Abstract
The centriole is a minute cylindrical organelle present in a wide range of eukaryotic species. Most centrioles have a signature ninefold radial symmetry of microtubules that is imparted to the axonemes of the cilia and flagella they template, with nine centriolar microtubule doublets growing into nine axonemal microtubule doublets. There are exceptions to the ninefold symmetrical arrangement of axonemal microtubules in some species, with lower or higher fold symmetries. In the few cases where this has been examined, such alterations in axonemal symmetries are grounded in similar alterations in centriolar symmetries. Here, we examine the question of microtubule number continuity between centriole and axoneme in flagellated gametes of the gregarine Lecudina tuzetae, which have been reported to exhibit a sixfold radial symmetry of axonemal microtubules. We used time-lapse differential interference microscopy to identify the stage at which flagellated gametes are present. Thereafter, using electron microscopy and ultrastructure-expansion microscopy coupled to stimulated emission depletion superresolution imaging, we uncover that a six- or fivefold radial symmetry in the axoneme is accompanied by an eightfold radial symmetry in the centriole. We conclude that the transition between centriolar and axonemal microtubules can be characterized by unexpected plasticity.
INTRODUCTION
Centrioles exhibit a nearly universal ninefold radial symmetry of microtubules (reviewed in Gönczy and Hatzopoulos, 2019). In ciliated and flagellated cells, centrioles mature into basal bodies, which seed the formation of a likewise ninefold radially symmetrical axoneme, the inner core of cilia and flagella (reviewed in Breslow and Holland, 2019). Thus, the symmetry of the centriole is thought to systematically dictate that of the axoneme, but experimental evidence supporting this view is sparse.
Phylogenetic considerations indicate that the nearly universal ninefold radial symmetry of centriolar and axonemal microtubules was present in the last eukaryotic common ancestor (reviewed in Azimzadeh, 2021). The strong evolutionary pressure to maintain this architecture is thought to reflect the critical importance of ciliary and flagellar motility across a vast range of organisms, including protists, algae, and animals, as well as some plants and fungi. Loss of this conserved arrangement is expected to result in reduced cell motility and thus usually selected against.
In most organisms, the proximal part of centrioles is characterized by nine microtubule triplets, dubbed A, B, and C microtubules; the A and B microtubules in each triplet extend until the distal end of the organelle. Upon cilium and flagellum formation, these nine microtubule doublets extend further to form the peripheral microtubule doublets of the axoneme. In most motile cilia and flagella, two centrally located axonemal microtubules are generated in addition, using a distinct mechanism (Jana et al., 2018; reviewed in Loreng and Smith, 2017).
Despite such general stereotypy, structural variations of the centriole have evolved multiple times independently. For instance, microtubule singlets – instead of the usual triplets and doublets – are present in some apicomplexans and fungi, as well as in Caenorhabditis elegans; in these cases, microtubule singlets are also arranged in a ninefold radially symmetrical manner (Dubremetz and Yvore, 1971; Pelletier et al., 2006; Sinden et al., 2010; Karpov et al., 2018, 2019). Because such centriolar microtubule singlets are accompanied by axonemal microtubule doublets, axonemal B microtubules must be generated in these cases by other mechanisms than simply a continuation of centriolar microtubule growth. The mechanisms by which B microtubules are added in such cases are not understood.
There are also instances that deviate from the canonical ninefold radially symmetrical arrangement of peripheral microtubules in the axoneme, which are usually accompanied by a lack of central microtubules and impaired cell motility. Examples of axonemes with a larger number of microtubule doublets, namely 10, 12, 14, 16, or 18, are encountered in certain protozoans, insects, arthropods and nematodes (Roggen et al., 1966; Ross, 1967; van Deurs, 1973, 1974; King and El-Hawawi, 1978; Dallai et al., 1992). The corresponding centriole symmetry has been investigated in two such cases, the proturan Acerentulus trägardhi and the sea spider Nymphon leptocheles (Baccetti et al., 1973; van Deurs, 1973). In both cases, the axoneme and the centriole exhibit the same 12-fold radial symmetry. An extreme instance occurs in the giant axoneme of the insect Sciara oprophila, where up to 70 microtubule doublets can be present (Phillips, 1966a,b). In this case, also, the radial symmetry of centriolar microtubules mirrors that of the axoneme.
Axonemes can also have radial symmetries of orders lower than nine. For instance, eightfold symmetrical axonemes are present in the coccidian Eimeria sp. (Reger and Florendo, 1970). A further reduction is encountered in the gregarines Diplauxis hatti and Lecudina tuzetae, in which three- and sixfold radial symmetry of axonemal microtubule doublets have been reported, respectively (Schrevel and Besse, 1975; Prensier et al., 1980). The underlying centriole symmetry has not been investigated in any organism with lower than ninefold symmetry of axonemal microtubules.
Here, we set out to investigate this point in the flagellated gametes of L. tuzetae, which have been reported to exhibit a sixfold radial symmetry of axonemal microtubule doublets, with one instance of five doublets plus one singlet also having been observed (Schrevel and Besse, 1975). Using electron microscopy (EM) and ultrastructure-expansion microscopy (U-ExM) coupled to stimulated emission depletion (STED) superresolution imaging of flagellated gametes, we show that most axonemal microtubule doublets exhibit a six- or fivefold radially symmetrical arrangement. Importantly, in addition, we establish that this is accompanied by an eightfold radial symmetry of microtubules in the centriole, thus uncovering unprecedented plasticity in the transition between centriolar and axonemal microtubules.
RESULTS
Lecudina tuzetae flagellated cells harbor the centriolar marker Centrin
Using time-lapse differential interference (DIC) microscopy, we monitored gametogenesis inside the ∼100 μm L. tuzetae gametocyte, which stems from a pair of trophozoite cells separated by a membrane and whose nuclei thereby occupy distinct sectors within the gametocyte (Figure 1A; Supplemental Video 1; Schrevel, 1969; Schrevel and Besse, 1975). After several rounds of nuclear division inside these two sectors (Figure 1A, 0 h 00 min), cellularization takes place, such that several hundred of larger flagellated as well as smaller nonflagellated gametes are then present in the gametocyte (Figure 1A, 5 h 34 min). The onset of flagellar movement is followed by the mixing of the two gamete types, a phase that has been dubbed “the dance of the gametes” (Schrevel, 1969; Schrevel and Besse, 1975), and then fertilization (Figure 1A, 6 h 13 min). Eventually the gametes stop moving and newly formed zygotes develop within the gametocyte (Figure 1A, 7 h 31 min).
