.005) | 6.859 (< .001) | ||
| TD | Imitation | Accuracy | 96.76 (4.51) | 90.98 (7.69) | 7.455 (< .001) |
| RT | 422.06 (45.01) | 456.70 (53.64) | 10.761 (< .001) | ||
| Drift rate | 0.015 (0.004) | 0.011 (0.004) | 8.915 (< .001) | ||
| Effector | Accuracy | 97.14 (3.24) | 93.33 (6.43) | 5.189 (< .001) | |
| RT | 434.48 (52.61) | 463.89 (55.54) | 8.633 (< .001) | ||
| Drift rate | 0.015 (0.004) | 0.012 (0.004) | 6.790 (< .001) | ||
df = 91
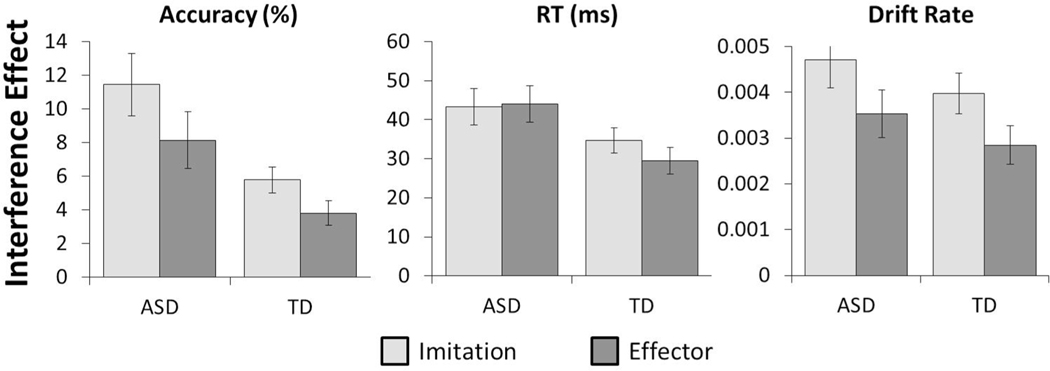
Fig. 2.
Mean interference effects for each of the three variables analyzed split by diagnosis ASD vs. TD) and stimulus type (Imitation vs. Effector). Error bars represent standard error of the mean
There was a significant effect of stimulus type on participants’ accuracy interference scores [F(1,90) = 10.379, p < .01, BF10 = 11.35] demonstrating that participants experienced greater interference on imitation (M = 8.610, SD = 9.04) relative to effector trials (M = 5.978, SD = 8.36). There was also a significant effect of stimulus type on drift rate interference scores [F(1,90) = 8.671, p < .01, BF10 = 6.07] demonstrating that interference on participants’ rate of information processing was reduced in the face of effector (M = 0.003, SD = 0.003), relative to imitation (M = 0.004, SD = 0.004), trials. There was no significant effect of stimulus type on corresponding RT [F(1,90) = 0.788, p = .377, BF01 = 4.35] interference scores.
As with stimulus type, there was a significant effect of diagnosis on participants’ accuracy interference scores [F(1,90) = 9.511, p < .01, BF10 = 11.59] indicating that ASD participants (M = 9.79, SD = 7.75) demonstrated larger interference scores than TD (M = 4.79, SD = 7.75). The effect of diagnosis on participants’ RT interference scores was also significant [F(1,90) = 5.569, p = .023, BF10 = 2.143] demonstrating that ASD participants (M = 43.71, SD = 23.66) exhibited greater interference to response latencies than did those with TD (M = 32.03, SD = 23.66). However, there was no significant effect of diagnosis on drift rate [F(1,90) = 1.480, p = .227, BF01 = 2.85] indicating that ASD and TD participants performed equivalently with regard to this measure.
Finally, and of most theoretical importance, there was no significant interaction between diagnosis and stimulus type on accuracy [F(1,90) = 0.670, p = .415, BF01 = 1.04], RT [F(1,90) = 1.339, p = .250, BF01 = 3.88], or drift rate [F(1,90) < 0.01, p = .954, BF01 = 4.12] interference scores. These data therefore clearly demonstrate that ASD participants did not display a specific imitative deficit relative to those with TD.
Top–Down Control of Automatic Imitation in ASD
In order to examine whether top–down control of imitative tendencies is uniquely impaired in ASD, interference scores were calculated for each diagnosis group, stimulus type, and block. We then calculated a composite proactive control benefit score (cf., Braver 2012) by subtracting interference scores in the mostly incongruent block from those in the mostly congruent block (MC-MI), larger scores therefore representing a greater adjustment of proactive control (see Table 3, for means broken down by congruence see supplementary Tables S4 and S5). For imitation trials there was a significant effect of block for all variables for ASD and TD participants, with the exception of drift rate for TD that was only marginally significant. For effector trials there was a significant effect of block for all variables for both ASD and TD participants. The proactive control benefit scores were submitted to three independent 2 (stimulus type: imitation vs. effector) × 2 (diagnosis: ASD vs. TD) ANOVAs (see Fig. 3).
