Abstract
Purpose
We investigated abnormalities and recovery in respiratory function after COVID-19 infection in an unvaccinated elite athlete population.
Methods
Measurements included maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and peak expiratory flow (PEF).
Results
The most frequent reported symptoms were fatigue with 80% and muscle/joint pain and headache with 50%, whereas only 10% reported dyspnoea and 30% cough. During follow-up, MIP was up to 13% and MEP up to 8% lower following COVID-19 infection. Likewise, FEV1 was up to 2% and FVC up to 5% lower. While MEP and FEV1 rapidly normalised, MIP and FVC still remained abnormal after 52 days of COVID-19 infection, thereby leading to a restrictive ventilatory pattern. PEF seemed unaffected during follow-up.
Conclusions
COVID-19 decreases respiratory function in unvaccinated athletes despite reporting few respiratory symptoms and having mild disease. An initiative aimed at reducing the long-term adverse effects following COVID-19 infection seems warranted, which perhaps may be avoided through vaccination.
Keywords: Athletes, Forced Expiratory Volume, Maximal Respiratory Pressures, Respiratory Function Tests, SARS-CoV-2
1. Introduction
The COVID-19 pandemic has become one of the most critical public health issues that the world is still trying to solve. Although it has been nearly two years since the World Health Organization (WHO) declared the first case with COVID-19 and effective vaccines have been developed (Polack et al., 2020), there are still incidences with severe disease and public events are still prohibited in many countries in order to contain the spread.
Infection with COVID-19 affects the respiratory system and triggers the host's immune response, which leads to the invasion of hyperinflammatory neutrophils, monocytes, and macrophages (Chen et al., 2020). This uncontrolled immune response produces the so-called “cytokine storm” with a large release of inflammatory-stimulating cytokines by hyperinflammatory cells (Chen et al., 2020). Recent evidence suggests that the degree of this cytokine storm determines the degree of respiratory functional impairment (Li et al., 2020a, Cascella et al., 2021). Indeed, the clinical presentation in COVID-19 infection has shown substantial variation ranging from asymptomatic carriers to severe disorders such as atypical pneumonia (Jung et al., 2020), hyperinflammatory phenotype (Lin et al., 2020), respiratory failure (Li et al., 2020b), and acute respiratory distress syndrome (Lin et al., 2020, Xu et al., 2020).
One of the most significant factors affecting exercise performance in professional athletes is respiratory function, and its functioning is mechanically dependent on respiratory muscle capacity. Numerous factors affect the athletes' respiratory function adversely such as exercise intensity, environmental factors, and acute respiratory disorders. Infection with COVID-19 that specifically targets the respiratory system may cause permanent damage in unvaccinated athletes even in mild disease, thereby affecting exercise performance and put an early end to their professional careers (Hull et al., 2020). Although more than half of patients have recovered from COVID-19 in the general population, little is known how COVID-19 has affected the athlete population (Shah et al., 2021, Smet et al., 2021, Anastasio et al., 2021). In the present study, we investigated abnormalities and recovery in respiratory function after COVID-19 infection in an unvaccinated elite athlete population.
2. Methods
2.1. Study population and design
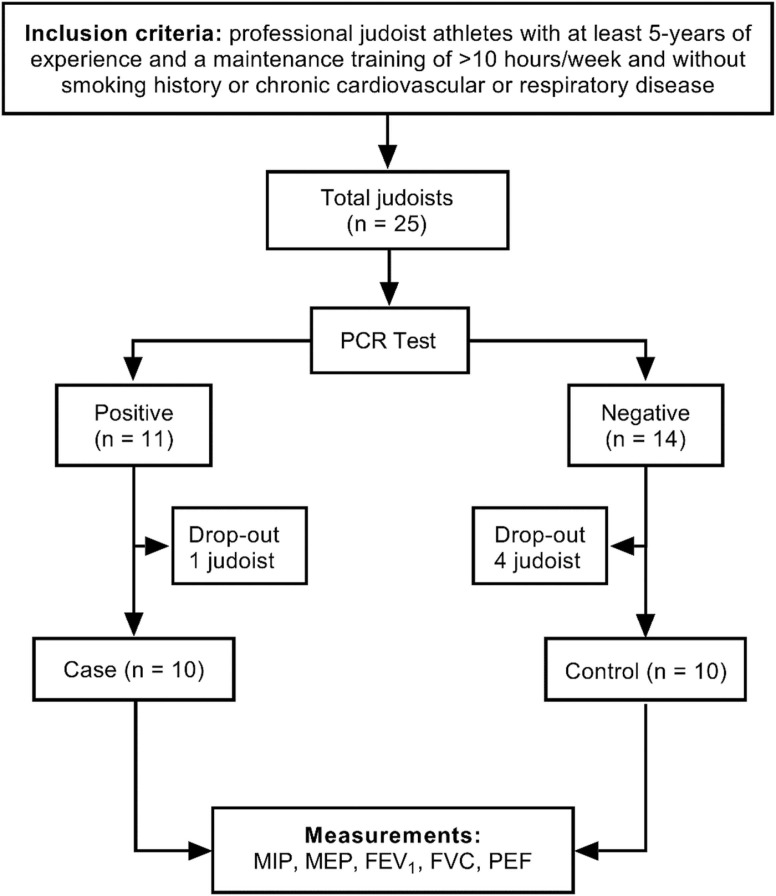
We recruited and prospectively followed a cohort of 25 unvaccinated elite judoists from the Turkish Olympic Preparation Centre in a case-control study design during 52 days in the period between February and April 2021 ( Fig. 1). Athletes were unvaccinated before COVID-19 infection, since vaccination was not available for this group of individuals during the pandemic. The respiratory function parameters were periodically followed by the researchers before and after COVID-19 infection. Polymerase Chain Reaction (PCR) test for COVID-19 was performed every 10 days. In total, 11 judoists (44%) were diagnosed with COVID-19. The PCR-positive judoists were included as the case group in the study (n = 11), and the PCR-negative judoists as the control group (n = 14). None of the PCR-positive judoists were admitted to the hospital or died during follow-up and were, thus, representing mild disease as intended. Both case and control groups were isolated in single rooms in the Turkish Olympic Preparation Centre in accordance with the WHO recommendations (WHO, 2020). All athletes did not perform any exercise or training during the 10-day isolation period. Athletes completing the isolation process returned to a low intensity training programme (i.e. judo-specific training) following a negative PCR-testing.