FIGURE 1:
Live imaging of flagellated L. tuzetae gametes. (A) Differential interference contrast (DIC) time-lapse microscopy of L. tuzetae gametocyte development in distinct sectors (dashed lines), reflecting territories occupied initially by the two parental trophozoites (Top: time from start of recording), with twofold magnified insets (Bottom); see also Supplemental Video 1. (0 h 00 min) Nuclei (dashed circles) are visible as areas devoid of granules during the nuclear divisions that occur within the two sectors. (5 h 34 min) Cellularization individualizes nuclei into larger flagellated (blue) and smaller nonflagellated (red) gametes within the two sectors. These two gamete types have also been referred to as male and female gametes, respectively (Schrevel and Besse, 1975). Note that flagellated and nonflagellated gametes each occupy approximately one-half of the gametocyst volume, which is not apparent in this single focal plane. (6 h 13 min) Swimming of flagellated gametes mixes the two gamete types, which eventually fuse during fertilization (purple). (7 h 31 min) After fertilization, the zygote stops swimming and becomes round (purple). (B) Schematic of encounter between a flagellated (blue) and a nonflagellated (red) gamete. (C) DIC time-lapse microscopy of L. tuzetae gamete fusion between flagellated and nonflagellated cells (time from start of recording); see also Supplemental Video 2. (0 min) Attachment: initially a narrow attachment between the two cells is observed. (9 min) Gamete fusion: the apposed region widens. (30 min) Postfertilization: the zygote has formed. The dashed line in the three panels highlights the flagellum located just above it.
Movie S1.
L. tuzetae gametocyst development. Differential interference contrast (DIC) time-lapse microscopy of L. tuzetae gametocyst development (time from start of recording). Images were acquired every 10 seconds and are played with 300 times acceleration. Related to Figure 1A.
To distinguish individual flagellated and nonflagellated gametes, as well as to better analyze fertilization events, gametes were set free from the gametocyte (Figure 1B). Time-lapse DIC microscopy enabled us to observe the movement of the flagellum (Figure 1C; Supplemental Video 2), as well as to monitor the encounter and the progressive fusion of the two gamete types, which results in zygote formation (Figure 1C).
Movie S2.
L. tuzetae gamete fusion. DIC time-lapse microscopy of gamete fusion (time from start of recording). L. tuzetae gametes were set free from the gametocyst when they started moving vigorously; arrowhead marks actively beating flagellum. Cells were immobilized on the glass and monitored until zygote formation. Images were acquired every second and are played with 10 times acceleration. Related to Figure 1C.
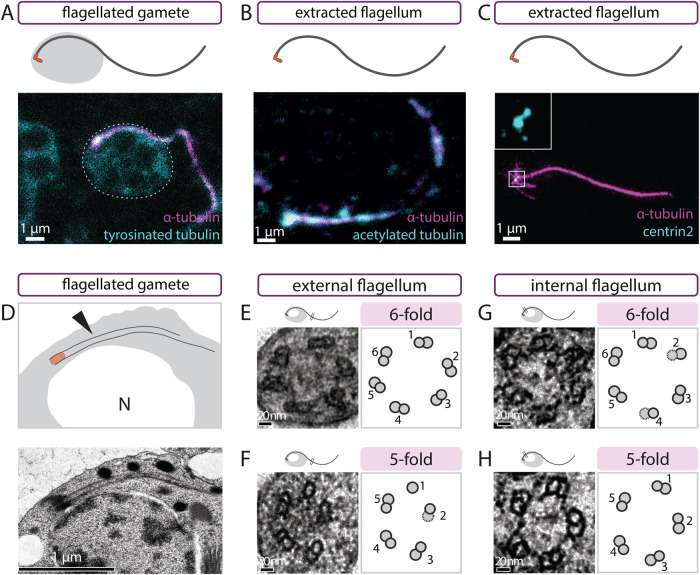
As in other gregarines, the axoneme of L. tuzetae consists of two parts: an internal cytoplasmic segment and an external segment, which is motile (Schrevel and Besse, 1975; reviewed in Francia et al., 2015). We analyzed flagellated gametes using indirect immunofluorescence microscopy with antibodies against α-tubulin and tyrosinated α-tubulin. Both internal cytoplasmic and external segments could be visualized with these antibodies (Figure 2A; white outline indicates cell body). We likewise detected the axoneme in flagella extracted with their centrioles (hereafter referred to as extracted flagella) using antibodies against α-tubulin and acetylated tubulin (Figure 2B). Importantly, antibodies against human Centrin-2, which mark centrioles in a large range of organisms, also localized to the base of extracted flagella, at the center of microtubule asters (Figure 2C), where γ-tubulin was shown to localize (Kuriyama et al., 2005). We conclude that Centrin proteins, and therefore centrioles, are likely present at the base of the axoneme in L. tuzetae flagellated gametes.
FIGURE 2:
Flagellated gametes harbor Centrin and exhibit six- or fivefold symmetry. (A) Schematic (top) and corresponding maximum intensity projection confocal image of L. tuzetae flagellated gamete stained with antibodies against α-tubulin (magenta) and tyrosinated tubulin (cyan). Dashed line indicates the cell body. (B) Schematic (top) and corresponding single-plane confocal image of extracted L. tuzetae flagellum stained with antibodies against α-tubulin (magenta) and acetylated tubulin (cyan). (C) Schematic (top) and corresponding maximum-intensity projection STED image of extracted L. tuzetae flagellum stained with antibodies against α-tubulin (magenta) and Centrin-2 (cyan), with fourfold magnified inset showing two Centrin-2 foci at the base of the flagellum. (D) Schematic representation (top) and corresponding transmission EM of L. tuzetae flagellated gamete with side view of centriole and internal axoneme next to the nucleus. Arrowhead points to the internal part of the flagellum. Note that the daughter centriole is not discernable in this image, perhaps because it is located in a different section. (E–H) Transmission EM transversal view of L. tuzetae external—E, F—or internal—G, H—axonemal segment with sixfold—E, G—or fivefold—F, H—arrangement of microtubule doublets (left) and corresponding schematics (right). Note that the average distance between adjacent A-microtubules is similar in the two types of arrangements (sixfold: 54 nm [± 8 nm SD], fivefold: 51 nm [±7 nm SD]).
