Abstract
The term M-phase supershift denotes the phosphorylation-dependent substantial increase in the apparent molecular weight of numerous proteins of varied biological functions during M-phase induction. Although the M-phase supershift of multiple key mitotic regulators has been attributed to the multisite phosphorylation catalyzed by the Cdk1/cyclin B/Cks complex, this view is challenged by multiple lines of paradoxical observations. To solve this problem, we reconstituted the M-phase supershift of Xenopus Cdc25C, Myt1, Wee1A, APC3, and Greatwall in Xenopus egg extracts and characterized the supershift-producing phosphorylations. Our results demonstrate that their M-phase supershifts are each due to simultaneous phosphorylation of a considerable portion of S/T/Y residues in a long intrinsically disordered region that is enriched in both S/T residues and S/TP motifs. Although the major mitotic kinases in Xenopus egg extracts, Cdk1, MAPK, Plx1, and RSK2, are able to phosphorylate the five mitotic regulators, they are neither sufficient nor required to produce the M-phase supershift. Accordingly, inhibition of the four major mitotic kinase activities in Xenopus oocytes did not inhibit the M-phase supershift in okadaic acid-induced oocyte maturation. These findings indicate that the M-phase supershift is produced by a previously unrecognized category of mitotic phosphorylation that likely plays important roles in M-phase induction.
INTRODUCTION
Entry into mitosis and meiosis (M-phase) in the eukaryotic cell cycle is a sudden and all-or-none process that consists of a large array of dramatic cellular changes that prepare cells for the process of mitotic division (Morgan, 2007). A hallmark biochemical event of this process is a sudden and substantial increase in the apparent molecular weight of a large subset of mitotic phosphoproteins (Stukenberg et al., 1997; Georgi et al., 2002), which we collectively term the M-phase supershift. Phosphatase treatment of different M-phase super-shifted proteins completely eliminated their gel mobility shifts (Stukenberg et al., 1997), indicating that protein phosphorylation is a direct cause of the M-phase supershift. The M-phase supershift of key mitotic regulators correlates with their activity changes that are suitable for their functions in mitosis as described later, implying that the protein phosphorylation that produces the M-phase supershift plays important roles in M-phase induction. However, although numerous studies have been performed to define the protein phosphorylation that produces the M-phase supershift of key mitotic regulators, a satisfactory explanation for the molecular basis of the M-phase supershift has not been forthcoming.
Xenopus Cdc25C, Wee1, Myt1, APC3, and Gwl are among the most studied key mitotic regulators that undergo the M-phase supershift. Cdc25C is a protein phosphatase that removes inhibitory phosphorylations in Cdk1 (Gautier et al., 1991; Kumagai and Dunphy, 1991), and its M-phase supershift correlates with a substantial increase in its Cdk1 dephosphorylating activity (Kumagai and Dunphy, 1992). In contrast with Cdc25C, Myt1 and Wee1 are the protein kinases that catalyze the inhibitory phosphorylations in Cdk1, and their M-phase supershifts correlate with a great decrease in the Cdk1 phosphorylating activity (Mueller et al., 1995a, b). APC3, also called Cdc27, is a component of anaphase-promoting complex (APC), and its M-phase supershift associates with activation of APC (King et al., 1995; Peters et al., 1996). Gwl is a recently characterized mitotic kinase that inactivates the Cdk1 opposing phosphatase PP2A-B55δ through activating its inhibitor Ensa/ARPP-19 (Gharbi-Ayachi et al., 2010; Mochida et al., 2010), and its M-phase supershift correlates with a great increase in Gwl kinase activity (Vigneron et al., 2011; Blake-Hodek et al., 2012). Interestingly, despite their different structures and functions, all five of these proteins contain multiple S/TP motifs, the minimal phosphorylation consensus sequences for Cdk1/2 (Nigg, 1991), in their regulatory regions. Consistent with this hint, each of these proteins can be phosphorylated by purified Cdk1/2 complexes (Izumi and Maller, 1993; Mueller et al., 1995a; Patra and Dunphy, 1998; Kim et al., 2005; Yu et al., 2006; Ruiz et al., 2008; Blake-Hodek et al., 2012; Fujimitsu et al., 2016). Although the Cdk1/2-induced gel mobility shifts of these proteins were often less than their M-phase supershifts, this gap has been filled by the finding that adding stochiometric levels of the Cdk1/2 binding protein Suc1/Cks, which also binds certain phosphorylated TP motifs to enable processive phosphorylations (Kõivomägi et al., 2013; McGrath et al., 2013), significantly enhanced the Cdk1/2-catalyzed gel mobility shifts of the five key mitotic regulators (Patra and Dunphy, 1998; Patra et al., 1999; Blake-Hodek et al., 2012). Further strengthening the role of Cdk1, phosphodefective mutation of some or all S/TP motifs in Cdc25C, Wee1A and Myt1 inhibited their M-phase supershifts (Izumi and Maller, 1993; Kim et al., 2005; Wang et al., 2007; Ruiz et al., 2008). Inhibition of Cdk1 activity in Xenopus oocytes or egg extracts always inhibited the M-phase supershifts of these proteins (Inoue and Sagata, 2005; Wang et al., 2007; Ruiz et al., 2008; Zhao et al., 2008; van Zon et al., 2010; Hara et al., 2012). Conversely, the addition of a single mitotic cyclin or a constitutively active Cdk1/cyclin B complex to interphase-arrested Xenopus egg extracts induced the M-phase supershift of these proteins (Kuang et al., 1994; Patra and Dunphy, 1998; Kim et al., 2005; Mochida et al., 2009; Trunnell et al., 2011). These mutually supportive observations generated a prevalent thinking that the M-phase supershift of these key mitotic regulators is primarily due to the phosphorylation of multiple S/TP motifs by the trimeric Cdk1/cyclin B/Cks complex.
Although most of the experimental observations that generated the Cdk1-centric model of the M-phase supershift were solid, there are also solid experimental observations that were incompatible with this model. For example, while phosphodefective mutations of certain S/TP motifs in Xenopus Cdc25C and Myt1 abolished their M-phase supershifts, specific phosphorylation of these S/TP motifs by purified kinases alone did not reconstitute their M-phase supershifts (Izumi and Maller, 1993; Wang et al., 2007; Ruiz et al., 2008; Wang et al., 2010). Depletion of Cdk1 from CSF extract did not eliminate its ability to induce the M-phase supershift of Wee1 proteins (Tang et al., 1993; Mueller et al., 1995a). Removal of Cks/p9 from interphase-arrested Xenopus egg extracts did not prevent a constitutively active Cdk1/cyclin B from inducing the M-phase supershifts of Cdc25C, Myt1, and Wee1 (Patra and Dunphy, 1998). Further, a nearly full extent of the M-phase supershift of endogenous Cdc25C, Gwl, and APC3 and exogenous fission yeast Wee1 can be induced in interphase-arrested Xenopus egg extracts in the absence of either mitotic cyclins or both mitotic cyclins and Cdk1/Cdk2 proteins by adding either a phosphatase inhibitor (Kumagai and Dunphy, 1992; Tang et al., 1993; Izumi and Maller, 1995; Mochida et al., 2009; Wu et al., 2010) or high concentrations of activated Gwl (Zhao et al., 2008). These paradoxical observations signify that our current understanding of mitotic regulation is missing critical components.
In this study, we reconstituted the M-phase supershifts of Xenopus Cdc25C, Myt1, Wee1A, Gwl, and APC3 in M-phase stabilized Xenopus egg extracts and made a comprehensive analysis of the protein phosphorylation that produced their M-phase supershifts. In particular, we mapped the supershift-producing regions, identified the phosphorylation sites, estimated the phosphorylation intensities, defined the cause of the substantial gel mobility shifts, and characterized the kinase involvement. Together, findings in this study indicate that the M-phase supershift of the five key mitotic regulators is produced by a previously unrecognized category of mitotic phosphorylation that likely plays important roles in M-phase induction.
RESULTS
Reconstitution of the M-phase supershift of the five key mitotic regulators by phosphorylation of recombinant proteins with MEE
To define the protein phosphorylation that generates the M-phase supershift of the five mitotic regulators, we first established an experimentally conducive and physiologically relevant cell-free system that recapitulates the protein phosphorylation that produces the M-phase supershift. For this objective, unfertilized Xenopus eggs, which are naturally arrested at the second meiotic metaphase and can be obtained in large quantities, were extracted with an equal volume of the classical MPF extraction buffer (EB) (Wu and Gerhart, 1980), which was supplemented with 1 mM ATP-γ-S and 1 μM okadaic acid (OA). The addition of ATP-γ-S was based on its unique ability to stabilize MPF activity in Xenopus egg extracts (Cyert et al., 1988; Dunphy and Newport, 1988; Kuang et al., 1991a). The addition of OA was based on accumulative evidence that OA or its related phosphatase inhibitor microcystin (MC) promotes or stabilizes the M-phase supershift of multiple key mitotic regulators in Xenopus egg extract (Kumagai and Dunphy, 1992; Tang et al., 1993; Izumi and Maller, 1995; Mochida et al., 2009; Wu et al., 2010). This M-phase arrested/stabilized Xenopus egg extract has been abbreviated as MEE in our previous studies (Wang et al., 2007; Wu et al., 2010). Because MEE reproducibly induced a supershift of recombinant Cdc25C and could be stored at –80°C for years without losing this activity, MEE was used to set up the cell-free system for the purpose of this study.