Table 3.
Means and standard deviations of interference scores (congruent-incongruent) across the factors diagnosis, stimulus type, and block
| Diagnosis | Stimulus type | Measure | MC mean (SD) | MI mean (SD) | Comparison t* (p) |
|---|---|---|---|---|---|
|
| |||||
| ASD | Imitation | Accuracy | 16.31 (16.91) | 6.58 (12.45) | 3.571 (< .001) |
| RT | 57.65 (44.19) | 29.08 (43.88) | 2.886 (< .01) | ||
| Drift rate | 0.006 (0.005) | 0.004 (0.004) | 2.418 (< .05) | ||
| Effector | Accuracy | 11.81 (13.08) | 4.48 (14.89) | 2.715 (< .01) | |
| RT | 58.25 (43.47) | 29.87 (34.19) | 3.643 (< .001) | ||
| Drift rate | 0.004 (0.004) | 0.003 (0.005) | 2.080 (< .05) | ||
| TD | Imitation | Accuracy | 7.60 (8.53) | 3.95 (4.48) | 3.191 (< .01) |
| RT | 47.41 (35.37) | 21.88 (26.48) | 4.218 (< .001) | ||
| Drift rate | 0.004 (0.004) | 0.003 (0.004) | 1.808 (.077) | ||
| Effector | Accuracy | 6.08 (7.93) | 1.54 (5.42) | 3.662 (< .001) | |
| RT | 41.52 (32.24) | 17.30 (27.49) | 4.806 (< .001) | ||
| Drift rate | 0.004 (0.004) | 0.002 (0.003) | 2.699 (< .01) | ||
df = 91
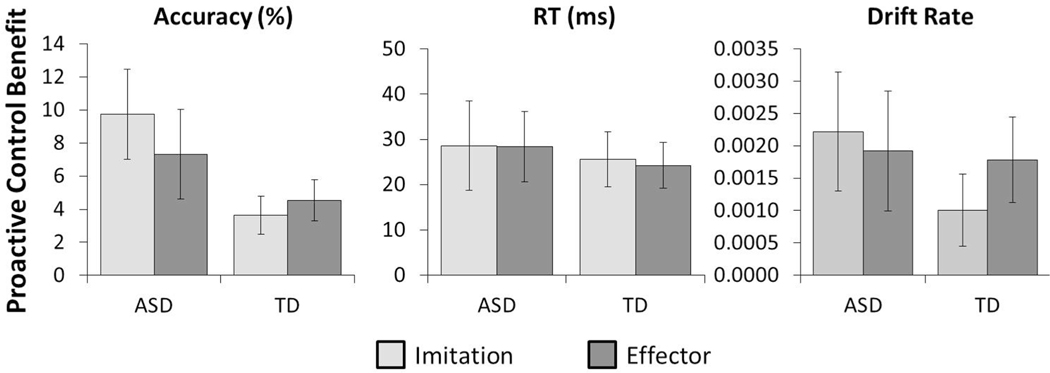
Fig. 3.
Mean proactive control benefit effects for each of the three variables analyzed split by diagnosis (ASD vs. TD) and stimulus type (Imitation vs. Effector). Error bars represent standard error of the mean
There was no significant effect of stimulus type on proactive control scores for accuracy [F(1,90) = 0.214, p = .645, BF01 = 7.41], RT [F(1,90) = 0.011, p = .917, BF01 = 9.25], or drift rate [F(1,90) = 0.119, p = .731, BF01 = 8.13], indicating that the level of proactive control did not differ between imitation and effector trials.
There was a marginally significant effect of diagnosis on proactive control scores for accuracy [F(1,90) = 3.832, p = .053, BF01 = 1.28], indicating that ASD participants (M = 8.53, SD = 10.82) demonstrated marginally greater proactive control benefit than TD (M = 4.09, SD = 10.82), although the evidence for this effect was weak. There was no significant effect of diagnosis on proactive control scores for RT [F(1,90) = 0.249, p = .619, BF01 = 6.94], or drift rate [F(1,90) = 0.693, p = .407, BF01 = 5.29], indicating that ASD and TD participants both engaged in modification of imitative tendencies in response to changes in the ratio of incongruent-to-congruent trials.
Finally, we also observed no significant interactions between stimulus type and diagnosis for accuracy [F(1,90) = 0.987, p = .323, BF01 = 9.52], RT [F(1,90) = 0.006, p = .938, BF01 = 50.00], or drift rate [F(1,90) = 0.590, p = .444, BF01 = 29.41], clearly demonstrating that ASD participants did not display a specific deficit in the control of imitation relative to those with TD on these measures.
Autism Symptom Severity and Imitation
To examine the relationship between ASD symptom severity and imitation we correlated both interference scores (i.e., AI effects) and proactive control benefit scores (i.e., control of AI effects) for each of our behavioral measures for imitation trials with scores on the subscales of two ASD symptom-severity measures: ADOS [subscales: social affect severity (ADOS-SA) and restricted and repetitive behaviors severity (ADOS-RRB)] and SRS [subscales: social communication severity (SRS-SCI) and restricted and repetitive behaviors severity (SRS-RRB)]. Although SRS measures were collected for both ASD and TD, the distribution of scores on the SRS measure was extremely narrow and clustered at the low end of the scale for TD participants. The scores in this group were unsuitable for a correlational analysis, meaning that the following analyses were only conducted for the ASD sample.