Axonemal microtubules exhibit six- or fivefold radial symmetry
To confirm the ultrastructure of the axoneme in L. tuzetae flagellated gametes, we conducted EM on resin-embedded specimens. As for the immunostainings, the internal flagellum was observed by EM to emanate from the basal body and to extend along the nuclear envelope (Figure 2D). To analyze the fold radial symmetry of microtubules, we focused on transverse views of axonemes. This enabled us to detect in some cases a sixfold radially symmetrical arrangement of microtubule doublets in the external segment of the axoneme, without any central microtubule, in line with previous findings (Figure 2E, N = 3; Schrevel and Besse, 1975). Moreover, we observed a fivefold symmetrical arrangement of microtubule doublets in the external axonemal segment in other cases (Figure 2F, N = 13). In addition, when examining the internal segment of the axoneme, we likewise found both six- and fivefold symmetries (Figure 2G, sixfold N = 2; Figure 2H, fivefold N = 4). We sought to confirm such six- and fivefold symmetrical arrangements through radial image symmetrization analysis, a classical approach to determining the fold symmetry of objects such as centrioles and axonemes (Friedman, 1970; van Deurs, 1974; Guichard et al., 2013). As shown in Supplemental Figure S1, A and B, by applying incremental 4- to 11-fold symmetrization, we found that six- and five-, respectively, fold symmetrization bears the most resemblance to the arrangement of microtubules in the original images. We noted also that flagellated gametes with both sixfold and fivefold symmetry were present in the same gametocyte, demonstrating that difference between the two types does not reflect genetic heterogeneity between gametocysts. Overall, we conclude that all internal and external segments of L. tuzetae axonemes analyzed deviate from the canonical ninefold symmetry.
Widening of the microtubule-bearing area toward the centriole
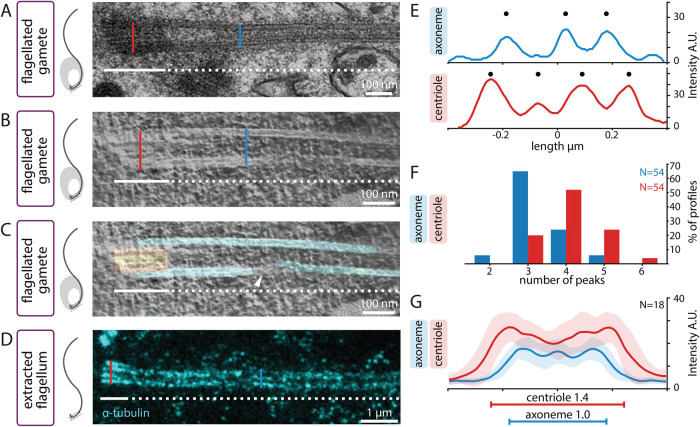
Further inspection of rare longitudinal views of resin-embedded flagellated gametes analyzed by EM uncovered a widening of the area corresponding to microtubules in the region where the centriole is located (Figure 3A, compare blue and red lines, N = 3). Tomographic reconstruction of such longitudinal EM sections confirmed a widening of the microtubule wall in the centriolar region (Figure 3B, compare blue and red lines, Figure 3C, N = 2). Interestingly, in addition, this analysis indicated that whereas some of the microtubules from the centriole appear to be continuous with those of the axoneme, others do not exhibit such continuity (Figure 3C, arrowhead; Supplemental Video 3).
FIGURE 3:
Centrioles are wider than axonemes in L. tuzetae. (A) Schematic (left) and corresponding side view transmission EM of L. tuzetae internal flagellum embedded in resin after chemical fixation, with centriolar part (white line) and axonemal part (white dashed line). Width of centriole (red line) and axoneme (blue line) are indicated for illustration purposes here, as well as in B and D. (B, C) Side view electron tomogram of 50-nm section embedded in resin after cryofixation, B, and corresponding model superimposition, C (microtubules in cyan, centriolar region in orange) of L. tuzetae internal flagellum, with centriolar part (white line) and axonemal part (white dashed line). Arrowhead points to discontinuity in one of the microtubules. (D) Side view U-ExM STED image from extracted L. tuzetae flagellum containing centriole (white line) and axoneme (white dashed line) upon 5.2-fold expansion and after staining with antibodies against α-tubulin. (E) Intensity plot profiles measured at the level of the centriole (red) and the axoneme (blue) from extracted L. tuzetae flagellum upon U-ExM STED (from specimen shown in D). In the axoneme, the full width at half maximum (FWHM) is 0.44 µm and three intensity peaks (discs) are visible. In the corresponding centriole, the FWHM is 0.58 µm and four intensity peaks (discs) are visible. (F) Line profile peak analysis from population of intensity plot profiles measured at axoneme (blue) and centriole (red). Plot profiles were determined in three positions for the axoneme and three positions for the centriole in 18 U-ExM STED images (hence N = 54 for both categories). (G) Mean intensity plot profiles measured in three positions for each centriole (red) and axoneme (blue; N = 18 images), with corresponding standard deviations (shaded) from extracted L. tuzetae flagella upon U-ExM STED (N = 54 plot profile per category). The FWHM of axoneme and centriole is statistically different (two-sample Student’s t test p = 1.22 × 10–7) and is indicated by horizontal lines (axoneme: blue, centriole: red).
Movie S3.
Tomogram with model of microtubules. Movie rendered in Chimera of the tomographic data from L. tuzetae with a superimposed model generated in IMOD. The video shows individual slices of the reconstructed tomogram of a 50-nm thick section through a L. tuzetae flagellum parallel to the section. Individual microtubules are represented with cylindrical models in cyan and the centriole in orange.
To quantify the apparent width difference uncovered through EM, we set out to analyze extracted flagella using U-ExM (Chen et al., 2015; Gambarotto et al., 2019). We reasoned that the ∼5.2 expansion factor afforded by U-ExM, coupled with STED superresolution, should enable us to probe potential width differences in longitudinal views readily by immunostaining. U-ExM STED of such specimens stained with α-tubulin antibodies indeed confirmed the widening toward the centriole (Figure 3D, compare blue and red lines). Linescans perpendicular to the flagellum revealed that three peaks of α-tubulin signal were present in most cases for the axonemal part, irrespective of the peak calling threshold applied, presumably corresponding to the side view of six- or five-fold microtubule doublets (Figure 3E; Figure 3F blue). By contrast, four intensity profile peaks were most frequently observed for the centriolar part (Figure 3E; Figure 3F, red). Importantly, measuring the full width at half maximum (FWHM) of the centriolar and axonemal line profiles revealed that the width at the centriole is on average ∼1.4× the width at the axoneme (Figure 3G; N = 18). Overall, we conclude that the width of the region corresponding to microtubules is consistently greater at the level of the centriole than at the level of the axoneme emanating from it.
