Peanut allergy affects about 2% of children in Western countries and is likely increasing in incidence.1 Peanut allergy typically develops during infancy and usually persists throughout life; therefore, early-life interventions aimed at peanut allergy prevention are essential for reducing disease burden. There is increasing evidence that nonoral exposure to environmental peanut, such as through the skin or respiratory tract, is a risk factor for peanut allergy development, whereas ingestion of peanut is associated with tolerance.2 The Learning Early About Peanut Allergy (LEAP) study was the first randomized controlled trial to demonstrate the importance of early introduction of dietary peanut to prevent peanut allergy in at-risk infants.3 In this landmark study, 640 children aged 4 to 11 months with severe eczema and/or egg allergy were randomized to either peanut consumption or avoidance. The primary outcome of the study was prevalence of peanut allergy at age 60 months as determined by oral food challenge. Among the 530 subjects evaluated for the primary outcome, the prevalence of peanut allergy was 13.7% in the avoidance group and 1.9% in the consumption group, indicating an 86% relative risk reduction with peanut consumption. To determine whether unresponsiveness to peanut was sustained, a follow-on study (LEAP-on) asked 550 participants who completed the LEAP trial to avoid peanut for 12 months.4 Peanut avoidance was not associated with an increase in peanut allergy, suggesting that tolerance of peanut had been established in the early-consumption group. The striking results from these randomized trials, along with observational data indicating that peanut allergy rates are low in societies in which early-life peanut consumption is common, prompted an addendum to the guidelines for management of food allergy in the United States.5 An expert panel sponsored by the National Institute of Allergy and Infectious Diseases recommended that infants with severe eczema, egg allergy, or both have peanut-containing foods introduced into their diet at 4 to 6 months of age to reduce the risk of peanut allergy.
Although the LEAP and LEAP-on studies provided strong evidence that early dietary introduction of peanut is protective against peanut allergy, the immune mechanisms responsible for the induction of tolerance of ingested peanut remain unclear. It has long been known that ingestion of food antigens can result in a systemic state of immunologic unresponsiveness to the antigen, a phenomenon known as oral tolerance. Several immune mechanisms for oral tolerance have been proposed, including clonal deletion of antigen-specific T cells, induction of T-cell anergy, and the generation of antigen-specific regulatory T (Treg) cells.6 Because of the inability to perform in-depth mechanistic studies in human infants, the relative contribution of these proposed mechanisms to tolerance induction during early dietary introduction of peanut is unknown. Elucidating the key cellular and molecular pathways responsible for tolerance induction is not only important for improving interventions aimed at peanut allergy prevention but will also likely improve the efficacy of peanut-specific immunotherapies.
In this issue of the Journal of Allergy and Clinical Immunology, Krempski et al7 have developed a mouse model simulating the clinical findings of the LEAP study as a tool for investigating the immune mechanisms by which early peanut introduction protects against peanut allergy. In their model, 7- to 12-week-old mice were fed a total of 1 g of a commercial peanut butter product (equivalent to 220 mg of peanut protein) by oral gavage 3 to 5 times per week for a total of 6 weeks. Krempski et al7 estimated that this feeding regimen would be similar to a human infant consuming 6 of 7 grams of peanut protein per week, which is the recommended dose for infants at high-risk of peanut allergy.5 To determine whether feeding peanut protected mice against developing peanut allergy, peanut flour was administered to the respiratory tract or skin weekly for 4 weeks to mimic environmental exposure to peanut. As expected, mice fed control buffer developed peanut-specific IgE and IgG1 following inhalational or epicutaneous exposure to peanut flour and developed anaphylaxis after peanut challenge. In contrast, the peanut-fed mice had low levels of peanut-specific immunoglobulins following airway or skin exposure to peanut and were protected from peanut-induced anaphylaxis. Mice allowed to consume peanut butter ad libitum before airway exposure to peanut also did not develop peanut allergy, indicating that natural feeding of peanut butter was also protective. Moreover, peanut-fed mice that were switched to a peanut-free diet for 4 weeks after inhalational peanut exposure were still protected against peanut allergy, suggesting that feeding peanut appeared to induce long-term tolerance rather than temporary desensitization in mice.