Fig. 1.
Flowchart. FEV1 =forced expiratory volume in 1 s. FVC=forced vital capacity. MEP=maximal expiratory pressure. MIP=maximal inspiratory pressure. PEF=peak expiratory flow.
All participants visited the laboratory regularly for respiratory muscle strength and pulmonary function measurements: a measurement before COVID-19 (baseline) and four measurements after infection at day 31, 38, 45, and 52 (post-COVID-19). The athletes were required to avoid strenuous exercise, not to consume caffeinated beverages, and have their last meal at least three hours before each visit. The measurements were made at the same time of the day. Their diet and training programmes were similar because the participants stayed and were followed in the Turkish Olympic Preparation Centre throughout the study period in a controlled environment. The athletes that had a positive PCR test result and manifested any COVID-19 symptoms or fever with > 38.0 °C on measurement days were excluded from the study, corresponding to one case (9%) and four controls (29%); a single participant wished to discontinue for personal reasons. The majority of the discontinued (16%) cases was due to cough, fever, muscle/joint tenderness, and/or fatigue.
The study was approved by the national Turkish Scientific Research Commission established by the Turkish Ministry of Health (project number: T15_40_27) and the Clinical Research Ethics Committee at the University of Ondokuz Mayıs (approval number: 2021/126), and was conducted according to the Declaration of Helsinki. All participants provided written informed consent.
2.2. Respiratory muscle strength and pulmonary function
Respiratory muscle strength was determined using maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP), measured with a MicroRPM (CareFusion Micro Medical, Kent, UK) and assessed in accordance with available guidelines in the field (Laveneziana et al., 2019). All measurements were obtained with the participants in a seating position with the use of a nose clip. Participants were carefully instructed to exhale to their residual volume and then to inhale to their total lung capacity. Pressure was sustained for at least 1.5 s before MIP and MEP were recorded. At least three measurement were performed before the highest values were selected (McConnell, 2007). Values were expressed as cmH2O and as percentage of predicted (measured value/predicted value x 100) (Miller and Pincock, 1988) using previously developed prediction equations (Evans and Whitelaw, 2009).
Pulmonary function was determined using forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and peak expiratory flow (PEF), measured with an electronic spirometer (Clement Clarke, Harlow, UK) and assessed in accordance with available guidelines in the field (Graham et al., 2019). Participants were carefully instructed before and during manoeuvre and the procedure was also shown on video. Spirometry was performed with the participants in a seating position with the use of a nose clip. At least three measurement were performed before the highest values were recorded (McConnell, 2007).
2.3. COVID-19 precautions during data collection
The preventive measures for the COVID-19 pandemic within the laboratory and during the tests were applied, as suggested by the national Turkish Society Experts Consensus Report and the European Respiratory Society (ERS) (Gemicioğlu et al., 2020, McGowan et al., 2020). Accordingly, COVID-19 infected patients must not be lung function tested for a minimum of 30 days (McGowan et al., 2020). We therefore began lung function measurements 31 days following COVID-19 infection. Surfaces touched by both the participants and researchers were regularly disinfected every 60 min. A sodium hypochlorite solution with a concentration of 0.1–0.5% (5% sodium hypochlorite prepared with 1:10 dilution) was used to eliminate COVID-19 (Kampf et al., 2020). A single-use antiviral and antibacterial filter (low-resistance and with filtration efficacy of 99.9%), mouthpieces, and nasal clips were used for each participant during respiratory muscle strength and pulmonary function measurements. The devices and equipment were sterilised using a disinfectant involving 70% isopropyl alcohol before and after each measurement and after each calibration.