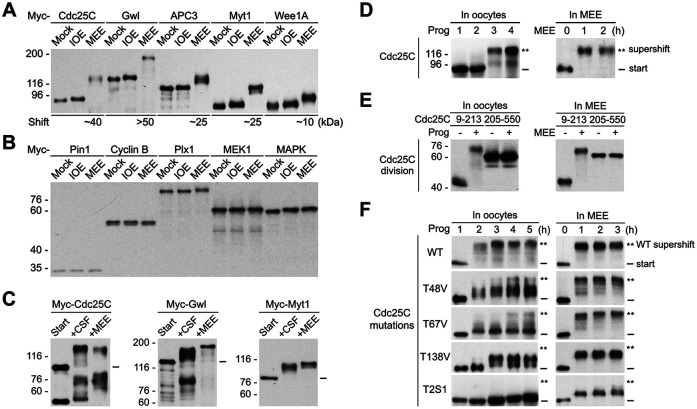
To characterize the ability of MEE to reconstitute the M-phase supershift by phosphorylation of recombinant proteins, we produced myc-tagged Cdc25C, Gwl, APC3, Myt1, and Wee1A by in vitro transcription coupled translation (TNT) and phosphorylated the TNT products with either MEE or interphase-arrested immature Xenopus oocyte extract (IOE) prepared similarly. Immunoblotting with anti-myc antibodies showed that all five of the tested proteins underwent a substantial increase in the apparent molecular weight upon MEE treatment but little or no shift upon IOE treatment (Figure 1A). Notably, relative magnitudes of the MEE-induced gel mobility shifts of the five proteins were similar to those of their M-phase shifts during M-phase induction (Patra and Dunphy, 1998; Georgi et al., 2002; Yu et al., 2006). To evaluate the substrate specificity of the MEE-induced supershifts, we produced myc-tagged human Pin1 (Yaffe et al., 1997), sea urchin cyclin B ∆90 (Murray et al., 1989), Xenopus Plk1 (Plx1) (Kumagai and Dunphy, 1996), Xenopus MEK1 (Kosako et al., 1993), and Xenopus MAPK (Gotoh et al., 1993), which undergo no or only a slight shift during M-phase induction (Murray et al., 1989; Qian et al., 1998; Shen et al., 1998; Yue and Ferrell, 2004; Wang et al., 2007), and analyzed their gel mobility shifts in IOE and MEE similarly. Of note, the use of the five nonshift proteins from different species was simply due to the available expression constructs for myc-tagged proteins in our reagent stock. While a slight gel mobility shift was observed with Plx1 upon MEE treatment and with MAPK upon both IOE and MEE treatments, none of these proteins underwent a substantial gel mobility shift upon IOE or MEE treatment (Figure 1B). These results indicate that the MEE-induced substantial gel mobility shifts only occur on proteins that undergo the M-phase supershift during M-phase induction.
FIGURE 1:
Reconstitution of the M-phase supershift of the five key mitotic regulators by phosphorylation of recombinant proteins with MEE. (A) Phosphorylation of myc-tagged Xenopus Cdc25C, Gwl, APC3, Myt1, and Wee1A with IOE or MEE for 2 h, followed by immunoblotting with anti-myc tag antibodies. (B) Phosphorylation of myc-tagged human Pin1, sea urchin cyclin B ∆90, Xenopus Plk1 (Plx1), Xenopus MEK1, and Xenopus MAPK with IOE or MEE for 2 h, followed by myc-tag immunoblotting. (C) Phosphorylation of myc-tagged Cdc25C, Gwl or Myt1 with CSF extract or MEE, followed by myc-tag immunoblotting. (D–F) Left panels show results from progesterone stimulation of Xenopus oocytes that ectopically expressed each of the indicated myc-tagged Cdc25C proteins for the indicated hours, followed by oocyte extraction and myc-tag immunoblotting. Right panels show results from phosphorylation of the TNT products of the same myc-tagged Cdc25C proteins with MEE for the indicated hours, followed by myc-tag immunoblotting.
To further characterize the physiological relevance of the MEE-induced supershifts, we phosphorylated myc-tagged Cdc25C, Gwl, and Myt1 with MEE and CSF extract in parallel and compared the gel mobility shifts induced by side-by-side immunoblotting. Since CSF extract was made of Xenopus eggs with very little buffer added (Lohka and Maller, 1985; Murray and Kirschner, 1989), it was close to pure cytosol of Xenopus eggs. However, CSF extract was not experimentally conducive because its M-phase status was sensitive to freezing/thawing, extract dilution, lengthy experimental manipulations, and the addition of exogenous Myt1 or Wee1 protein. As shown in Figure 1C, MEE induced a slightly less gel mobility shift of Cdc25C but a slightly greater gel mobility shift of Gwl and Myt1 than did CSF extract. The similar gel mobility shifts induced by MEE and CSF indicate that MEE induced a physiologically relevant level of protein phosphorylation in the five mitotic regulators examined.
Finally, we determined whether the MEE-based cell-free system preserves the fundamental rules that govern the M-phase supershift induction during M-phase induction of Xenopus oocytes. For this objective, we first performed time course examination of the gel mobility shift of Cdc25C in progesterone-matured oocytes and in MEE. In both systems, Cdc25C underwent a supershift of ∼40 kDa in an abrupt manner (Figure 1D). Although the supershift was sometimes less stoichiometric in progesterone-matured oocytes than in MEE, this difference could be explained by inhibition of phosphatase activity in MEE. Second, we compared the gel mobility shifts of myc-tagged Cdc25C9-213 and Cdc25C205-550 in the two systems. In both systems, the supershift specifically associated with the N-terminal fragment Cdc25C9-213 (Figure 1E), consistent with results in previous studies (Kumagai and Dunphy, 1992). Third, we compared the effects of the T-to-V mutation of the three conserved TP motifs in Cdc25C on the gel mobility shift of Cdc25C in the two system. In progesterone-matured oocytes, the T48V and T67V mutations each significantly decreased the stoichiometry of the Cdc25C supershift, whereas the T138V mutation significantly deceased the magnitude of the Cdc25C shift, consistent with results in previous studies (Izumi and Maller, 1993). In MEE, the three mutations produced qualitatively similar effects although the severity of inhibition was less than that observed in progesterone-matured oocytes (Figure 1F). Again, this difference could be accounted for by the inhibition of phosphatase activity in MEE. Fourth, we compared the inhibitory effects in the two systems of the well-characterized T2S1 mutation (T48V-T138V-S205A), which dramatically decreased the magnitude of the gel mobility shift of Cdc25C in progesterone-induced oocyte maturation (Wang et al., 2007). In both systems, the T2S1 mutation dramatically decreased the magnitude of the gel mobility shift of Cdc25C (Figure 1F). Together, these results indicate that the MEE-induced supershifts follow the fundamental rules that govern the M-phase supershift in Xenopus oocytes.
Association of the M-phase supershift with specific protein regions
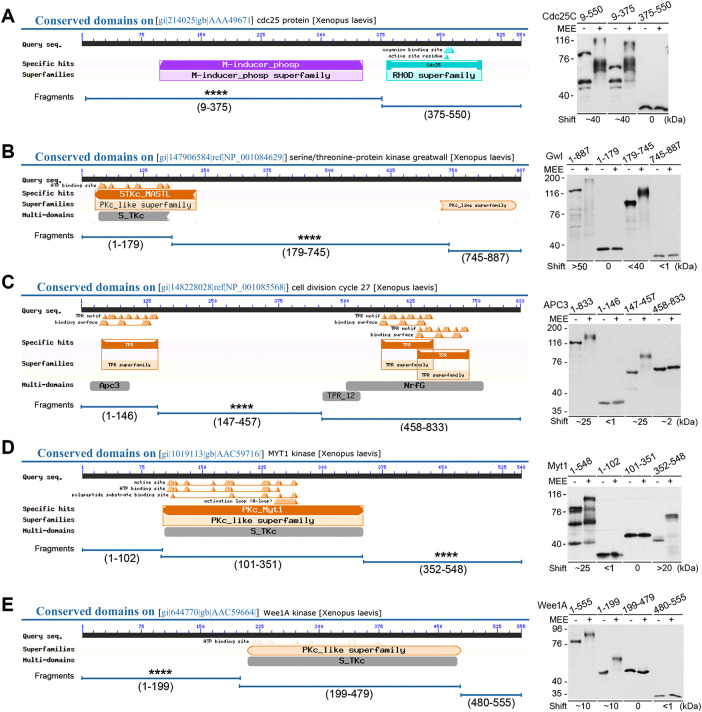
Previous studies suggested that the M-phase supershift of Xenopus Cdc25C and Myt1 associates with specific protein regions (Kumagai and Dunphy, 1992; Ruiz et al., 2008). To systematically define regions in the five key mitotic regulators that generate the M-phase supershift, we first divided each of them into two or three fragments according to their functional domains from NCBI search (Figure 2, A–E, left) and assayed their gel mobility shifts upon MEE treatment (Figure 2, A–E, right). Clearly, only one fragment from each of these proteins underwent a substantial gel mobility shift that was similar or comparable to that of the nondivided protein. In particular, Cdc25C9-375, the N-terminal regulatory region, underwent a shift of ∼40-kDa as did the full-length protein (Figure 2A). Gwl179-745, the middle regulatory region, underwent a shift of <40-kDa, which was appreciably less than the >50-kDa shift of the full-length Gwl (Figure 2B). This difference could be accounted for by autophosphorylation of Gwl suggested in previous studies (Blake-Hodek et al., 2012). APC3147-457, the middle regulatory region, underwent a shift of ∼25-kDa as did the full-length protein (Figure 2C). Myt1352-548, the C-terminal regulatory region, underwent a shift of ∼25-kDa as did the full-length protein (Figure 2D). Wee1A1-199, the N-terminal regulatory region, underwent a shift of ∼10-kDa as did the full-length protein (Figure 2E). Second, we made sequential C-terminal truncations of Cdc25C9-375 (Supplemental Figure S1A) and determined the effects on the gel mobility shift both in MEE (Supplemental Figure S1B) and in progesterone-matured oocytes (Supplemental Figure S1C). In both systems, the sequential C-terminal truncations progressively reduced the magnitude of the gel mobility shift of Cdc25C without a correlation between the number of S/TP motifs and the amount of the shift. We also made C-terminal sequential truncations of Gwl179-745 (Supplemental Figure S1D) and determined the effects on its gel mobility shift in MEE (Supplemental Figure S1E). Again, the sequential C-terminal truncations of Gwl179-745 progressively reduced the magnitude of the gel mobility shift without a correlation between the number of S/TP motifs and the amount of the shift. Together, these results indicate that the M-phase supershift is due to wide-ranging phosphorylation of particular protein regions, which we collectively call the supershift domain.