Relationship to Imitation
ADOS-SA severity scores showed a marginally significant correlation with RT interference scores (rs = − .305, p = .049), with higher ADOS-SA relating to lower levels of interference. In order to examine whether the relationship was unique to imitative trials, we also examined the relationship between ADOS-SA severity and RT interference on effector trials (rs = − .237, p = .132) and found it to be equivalent to that for imitation trials (z = 0.32, p = .749). ADOS-SA severity was not significantly related to accuracy or drift rate interference scores. ADOS-RRB severity was not significantly associated with interference scores on any dependent variable.
SRS-SCI severity scores showed a significant correlation with accuracy interference scores on imitation trials (rs = − .342, p = .041), with higher SRS-SCI relating to lower levels of interference. However, when comparing this relationship to that observed for effector trials (rs = − .146, p = .396) we found the two to be equivalent (z = 0.9, p = .368). SRS-SCI severity scores also showed a significant correlation with drift rate interference scores on imitation trials (rs = − .365, p = .029), once again with higher SRSSCI relating to lower levels of interference. However, this relationship was also not unique to imitation trials (effector trials: rs = − .133, p = .440; z = 1.01, p = .313). SRS-SCI severity was not significantly related to RT interference scores. SRS-RRB severity was not significantly associated with interference scores on any dependent variable.
Relationship to Control of Imitation
No significant relationship emerged between ADOS-SA or ADOS-RRB severity scores and any of our dependent variables. Similarly, no significant relationships were observed between SRS-SCI or SRS-RRB severity scores and any of the dependent variables, suggesting that ASD symptom severity did not relate to participants’ ability to exert proactive control of imitation.
Discussion
Previous research has proved equivocal with regards to the possibility of a specific imitation deficit in autism spectrum disorders (ASD). While studies of voluntary imitation have tended to provide evidence in favor of a deficit (Williams et al. 2001, 2004), research using stimulus–response compatibility paradigms to investigate automatic imitation (AI) have, by and large, shown AI to be unimpaired in ASD (Hamilton et al. 2007; Press et al. 2010; Schunke et al. 2016, Sowden et al. 2016). The results of the current work lend further support to the notion that AI is unimpaired in ASD. Specifically, our results suggest that while ASD participants were less accurate and exhibited longer response latencies (although drift rate and response conservativeness were unaffected), they experienced the same degree of interference due to incongruent action stimuli as those with typical development (TD) across our three behavioral measures. These data align strongly with the results of experiment 1 of Sowden et al. (2016), where, using an identical task (although without the proportion congruency manipulation used in the current work), no differences in AI were observed between ASD and TD. Importantly, other than Sowden and colleagues’ study, these data represent one of the largest ASD samples investigated to date, adding significant weight to a growing corpus of research that suggests that automatic imitation remains unimpaired in ASD. However, although previous research has promoted the lack of impairment on AI tasks in ASD as proof against the notion of a ‘broken’ mirror neuron system (MNS, cf., Southgate and Hamilton 2008; Sowden et al. 2016), we acknowledge that no strong claim can be made about the normative functioning of the MNS from a neural standpoint on the basis of behavioral data alone, as no one-to-one mapping between brain systems and behavior exists (Iacoboni 2017). Rather, these data demonstrate that the observation of motor actions incongruent with one’s own required motor action impairs task performance regardless of diagnosis.
Despite this equivalence in AI between the diagnosis groups we did observe tentative evidence that AI in ASD was related to individual variations in disorder severity as measured by the social-affect (SA) subscale of the ADOS-2, suggesting that those with more severe autistic traits experienced less interference from incongruent imitative stimuli. This finding stands in contrast to previous research using AI paradigms (Sowden et al. 2016, experiment 2), and suggests that more severe autistic-impairments lead to a concomitant reduction in levels of AI (cf., Zachor et al. 2010). Although at first glance these data appear to support the notion of impaired imitation in ASD, it is critical to note that we also observed a similar correlation with ADOS-2 severity scores for effector trials (a relationship which does not appear to have been examined in Sowden and colleagues paper), which, given their ostensibly non-imitative nature, suggests that these relationships were not unique to imitation. Instead, our results may suggest that participants with more severe autistic-traits engaged with the task in a different manner to those with a less severe presentation. Specifically, prior research has demonstrated a clear relationship between ASD and circumscribed attentional processes (e.g., Liss et al. 2006), as well as showing that perseveration of attention increases with ASD symptom severity (Sasson et al. 2008). Thus, our findings may indicate that participants with more severe ASD in the current study perseverated more on the numeric cue (consistent with task demands), and therefore devoted less attention to the task-irrelevant stimulus (i.e., the hand), leading to a reduction in interference. Although this notion remains entirely speculative, it is supported by the equivalent analysis for the IQ case-matched sample in which we did not observe any relationships between imitation and ADOS-2 severity measures. Given that TD-ASD IQ case-matching necessarily involves the removal of the most impaired ASD participants, it makes logical sense that any correlations between severity and task performance may disappear. These data may therefore provide a fertile ground for future work in this area examining the role of attentional processes in AI paradigms specifically involving ASD participants.