2.4. Statistical analysis
SPSS 22.0 for Windows (IBM Inc., Chicago, IL, USA) was used for statistical analyses, and a two-sided P-value < 0.05 was considered as statistical significance. Descriptive data was presented as mean and standard deviation. Normality assumption was evaluated by visual inspection of the histograms (approximating a normal distribution) and applying a Shapiro-Wilk test. Differences in baseline characteristics were assessed using the independent sample t-test analysis. Comparisons between groups over time were determined using mixed repeated-measures analysis of variance (RM ANOVA) with a Bonferroni correction for post hoc analyses. ‘Time’ for respiratory muscle strength and pulmonary function parameters at baseline and post-COVID-19 (day 31, 38, 45, and 52) and ‘group’ (case and control) were considered as within-subjects and between-subjects factors at five ‘time’ and two ‘group’ levels, respectively. As a key assumption, we tested for interaction between time and group. An interaction may suggest that time changes between cases and controls may not be equivalent; however, no substantial violations were detected. The percentage of the mean differences for all respiratory muscle strength and pulmonary function parameters were calculated according to baseline.
3. Results
A total of 20 unvaccinated male elite judoists were included, 10 cases and 10 controls. At baseline examination, controls had slightly higher height, weight, and body mass index (BMI), but there were no overall differences in age or sport experience ( Table 1). The three most common reported symptoms in these unvaccinated cases were fatigue with 80%, muscle/joint pain with 50%, and headache with 50%. Only 10% reported dyspnoea and 30% cough.
Table 1.
Baseline characteristics of case and control judoists from the Turkish Olympic Preparation Centre.
| Case (n = 10) | Control (n = 10) | Total (n = 20) | |
|---|---|---|---|
| Age, years | 18.2 ± 0.6 | 18.8 ± 1.5 | 18.5 ± 1.1 |
| Height, cm | 175.8 ± 6.9 | 180.5 ± 9 | 178.2 ± 8.2 |
| Weight, kg | 74.3 ± 15.5 | 80.2 ± 15.1 | 77.3 ± 15.2 |
| BMI, kg/m2 | 24 ± 4.2 | 25.1 ± 3.7 | 24.5 ± 3.9 |
| Sport experience, years | 7.4 ± 1.7 | 7.5 ± 2.1 | 7.5 ± 1.8 |
| Weight class, kg | 69.5 ± 12.5 | 75.1 ± 12.4 | 72.3 ± 12.5 |
Data are presented as mean±standard deviation. No differences were observed between cases and controls. All comparisons had P-values ≥ 0.05. BMI=body mass index.