FIGURE 2:
Association of the M-phase supershift with specific protein regions. Cdc25C (A), Gwl (B), APC3 (C), Myt1 (D), and Wee1A (E) were each divided into indicated fragments according to their functional domains from NCBI search, with indication of the supershift fragments identified by four asterisks (left). Indicated myc-tagged full-length and fragment proteins were phosphorylated with MEE for 2 h, followed by myc-tag immunoblotting (right). Estimated magnitudes of the MEE-induced gel mobility shifts for different proteins are indicated.
Common features of the supershift domain
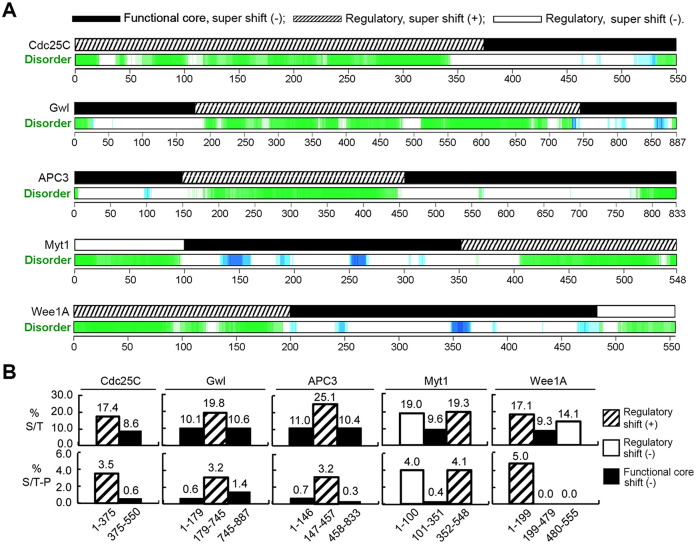
To characterize the supershift domain, we analyzed the structural and compositional properties of the five supershift fragments by informatics. Online search of the Database of Disordered Protein Predictions (Oates et al., 2013) for Xenopus Cdc25C, Gwl, APC3, Myt1, and Wee1A revealed that all five of the supershift fragments are structurally disordered for the most part, whereas their adjacent core functional domains form globular structures (Figure 3A). Analyses of amino acid compositions of the five proteins showed that the supershift domain was about twofold higher than their adjacent globular domains in the abundance of S/T residues (Figure 3B, upper). Further analyses of their potential phosphorylation sites revealed that the five supershift fragments each contain a much greater abundance of S/TP motifs than their adjacent globular domains (Figure 3B, lower). The perfect positive correlations of these three features with the supershift domain indicate that they are important determinants for the supershift-producing phosphorylation.
FIGURE 3:
Common features of the supershift fragments. (A) Prediction of disordered regions in Xenopus Cdc25C, Gwl, APC3, Myt1, and Wee1A (green and blue) by d2p2 with parallel indication of the supershift ability of tested fragments. (B) The percentage of S/T residues (upper) and the number of S/TP motifs per 100 residues (lower) were determined for indicated fragments of the five proteins and graphed.
To evaluate the correlation of the lack of supershift capability with the absence of the three common features of the supershift domain, we made similar analyses of the two remaining nonsupershift regulatory regions in the five mitotic regulators, Myt11-100 and Wee1A480-555, and also in five nonsupershift Xenopus proteins, including cyclin B1 (NCBI ACCESSION P13350), Plx1 (NCBI ACCESSION P70032), MAPK (NCBI Accession P27638), MEK1 (NCBI Accession Q05116), and Pin1 (NCBI Accession AAF43897). Interestingly, Myt11-100, but not Wee1A480-555, has all three of the common features of the supershift domain (Figure 3A and B). Nonetheless, none of the five nonsupershift proteins regions contain a long intrinsically disordered region that is substantially enriched in both S/T residues and S/TP motifs, even though Plx1 and Pin1 each contain a long intrinsically disordered region that has significant enrichment of S/T residues, and cyclin B and MEK1 each contain a long intrinsically disordered region that has significant enrichment of S/TP motifs (Supplemental Figure S2). While the almost perfect negative correlation strengthens the importance of the three common features of the supershift domain in the supershift-producing phosphorylation, the presence of an exception in the negative correlation predicts that the supershift domain contains additional cis-operating determinants that enable the supershift-producing phosphorylation.
Although identification of the additional determinants in the supershift domain is beyond the scope of the present study, an N-terminal truncation of the conserved 9-79 region of Cdc25C, which itself shifted only by ∼5 kDa (Supplemental Figure S1A), disproportionately reduced the magnitude of the Cdc25C shift from 40 to 10 kDa in MEE and from 40 to 5 kDa in progesterone-matured oocytes (Figure S3, A and B). Further, while both end truncations of Gwl179-745 to Gwl227-712 did not disproportionally decrease the magnitude of the supershift in MEE, further both end truncations to Gwl321-609 almost eliminated the supershift. In contrast, either end truncation of Gwl227-712 to Gwl321-712 or Gwl227-609 did not have this inhibitory effect (Supplemental Figure S3, C and D). These results indicate that the supershift domain contains specific regulatory regions that promote the supershift-producing phosphorylation of the entire supershift domain.
Heterogeneous phosphorylations of most of the S/T/Y residues in the supershift domain
To characterize the supershift-producing phosphorylation in the supershift domain, we phosphorylated GST-tagged Cdc25C9-374, Gwl179-745, APC3147-457, Myt1352-548, and Wee1A1-199, which were immobilized on glutathione resins, with MEE and analyzed super-shifted proteins by LC-MS/MS without phosphopeptide enrichment. While GST-Cdc25C9-374, GST-Gwl179-745, GST-Myt1352-548 and GST-Wee1A1-199 were each phosphorylated with 2 volumes of MEE for 3 h, GST-APC3147-457 was phosphorylated with 2 volumes of MEE for 15 h due to its much delayed M-phase supershift as compared to Cdc25C and Wee1 (Georgi et al., 2002). It should be noted that GST tag, which facilitated affinity purification of substrates, was not phosphorylated by MEE as determined by 32P-incorporation (data not shown). Also, immobilized GST-Cdc25C9-374 shifted to moderately lower magnitudes and stoichiometry than soluble myc-Cdc25C9-375 upon phosphorylation with MEE for 3 h (data not shown), probably due to much higher concentrations and lower mobilities of GST-tagged substrate proteins.
Several micrograms (∼50 pmol) of each supershift fragment were used in the analysis by mass spectrometry (Supplemental Figure S4A) as suggested (Gropengiesser et al., 2009) in order to obtain improved sequence coverage and phosphorylation site identification over previous studies (Kim et al., 2005; Casado-Vela et al., 2007; Vigneron et al., 2011; Blake-Hodek et al., 2012). Despite this improvement, a standard mass spectrometry typically identifies multi-phosphorylated peptides much less efficiently than their nonphosphorylated or singly phosphorylated counterparts (Gropengiesser et al., 2009; Dephoure et al., 2013). On top of this limitation, output data from our mass spectrometry only contained unambiguously identified peptide sequence matches (PSMs), in which only the most abundant phosphorylation species from each inquiry was selected for presentation, as exemplified in Supplemental Figure S5. Consequently, results from our mass spectrometry were an inevitably significant under-representation of the actual phosphorylation status in the supershift domain.
A vast number (1399–11831) of PSMs were obtained from each super-shifted protein (Supplemental Figure S4A). Strikingly, 74–91% the S/T/Y-containing PSMs from the five supershift fragments were phosphorylated, whereas only 0.5–1.6% of the S/T/Y-containing PSMs in the GST region were phosphorylated (Supplemental Figure S4B). PSMs obtained from each of the five supershift fragments were then sorted and aligned (Supplemental Tables S1–S5). Clearly, the identified phosphopeptides distributed throughout the supershift fragments without significant gaps, consistent with involvement of the entire supershift domain in the supershift (Supplemental Figure S1). Also observed was a great deal of heterogeneity in the number and location of the phosphorylation sites in different regions of the supershift fragments without signs of specific orders, indicating great flexibilities in the phosphorylation site selection.