Turning to our primary research question, the lack of evidence for an AI deficit as well as evidence of hyper-imitation in AI paradigms has led some researchers to suggest that, rather than a general imitative deficit, ASD participants exhibit a specific deficit in the top-down control of imitative behavior (Sowden et al. 2016; Spengler et al. 2010). Previous work has provided evidence for this theory by demonstrating that AI effects are negatively correlated with self-other processing in ASD (as measured by theory of mind tasks, Spengler et al. 2010). Researchers have thus inferred that difficulties in dissociating the representations of one’s own actions from those of another are hallmarks of a faulty top-down control of imitative tendencies (Bird et al. 2006; Cook and Bird 2012). As such, it has been suggested that deficits in social functioning in ASD are driven by an inability to exert top-down control of imitative tendencies. Constituting a distinct advantage over previous research, the current study included an experimental manipulation of the ratio of incongruent-to-congruent trials, allowing us to directly influence the requirement for control of imitation. Consistent with the notion of preserved imitation control in ASD we observed that both ASD and TD participants were able to reduce the level of interference from incongruent imitative stimuli to a similar degree when presented with a higher ratio of incongruent-to-congruent trials (although we acknowledge that in a few cases this effect was only at the trend level). As such these data stand in contrast to the notion of impairments in self-other processing in ASD (e.g., Spengler et al. 2010); instead demonstrating that ASD participants are able to exert appropriate control of imitative tendencies. These results are broadly consistent with the findings of a recent meta-analysis indicating no clear evidence of hyper-imitation in ASD and concluding that the disorder is not associated with deficits in imitation or imitative control (Cracco et al. 2018). However, although imitative control may be spared in ASD, another view suggests that it is the ability to adapt control of imitative tendencies to the particular context that is impaired in ASD (Cracco et al. 2018; Forbes et al. 2017; Hamilton 2013). Our results contradict this view by providing compelling evidence that imitative tendencies are modulated by contextual factors in ASD. Specifically, heightening participants’ expectations of incongruence by presenting a higher ratio of incongruent-to-congruent trials comprised a contextual cue for a shift in strategy to a more proactive form of control (Braver et al. 2007) that was accomplished by ASD and TD participants alike.
At the more general level, the finding that a block-wise manipulation of proportion congruency resulted in modulation of control of AI for TD participants is deserving of further inquiry. Specifically, this finding raises the question of whether AI can truly be described as an automatic process. Processes are generally described as automatic when they occur without endogenous processing (i.e., cognitive processes not under explicit control). In the context of AI, the traditional notion of it being an automatic process largely rests upon the finding that participants struggle to overcome the influence of a task irrelevant stimulus even when it impairs response selection; as such, it can be surmised that AI does not require an intention to imitate, cannot be controlled and is therefore involuntarily (Cracco and Brass 2019; Heyes 2011; Moors and De Houwer 2006). Although the aim of our current work was not to analyze AI effects in TD, our paradigm and sample size allowed us to test the ‘automaticity’ assumption in this sample. Only a single previous study has sought to address automaticity of AI using such a manipulation in TD; Hogeveen and Obhi (2013) found that TD participants did not show any proportion congruency based manipulation of AI, concluding that this was evidence in favor of the assumption of automaticity. However, the current data provide persuasive evidence against this view by demonstrating that AI effects can indeed be attenuated in the context of a heightened expectation of incongruent stimuli. Given that this effect was clear for both ASD and TD groups separately, and considering the significant increase in sample size and therefore power over that prior work, our data therefore suggest that AI may be no more automatic than other SRC paradigms that are sensitive to proportion congruence manipulations (e.g., Stroop; Tzelgov et al. 1992). One possible reason for this disparity between these two experiments is that Hogeveen and Obhi (2013) provided explicit task instruction to the participants that the background hand would either be likely to match the number cue (MC block), or would be unlikely to match the number cue (MI block), whereas the current work provided no such instruction. In doing this, the authors may have biased participants processing towards the relevant stimulus dimension (i.e., the numeric cue), resulting in a reduced interference effect (as the irrelevant stimulus dimension would exert less influence on participants’ responses). Indeed, explicit task instruction may thus have engaged a form of proactive control that operated across the entire experiment rendering responses insensitive to the manipulation of proportion congruence across experimental blocks.