Next, Krempski et al7 used their model to investigate the immune mechanisms by which feeding peanut protected mice against sensitization to environmental peanut exposure; they found that feeding peanut butter inhibited development of IL-4–producing follicular helper T cells in lung-draining lymph nodes, which are essential for inducing peanut-specific IgE.8 Because inducible Treg cells have been shown to mediate oral tolerance of food antigens,9 Krempski et al7 next evaluated how feeding peanut affected Treg cell development. Interestingly, they did not observe an increase in the number or frequency of Treg cells in lung-draining or mesenteric lymph nodes from peanut-fed mice compared with the number or frequency in mice fed control buffer. To determine whether feeding peanut affected Treg cell phenotype, Krempski et al7 performed single-cell RNA sequencing on CD4+ T cells from lung-draining lymph nodes of peanut-fed mice after airway sensitization to peanut. They identified a cluster of CD4+ T cells with a typical Treg cell transcriptional profile that expressed high levels of the inhibitory molecule cytotoxic T lymphocyte–associated protein 4 (CTLA-4), but not the immunosuppressive cytokines IL-10 or TGF-β. To investigate the role of CTLA-4 in mediating protection against peanut allergy, Krempski et al7 administered anti–CTLA-4 blocking antibody to peanut-fed or control mice during airway sensitization to peanut. Treatment with anti–CTLA-4 antibody resulted in increased numbers of follicular helper T cells and germinal center B cells in lung-draining lymph nodes, which corresponded with elevated levels of peanut-specific antibodies. Furthermore, blocking CTLA-4 reversed the protective effects of feeding peanut on anaphylaxis, indicating that the CTLA-4 pathway plays an important role in preventing sensitization to environmental peanut (Fig 1).
FIG 1.
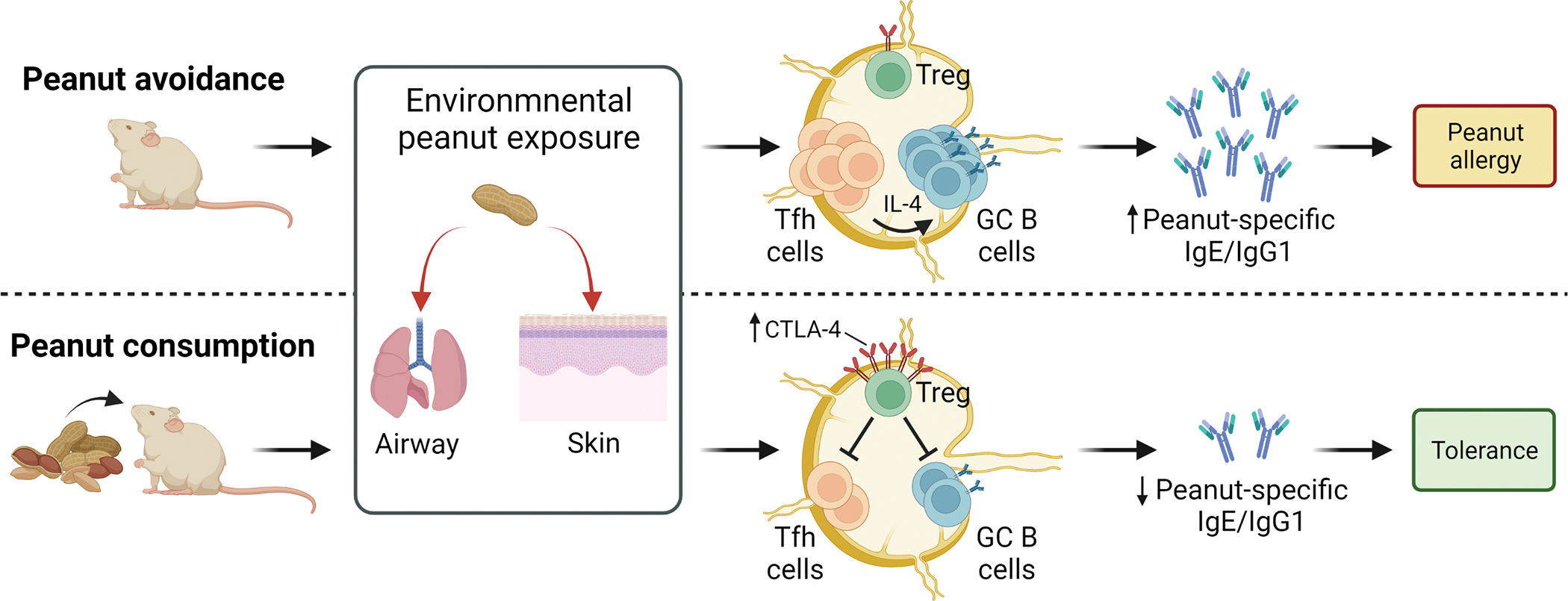
Ingestion of peanut induces CTLA-4-expressing Treg cells that protect mice from peanut allergy caused by environmental peanut exposure. During peanut avoidance, exposure to environmental peanut through the airway or skin results in increased numbers of IL-4–producing follicular helper T (TFH) cells and germinal center (GC) B cells in tissue-draining lymph nodes, leading to increased levels of peanut-specific immunoglobulins and development of peanut allergy. In contrast, peanut consumption induces a Treg cell population that expresses high-levels of CTLA-4, which suppress TFH and GC B-cell expansion following environmental peanut exposure. This results in decreased production of peanut-specific immunoglobulins and development of tolerance to peanut. Created with BioRender.com.