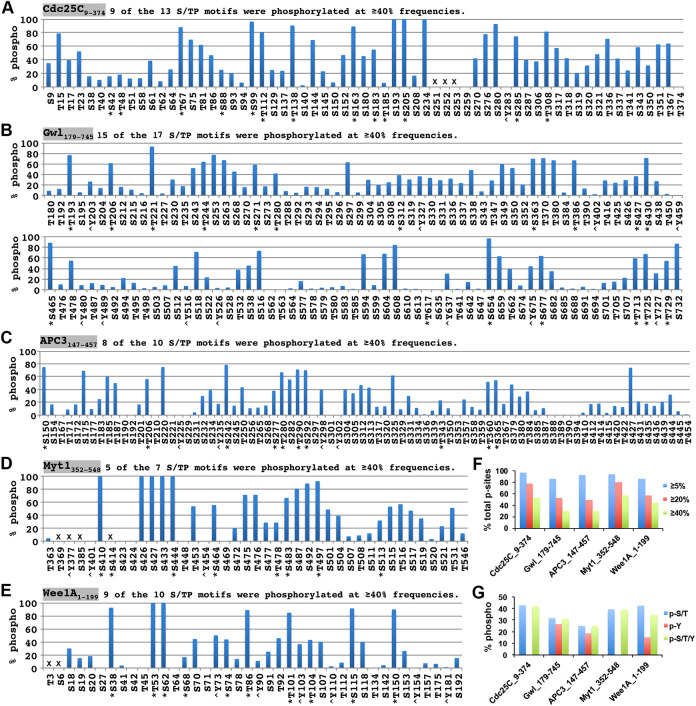
Figure 4, A–E summarizes all of the phosphorylation sites identified in the five supershift fragments, which led to the calculations that 82–96% of the recovered S/T/Y residues were phosphorylated (Figure 4F). Since about half of the S/T/Y residues in the supershift fragments existed in clusters (underlined), which contained false negatives, as exemplified for Cdc25C-Y283 in Supplemental Figure S4C, the actual percentages of the S/T/Y phosphorylations in the supershift domain could be even higher. Such a high percentage of S/T/Y phosphorylation in the supershift domain was initially concerning since it is against the deeply rooted thinking that protein phosphorylation should be site specific. Conceivably, the site-comprehensive phosphorylation observed in the supershift domain might result from boosted promiscuous actions of site-specific protein kinases in MEE due to the lack of substrate competitors and protein phosphatase activities. However, while promiscuous phosphorylations are characterized by low stoichiometry, most of the phosphorylation sites in the supershift domain were identified in >5% of the total relevant PSMs analyzed (red nonshaded in Figure 4, A–E), which was a pretty stringent cutoff to rule out noise level phosphorylations or false identifications considering the above-described limitation of mass spectrometry. Further, 49–69% of the phosphopeptides identified from the five supershift fragments were phosphorylated at ≥2 sites (Supplemental Figure S4D), indicating high frequencies of multisite phosphorylations. It thus seems unlikely that promiscuous actions of protein kinases in MEE account for the site-comprehensive phosphorylation observed in the supershift domain.
FIGURE 4:
Significant phosphorylation of most of the S/T or S/T/Y residues in the supershift domain. (A–E) Phosphorylation sites identified in the supershift fragments of Cdc25C (A), Gwl (B), APC3 (C), Myt1 (D), and Wee1A (E). Strikethroughs indicate nonrecovered sequences. Red and blue types indicate phosphorylated and nonphosphorylated S/T/Y residues determined by mass spectrometry, respectively. Underlines are S/T/Y clusters. Shaded S/T/Y residues in red types indicate <5% of phosphorylation identification frequencies. (F) A summary of recovered S/T/Y residues and identified phosphorylation sites from the five supershift fragments.
Simultaneous phosphorylations of a considerable portion of the S/T or S/T/Y residues in the supershift domain
To further characterize the phosphorylation intensity of the supershift domain, we estimated the individual site phosphorylation occupancies and the degree of simultaneous phosphorylations in the supershift domain. Due to the complexity and heterogeneity in the phosphorylation of the supershift domain, it was impossible to achieve these objectives by routinely available techniques of mass spectrometry. As a practical alternative to address the first issue, we calculated the phosphorylation identification percentage for individual S/T/Y residues in the five supershift fragments and conservatively used ≥5%, ≥20%, and ≥40% phosphorylation identification frequencies to indicate significant, above-medium-occupancy and high-occupancy phosphorylation sites, respectively. As shown in Figure 5, A–E and summarized in Figure 5F, 86–98% of the identified phosphorylation sites were phosphorylated at ≥5% of the PSMs analyzed, indicating significant phosphorylations of most of the sites. Further, 50–81% and 31–58% of the identified phosphorylation sites were phosphorylated at ≥20% and ≥40% identification frequencies, respectively, indicating a widespread medium to high occupancy phosphorylation sites. Notably, upon combining the five supershift fragments, 81% of the S/TP motifs (46/57) were phosphorylated at ≥40% frequencies, whereas 31% of nonproline-directed S/T residues (71/230) and 12% of Y residues (2/17) were phosphorylated at ≥40% frequencies, respectively. It thus seems that S/TP motifs were phosphorylated more favorably than nonproline-directed S/T residues and Y residues in the supershift domain.
FIGURE 5:
Widespread medium to high phosphorylation stoichiometries in the supershift domain. (A–E) Phosphorylation identification frequencies for individual S/T/Y residues in Cdc25C9-374 (A), Gwl179-745 (B), APC3147-457 (C), Myt1352-548 (D), and Wee1A1-199 (E). Asterisks indicate proline-directed S/T residues, and < symbols indicate tyrosine residues. Crosses indicate the S/T/Y residues in nonrecovered regions. (F) Percentages of the phosphorylation sites that were identified at ≥5%, ≥20%, and ≥40% frequencies by mass spectrometry for the five supershift fragments. (G) Percentages of total S/T, Y, or S/T/Y residues recovered from each of the five supershift fragments that were phosphorylated.
As a practical alternative to address the second issue, we calculated the percentage of total recovered S/T/Y residues in each supershift fragment that were phosphorylated. In ideal situations, the total S/T/Y phosphorylation percentage in a particular supershift fragment should correlate with the extent of simultaneous phosphorylation of S/T/Y residues in this supershift fragment. As summarized in Figure 5G, 25–43% of the total recovered S/T residues in the five supershift fragments were phosphorylated, whereas 15–27% of the total recovered Y residues in Gwl179-745, APC3147-457 and Wee1A1-199 were phosphorylated. When combined, 25–42% of the total S/T/Y residues were phosphorylated. If we conservatively assume that these percentages were at least twofold under-representations of the actual total S/T/Y phosphorylations, these results indicate that about half or more than half of the total S/T/Y residues in the supershift domain were simultaneously phosphorylated.
To further examine the above estimation, we phosphorylated Xenopus Cdc25C375-550 (an example of no phosphorylation), MEK1 (an example of phosphorylation of mainly one site), and Myt11-102 (an example of the supershift domain property without supershift) with MEE for 1 or 2 h in parallel with Xenopus Cdc25C9-213, Gwl179-324, APC147-457, Myt1406-548, and Wee1A1-199 (examples of supershift fragments) and analyzed their phosphorylations by Phos-tag SDS–PAGE (Kinoshita et al., 2006). As expected, Cdc25C375-550 underwent no shift, whereas MEK1 underwent one distinct shift (Supplemental Figure S6A). Interestingly, Myt11-102 underwent a ladder of shifts, consisting of seven to eight steps (Supplemental Figure S6B), indicative of heterogenous low stoichiometry phosphorylation of multiple sites. Strikingly, Cdc25C9-213, Gwl179-324, APC147-457, Myt1406-548, and Wee1A1-199 either did not enter the gel (most of Cdc25C9-213 and all of Myt1406-548) or showed up as one fat band near the top of the gel (Gwl179-324, APC147-457, and Wee1A1-199; Supplemental Figure S6C), consistent with simultaneous phosphorylation of a considerable portion of the S/T/Y residues in these proteins. To further examine the difference between Myt11-102 and supershift proteins, we phosphorylated Myt11-102 and Gwl179-324 for up to 32 min and examined the products by Phos-tag SDS–PAGE. As shown in Figure S6, D and E, both Myt11-102 and Gwl179-324 underwent a ladder of shifts, consisting of eight major steps. However, the ladder of Gwl179-324 moved up much more quickly than Myt11-102 and uniquely contained big jumps at certain steps. In the end, the ladder of Gwl179-324 quickly merged into one fat band near the top of the gel at 4 min and then moved up further gradually, whereas the ladder of Myt11-102 slowly moved up only by one step and remained as a ladder of shifts throughout the time course. These results indicate that while phosphorylation of numerous sites is a common characteristic of protein regions that contain the three common features of the supershift domain, simultaneous phosphorylation of a considerable portion of the potential phosphorylation sites, which we call high stoichiometry comprehensive phosphorylation, is a unique property of the supershift domain.
Similar phosphorylation patterns of human Cdc25C, Gwl, APC3, Myt1, and Wee1 in mitotic cells
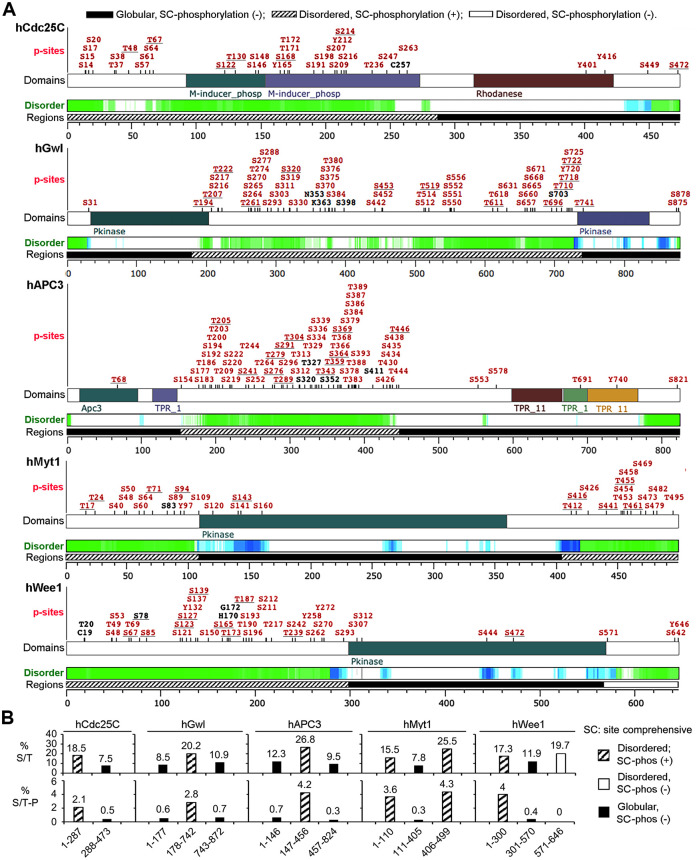
To investigate the significance of the above findings in mitotic cell cycles, we made informatic analysis of phosphorylation patterns of human Cdc25C, Gwl, APC3, Myt1, and Wee1, which undergo the M-phase supershift in mitotic cell cycles (Watanabe et al., 1995; Booher et al., 1997; Gabrielli et al., 1997; Kraft et al., 2003; Voets and Wolthuis, 2010). For this objective, we searched the phosphorylation site database PhosphoSitePlus (Hornbeck et al., 2012) for a curated compilation of their identified phosphorylation sites from previous studies and aligned the phosphorylation sites with protein disorder prediction obtained by search of the Database of Disordered Protein Predictions (d2p2). Although the phosphorylation sites listed in PhosphoSitePlus do not specify their cell cycle status, all five of the proteins in question were specifically or preferentially phosphorylated in mitotic cells in high-throughput mass spectrometry of interphase versus mitotic HeLa cell lysates (Daub et al., 2008; Dephoure et al., 2008). Clearly, the human counterparts of the five supershift fragments of the Xenopus proteins are also long intrinsically disordered regions that contain clustered phosphorylation sites, whereas their adjacent globular regions are scarcely phosphorylated (Figure 6A). These heavily phosphorylated regions are also enriched in both S/T residues and S/TP motifs (Figure 6B). In addition, the human counterpart of the Xenopus exceptional protein region Myt11-100 also contains clustered phosphorylation sites. These results indicate that human Cdc25C, Gwl, APC3, Myt1, and Wee1 follow similar phosphorylation patterns in mitotic cell cycles as their Xenopus counterparts in meiotic cell cycles.