Centriolar microtubules exhibit eightfold radial symmetry
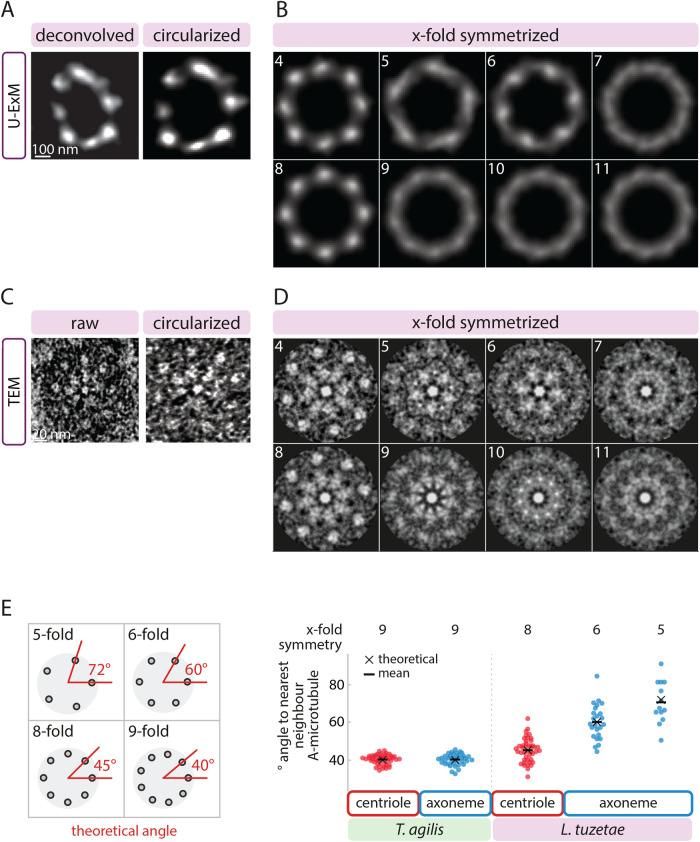
The above observations are compatible with at least two possibilities. First, microtubules with six- or fivefold radial symmetry could also be present in the centriole but be positioned at a greater distance from one another than in the axoneme, resulting in a greater overall width. Second, the centriole may contain a larger number of peripheral microtubules than the six or five sets present in the axoneme. Analyzing transverse sections of centrioles should distinguish between these possibilities. To this end, we conducted U-ExM followed by STED of extracted flagella probed with α-tubulin antibodies. The rare transverse centriolar views obtained in this manner clearly showed more peripheral microtubules than the six or five that are present in axonemes (see below, N = 4). We set out to uncover the symmetry of these centriolar microtubules. To compensate for slight distortions, images of deconvolved transverse views were first circularized (Figure 4A) before applying incremental 4- to 11-fold symmetrization, as was done for the axoneme. As is apparent from Figure 4B, eightfold radial symmetrization yielded the best resemblance to the arrangement of microtubules in the original images of the centriole (see also Supplemental Figure S1C and Supplemental Figure S2A). This conclusion was further supported by the fourfold symmetrization pattern, which resembles that of the eightfold patterns, as expected from it being half that (Figure 4B; Supplemental Figures S1C, S2A). For all four centrioles analyzed in this manner, eight- and fourfold symmetrization correlated best with the original images (Supplemental Figure S2, B and C). All other applied symmetries resulted in unresolvable microtubule signals that did not resemble the original images (Figure 4B).
FIGURE 4:
L. tuzetae centrioles exhibit 8-fold radial symmetry. (A) Transverse view of deconvolved U-ExM STED image of L. tuzetae centriole from extracted flagellum stained with antibodies against α-tubulin (left), and corresponding circularized image (right). (B) X-fold symmetrization (4–11, as indicated) of circularized centriole from A. (C) Transverse view of transmission EM of L. tuzetae centriole embedded in resin after chemical fixation (left), and corresponding circularized image (right). Note presence of central hub, as well as several spokes emanating from this hub toward the microtubules, reminiscent of the cartwheel architecture in other systems. Such structures were not clearly discerned in all cases. (D) X-fold symmetrization (4–11, as indicated) of circularized centriole from C. (E) Schematic of theoretical angles in wedges between centriole center and two neighboring peripheral A-microtubules for the indicated fold-symmetries (left). Theoretical angles (black cross) and corresponding measurements of observed angles (disks) with mean (black bar) in Trichonympha agilis ninefold symmetrical centriole (N = 45 angles between neighboring microtubules of n = 5 centrioles) and axoneme (N = 36, n = 4 axonemes), as well as in L. tuzetae centriole (N = 43, n = 9) and axoneme (sixfold: N = 24, n = 4; fivefold: N = 13, n = 3), as indicated.
To probe the radial symmetry of centriolar microtubules in a complementary manner, we also analyzed transverse EM sections of the L. tuzetae centriole. Not all microtubules could be discerned clearly in a single section in these cases (Figure 4C), likely because the sections are not perfectly orthogonal to the longitudinal axis of the centriole. Regardless, those centriolar microtubules that were clearly discernible appeared as singlets arranged around a central hub, although we cannot exclude the possibility that the electron-dense cloud surrounding centrioles or microtubule inner proteins might have obscured B-microtubules. Applying again incremental 4- to 11-fold symmetrization of the EM images, we uncovered that the radial arrangement of centriolar microtubules was most compatible with an eightfold symmetry, a view further supported by the fourfold symmetrization data (Figure 4D). Again, for all eight images analyzed in this manner, eight- and fourfold symmetrization correlated best with the original images (Supplemental Figure S1D; Supplemental Figure S2, D–F). To corroborate this point in the nonsymmetrized raw data, we measured the inner angle in wedges formed between the center of the centriole and two clearly discernible neighboring peripheral microtubules (Figure 4E). A given fold symmetrical arrangement of microtubules should yield a predictable angle in such wedges, given the 360° present in a circle. Thus, an eightfold symmetrical arrangement of microtubules results in a theoretical angle of 45°, whereas a ninefold arrangement yields a theoretical angle of 40°. Utilizing TEM data set from Trichonympha agilis (Nazarov et al., 2020), in which microtubules exhibit a canonical ninefold radially symmetrical arrangement in both centriole and axoneme, we found as expected that the average angle is ∼40° in both compartments (centriole: 40.1° ± 2.5° SD (SD); axoneme: 40.0° ± 3.0° SD; Figure 4E). This confirms that measuring the angle between neighboring microtubules can be used as a proxy for the fold symmetry. In L. tuzetae, we found in contrast that the average angle measured for all centrioles taken together is 45.1° (±6.3° SD; Figure 4E), which is fully compatible with an eightfold symmetrical arrangement. In addition, the average angle between neighboring microtubules within individual centrioles was invariably closer to 45° than to 40° (N = 9 centrioles). As anticipated, transverse views of the six- and fivefold axonemes yielded average angles of 60.1° (±9.4° SD) and 70.6° (±11.2° SD), respectively, close to the theoretical angles (Figure 4E). Together, despite the relatively small number of images analyzed, these findings lead us to conclude that the centriole in L. tuzetae exhibits an eightfold symmetrical arrangement of microtubules underlying six- and fivefold axonemes.