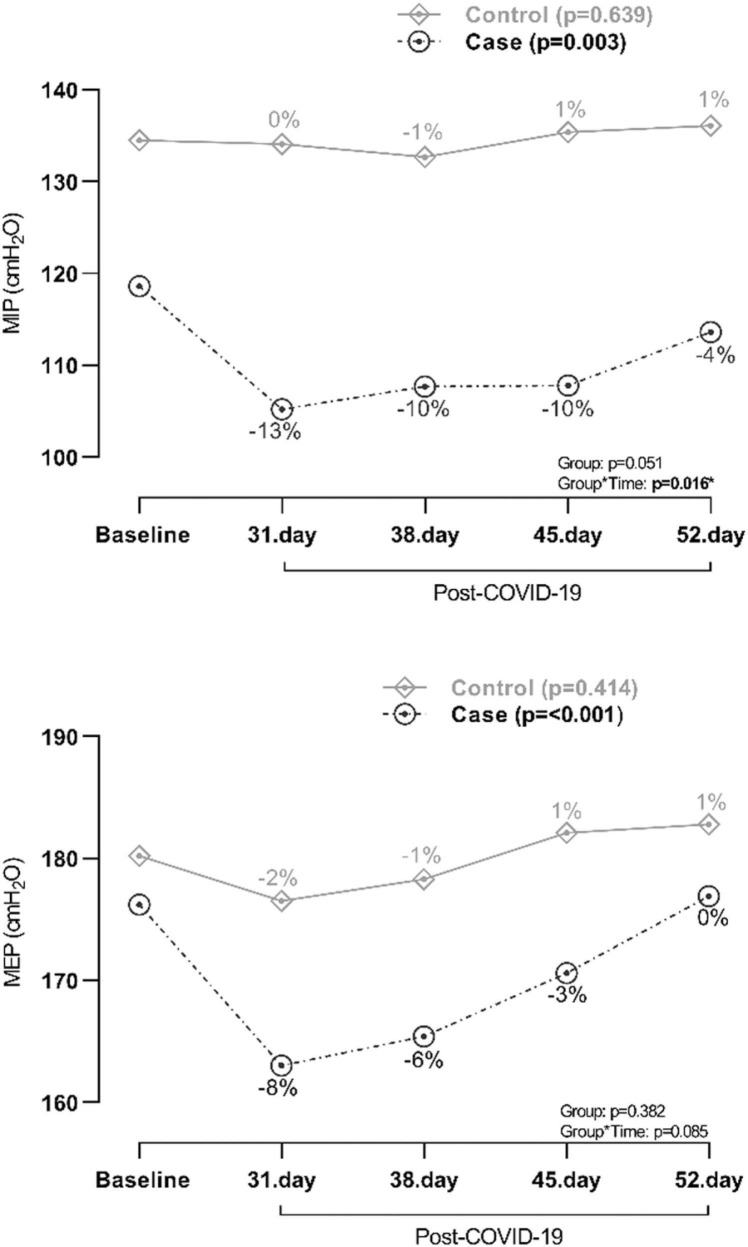
Respiratory muscle strength as measured with MIP and MEP was reduced in cases subsequently after infection with COVID-19, but gradually increased during 52 days of follow-up ( Fig. 2). While MEP normalised between day 45 and 52, MIP was still reduced at day 52. As expected, respiratory muscle strength in controls were unaffected. MIP in cases was 118.6 cmH2O (corresponding to baseline value) before infection and 105.2 cmH2O (corresponding to −13% from baseline value) at day 31, 107.7 cmH2O (−10%) at day 38, 107.8 cmH2O (−10%) at day 45, and 113.6 cmH2O (−4%) at day 52 after infection (p = 0.003) (Fig. 2). MEP in cases was 176.2 cmH2O (corresponding to baseline value) before infection and 163 cmH2O (corresponding to −8% from baseline value) at day 31, 165.4 cmH2O (−6%) at day 38, 170.6 cmH2O (−3%) at day 45, and 176.9 cmH2O (0%) at day 52 after infection (p = <0.001) (Fig. 2). Corresponding value for MIP as percentage of predicted was 105.4% (baseline), 93.5%, 95.7%, 95.8% and 100.9%, and for MEP as percentage of predicted was 110.9% (baseline), 102.6%, 104.1%, 107.4% and 111.3%, respectively.
Fig. 2.
Respiratory muscle strength in case and control judoists before and 31, 38, 45, and 52 days after COVID-19 infection. Percentages indicate change from baseline value. MIP=maximal inspiratory pressure, MEP=maximal expiratory pressure.
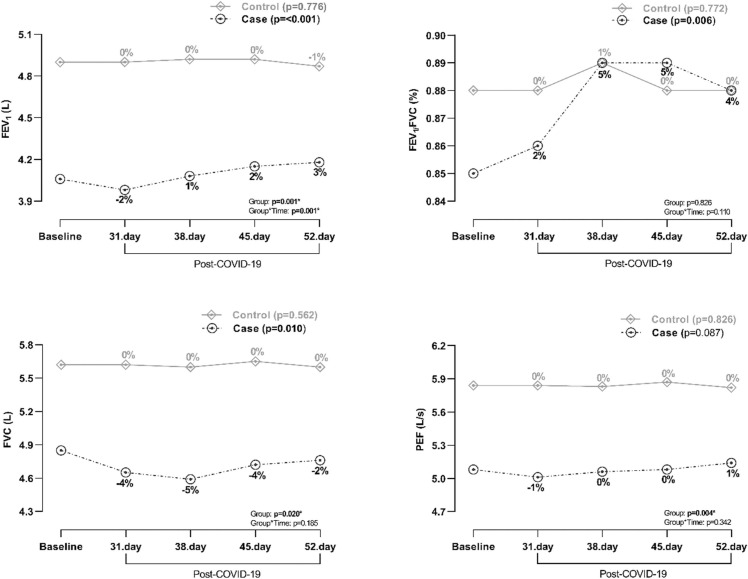
Pulmonary function as measured with FEV1 and FVC was also reduced in cases subsequently after infection with COVID-19, and gradually increased during 52 days of follow-up ( Fig. 3). Interestingly, FVC was much more reduced than FEV1 and as a consequence an increment in FEV1/FVC could be observed. While FEV1 normalised already between day 31 and 38, FVC was still reduced at day 52. In contrast, PEF seemed unaffected during the whole 52 days of follow-up period. As expected, pulmonary function in controls were unaffected. FEV1 in cases was 4.06 L (corresponding to baseline value) before infection and 3.98 L (corresponding to −2% from baseline value) at day 31, 4.08 L (1%) at day 38, 4.16 L (2%) at day 45, and 4.18 L (3%) at day 52 after infection (p = <0.001). Corresponding values for FVC in cases was 4.86 L (baseline), 4.65 L (−4%), 4.59 L (−5%), 4.72 L (−3%) and 4.76 L (−2%), respectively (p = 0.010) (Fig. 3).
Fig. 3.