It is critical to note a number of limitations to the current work so that these data can be interpreted with the appropriate caution. First, our results highlighted the rather counterintuitive finding that AI effects appeared to be equivalent in both imitative and non-imitative effector conditions for reaction time. However, there was a significant effect on participants’ accuracy drift rate meaning that we must refrain from an over-interpretation of this null-effect. Nevertheless, we cite two possible reasons for this finding. On the one hand, while imitation is generally thought to rely upon observed action (Leighton and Heyes 2010), it is possible that the provision of a non-imitative cue alone was sufficient to engage an action priming effect and thus affect task performance. This is consistent with research showing that action priming effects can be observed even when participants are only presented with single images that only imply action (Craighero et al. 2002; Mele et al. 2014; Vogt et al. 2003). This notion is also consistent with neuroimaging research demonstrating that brain networks active during imitation control are also activated when required to inhibit imitation of an abstract spatial cue, suggesting that both explicitly imitative and non-imitative cues engaged similar cognitive mechanisms (Cross and Iacoboni 2013). On the other hand, while the difference between imitation and effector trials did not reach significance, it is clear that there was at least a numeric difference in the expected direction (i.e., lower interference on non-imitative effector trials), suggesting that the null difference may simply reflect a lack of statistical power to detect a difference.
On a related point, to minimize the effect of spatial compatibility, we and other authors present the hand stimulus rotated 90 degrees with respect to the participant’s hand (Cook and Bird 2012; Hogeveen et al. 2014; Sowden et al. 2016). This approach introduces the potential confound of ‘orthogonal spatial compatibility’: stimulus features occurring in the upper portion of space can facilitate right button responses, and vice versa (Cho and Proctor 2004). Therefore, a limitation of the current study is that complex orthogonal spatial compatibility might have influenced task performance. However, it is improbable that orthogonal spatial compatibility drove the null group differences observed in the current study. This pattern of results would necessitate ASD participants presenting with a combination of reduced spatial interference and increased AI interference, and this is unlikely given an elegant recent study showing matched spatial compatibility effects across ASD and TD (Sowden et al. 2016). A further question is whether our failure to identify a specific relationship between imitative capability and ASD symptom severity may have been partly driven by the orthogonal spatial compatibility effect. Sowden et al. (2016) observation of a positive association between the two in experiment 2 was apparent after explicitly controlling for orthogonal spatial compatibility effects, an approach that was not possible in the current work. Further research should seek to directly test to what degree such associations may be affected by orthogonal spatial compatibility.
Second, our experimental paradigm did not include a block in which there were an equal number of congruent and incongruent trials, as has been included in previous research on AI (Hogeveen and Obhi 2013) and other SRC paradigms (see Bugg and Crump 2012). Our reasoning for this omission was that any adjustment in control due to proportion congruence should be identifiable with only the blocks we used in the current work (i.e., MC vs. MI), the inclusion of a ‘50/50’ block was therefore unnecessary to examine the effect of interest. Nevertheless, this omission leaves us unable to specifically analyze whether AI effects in either of the included blocks were significantly different to such a ‘50/50’ block.
Finally, as with any research involving ASD individuals, we must note certain specific sample-focused limitations to the generalizability of these findings. In particular, our sample of ASD participants consisted of individuals without intellectual disability (ID, i.e., had an FSIQ > 70). While this is not uncommon in this sphere of work (indeed most previous research on AI in ASD has used a similar, if not more capable sample of ASD participants—see Sowden et al. 2016; Spengler et al. 2010) it is conceivable (and indeed likely given the results of our correlational analyses) that the observed pattern of data may change when ASD participants who also exhibit intellectual disability (ID) are examined. Nevertheless, considering that ASD is the disorder most likely to overlap with ID (Bartak and Rutter 1976; Wilkins and Matson 2009), and that much of the literature examines ASD participants with average or better intellectual abilities (Hurley and Levitas 2007), future research should seek to delineate more clearly whether imitative capability as measured by AI is fundamentally linked to disorder severity. We also note a specific limitation to the generalizability of our findings, regarding the age of the participants in the current work, who were for the most part adolescents. With the notable exception of Hamilton et al. (2007), all prior research on AI in ASD using similar methods (i.e., manual stimuli) to those used here has used older adult samples, typically between 30 and 40 years of age. To our knowledge, the only research to examine the effect of age on AI in ASD found a negative correlation between the two, suggesting that older ASD individuals experience less AI (Schulte-Rüther et al. 2017). However, that research used emotive facial stimuli and was therefore likely to engage different cognitive processes to those involved in manual AI. Schulte-Rüther et al. (2017) results’ also contrast to data gathered in TD individuals showing evidence for an increase in AI with experience (Heyes et al. 2005) and therefore, presumably, with age. One major drawback of extant work is that it has been almost entirely cross-sectional, limiting the generalizability of the findings. Future work should seek to adopt longitudinal approaches to address the question of whether AI effects are modulated by age, and whether potential age effects differ between ASD and TD individuals.
In summary, in contrast to the notion of impairments in imitation in ASD, the current work found no evidence for a specific deficit in imitative capability in ASD as measured by AI. Instead, we found that the presentation of an action incongruent to that required by a cue resulted in an equivalent degree of interference for both ASD and TD participants. In addition, the current work provided strong evidence that those with ASD are able to exert top-down proactive control over AI and that the level of this control is no different from that observed in TD, calling into question the notion of diminished imitative control being a hallmark of ASD.