FIGURE 6:
Informatic analysis of phosphorylation patterns of human Cdc25C, Gwl, APC3, Myt1, and Wee1. (A) Curated compilation of previously identified phosphorylation sites in human Cdc25C, Gwl, APC3, Myt1, and Wee1 with parallel indications of intrinsically disordered regions (green and blue) and similar divisions as done for their Xenopus orthologs. Proline-directed phosphorylation sites are underlined. Black sites indicate phosphorylated S/T residues in equivalent positions of rodent orthologs. (B) The percentage of S/T residues (upper) and the number of S/TP motifs per 100 residues (lower) are graphed for indicated fragments of the five proteins.
The M-phase supershift occurs not only on the five key mitotic regulators but also numerous other proteins of varied biological functions (Stukenberg et al., 1997). To explore a potential applicability of the above findings to the M-phase supershift in general, we analyzed the phosphorylation patterns of human anillin, GTSE1, INCENP, Ki-67, and NuMA-1 by informatics since these proteins also both undergo the M-phase supershift in mitotic cell cycles (Sparks et al., 1995; Collavin et al., 2000; Endl and Gerdes, 2000; Honda et al., 2003; Monzo et al., 2005) and are specifically or preferentially phosphorylated in mitotic cells in high-throughput mass spectrometry of interphase versus mitotic HeLa cell lysates (Dephoure et al., 2008). Similar to human Cdc25C, Gwl, APC3, Myt1, and Wee1, these five additional supershift proteins all contain long intrinsically disordered regions that contain clustered phosphorylation sites (Supplemental Figure S7). Sequence analysis of these proteins showed that the heavily phosphorylated regions are also enriched in both S/T residues and S/TP motifs (data not shown). These results indicate that the type of protein phosphorylation that produces the M-phase supershift of the five key mitotic regulators may also produce the M-phase supershift of other proteins.
Generation of supershifts by additive numerous small shifts
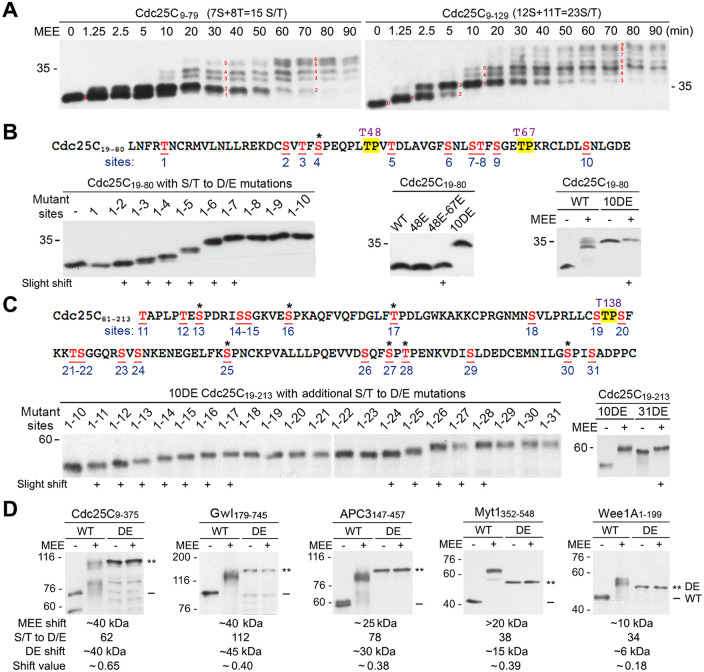
To determine whether the high stoichiometry comprehensive phosphorylation of the supershift domain generates a supershift through big effects from phosphorylation of particular motifs or additive numerous small shifts from phosphorylation of diverse sites, we examined the process of MEE-induced gel mobility shifts of Cdc25C9-79 and Cdc25C9-129, which contain 15 and 23 S/T residues, respectively. Both Cdc25C9-79 and Cdc25C9-129 gradually reached the maximal shift through a ladder of six and nine steps, respectively (Figure 7A), indicating generation of a supershift by additive numerous small shifts. To further examine this possibility, we characterized the small shift-producing phosphorylation sites in Cdc25C. Inspired by the previous finding that phosphomimetic mutation of a shift-producing phosphorylation site mimics the actual phosphorylation to generate a gel mobility shift (Lee et al., 2013; Lee et al., 2019), we made cumulative phosphomimetic mutations in the Cdc25C19-80 and Cdc25C81-213 regions, respectively, and determined the effects on the gel mobility. As shown in Figure 7B, Cdc25C19-80 contains 12 S/T residues, which are designated sites 1 to 10 plus two conserved TP motifs at T48 and T67 (upper). Cumulative T-to-E and S-to-D mutations of sites 1 to 10 progressively increased the apparent molecular weight of Cdc25C19-80 through six discernible steps and only one of the shifting sites was at an S/TP motif. The shift occurred at both clustered and isolated sites without a common rule found (lower left). In contrast, the T48E single mutation or the T48E-T67E double mutation generated no or only a slight shift, respectively (lower middle). Accordingly, the 10DE mutation produced a similar supershift as did the MEE treatment, whereas phosphorylation of the remaining T48 and T67 residues by MEE only produced a slight shift (lower right). As shown in Figure 7C, Cdc25C81-213 contains 22 S/T residues, which are designated sites 11 to 31 plus a conserved TP motif at T138 (upper). Cumulative phosphomimetic mutations of sites 11 to 31 in the fusion protein between 10DE Cdc25C19-80 and Cdc25C81-213 (10DE Cdc25C19-213) progressively increased the apparent molecular weight through 12 discernible steps, and 6 of the 12 shift-producing sites were at S/TP motifs. Again, the shift occurred at both isolated and clustered S/T residues without a common rule found (lower panel left). Accordingly, the 21DE mutation in 10DE Cdc25C19-213 produced a similar supershift as did the MEE treatment, whereas phosphorylation of the remaining T48, T67 and T138 residues by MEE only produced a slight shift (lower panel right). Consistent with each other, these results indicate that numerous small shifts of Cdc25C are generated by phosphorylation of diverse sites, including both S/TP and non-S/TP motifs. Based on these new results, previously observed inhibition of the M-phase supershift of Cdc25C by phosphodefective mutations of the three conserved TP motifs (Izumi and Maller, 1993; Wang et al., 2007; Wu et al., 2010) should be explained by regulatory roles of their phosphorylations in the supershift-producing phosphorylation of Cdc25C. To extend the above findings to all five of the supershift fragments, we mutated all S/T residues in the five supershift fragments to D/E residues and examined the effects on the gel mobility. In each case, all DE mutations substantially increased the apparent molecular weight, and the mutant proteins did not shift further upon MEE treatment (Figure 7D), suggesting that the five supershift fragments follow a similar rule in generating the supershift.
FIGURE 7:
Generation of supershifts by additive numerous small shifts. (A) Phosphorylation of myc-tagged Cdc25C9-79 (left) and Cdc25C9-129 (right) for the indicated minutes, followed by myc-tag immunoblotting. Red numbers indicate discernible steps in the process of developing the supershift. (B) The upper panel shows the amino acid sequence of Cdc25C19-80 with numbering of the 12 S/T residues (red underlined), with conserved TP motifs at T48 and T67 highlighted by yellow, and the additional proline-directed S/T residue is indicated by an asterisk. The lower panel shows myc-tag immunoblots of different mutant forms of Cdc25C19-80 without MEE treatment (left and middle) or with MEE treatment (right). (C) The upper panel shows the amino acid sequence of Cdc25C81-213 with numbering of the 22 S/T residues, with the conserved TP motifs at T138 highlighted by yellow and the remaining six proline-directed S/T residues indicated each by an asterisk. The lower panel shows myc-tag immunoblots of different mutant forms of 10DE Cdc25C19-213 without MEE treatment (left) or with MEE treatment (right). (D) Phosphorylation of myc-tagged wild type (WT) or all ST-to-DE mutant forms (DE) of the five supershift fragments with MEE for 2 h, followed by myc-tag immunoblotting. The shift value was calculated by dividing the shift kDa from all DE mutations by the total number of DE mutations.