The investigations by Krempski et al7 introduce a novel mouse model for simulating early peanut introduction and provide new insights into how feeding peanut protects against peanut allergy development. Although any preclinical animal model of human disease has its limitations, the current studies are strengthened by a rigorous attempt to use relevant sources and quantities of peanut protein for feeding, demonstrating protection against peanut sensitization by using multiple exposure routes and confirming experimental results in different mouse genotypes and sexes. Still, the use of adult mice is a limitation regarding the translation of study findings to human infants. Although Krempski et al7 observed that the mice used for their experiments have an immature immune system based on CD8+ T cell immunophenotyping, it is likely that other aspects of the adaptive immune response differ significantly between adult mice and human infants. Future studies using neonatal mice would help address this concern and increase the translational impact of the animal model presented by Krempski et al.7 This study also highlights an important regulatory role for CTLA-4 in mediating tolerance of food allergens and provides preclinical evidence that augmenting CTLA-4 signaling may increase the efficacy of allergen-specific immunotherapy. Indeed, abatacept, a soluble CTLA-4–Ig fusion protein, is being investigated as an adjuvant to peanut oral immunotherapy (ClinicalTrials.gov identifier NCT04872218). Still, several questions remain, including the mechanism by which CTLA-4 protects mice against airway sensitization to peanut and where CTLA-4–expressing Treg cells are induced in response to peanut feeding. Interestingly, Krempski et al7 found that adoptive transfer of Treg cells from mesenteric lymph nodes of peanut-fed mice did not confer protection against airway sensitization to peanut, raising the possibility that peanut-specific Treg cells might increase in number and become activated in the gut mucosa, as described in other models of oral tolerance.10 Another limitation is that because the protective role of CTLA-4 was demonstrated only in an airway sensitization model, whether a similar mechanism also mediates protection against sensitization through the skin or gut is unclear. The mouse model presented by Krempski et al7 will provide a valuable tool to address these questions and others regarding the regulatory mechanisms that mediate tolerance of food allergens.
Acknowledgments
Supported by grant funding from the National Institutes of Health (to T.P.M. and M.K.) and the US Department of Defense (to M.K.).
Footnotes
Disclosure of potential conflict of interest: M. D. Kulis receives consulting fees from UKKO. The remaining author declares that he has no relevant conflicts of interest.
REFERENCES
- 1.Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018;141:41–58. [DOI] [PubMed] [Google Scholar]
- 2.Kulis MD, Smeekens JM, Immormino RM, Moran TP. The airway as a route of sensitization to peanut: an update to the dual allergen exposure hypothesis. J Allergy Clin Immunol 2021;148:689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015;372:803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med 2016;374:1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Togias A, Cooper SF, Acebal ML, Assa’ad A, Baker JR Jr, Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol 2017;139:29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tordesillas L, Berin MC. Mechanisms of oral tolerance. Clin Rev Allergy Immunol 2018;55:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krempski JW, Lama JK, Iijima K, Kobayashi T, Matsunaga M, Kita H. A mouse model of the LEAP study reveals a role for CTLA-4 in preventing peanut allergy induced by environmental peanut exposure. J Allergy Clin Immunol 2022;150:425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolence JJ, Kobayashi T, Iijima K, Krempski J, Drake LY, Dent AL, et al. Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol 2018;142:1144–11458 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 2005;115:1923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 2011;34:237–46. [DOI] [PubMed] [Google Scholar]