DISCUSSION
Our findings further establish that the L. tuzetae axoneme diverges from the canonical ninefold radial arrangement observed in most species and uncovers the symmetry of the corresponding centriole (Figure 5). We find that the axoneme of the flagellated gamete harbors six or five radially arranged sets of microtubules, in both internal and external segments of the axoneme, instead of the usual nine. Importantly, in addition, our findings indicate that the centriole from which this axoneme emanates contains eight microtubules, instead of the usual nine present in most other species, or of the six or five that might have been anticipated from the axonemal configuration.
FIGURE 5:
Schematic summary of centriolar and axonemal microtubules in species with canonical ninefold symmetry and in L. tuzetae. (A) Schematic of microtubule arrangement in the canonical situation, where 9-fold symmetrical microtubule doublets in the centriole yield a likewise ninefold symmetrical arrangement in the axoneme of the flagellum (middle). (B) Corresponding schematic in L. tuzetae, where eightfold symmetrical microtubule singlets in the centriole are followed by five- or sixfold symmetrical microtubule doublets in the axoneme.
How can an eightfold radially symmetrical centriole be built?
What mechanisms could explain the formation of eightfold radially symmetrical centrioles in L. tuzetae? Across the eukaryotic tree of life, proteins of the SAS-6 family are critical for scaffolding the onset of centriole assembly (reviewed in Gönczy, 2012; Hirono, 2014). SAS-6 forms homodimers, which possess an intrinsic ability to assemble into 9-fold symmetrical ring structures. These ring structures then stack to form a ∼100 nm–high cartwheel, which is thought to act as a scaffold for centriole assembly, including contributing to impart the fold symmetry of peripheral microtubules.
How could L. tuzetae SAS-6 (LtSAS-6) self-assembly yield an eightfold symmetrical structure? In the absence of molecular information regarding LtSAS-6, we speculate that the protein might harbor amino acid changes that favor assembly of an eightfold symmetrical structure. Compatible with this notion, rational mutagenesis of Chlamydomonas reinhardtii SAS-6 (CrSAS-6) can alter the fold symmetry of structures self-assembled in vitro, including toward eightfold (Hilbert et al., 2016). Another possibility is based on the finding that although wild-type CrSAS-6 favors self-assembly of 9-fold symmetrical structures, in vitro, 8-fold and 10-fold symmetrical ring structures can be observed in addition to 9-fold symmetrical ones (Hilbert et al., 2016; Banterle et al., 2021). Such observations have led to the suggestion that peripheral elements must function together with SAS-6 ring-containing structures to select strictly ninefold radially symmetrical entities (Hilbert et al., 2016; Banterle and Gönczy, 2017). Perhaps in L. tuzetae such peripheral components are absent or different from those in most other species, thus favoring eightfold symmetrical assemblies.
Diminution of microtubule numbers from centriole to axoneme
The finding that there are distinct fold symmetries in the centriole and in the axoneme that emanates from it is unprecedented to our knowledge. It appears that some of the microtubules present in the centriole do not extend further into axonemal microtubules in L. tuzetae, indicative of plasticity in the processes at the transition between the two cellular compartments. What could be the basis for such plasticity? Although the underlying molecular mechanisms remain to be discovered, we note that axonemes assemble rapidly in the cytoplasm of the related apicomplexan Plasmodium falciparum (reviewed in Sinden et al., 2010). Perhaps likewise rapid assembly kinetics occur in L. tuzetae, which we speculate could result in loss of continuity for two or three microtubules between centriole and axonemal compartments, leading to axonemes with six or five microtubule doublets. Axoneme architecture can also exhibit plasticity over time, as evidenced by specific sensory cilia in C. elegans, where the number of microtubule doublets is remodeled from ninefold in larvae to sixfold in adults (Akella et al., 2019). Such temporal remodeling could conceivably also explain why some axonemes exhibit six- and others fivefold symmetry in L. tuzetae.
How could an otherwise very constrained architecture be relaxed in L. tuzetae and other apicomplexans? A plausible answer lies in the underlying biology. In contrast to the situation in most other species, the flagellated cells in L. tuzetae are enclosed with the nonflagellated cells in a common gametocyte, thus facilitating fertilization. Moreover, since all flagellated cells derive from a single trophozoite, they are likely in competition only with their genetically identical clones. Therefore, the evolutionary pressure for flagellar motility may be minimal in L. tuzetae. Compatible with this view, L. tuzetae flagellated gametes exhibit a slow tumbling forward motion and are poor swimmers compared with sperm cells from other species (Schrevel and Besse, 1975; Goldstein, 1982). Regardless, such diversity in axonemal and centriolar architecture highlights striking biodiversity in otherwise strictly constrained cellular structures of critical importance for the successful reproduction of many species.
MATERIALS AND METHODS
Live imaging of Lecudina tuzetae gametocysts
Work in the laboratory of PG is conducted under authorization number A1821681 2 delivered by the Swiss Federal Office for Public Health. Hediste diversicolor worms, which host Lecudina tuzetae intestinal parasites, were collected in 2017, 2018 and 2021 between April and October from location 48°37’8.20’’N 3°57’9.03’W by the collection service of the Station Biologique de Roscoff (France). After courier shipping, worms were maintained without feeding in sea water (22 g/L sea salt) at room temperature, one per 10 cm Petri dish. The Petri dish was aerated on the first day and worms were allowed to recover for 48 h after shipping, before being transferred into fresh sea water. This enabled collection during the following 16–20 h of partially synchronous L. tuzetae gametocysts from the Petri dish with a pipette (200 µl tip) under a dissecting microscope with transmitted light.