Pulmonary function in case and control judoists before and 31, 38, 45, and 52 days after COVID-19 infection. Percentages indicate change from baseline value. FEV1 =forced expiratory volume in 1 s. FVC=forced vital capacity. PEF=peak expiratory flow.
4. Discussion
In the present study, we investigated abnormalities and recovery in respiratory function after COVID-19 infection in an unvaccinated elite athlete population. We found that COVID-19 infection decreases respiratory muscle strength and pulmonary function in unvaccinated athletes despite reporting few respiratory symptoms and having mild disease. While MEP and FEV1 began to rapidly normalise after 31 days and recovered almost fully during 52 days following a COVID-19 infection, MIP and FVC were still affected. These findings suggest that athletes may need a longer recovery time before being able to return to training and competition following a COVID-19 infection, which perhaps may be avoided through vaccination.
Despite significant reductions in respiratory capacity already observed in the early days following COVID-19 infection, only 10% of the unvaccinated athletes reported dyspnoea and 30% cough. Instead, the most typical symptoms were fatigue (80%), headache (50%), and muscle/joint pain (50%) suggesting mild disease, which at first glance seems different from the symptoms reported in the general population (Rodriguez-Morales et al., 2020). In our cohort, age and physical activity may be the most important factors contributing to the mild symptoms encountered by the participants. It has been reported that age-related structural differences may affect the altered immune responses to SARS-CoV-2 and thus the severity of disease or symptoms (Mancilla-Galindo et al., 2021, Bajaj et al., 2021). However, it is also important to note that symptoms at the onset or after COVID-19 infection vary widely in the general population and seems among others to be affected by how the immune system responds (Assaf et al., 2020, Corse et al., 2020). Moderate exercise has been shown to boost the immune system, whereas strenuous exercise seems to suppress it and increases inflammatory response during training sessions (Rahmati-Ahmadabad and Hosseini, 2020, Simpson et al., 2020). Impaired immune system during strenuous exercise may have increased the amount of viral load in these unvaccinated athletes infected with COVID-19 and may also explain some of the differences in symptoms when compared to the general population (Liu et al., 2020). Interestingly, a study of 48,440 adult patients with COVID-19 reported that regular physical activity is less likely to be hospitalised, admitted to intensive care, and die from COVID-19 than those reporting a sedentary lifestyle (Sallis et al., 2021). Furthermore, physically active lifestyle seems to minimise or protect against the incidence, duration, or severity of COVID-19 (de Souza et al., 2021, Zbinden-Foncea et al., 2020, Salgado-Aranda et al., 2021). Physical activity may affect susceptibility to infection by modifying immunological response and cellular immunity (Amatriain-Fernández et al., 2020, Nieman and Wentz, 2019, da Silveira et al., 2021). Different mechanisms (aerobic capacity, regulated C-reactive protein concentration, immunoglobulin level) may be in play in relation to moderate and strenuous exercise.
To our knowledge, the impact of COVID-19 infection on respiratory muscles has not previously been followed periodically in unvaccinated athletes, however, small studies have demonstrated respiratory muscle weakness in the post-COVID-19 chronic period. Since respiratory muscles drive alveolar ventilation, and their weakness may result in imbalances in the demands required for ventilation, it is an important issue to clarify as it may affect the performance of the athletes despite mild disease, not only temporarily but also in the long term. Interestingly, MIP normalised more slowly than MEP and did not recover completely following COVID-19 infection in the present study with up to 52 days follow-up. This may to some part be explained by muscle deconditioning and/or diaphragmatic myopathy (Anastasio et al., 2021, Shi et al., 2021). Assuming that inspiration is an active action (muscle contraction) compared to expiration, inspiratory structures with muscle deconditioning and/or myopathy may take longer time to recover. A post mortem study revealed that angiotensin-converting enzyme 2 is expressed in the human diaphragm, and viral infiltration of SARS-CoV-2 was present in the diaphragm myofibers of patients infected with COVID-19 (Shi et al., 2021). In the same study, there was also an increased expression of genes involved in fibrosis as well as histologically verified development of fibrosis in the diaphragm (Shi et al., 2021). This myopathic phenotype may lead to reductions in inspiratory muscle force-generating capacity of the diaphragm that could trigger respiratory dysfunction through various mechanisms, perhaps also be responsible for the deterioration in pulmonary function. While FEV1 normalised rapidly between days 31 and 38, FVC was still affected at day 52, thereby leading to a slightly restrictive ventilatory pattern. A systematic review has suggested that a restrictive ventilatory pattern may be more common following a COVID-19 infection than an obstructive ventilatory pattern (Boutou et al., 2021). COVID-19 infected patients often have signs of acute exudative lesions with diffuse alveolar damage and fibrotic changes, which may lead to a decrease in pulmonary compliance and a restrictive ventilatory pattern (Carsana et al., 2020, Yao et al., 2020).