Supplementary Material
Acknowledgments
This work was supported by a National Institute of Mental Health Grant (R01MH10651802) awarded to Marjorie Solomon. The authors would like to thank Matthew Elliott, Garrett Gower, Ashley Tay, and Rachel Wulff for their assistance with this study.
Footnotes
The length of presentation was determined by randomly selecting a time within this range (in 50 ms increments). During the first trial of each block this image was displayed for 2000–3000 ms to give participants extra time to prepare for the task.
Median RTs were used instead of mean RTs in order to provide an unbiased measure in the presence of skewed RT distributions (see Brenner and Smeets 2019; Rousselet and Wilcox 2019; Whelan 2008). In order to ascertain that this choice did not unduly affect the pattern of data observed we re-ran the analyses using mean RTs and observed results equivalent to those using median RTs.
In addition, and in order to provide data consistent with prior work (e.g., 47) we re ran our analyses on inverse efficiency scores (IES: calculated as the reaction time divided by the accuracy) that are also designed to address the speed-accuracy trade-of. The results of these analyses did not differ from those using drift rate and we therefore only report the drift rate analysis in this manuscript.
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of UCD IRB Administration (IRB ID: 254439–35) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest The authors declare that they have no conflict of interest.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Bartak L, & Rutter M (1976). Differences between mentally retarded and normally intelligent autistic children. Journal of Autism and Childhood Schizophrenia, 6(2), 109–120. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, & Bailey A (1999). Autism screening questionnaire: Diagnostic validity. The British Journal of Psychiatry, 175(5), 444–451. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, & Frith U (2006). Attention does not modulate neural responses to social stimuli in autism spectrum disorders. NeuroImage, 31(4), 1614–1624. [DOI] [PubMed] [Google Scholar]
- Bird G, Leighton J, Press C, & Heyes C (2007). Intact automatic imitation of human and robot actions in autism spectrum disorders. Proceedings of the Royal Society B: Biological Sciences, 274(1628), 3027–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Bekkering H, & Prinz W (2001). Movement observation affects movement execution in a simple response task. Acta Psychologica, 106(1–2), 3–22. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschläger A, & Prinz W (2000). Compatibility between observed and executed finger movements: Comparing symbolic, spatial, and imitative cues. Brain and Cognition, 44(2), 124–143. [DOI] [PubMed] [Google Scholar]
- Braver TS (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Gray JR, & Burgess GC (2007). Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Variation in Working Memory, 75, 106. [Google Scholar]
- Braver TS, Paxton JL, Locke HS, & Barch DM (2009). Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences, 106(18), 7351–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner E, & Smeets JB (2019). How can you best measure reaction times? Journal of Motor Behavior, 51(5), 486–495. [DOI] [PubMed] [Google Scholar]
- Bugg JM, & Crump MJ (2012). In support of a distinction between voluntary and stimulus-driven control: A review of the literature on proportion congruent effects. Frontiers in Psychology, 3, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catmur C, Walsh V, & Heyes C (2007). Sensorimotor learning configures the human mirror system. Current Biology, 17(17), 1527–1531. [DOI] [PubMed] [Google Scholar]
- Chartrand TL, & Lakin JL (2013). The antecedents and consequences of human behavioral mimicry. Annual Review of Psychology, 64, 285–308. [DOI] [PubMed] [Google Scholar]
- Cho YS, & Proctor RW (2004). Influences of multiple spatial stimulus and response codes on orthogonal stimulus—response compatibility. Perception & Psychophysics, 66(6), 1003–1017. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale–second edition (SRS-2). Torrance: Western Psychological Services. [Google Scholar]
- Cook JL, & Bird G (2012). Atypical social modulation of imitation in autism spectrum conditions. Journal of Autism and Developmental Disorders, 42(6), 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracco E, Bardi L, Desmet C, Genschow O, Rigoni D, De Coster L, et al. (2018). Automatic imitation: A meta-analysis. Psychological Bulletin, 144(5), 453. [DOI] [PubMed] [Google Scholar]
- Cracco E, & Brass M (2019). Reaction time indices of automatic imitation measure imitative response tendencies. Consciousness and Cognition, 68, 115–118. [DOI] [PubMed] [Google Scholar]
- Craighero L, Bello A, Fadiga L, & Rizzolatti G (2002). Hand action preparation influences the responses to hand pictures. Neuropsychologia, 40(5), 492–502. [DOI] [PubMed] [Google Scholar]