Although Gwl179-745 is much larger and contains many more phosphorylation sites than Cdc25C9-375, both proteins shifted by ∼40 kDa. And also, although Myt1352-548 and Wee1A1-199 are of similar lengths and numbers of phosphorylation sites, Myt1352-548 shifted 10 kDa more than Wee1A1-199. Thus the abundance of shift-producing phosphorylation sites may significantly differ among different supershift domains. To test this prediction, we divided the shift amount from all DE mutations by the number of DE mutations and compared the values for different supershift fragments. As speculated, the value varied greatly for the five supershift fragments, which largely correlates with the differences in the average degree of the gel mobility shift (Figure 7D).
Phosphorylation of the five key mitotic regulators by the four major mitotic kinases produced by TNT is insufficient to produce the M-phase supershift
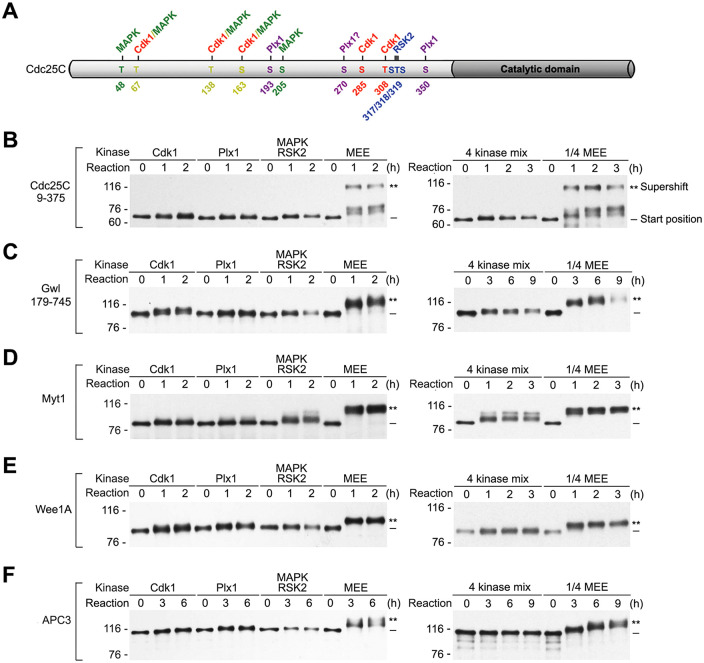
Cdk1, MAPK, Plx1, and RSK2 are the major proline-directed, acidophilic, and basophilic kinases that are activated during M-phase induction of Xenopus oocytes (Wang et al., 2007; Wang et al., 2010). Consistent with the presence of multiple putative phosphorylation sites for these four major mitotic kinases in the five supershift fragments, each of these four kinases has been reported to phosphorylate one or multiple key mitotic regulators by in vitro kinase assays (Izumi and Maller, 1993; Mueller et al., 1995a; Kumagai and Dunphy, 1996; Palmer et al., 1998; Inoue and Sagata, 2005; Wang et al., 2007, 2010; Vigneron et al., 2011; Blake-Hodek et al., 2012). Furthermore, the Cdc25C phosphorylating activity in MEE has been primarily attributed to these four major mitotic kinases by protein purification (Wang et al., 2007, 2010), and each of the four kinases has been shown to phosphorylate a unique set of sites in the supershift domain of Cdc25C, as diagrammed in Figure 8A, by substrate fragmentation and mutagenesis. In particular, Cdk1 primarily phosphorylated the proline-directed sites T138/S285/T308, whereas MAPK specifically phosphorylated the proline-directed sites T48/T138/S205(Wang et al., 2007). Additional studies indicated that MAPK and Cdk1 also phosphorylate the proline-directed sites T67 and S163 (unpublished results). In contrast, RSK2 primarily phosphorylated the basophilic sites S317/T318/S319 (Wang et al., 2010), whereas Plx1 primarily phosphorylated the acidophilic sites S193/S350 and likely also S270. Since the results on Plx1 sites had never been published prior to this study, they are presented in Supplemental Figure S8. The phosphorylation of four different sets of sites in the supershift of domain of Cdc25C by the four major mitotic kinases fits the hypothesis that the four major mitotic kinases act in concert to induce the M-phase supershift of Cdc25C and possibly other four mitotic regulators.
FIGURE 8:
Phosphorylation of the five mitotic regulators with the four major mitotic kinases produced by TNT is insufficient to produce the M-phase supershift. (A) A schematic diagram of Cdc25C with illustration of the major Cdk1, MAPK, Plx1, and RSK2 phosphorylation sites identified in the supershift domain. (B–F) Myc tagged Cdc25C9-375 (B), Gwl179-745 (C), Myt1 (D), Wee1A (E), and APC3 (F) were each phosphorylated either with the four major mitotic kinases individually in parallel with MEE (left panels) or with the four kinases collectively (4 kinase mix) in parallel with 1:4 MEE (right panels) for the indicated hours, followed by myc-tag immunoblotting.
For testing the above hypothesis, we produced active forms of Cdk1, Plx1, MAPK, and RSK2 by TNT for phosphorylation reactions. It should be emphasized that 1 µM OA or MC was routinely added to TNT-produced kinases in order to inhibit phosphatase activities in the system. The active Cdk1 complex was produced by mixing myc-tagged human Cdk1-T161E, sea urchin ∆CycB, and Xenopus Cks/p9, and the three proteins were expressed at approximately equal molar concentrations (Supplemental Figure S9A). By immunoblotting with phosphospecific antibodies, the mixture not only had similar levels of histone H1 kinase activity as did MEE (Supplemental Figure S9B) but also robustly phosphorylated Cdc25C at both T138 and S163 (Supplemental Figure S9C). The active Plx1 was produced by expressing a HA-tagged constitutively active form of Plx1 (CA-Plx1; Qian et al., 1999), and its final protein concentration was similar to that of endogenous Plx1 in MEE (Supplemental Figure S9D). Consistent with phosphorylation of S193 in Cdc25C by Plx1 (Supplemental Figure S8), phosphorylation of myc-Cdc25C126-213 with CA-Plx1 generated one distinct shift by Phos-tag SDS–PAGE (Supplemental Figure S9E). Further, consistent with phosphorylation of Myt1 by Plx1 in previous studies (Inoue and Sagata, 2005), phosphorylation of myc-Myt1 with CA-Plx1 reproducibly generated a readily detectable gel mobility shift of myc-Myt1 (Supplemental Figure S9F). Active MAPK and RSK2 were produced together by mixing HA-tagged MAPK and RSK2 with HA-tagged constitutively active MEK1 (Brunet et al., 1994), as MAPK and RSK2 are sequentially activated by MEK1 (Bhatt and Ferrell, 2000). The levels of active MAPK and RSK2 were similar to that of endogenous proteins in MEE, as determined by immunoblotting with phosphospecific antibodies that recognize activation-associated phosphorylations of MAPK and RSK2 (Supplemental Figure S9G). The MAPK/RSK2 mix robustly phosphorylated both the MAPK sites T48/T138/S163 and the RSK2 sites S317/T318/S319 in GST-Cdc25C (Supplemental Figure S9H).
Having characterized TNT-produced Cdk1, Plx1, and MAPK/RSK2, we then determined their individual and collective abilities to induce the M-phase supershift of the five mitotic regulators. As the M-phase supershift is a unique property of the supershift domain, we originally planned to use the five supershift fragments as substrates. However, the supershift fragments of Myt1, Wee1A, and APC3 overlapped or ran too closely with Cdk1 and CycB proteins on myc-tag immunoblotting, making us use full-length proteins of Myt1, Wee1A, and APC3 instead. To have a reference point for the M-phase supershift, phosphorylation of substrate proteins with individual kinase samples was performed in parallel with undiluted MEE, whereas the phosphorylation with the three kinase samples collectively (4 kinase mix), which evenly reduced each kinase concentration by fourfold, was all performed in parallel with 1:4-diluted MEE, which still induced the M-phase supershift of the five mitotic regulators. To give enough time for TNT-produced kinases to act, duration of each time course examination was set to greatly exceed the minimal amount of time required for control MEE to quantitatively induce the M-phase supershift.
Results from phosphorylation with individual kinase samples are shown in the left panels of Figure 8, B–F. In each of the five proteins tested, Cdk1, Plx1, and MAPK/RSK each induced a detectable slight shift, which was minor as compared with the supershift induced by MEE. On top of these common effects, MAPK/RSK induced an additional shift in a very small portion of Myt1, which was still much below the MEE-induced supershift. Results from phosphorylation with the 4 kinase mix are shown in the right panels of Figure 8, B–F. For Cdc25C9-375, Gwl179-745, and Wee1A, the 4 kinase mix induced a slight shift similarly as did phosphorylation with individual kinase samples. For Myt1, the 4 kinase mix induced a double shift that was comparable with the double shift induced by MAPK/RSK2. For APC3, however, the 4 kinase mix did not induce a detectable slight shift. Together, these results indicate that physiologically relevant levels of the four major mitotic kinases are insufficient to catalyze the high stoichiometry comprehensive phosphorylation of the supershift domain that produces the M-phase supershift.
Activation of all four of the major mitotic kinases in immature oocyte extracts did not induce the M-phase supershift of Cdc25C
One potential caveat of the above-described experiments was the presence of fortuitous inhibitory factors in the TNT system that significantly reduced the phosphorylation efficiency of produced mitotic kinases. To avoid this problem, we developed a protocol that robustly activated all four of the major mitotic kinases in immature oocyte extract without inducing M-phase entry and determined the effect on the M-phase supershift. As described in Supplemental Figure S10A, the protocol involved preparation of 400,000 × g cytosolic extract from manually defolliculated stage VI Xenopus oocytes (IOE*), immunoprecipitation of myc-tagged Cdc25C, phosphorylation of the myc-Cdc25C immunoprecipiates (IP) with MEE or control buffer, and incubation of 1:10-diluted IOE* supplemented with OA with the myc-Cdc25C IP. To evaluate kinase activations, the input myc-Cdc25C IP was transiently pelleted at different intervals, and supernatant (SN) proteins were immunoblotted with antibodies that recognize total and phosphorylated Cdk1, Plx1, MAPK, and RSK2.