Previous studies have demonstrated poor respiratory outcomes in unvaccinated patients with COVID-19 (Mo et al., 2020, Zhao et al., 2020). In a cohort of 57 hospitalised patients with COVID-19, more than half showed impairment in respiratory muscle strength during early convalescence phase (Huang et al., 2020). A prospective cohort study of 55 survivors with Severe Acute Respiratory Syndrome (SARS) showed permanently abnormal MEP values, and MIP values notably decreased over a duration of 24 months (Ngai et al., 2010). Çelik et al. found inspiratory and expiratory muscle-weakening in female volleyball players in the chronic period after COVID-19 infection (Çelik et al., 2021). In an unvaccinated athlete population, we now demonstrate that MEP recovers rapidly, whereas MIP still seems to be reduced after 52 days of COVID-19 infection.
A few reports retrospectively evaluated the pulmonary function of unvaccinated COVID-19 survivors a month after hospital discharge, which showed that some patients continued to have significant reductions in FEV1 and FVC (Mo et al., 2020, Huang et al., 2020). Fumagalli et al. demonstrated that FEV1 and FEV1/FVC recovered to normal, whereas FVC was still low 6 weeks after hospital discharge in 13 patients with COVID-19 (Fumagalli et al., 2021). However, another study showed that anomalies in FEV1 and FVC continued for 11% and diffusing capacity for 16% at follow-up 3 months after hospital discharge (Zhao et al., 2020). In contrast to all other studies, Frija-Masson et al. found that FEV1 and FVC were normal for 12 unvaccinated non-critical patients only 1 month after symptom onset (Frija-Masson et al., 2020). In addition, previous studies have revealed that pulmonary function may be normalised in the long term 3–12 months after in SARS survivors (Ng et al., 2004, Ong et al., 2005); however, decreased diffusing capacity may still persist in up to 45% (Ngai et al., 2010, Su et al., 2007). In another study, abnormal pulmonary function continued for nearly up to ten weeks in more than 50% of unvaccinated COVID-19 survivors (Shah et al., 2021, Smet et al., 2021). In a small cohort of unvaccinated soccer players, post-infection (at least 15 days after clinical resolution) FVC, FEV1, and PEF were decreased (Gervasi et al., 2021). In an unvaccinated athlete population, we now demonstrate that FEV1 recovers rapidly, whereas FVC still seems to be reduced after 52 days of COVID-19 infection leading to a restrictive ventilatory pattern. Both the case and control groups did not perform any exercise or training during the 10-day isolation period, but they returned to a low-intensity training programme immediately after the isolation period. Moreover, the athletes continued their normal-intensity training programmes after the low-intensity training period (approximately 7 days after). We think that the decreased activity level due to infection cannot affect the respiratory capacity, since the cases shared same training/detraining conditions as the controls, and the controls did not experience decrease in their respiratory capacity as the cases (Fig. 2, Fig. 3). These results show us that it may be due to the COVID-19-specific infection rather than the decreased levels of activity. More research is required to identify mechanisms for rapid recovery and permanent damage related to respiratory muscle strength and pulmonary following a COVID-19 infection.
Strengths of the present study include a professional unvaccinated athlete population at an Olympic-level with important respiratory physiology measurements before and after a COVID-19 infection in a well-controlled environment. Furthermore, it is also a strength to have repeated measurements.
This study has several limitations. Firstly, we were not able to investigate the relationship between respiratory abnormalities and performance, since we avoided complex measurements such as aerobic capacity and gas analysis due to contamination risk during the COVID-19 pandemic and precautions. Secondly, we were unable to establish a vaccinated group within the same population because vaccination was not available at the time of data collection. Thirdly, we had a relatively small sample size, which may affect generalisability to other athlete populations. However, our findings may still contribute to understand the changes in respiratory muscle strength and pulmonary function in professional unvaccinated athlete population during and after COVID-19 infection. Finally, we were unable to capture a full recovery in all participants, and it would be preferable to have a longer follow-up time, which is not possible, and this could therefore be regarded as a limitation.
5. Conclusion
In conclusion, we found that COVID-19 decreases respiratory muscle strength and pulmonary function in unvaccinated athletes despite reporting few respiratory symptoms and having mild disease. Athletes may need a longer recovery time before being able to return to training and competition. An initiative aimed at reducing the long-term adverse effects following COVID-19 infection seems warranted, which perhaps may be avoided through vaccination.
Funding
This study was funded by University of Ondokuz Mayıs. The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Contributors
ÖB and EK had full access to all data in the study and had final responsibility for the decision to submit. ÖB and EK contributed to the study concept and design. ÖB, EK, and AKY collected, analysed, or interpreted the data. ÖB and AKY wrote the draft manuscript. YÇ , MK, and SB revised the manuscript for important intellectual content. ÖB provided administrative, technical, and/or material support. All authors have read and approved the final version of the manuscript.
Disclosure statement
YÇ reports personal fees from Boehringer-Ingelheim, AstraZeneca, and Sanofi Genzyme outside the submitted work. ÖB, EK, AKY, MK, and SB have nothing to disclose. The views expressed are those of the authors.