- Cross KA, & Iacoboni M (2013). Optimized neural coding? Control mechanisms in large cortical networks implemented by connectivity changes. Human Brain Mapping, 34(1), 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschrijver E, Wiersema JR, & Brass M (2017). The influence of action observation on action execution: Dissociating the contribution of action on perception, perception on action, and resolving conflict. Cognitive, Affective, & Behavioral Neuroscience, 17(2), 381–393. [DOI] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, & Rizzolatti G (1992). Understanding motor events: A neurophysiological study. Experimental Brain Research, 91(1), 176–180. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, & Rizzolatti G (2014). Mirror neuron research: The past and the future. Philosophical Transactions of the Royal Society B: Biological Sciences. 10.1098/rstb.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I, Keown CL, Lincoln AJ, Pineda JA, & Müller RA (2014). Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry, 71(7), 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes PA, Wang Y, & Hamilton AFDC (2017). STORMy interactions: Gaze and the modulation of mimicry in adults on the autism spectrum. Psychonomic Bulletin & Review, 24(2), 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantman A, Kapp SK, Orenski K, & Laugeson EA (2012). Social skills training for young adults with high-functioning autism spectrum disorders: A randomized controlled pilot study. Journal of Autism and Developmental Disorders, 42(6), 1094–1103. [DOI] [PubMed] [Google Scholar]
- Gowen E, Stanley J, & Miall RC (2008). Movement interference in autism-spectrum disorder. Neuropsychologia, 46(4), 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecucci A, Brambilla P, Siugzdaite R, Londero D, Fabbro F, & Rumiati RI (2013). Emotional resonance deficits in autistic children. Journal of Autism and Developmental Disorders, 43(3), 616–628. [DOI] [PubMed] [Google Scholar]
- Hamilton AFDC (2013). Reflecting on the mirror neuron system in autism: A systematic review of current theories. Developmental Cognitive Neuroscience, 3, 91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AFDC, Brindley RM, & Frith U (2007). Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? Neuropsychologia, 45(8), 1859–1868. [DOI] [PubMed] [Google Scholar]
- Held L, & Ott M (2018). On p-values and Bayes factors. Annual Review of Statistics and Its Application, 5, 393–419. [Google Scholar]
- Heyes C (2011). Automatic imitation. Psychological Bulletin, 137(3), 463. [DOI] [PubMed] [Google Scholar]
- Heyes C, Bird G, Johnson H, & Haggard P (2005). Experience modulates automatic imitation. Cognitive Brain Research, 22(2), 233–240. [DOI] [PubMed] [Google Scholar]
- Hogeveen J, & Obhi SS (2013). Automatic imitation is automatic, but less so for narcissists. Experimental Brain Research, 224(4), 613–621. [DOI] [PubMed] [Google Scholar]
- Hogeveen J, Obhi SS, Banissy MJ, Santiesteban I, Press C, Catmur C, & Bird G (2014). Task-dependent and distinct roles of the temporoparietal junction and inferior frontal cortex in the control of imitation. Social Cognitive and Affective Neuroscience, 10(7), 1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S (1979). A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics, 6(2), 65–70. [Google Scholar]
- Hurley AD, & Levitas AS (2007). The importance of recognizing autism spectrum disorders in intellectual disability. Mental Health Aspects of Developmental Disabilities, 10(4), 157. [Google Scholar]
- Iacoboni M (2017). Neurobiology of imitation in autism. In Casanova MF, El-Baz A, & Suri JS (Eds.), Autism imaging and devices (pp. 75–94). Boca Raton, FL: CRC Press. [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, & Rizzolatti G (1999). Cortical mechanisms of human imitation. Science, 286(5449), 2526–2528. [DOI] [PubMed] [Google Scholar]
- Ingersoll B (2008). The social role of imitation in autism: Implications for the treatment of imitation deficits. Infants & Young Children, 21(2), 107–119. [Google Scholar]
- Kane MJ, & Engle RW (2003). Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General, 132(1), 47. [DOI] [PubMed] [Google Scholar]
- Kass RE, & Raftery AE (1995). Bayes factors. Journal of the American Statistical Association, 90(430), 773–795. [Google Scholar]
- Kornblum S, Hasbroucq T, & Osman A (1990). Dimensional overlap: Cognitive basis for stimulus-response compatibility—A model and taxonomy. Psychological Review, 97(2), 253. [DOI] [PubMed] [Google Scholar]
- Krause F, & Lindemann O (2014). Expyriment: A Python library for cognitive and neuroscientific experiments. Behavior Research Methods, 46(2), 416–428. [DOI] [PubMed] [Google Scholar]
- Lakens D, McLatchie N, Isager PM, Scheel AM, & Dienes Z (2018). Improving inferences about null effects with Bayes factors and equivalence tests. The Journals of Gerontology: Series B, 75, 45–57. [DOI] [PubMed] [Google Scholar]
- Lakin JL, & Chartrand TL (2003). Using nonconscious behavioral mimicry to create affiliation and rapport. Psychological Science, 14(4), 334–339. [DOI] [PubMed] [Google Scholar]
- Leighton J, & Heyes C (2010). Hand to mouth: Automatic imitation across effector systems. Journal of Experimental Psychology: Human Perception and Performance, 36(5), 1174. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, & Jacoby LL (1994). Stroop process dissociations: The relationship between facilitation and interference. Journal of Experimental Psychology: Human Perception and Performance, 20(2), 219. [DOI] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, & Kinsbourne M (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155–172. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. (2000). The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lowe DG, & Mitterer JO (1982). Selective and divided attention in a Stroop task. Canadian Journal of Psychology, 36(4), 684. [DOI] [PubMed] [Google Scholar]