For time-lapse imaging, gametocysts were mounted on 2% agarose pads immersed in sea water, covered with a coverslip, the edges of which were sealed with VaLaP (1:1:1 mixture of petroleum jelly:lanolin:paraffin wax) to prevent evaporation (see Figure 1A). To mechanically set free flagellated and non-flagellated gametes during fusion, gametocysts were mounted without agarose and pressure applied on the coverslip after initiation of swimming (Figure 1C). DIC time-lapse microscopy was performed by capturing an image every 10 s for gametocysts and every 1 s for released gametes with a 63× EC Plan-NEOFLUAR objective (NA 1.25) on a Zeiss Axioskop2 plus equipped with a DCC1545M-GL Thorlabs camera. Gametocysts were monitored using DIC time-lapse microscopy to identify the appropriate stage for fixation for subsequent EM and immunofluorescence experiments.
Immunostaining
For immunostaining of intact gametes and extracted flagella, gametes were mechanically set free from gametocysts by applying gentle pressure on the coverslip and removing excess water with a filter paper, followed by freeze-cracking on a poly-d-Lysine (2 mg/ml in water) coated slide. To isolate flagella, cysts were collected by centrifugation for 1 min at 100 g on a tabletop centrifuge and lysed in 10 mM K-Pipes pH 6.8, 1% NP-40 for 10 min in the presence of complete protease inhibitor cocktail (Roche, 1:1000) on ice, followed by 10–20 strokes with a pestle to break the cyst wall. The crude extract containing intact flagella was centrifuged at 10,000 g onto coverslips in a Corex tube with an adaptor in a Beckman JS 13.1 rotor. Fixation was carried out in methanol at −20°C for 5 min, followed by incubation for 1 h at room temperature with the following primary antibodies (all 1:500): mouse anti-α-tubulin (B-5-1-2 coupled to Alexa-488, Thermo Fisher or DM1A, Sigma), rat anti-tyrosinated tubulin (YL1/2, Merck), mouse anti-acetylated tubulin (T6793; Sigma), mouse anti-Centrin-2 (20H5, Millipore). Secondary antibodies were donkey anti-rabbit conjugated to Alexa 594 (Abcam, 150072) and goat anti-mouse conjugated to Alexa 488 (Thermo Fisher, A11001), both used 1:1000. Indirect immunofluorescence was imaged on LSM700 Zeiss or Leica TCS SP8 STED 3X microscopes with a 100 × 1.4 NA oil-immersion objective.
Transmission electron microscopy and tomography
For chemical fixation (Figures 2, G–H, 3A and 4, C–D), Supplemental Figure S1D), cells were released from gametocysts by applying pressure on the coverslip mounted on a poly-d-Lysine (Sigma, #P1024) coated slide and fixed overnight in 2% paraformaldehyde 1% glutaraldehyde in phosphate buffer 0.1 M, pH 7.4, washed in cacodylate buffer (0.1 M, pH 7.4) at 4°C, and post-fixed with 0.5% tannic acid in cacodylate buffer (0.1 M, pH 7.4) for 40 min at room temperature. After two washes in distilled water, cells were post-fixed in 1% osmium tetroxide in cacodylate buffer. Thereafter, samples were washed in distilled water and dehydrated in a graded ethanol series (1 × 50%, 1 × 70%, 2 × 96%, 2 × 100%), and finally embedded in epon resin before polymerization overnight at 65°C.
For cryo-fixation (Figures 2, D–F and 3, B–C), intact gametocysts bathed in 3% BSA were subjected to high-pressure freezing (HPM100, Leica Microsystems), before freeze-substitution using a temperature-controlled chamber (AFS, Leica Microsystems). Samples were first transferred into plastic tubes containing acetone with 0.2% glutaraldehyde at –90°C for 24 h, and then washed in pure acetone before transfer to 0.1 % tannic acid in acetone for a further 24 h. Next, the temperature was raised to –30°C over the course of 3 days in 2 % osmium, and the samples then transferred to 0.5 % uranyl acetate for 24 h while the temperature rose again from –30°C to –10°C. The samples were then rinsed in pure acetone three times, the temperature raised to 0°C, and a 50/50 acetone/epon resin mix added. The samples were left still for 2 h before being placed on a rotator at room temperature and increasing concentrations of resin added up to 100%. The samples were then left overnight, before hardening at 65°C for 48 hours.
50 nm sections were cut using a diamond knife on an ultramicrotome (Leica UC7), and collected on single slot copper grids with a formvar support film. Sections were then further stained with lead citrate and uranyl acetate before imaging inside a transmission electron microscope operating at 80 kV (Tecnai Spirit, FEI Company), using a CCD camera (Eagle, FEI Company).
Tilt-series from cryo-fixed sections were acquired on a Tecnai F20 operated at 200 kV (Thermo Fischer Scientific using Thermo Scientific Tomography software in continuous tilt scheme from –60º to +60º in 2º steps at –2.5 µm defocus. Data were recorded with a Falcon III DD camera (Thermo Fisher Scientific) in linear mode at 29,000 × magnification, corresponding to a pixel size of 3.49 Å. Tilt series alignment and tomogram reconstruction was done using EMAN 2.9 (Tang et al., 2007). 4× binned tomograms with a corresponding pixel size of 13.96 Å were corrected using IsoNet v.0.9 (Liu et al., 2021). Microtubule and central hub densities were traced in the corrected tomograms using Imod 4.9 (Kremer et al., 1996). Video visualization was done using Chimera 1.14.