Acknowledgements
We thank our athletes and their coaches for helping us to reveal the effects of COVID-19 infection on athletes.
Edited by Yu Ru Kou
Data Availability
No data was used for the research described in the article.
References
- Amatriain-Fernández S., Gronwald T., Murillo-Rodríguez E., Imperatori C., Solano A.F., Latini A., et al. Physical exercise potentials against viral diseases like COVID-19 in the elderly. Front Med (Lausanne) 2020;7:379. doi: 10.3389/fmed.2020.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio F., Barbuto S., Scarnecchia E., Cosma P., Fugagnoli A., Rossi G., et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur. Respir. J. 2021;58(3):2004015. doi: 10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf G., Davis H., McCorkell L., et al. An analysis of the prolonged COVID-19 symptoms survey by patient-led research team. Patient Led. Res. 2020 https://patientresearchcovid19.com/research/report-1/ (Accessed 10 April 2021) [Google Scholar]
- Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections. Front. Physiol. 2021;11 doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutou A.K., Georgopoulou A., Pitsiou G., Stanopoulos I., Kontakiotis T., Kioumis I. Changes in the respiratory function of COVID-19 survivors during follow-up: a novel respiratory disorder on the rise? Int J. Clin. Pr. 2021;75(10) doi: 10.1111/ijcp.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Aleem A., Dulebohn S.C., Di Napoli R. In: StatPearls 2021. 2021. Features, evaluation, and treatment of coronavirus (COVID-19)www.statpearls.com/ArticleLibrary/viewarticle/52171 (Accessed 10 June 2021) [PubMed] [Google Scholar]
- Çelik Z., Güzel N.A., Kafa N., Köktürk N. Respiratory muscle strength in volleyball players suffered from COVID-19. Ir. J. Med Sci. (1971-) 2021:1–7. doi: 10.1007/s11845-021-02849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corse T., Dayan L., Kersten S., Battaglia F., Terlecky S.R., Han Z. Clinical outcomes of COVID-19 patients with pre-existing, compromised immune systems: a review of case reports. Int J. Med Sci. 2020;17(18):2974–2986. doi: 10.7150/ijms.50537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silveira M.P., da Silva Fagundes K.K., Bizuti M.R., Starck É., Rossi R.C., de Resende E., Silva D.T. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin. Exp. Med. 2021;21(1):15–28. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.A., Whitelaw W.A. The assessment of maximal respiratory mouth pressures in adults. Respir. care. 2009;54(10):1348–1359. [PubMed] [Google Scholar]
- Frija-Masson J., Debray M.P., Gilbert M., Lescure F.X., Travert F., Borie R., et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur. Respir. J. 2020;56(2):2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli A., Misuraca C., Bianchi A., Borsa N., Limonta S., Maggiolini S., et al. Long-term changes in pulmonary function among patients surviving to COVID-19 pneumonia. Infection. 2021;49(1):153–157. doi: 10.1007/s15010-021-01718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemicioğlu B., Börekçi Ş., Dilektaşlı A.G., Ulubay G., Azap Ö., Saryal S. Turkish thoracic society experts consensus report: recommendations for pulmonary function tests during and after COVID 19 pandemic. Turk. Thorac. J. 2020;21(3):193–200. doi: 10.5152/TurkThoracJ.2020.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi S.F., Pengue L., Damato L., Monti R., Pradella S., Pirronti T., Palmieri V. Is extensive cardiopulmonary screening useful in athletes with previous asymptomatic or mild SARS-CoV-2 infection. Br. J. Sports Med. 2021;55(1):54–61. doi: 10.1136/bjsports-2020-102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B.L., Steenbruggen I., Miller M.R., Barjaktarevic I.Z., Cooper B.G., Hall G.L., et al. Standardization of spirometry 2019 update. an official american thoracic society and european respiratory society technical statement. Am. J. Respir. Crit. Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;21(1):163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J.H., Loosemore M., Schwellnus M. Respiratory health in athletes: facing the COVID-19 challenge. Lancet Respir. Med. 2020;8(6):557–558. doi: 10.1016/S2213-2600(20)30175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S.M., Kinoshita R., Thompson R.N., Linton N.M., Yang Y., Akhmetzhanov A.R., et al. Epidemiological Identification of A Novel Pathogen in Real Time: Analysis of the Atypical Pneumonia Outbreak in Wuhan, China, 2019-2020. J. Clin. Med. 2020;9(3):637. doi: 10.3390/jcm9030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laveneziana P., Albuquerque A., Aliverti A., Babb T., Barreiro E., Dres M., Verges S. ERS statement on respiratory muscle testing at rest and during exercise. Eur. Respir. J. 2019;53(6) doi: 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- Li X., Wang C., Kou S., Luo P., Zhao M., Yu K. Lung ventilation function characteristics of survivors from severe COVID-19: a prospective study. Crit. Care. 2020;24(1):300. doi: 10.1186/s13054-020-02992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.H., Zhao Y.S., Zhou D.X., Zhou F.C., Xu F. Coronavirus disease 2019 (COVID-19): cytokine storms, hyper-inflammatory phenotypes, and acute respiratory distress syndrome. Genes Dis. 2020;7(4):520–527. doi: 10.1016/j.gendis.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla-Galindo J., Kammar-García A., Martínez-Esteban A., Meza-Comparán H.D., Mancilla-Ramírez J., Galindo-Sevilla N. COVID-19 patients with increasing age experience differential time to initial medical care and severity of symptoms. Epidemiol. Infect. 2021;149 doi: 10.1017/S095026882100234X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell A. In: Sport and exercise physiology testing guidelines, the British Association of Sport and Exercise Sciences Guide (1st ed.) Winter E.M., Jones A.M., Davison R.C.R., et al., editors. Routledge; Oxford, UK: 2007. Lung and respiratory muscle function; pp. 63–75. [Google Scholar]
- McGowan A., Sylvester K., Burgos F. (2020) Recommendation from ERS Group 9.1 (Respiratory function technologists/Scientists): Lung function testing during COVID-19 pandemic and beyond. https://ers.app.box.com/s/zs1uu88wy51monr0ewd990itoz4tsn2h. (Accessed 15 April 2021).