- Mele S, Mattiassi AD, & Urgesi C (2014). Unconscious processing of body actions primes subsequent action perception but not motor execution. Journal of Experimental Psychology: Human Perception and Performance, 40(5), 1940. [DOI] [PubMed] [Google Scholar]
- Merhoum N, Mengarelli F, Mottolese R, Andari E, & Sirigu A (2015). Social functioning in autism. In Autism spectrum disorders (Vol. 180, pp. 46–53). Karger Publishers, Basel. [Google Scholar]
- Moors A, & De Houwer J (2006). Automaticity: A theoretical and conceptual analysis. Psychological Bulletin, 132(2), 297. [DOI] [PubMed] [Google Scholar]
- Oberman LM, & Ramachandran VS (2007). The simulating social mind: The role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychological Bulletin, 133(2), 310. [DOI] [PubMed] [Google Scholar]
- Obhi SS, Hogeveen J, Giacomin M, & Jordan CH (2014). Automatic imitation is reduced in narcissists. Journal of Experimental Psychology: Human Perception and Performance, 40(3), 920. [DOI] [PubMed] [Google Scholar]
- Press C, Richardson D, & Bird G (2010). Intact imitation of emotional facial actions in autism spectrum conditions. Neuropsychologia, 48(11), 3291–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, & Pennington BF (1991). A theoretical approach to the deficits in infantile autism. Development and Psychopathology, 3(2), 137–162. [Google Scholar]
- Rousselet GA, & Wilcox RR (2019). Reaction times and other skewed distributions: Problems with the mean and the median. Meta-Psychology. 10.1101/383935. [DOI] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The social communication questionnaire: Manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KS, & Bodfish JW (2008). Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research, 1(1), 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten JF, & Bekker JAM (1967). Reaction time and accuracy. Acta Psychologica, 27, 143–153. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M, Otte E, Adigüzel K, Firk C, Herpertz-Dahlmann B, Koch I, et al. (2017). Intact mirror mechanisms for automatic facial emotions in children and adolescents with autism spectrum disorder. Autism Research, 10(2), 298–310. [DOI] [PubMed] [Google Scholar]
- Schunke O, Schöttle D, Vettorazzi E, Brandt V, Kahl U, Bäumer T, et al. (2016). Mirror me: Imitative responses in adults with autism. Autism, 20(2), 134–144. [DOI] [PubMed] [Google Scholar]
- Smeekens I, Didden R, & Verhoeven EWM (2015). Exploring the relationship of autonomic and endocrine activity with social functioning in adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 495–505. [DOI] [PubMed] [Google Scholar]
- Southgate V, & Hamilton AFDC (2008). Unbroken mirrors: Challenging a theory of autism. Trends in Cognitive Sciences, 12(6), 225–229. [DOI] [PubMed] [Google Scholar]
- Sowden S, Koehne S, Catmur C, Dziobek I, & Bird G (2016). Intact automatic imitation and typical spatial compatibility in autism spectrum disorder: Challenging the broken mirror theory. Autism Research, 9(2), 292–300. [DOI] [PubMed] [Google Scholar]
- Spengler S, Bird G, & Brass M (2010). Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biological Psychiatry, 68(12), 1148–1155. [DOI] [PubMed] [Google Scholar]
- Tzelgov J, Henik A, & Berger J (1992). Controlling Stroop effects by manipulating expectations for color words. Memory & Cognition, 20(6), 727–735. [DOI] [PubMed] [Google Scholar]
- Vogt S, Taylor P, & Hopkins B (2003). Visuomotor priming by pictures of hand postures: Perspective matters. Neuropsychologia, 41(8), 941–951. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, Van Der Maas HL, & Grasman RP (2007). An EZ-diffusion model for response time and accuracy. Psychonomic Bulletin & Review, 14(1), 3–22. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. [Google Scholar]
- West R, & Baylis GC (1998). Effects of increased response dominance and contextual disintegration on the Stroop interference effect in older adults. Psychology and Aging, 13(2), 206. [DOI] [PubMed] [Google Scholar]
- Whelan R (2008). Effective analysis of reaction time data. The Psychological Record, 58(3), 475–482. [Google Scholar]
- Wickelgren WA (1977). Speed-accuracy tradeoff and information processing dynamics. Acta Psychologica, 41(1), 67–85. [Google Scholar]
- Wilkins J, & Matson JL (2009). A comparison of social skills profiles in intellectually disabled adults with and without ASD. Behavior Modification, 33(2), 143–155. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, & Singh T (2004). A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders, 34(3), 285–299. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, & Perrett DI (2001). Imitation, mirror neurons and autism. Neuroscience and Biobehavioral Reviews, 25(4), 287–295. [DOI] [PubMed] [Google Scholar]
- Zachor DA, Ilanit T, & Itzchak EB (2010). Autism severity and motor abilities correlates of imitation situations in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 4(3), 438–443. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.