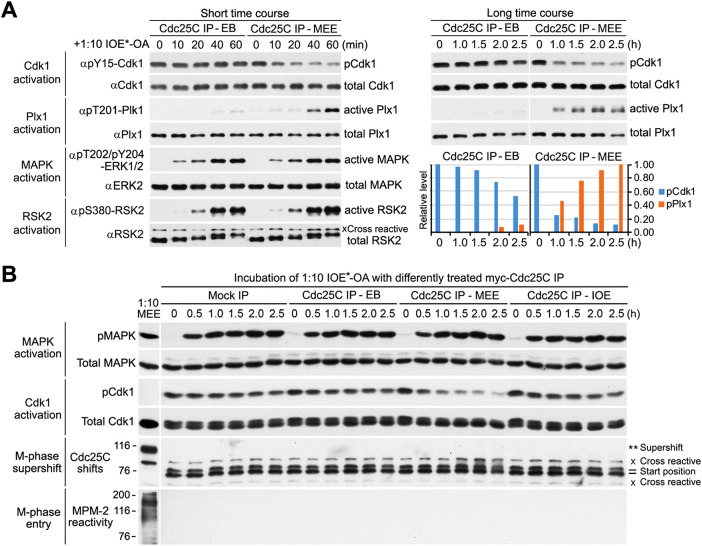
As shown in Figure 9A, MEE-treated myc-Cdc25C IP progressively decreased the inhibitory phosphorylation of Cdk1 to ∼20% of its start level within 60 min, and this activation-associated Cdk1 dephosphorylation was followed by activation-associated Plx1 phosphorylation with a 30-min delay, consistent with indirect regulation of Plx1 by Cdk1 in Xenopus egg extracts (Vigneron et al., 2018). A longer time course examination showed that the Plx1 phosphorylation further increased by >2-fold from 1.0 to 2.5 h, whereas the Cdk1 phosphorylation further decreased only by 10%. In contrast with MEE-treated myc-Cdc25C IP, EB buffer-treated myc-Cdc25C IP had little effect on the Cdk1 or Plx1 phosphorylation within the first 60 min but moderately decreased the Cdk1 phosphorylation and weakly induced the Plx1 phosphorylation from 1.0 to 2.5 h, indicating that robust activation of Cdk1 was required for strong Plx1 activation. In contrast with the distinctive activation states of Cdk1 and Plx1 under the two Cdc25C IP conditions, activation-associated phosphorylations of MAPK and RSK2 unanimously started in 10 and 20 min and completed in 40 and 60 min, respectively, consistent with their independence of Cdk1 activation in cell-free extracts of Xenopus oocytes (Nebreda and Hunt, 1993; Karaiskou et al., 1998). These results established that OA plus MEE-activated Cdc25C robustly activated all four of the major mitotic kinases in 1:10-IOE* in 1.5–2.5 h.
FIGURE 9:
Activation of all four of the major mitotic kinases in IOE is insufficient to induce the M-phase supershift. (A) 1:10-IOE*-OA was incubated with EB-treated or MEE-phosphorylated myc-Cdc25C immunocomplex at indicated intervals for up to 60 min (short time course) or for up to 2.5 h (long time course), followed by immunoblotting with indicated antibodies to visualize activations of Cdk1, Plx1, MAPK, and RSK2. (B) 1:10-IOE*-OA was incubated for the indicated hours with either control IgG immunoprecipitate (mock IP) or myc-Cdc25C immunoprecipitate (Cdc25 IP) that had been treated with EB, MEE, or IOE. After beads were pelleted down at indicated time points, SN proteins were immunoblotted in parallel with 1:10 MEE with the same antibodies as used in A and B to visualize activations of MAPK and Cdk1, with anti-Cdc25C antibodies to visualize gel mobility shifts of endogenous Cdc25C, and with MPM-2 to assess M-phase entry.
To evaluate the effects of activations of the four major mitotic kinases in 1:10 IOE* on the M-phase supershift, the longer time course incubation was repeated with two more negative controls, i.e., mock IP and IOE-treated myc-Cdc25C IP, and SN proteins collected were immunoblotted in parallel with 1:0-diluted MEE (positive control) with both antibodies that recognize endogenous Cdc25C and the mitosis specific phosphoprotein monoclonal antibody MPM-2 (Davis et al., 1983). The burst of MPM-2 reactivity in numerous proteins is a reliable M-phase specific marker that tightly associates with the M-phase supershift (Wu et al., 2010). Supplemental Figure S10B shows myc-tag immunoblotting of the four different input IPs. Results in Figure 9B show that robust activation of Cdk1 and presumably also Plx1 occurred only with addition of MEE-treated myc-Cdc25C IP, whereas robust activation of MAPK and presumably also RSK2 occurred under all four of the conditions. Consistent with results from phosphorylation with TNT-produced kinases, under none of the four conditions was the M-phase supershift of endogenous Cdc25C or the burst of MPM-2 reactivity induced; however, readily detectable slight gel mobility shifts of Cdc25C were induced under the condition of robust activations of all four of the major mitotic kinases (MEE-Cdc25C IP, 1.5–2.5 h). In contrast, even 1:16- and 1:32-diluted MEE still quantitatively and partially induced the M-phase supershift of myc-Cdc25C, respectively (Supplemental Figure S10C), eliminating the possibility that the inability of the four activated mitotic kinases in 1:10 IOE* to induce the M-phase supershift of Cdc25C was due to the dilution of IOE*. Together, these results lend a strong support to the conclusion that the site-specific phosphorylation catalyzed by the four major mitotic kinases is unable to generate the M-phase supershift.
None of the four major mitotic kinases are required for MEE to produce the M-phase supershift
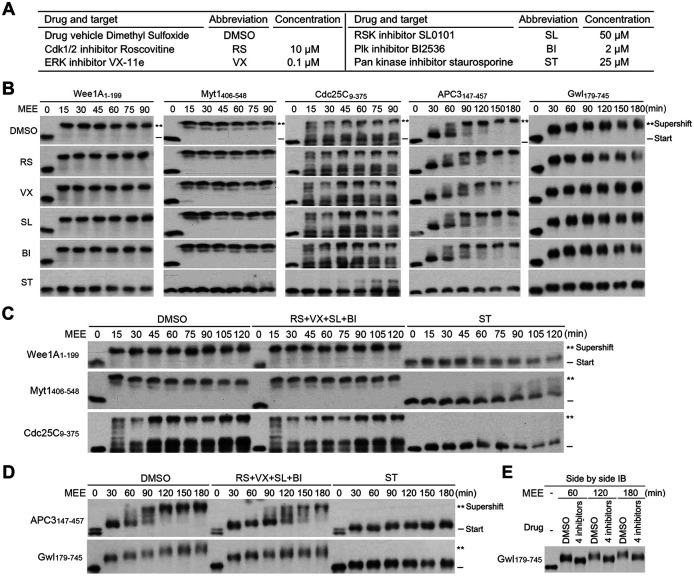
Although not sufficient, the four major mitotic kinases may be required for the M-phase supershift by priming the supershift domain to undergo the high stoichiometry comprehensive phosphorylation. To test this possibility, we inhibited Cdk1, MAPK, Plx1, and/or RSK2 activities in MEE and determined the effects on MEE-induced supershifts of the five supershift fragments by time course examination. Figure 10A lists the specific and pan kinase inhibitors and their concentrations used in MEE. Results in Figure S11, A–D show that 20% of each of their concentrations used in MEE was sufficient to potently inhibit the activity of the corresponding TNT-produced kinase, making it likely that each of their concentrations used in MEE is sufficient to potently inhibit the corresponding kinase activity. In support of this prediction, 10 µM roscovitine (RS; Cdk1/Cdk2 inhibitor) potently inhibited histone H1 kinase activity in MEE (Figure S11E). Gel mobility shift assays showed that none of the four specific kinase inhibitors used individually affected the magnitude or kinetics of the MEE-induced supershift of the five supershift fragments, while the pan kinase inhibitor abolished or dramatically reduced their gel mobility shifts (Figure 10B). When the four specific kinase inhibitors were used together, the magnitude or kinetics of the MEE-induced quick supershifts of Wee1A1-199, Myt1406-548, and Cdc25C9-375 were still not affected (Figure 10C), although the MEE-induced slower supershift of APC3147-457 was moderately retarded, and the MEE-induced gradual shift of Gwl179-745 showed a slightly decreased slope (Figure 10D). The minor effect on Gwl179-745 was further demonstrated by side-by-side immunoblotting of samples from three different time points (Figure 10E). Whether the moderate effects of the 4 kinase inhibition on the supershifts of APC3147-457 and Gwl179-745 were due to off target effects of kinase inhibitors or nonessential involvement of the four kinases in the process remains to be determined. Nonetheless, these results are sufficient to indicate that none of the four major mitotic kinases in MEE is required for the protein phosphorylation that generates the M-phase supershift.
FIGURE 10:
Inhibition of the four major mitotic kinase activities had little effect on MEE-induced supershifts of the five supershift fragments. (A) Kinase inhibitors and their concentrations used in MEE. (B) Phosphorylation of indicated supershift fragments with MEE in the presence of individual kinase inhibitors for the indicated minutes, followed by myc-tag immunoblotting. (C, D) Phosphorylation of indicated supershift fragments with MEE in the presence of all four of specific kinase inhibitors or ST for the indicated minutes, followed by myc-tag immunoblotting. (E) Side-by-side myc-tag immunoblotting of the Gwl179-745 samples from three different time points in D.