- Miller M.R., Pincock A.C. Predicted values: how should we use them. Thorax. 1988;43(4):265. doi: 10.1136/thx.43.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo X., Jian W., Su Z., Chen M., Peng H., Peng P., et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;55(6):2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C.K., Chan J.W., Kwan T.L., To T.S., Chan Y.H., Ng F.Y. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59(10):889–891. doi: 10.1136/thx.2004.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai J.C., Ko F.W., Ng S.S., To K.W., Tong M., Hui D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman D.C., Wentz L.M. The compelling link between physical activity and the body's defense system. J. Sport Health Sci. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K.C., Ng A.W., Lee L.S., Kaw G., Kwek S.K., Leow M.K., et al. 1-year pulmonary function and health status in survivors of severe acute respiratory syndrome. Chest. 2005;128(3):1393–1400. doi: 10.1378/chest.128.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati-Ahmadabad S., Hosseini F. Exercise against SARS-CoV-2 (COVID-19): does workout intensity matter? (A mini review of some indirect evidence related to obesity. Obes. Med. 2020;19 doi: 10.1016/j.obmed.2020.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado-Aranda R., Pérez-Castellano N., Núñez-Gil I., Orozco A.J., Torres-Esquivel N., Flores-Soler J., et al. Influence of baseline physical activity as a modifying factor on COVID-19 mortality: a single-center, retrospective study. Infect. Dis. Ther. 2021;10(2):801–814. doi: 10.1007/s40121-021-00418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis R., Young D.R., Tartof S.Y., Sallis J.F., Sall J., Li Q., et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br. J. Sports Med. 2021;55(19):1099–1105. doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- Shah A.S., Wong A.W., Hague C.J., Murphy D.T., Johnston J.C., Ryerson C.J., et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76(4):402–404. doi: 10.1136/thoraxjnl-2020-216308. [DOI] [PubMed] [Google Scholar]
- Shi Z., de Vries H.J., Vlaar A.P.J., van der Hoeven J., Boon R.A., Heunks L.M.A., et al. Diaphragm pathology in critically ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med. 2021;181(1):122–124. doi: 10.1001/jamainternmed.2020.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R.J., Campbell J.P., Gleeson M., Krüger K., Nieman D.C., Pyne D.B., et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol. Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- Smet J., Stylemans D., Hanon S., Ilsen B., Verbanck S., Vanderhelst E. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir. Med. 2021;176 doi: 10.1016/j.rmed.2020.106276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza F.R., Motta-Santos D., Dos Santos Soares D., de Lima J.B., Cardozo G.G., Guimarães L.S.P., et al. Association of physical activity levels and the prevalence of COVID-19-associated hospitalization. J. Sci. Med Sport. 2021;24(9):913–918. doi: 10.1016/j.jsams.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M.C., Hsieh Y.T., Wang Y.H., Lin A.S., Chung Y.H., Lin M.C. Exercise capacity and pulmonary function in hospital workers recovered from severe acute respiratory syndrome. Respiration. 2007;74(5):511–516. doi: 10.1159/000095673. [DOI] [PubMed] [Google Scholar]
- WHO (2020) Clinical management of severe acute respiratory infection when COVID-19 disease is suspected: interim guidance. www.who.int/publications/i/item/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. (Accessed 18 March 2021).
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing. li xue za zhi ( Chin. ) 2020;49 doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- Zbinden-Foncea H., Francaux M., Deldicque L., Hawley J.A. Does high cardiorespiratory fitness confer some protection against proinflammatory responses after infection by SARS-CoV-2? Obes. (Silver Spring) 2020;28(8):1378–1381. doi: 10.1002/oby.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F., et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.