Ultrastructure expansion microscopy
Flagella were isolated and spun onto 10 mm coverslips as above. After methanol fixation, coverslips were incubated overnight at room temperature in an acrylamide/formaldehyde solution (1% AA and 0.7% FA in PBS) under mild agitation. Next, coverslips were incubated in 50 µl monomer solution (19% (wt/wt) SA, 10% (wt/wt) AA, 0.05% (wt/wt) BIS in PBS) supplemented with 0.5% Tetramethylethylenediamine (TEMED) and 0.5% Amonium Persulfate (APS) on a piece of Parafilm for 1 h at 37°C in a moist dark chamber for gelation. All subsequent steps were carried out with mild agitation at room temperature unless otherwise stated. For denaturation, gels were incubated for 15 min in denaturation buffer (200 mM SDS, 200 mM NaCl and 50 mM Tris in distilled water, pH = 9) in 5 cm Petri dishes followed by incubation for 1 h on a 95°C hot plate in fresh denaturation buffer. After denaturation, gels were washed extensively with distilled water in 10 cm Petri dishes. Water was exchanged 5 times every 20 min, followed by a wash in distilled water overnight at 4°C. After expansion, the gel size was measured with a ruler to determine the fold expansion, and the gel cut in pieces fitting into a 5 cm Petri dish. Prior to staining, gels were blocked for 1 h in blocking buffer (10 mM HEPES (pH = 7.4), 3% BSA, 0.1% Tween 20, sodium azide [0.05%]) followed by incubation with rabbit anti-α-tubulin antibodies (Abcam 18251) diluted 1:250 in blocking buffer. Thereafter, gels were again washed three times in blocking buffer for 10 min each, before incubation with secondary antibodies diluted in blocking buffer at 37°C in the dark for 3 h. Finally, gels were washed three times in blocking buffer for 10 min each before transfer into a 10 cm Petri dish for re-expansion by 6 washes, each 20 min in distilled water. For imaging, gels were cut and mounted on a 60 × 24 mm coverslip coated with poly-d-lysine diluted in water (2 mg/ml) and supported on both longitudinal sides with capillaries attached with superglue. To prevent drying, the edges of the gel were covered with VaLaP and the gel covered with Halocarbon oil 700 for imaging. STED images were acquired using the 775 pulsed laser for depletion on a Leica TCS SP8 STED 3× microscope with a 100 × 1.4 NA oil-immersion objective.
Image analysis
For quantification of axoneme and centriole width in α-tubulin UExM STED experiments, three 1 µm line scans with a width of 5 pixel perpendicular to the long axis of flagellum were measured per image. Peak calling in IgorPro8 software (Wavemetrics, USA) was used to first interpolate and then resample to obtain the same number of data points for all images regardless of the expansion factor. Further line profile peak analysis was done using its build-in PeakFind function. The parameters were set globally for all traces as follows: pBegin 30; pEnd 100; maxPeaks 6; minPeakPercent 5; noiselevel 1.4; smoothingfactor 5.2. The most frequent categories remained unchanged if the threshold was altered. Next, mean intensity line profiles for both the axoneme and the centriole were calculated, and full-width half maxima (FWHM) determined using a custom Python script, and the average of the FWHMs computed. Line profiles were aligned on the center of the FWHM to plot the mean intensity profile from all 54 profiles combined, together with the standard deviation.
In EM and UExM STED transverse views, centrioles with slight perspective distortion due to tilted orientations were circularized using the ImageJ plugin ‘Transform-Interactive Affine’ before symmetrization. The circularized input image and the symmetrized images from 4- to 11-fold symmetry were overlaid and both intensity profiles measured in a circular band along the microtubules, and the correlation of the signal analyzed using R2 values for each symmetrization.
The ‘angle tool’ in FIJI was used for quantification of angles in wedges between the center of the centriole and two clearly visible neighboring peripheral A-microtubules in centrioles and axonemes imaged in transverse view by EM.
Supplementary Material
Acknowledgments
We are grateful to Paul Guichard for contributing to initiating the project, as well as to Isabelle Florent, Graham Knott, Joseph Schrével, and Gérard Prensier for valuable advice and discussions. Michel Bornens and Gaia Pigino are acknowledged for further discussions. We thank Marie Croisier, and also Anaëlle Dubois from the Bio-EM Facility in the School of Life Sciences at EPFL headed by Graham Knott for help with ultrastructural analysis, as well as Sophie Booker and Ronan Garnier from the Station Biologique de Roscoff (France) for providing Hediste diversicolor animals. We are grateful to Georgios Hatzopoulos for help with drawing the schematics in Figure 5. We acknowledge Niccolò Banterle and Ella L. Müller for help with data processing, as well as Isabelle Florent, Paul Guichard, Nils Kalbfuss, and Joseph Schrével for useful comments on the manuscript. This work was supported in part by grants from the European Union (MCSA-IF 588594 to A.W. and COFUND-EuroPostdoc 588459 to F.S., as well as ERC AdG 340227 and 588437 to P.G.).
Abbreviations used:
- DIC
differential interference contrast
- EM
electron microscopy
- STED
stimulated emission depletion microscopy
- FWHM
full width at half maximum.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E22-04-0123) on May 11, 2022.
REFERENCES
- Akella JS, Silva M, Morsci NS, Nguyen KC, Rice WJ, Hall DH, Barr MM (2019). Cell type-specific structural plasticity of the ciliary transition zone in C. elegans. Biol Cell 111, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimzadeh J (2021). Evolution of the centrosome, from the periphery to the center. Curr Opin Struct Biol 66, 96–103. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Dallai R, Fratello B (1973). The spermatozoon of Arthropoda. XXII. The 12+0’, 14+0’ or aflagellate sperm of Protura. J Cell Sci 13, 321–335. [DOI] [PubMed] [Google Scholar]
- Banterle N, Gönczy P (2017). Centriole biogenesis: From identifying the characters to understanding the plot. Annu Rev Cell Dev Biol 33, 23–49. [DOI] [PubMed] [Google Scholar]
- Banterle N, Nievergelt AP, de Buhr S, Hatzopoulos GN, Brillard C, Andany S, Hübscher T, Sorgenfrei FA, Schwarz US, Gräter F, et al. (2021). Kinetic and structural roles for the surface in guiding SAS-6 self-assembly to direct centriole architecture. Nat Commun 12, 6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Holland AJ (2019). Mechanism and regulation of centriole and cilium biogenesis. Annu Rev Biochem 88, 691–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tillberg PW, Boyden ES (2015). Optical imaging. Expansion microscopy. Science 347, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallai R, Xué L, Yin W (1992). Flagellate spermatozoa of Protura (Insecta, Apterygota) are motile. Int J Insect Morphol Embryol 21, 137–148. [Google Scholar]
- Dubremetz JF, Yvore P (1971). Oocystic wall formation in the coccidia Eimeria necatrix Johnson 1930 (Sporozoa, Coccidiomorpha). Study with electronic microscope. C R Seances Soc Biol Fil 165, 862–866. [PubMed] [Google Scholar]
- Francia ME, Dubremetz J-F, Morrissette NS (2015). Basal body structure and composition in the apicomplexans Toxoplasma and Plasmodium. Cilia 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MH (1970). A reevaluation of the Markham rotation technique using model systems. J Ultrastruct Res 32, 226–236. [DOI] [PubMed] [Google Scholar]
- Gambarotto D, Zwettler FU, Le Guennec M, Schmidt-Cernohorska M, Fortun D, Borgers S, Heine J, Schloetel J-G, Reuss M, Unser M, et al. (2019). Imaging cellular ultrastructures using expansion microscopy (U-ExM). Nat Methods 16, 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein SF (1982). Motility of 9 + 0 mutants of Chlamydomonas rheinhardtii. Prog Clin Biol Res 80, 165–168. [DOI] [PubMed] [Google Scholar]
- Gönczy P (2012). Towards a molecular architecture of centriole assembly. Nat Rev Mol Cell Biol 13, 425–435. [DOI] [PubMed] [Google Scholar]
- Gönczy P, Hatzopoulos GN (2019). Centriole assembly at a glance. J Cell Sci 132, jcs228833. [DOI] [PubMed] [Google Scholar]
- Guichard P, Hachet V, Majubu N, Neves A, Demurtas D, Olieric N, Fluckiger I, Yamada A, Kihara K, Nishida Y, et al. (2013). Native architecture of the centriole proximal region reveals features underlying its 9-fold radial symmetry. Curr Biol CB 23, 1620–1628. [DOI] [PubMed] [Google Scholar]
- Hilbert M, Noga A, Frey D, Hamel V, Guichard P, Kraatz SHW, Pfreundschuh M, Hosner S, Flückiger I, Jaussi R, et al. (2016). SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture. Nat Cell Biol 18, 393–403. [DOI] [PubMed] [Google Scholar]
- Hirono M (2014). Cartwheel assembly. Philos Trans R Soc Lond B Biol Sci 369, 20130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana SC, Mendonça S, Machado P, Werner S, Rocha J, Pereira A, Maiato H, Bettencourt-Dias M (2018). Differential regulation of transition zone and centriole proteins contributes to ciliary base diversity. Nat Cell Biol 20, 928–941. [DOI] [PubMed] [Google Scholar]
- Karpov SA, López-García P, Mamkaeva MA, Klimov VI, Vishnyakov AE, Tcvetkova VS, Moreira D (2018). The chytrid-like parasites of algae Amoeboradix gromovi gen. et sp. nov, Sanchytrium tribonematis belong to a new fungal lineage. Protist 169, 122–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpov SA, Vishnyakov AE, Moreira D, López-García P (2019). The ultrastructure of Sanchytrium tribonematis (Sanchytriaceae, Fungi incertae sedis) confirms its close relationship to Amoeboradix. J Eukaryot Microbiol 66, 892–898. [DOI] [PubMed] [Google Scholar]
- King PE, El-Hawawi ASN (1978). Spermiogenesis in the pycnogonid Pycnogonum littorale (Ström). Acta Zool. 59, 97–103. [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR (1996). Computer visualization of three-dimensional image data using IMOD. J Struct Biol 116, 71–76. [DOI] [PubMed] [Google Scholar]
- Kuriyama R, Besse C, Gèze M, Omoto CK, Schrével J (2005). Dynamic organization of microtubules and microtubule-organizing centers during the sexual phase of a parasitic protozoan, Lecudina tuzetae (Gregarine, Apicomplexa). Cell Motil Cytoskeleton 62, 195–209. [DOI] [PubMed] [Google Scholar]
- Liu Y-T, Zhang H, Wang H, Tao C-L, Bi G-Q, Zhou ZH (2021). Isotropic reconstruction of electron tomograms with deep learning. 2021.07.17.452128. [DOI] [PMC free article] [PubMed]
- Loreng TD, Smith EF (2017). The central apparatus of cilia and eukaryotic flagella. Cold Spring Harb Perspect Biol 9, a028118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarov S, Bezler A, Hatzopoulos GN, Nemcˇíková Villímová V, Demurtas D, Le Guennec M, Guichard P, Gönczy P. (2020). Novel features of centriole polarity and cartwheel stacking revealed by cryo-tomography. EMBO J 39, e106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L, O’Toole E, Schwager A, Hyman AA, Müller-Reichert T (2006). Centriole assembly in Caenorhabditis elegans. Nature 444, 619–623. [DOI] [PubMed] [Google Scholar]
- Phillips DM (1966a). Fine structure of Sciara coprophila sperm. J Cell Biol 30, 499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DM (1966b). Observations on spermiogenesis in the fungus gnat Sciara coprophila. J Cell Biol 30, 477–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensier G, Vivier E, Goldstein S, Schrével J (1980). Motile flagellum with a “3 + 0” ultrastructure. Science 207, 1493–1494. [DOI] [PubMed] [Google Scholar]
- Reger JF, Florendo NT (1970). Observations on microgamonts and microgametes of the coccidian, Eimeria sp. parasitic in the ostracod, Cypridopsis sp. J Submicrosc Cytol 2, 69–78. [Google Scholar]
- Roggen DR, Raski DJ, Jones NO (1966). Cilia in nematode sensory organs. Science 152, 515–516. [DOI] [PubMed] [Google Scholar]
- Ross MM (1967). Modified cilia in sensory organs of juvenile stages of a parasitic nematode. Science 156, 1494–1495. [DOI] [PubMed] [Google Scholar]
- Schrevel J (1969). Recherches sur le cycle des Lecudinidae grégarines parasites d’annélides polychétes. Protistologica 5, 561–588. [Google Scholar]
- Schrevel J, Besse C (1975). A functional flagella with a 6 + 0 pattern. J Cell Biol 66, 492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden RE, Talman A, Marques SR, Wass MN, Sternberg MJE (2010). The flagellum in malarial parasites. Curr Opin Microbiol 13, 491–500. [DOI] [PubMed] [Google Scholar]
- Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, Rees I, Ludtke SJ (2007). EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 157, 38–46. [DOI] [PubMed] [Google Scholar]
- van Deurs B (1973). Azonemal 12+0 pattern in the flagellum of the motile spermatozoon of Nymphon leptocheles. J Ultrastruct Res 42, 594–598. [DOI] [PubMed] [Google Scholar]
- van Deurs B (1974). Pycnogonid sperm. An example of inter-and intraspecific axonemal variation. Cell Tissue Res 149, 105–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.