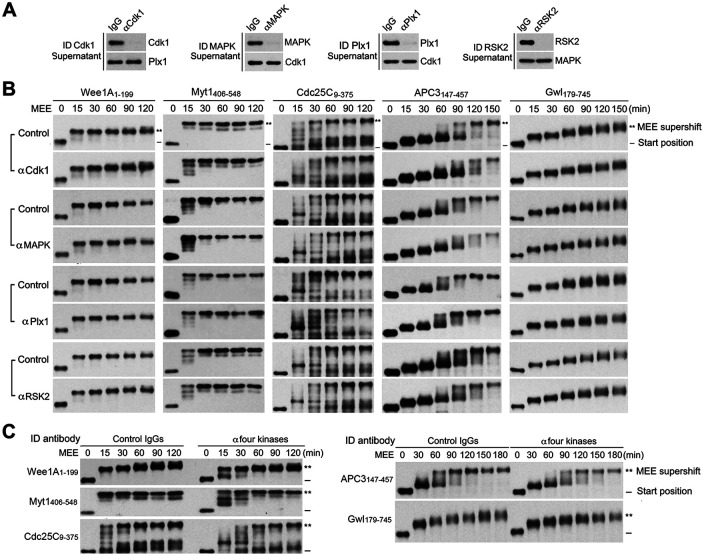
To further evaluate the above findings, we immunodepleted Cdk1, MAPK, Plx1, and/or RSK2 proteins from MEE with kinase-specific antibodies and determined the effects on MEE-induced supershifts of the five supershift fragments by time course examination. While each of the single kinase depletions reached at least 90% completion (Figure 11A), none of the single kinase depletions significantly affected the magnitude or kinetics of the MEE-induced supershift of the five supershift fragments (Figure 11B; Supplemental Figure S12A). Even when the four kinases were depleted together (Supplemental Figure S12B), completion of the MEE-induced supershifts of Cdc25C9-375, Myt1406-548, Wee1A1-199, and APC3147-457 was only moderately retarded, and the magnitude of the MEE-induced supershift of Gwl179-745 was only slightly decreased (Figure 11C; Supplemental Figure S12C). Again, whether the moderate inhibitory effects of the 4 kinase depletion were due to secondary or irrelevant effects of the kinase depletion or nonessential involvement of the four kinases in the process remains to be determined. Largely consistent with results from kinase inhibition, these results lend a strong support to noncritical roles of the four major mitotic kinases in the protein phosphorylation that produces the M-phase supershift.
FIGURE 11:
Immunodepletion of the four major mitotic kinases had no or minor effects on MEE-induced supershifts of the five supershift fragments. (A) Immunodepletion of MEE with each of indicated kinase-specific antibodies or control IgG, followed by immunoblotting of SNs with antibodies for each of indicated proteins. (B) Phosphorylation of each of myc-tagged supershift fragments with individual kinase-depleted or control IgG-absorbed MEE for the indicated minutes, followed by myc-tag immunoblotting. (C) Phosphorylation of each of myc-tagged supershift fragments with all four kinase-depleted or control IgG-absorbed MEE for the indicated minutes, followed by myc-tag immunoblotting.
p9-enhanced multisite phosphorylation by the Cdk1/CycB complex is unlikely to play a physiologically important role in the M-phase supershift
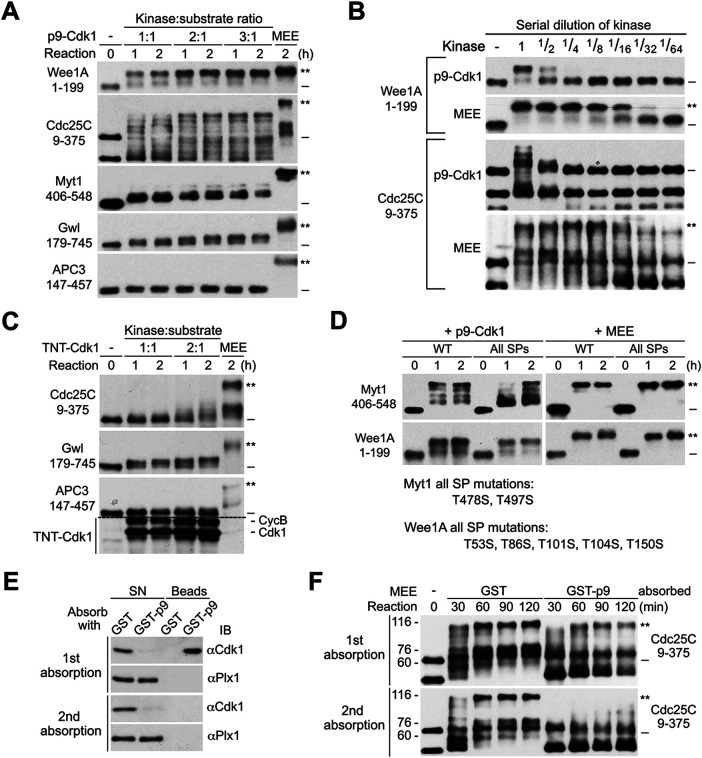
Previous studies demonstrated that the Cdk1/CycB complex that associates with stoichiometric levels of the Xenopus CKS protein p9 induced substantial gel mobility shifts of multiple key mitotic regulators (Patra and Dunphy, 1998; Patra et al., 1999). Since our results demonstrated that Cdk1 is not required for MEE-induced supershifts of the five key mitotic regulators, we speculated that although a high concentration of the Cdk1 complex that contains a high stoichiometry of p9 is able to induce substantial gel mobility shifts of multiple key mitotic regulators, this mechanism of protein phosphorylation does not play a physiologically important role in the M-phase supershift in M-phase cells. To test this possibility, we produced GST-tagged p9 to affinity absorb Cdk1 protein from excess amounts of MEE (Supplemental Figure S13A) and characterized the eluted GST-p9-Cdk1 complex (p9-Cdk1) for inducing gel mobility shifts of the five supershift fragments at 1:1, 2:1, and 3:1 kinase:substrate ratios (Figure 12A). Parallel immunoblotting showed that p9-Cdk1 contained a slightly higher concentration of Cdk1 than MEE (Supplemental Figure S13B). Parallel phosphorylation of histone H1 with p9-Cdk1 and MEE showed that they contained similar levels of H1 kinase activity (Supplemental Figure S13C). However, due to the quantitative binding of both free Cdk1 and the Cdk1/CycB complex in MEE, p9-Cdk1 prepared in this manner probably contained a higher stoichiometry of p9 than the Cdk1 complex produced by TNT (TNT-Cdk1).
FIGURE 12:
p9-enhanced multisite phosphorylation by the Cdk1/CycB complex does not play a physiologically important role in the M-phase supershift. (A) Phosphorylation of myc-tagged five supershift fragments with increased ratios of p9-Cdk1:substrate or MEE for the indicated hours, followed by myc-tag immunoblotting. (B) Parallel phosphorylation of myc-tagged Cdc25C9-375 or Wee1A1-199 with serial dilutions of p9-Cdk1 or MEE for 1 h, followed by myc-tag immunoblotting. (C) Parallel phosphorylation of each of the indicated myc-tagged supershift fragments with increased ratios of TNT-Cdk1:substrate or MEE for the indicated hours, followed by myc-tag immunoblotting. (D) Parallel phosphorylation of WT and all SP mutant form (All SPs) of myc-tagged Myt1406-548 or Wee1A1-199 with p9-Cdk1 or MEE for the indicated hours, followed by myc-tag immunoblotting. The mutant sites in the two proteins are specified. (E) MEE was absorbed with GST or GST-p9 once or twice, and the SN and bound proteins (beads) were immunoblotted with anti-Cdk1 or anti-Plx1 antibodies. (F) Phosphorylation of myc-Cdc25C9-375 for the indicated minutes with MEE that was absorbed with GST- or GST-p9 once or twice, followed by myc-tag immunoblotting.
Consistent with results in previous studies (Patra et al., 1999; Trunnell et al., 2011), p9-Cdk1 induced readily detectable or substantial gel mobility shifts of Myt1406-548, Gwl179-745, Cdc25C9-375, and Wee1A1-199 in a dose-dependent manner, although the shifts were less in magnitude and stoichiometry than that induced by MEE (Figure 12A). However, when Wee1A1-199 and Cdc25C9-375 were phosphorylated with serial dilutions of p9-Cdk1 and MEE for 1 h, MEE could be diluted up to 8- to 16-fold without losing the ability to induce a quantitative supershift of the two proteins, whereas just a twofold dilution of p9-Cdk1 eliminated or greatly reduced the stoichiometry of the substantial gel mobility shifts of Cdc25C9-375 or Wee1A1-199, respectively (Figure 12B). Thus the supershift-producing activity in MEE is at least a magnitude more potent than the Cdk1-mediated partial shift-producing activity. This result concurred with no or little effect of Cdk1 inhibition or depletion on MEE-induced supershifts (Figures 10B and 11B). We also phosphorylated Cdc25C9-375, Gwl179-745, and APC3147-457 with TNT-Cdk1 at both 1:1 and 2:1 kinase:substrate ratios. Clearly, doubling the amount of TNT-Cdk1 increased the gel mobility shifts of Cdc25C9-375 and Gwl179-745 (Figure 12C). It is conceivable that further increasing the concentrations of TNT-Cdk1 would achieve the substantial gel mobility shifts of multiple key mitotic regulators observed in previous studies. The high-concentration dependency of Cdk1-induced substantial gel mobility shifts argues against its physiological significance.
Previous studies demonstrated that Cks proteins enhance multisite phosphorylations by Cdk/cyclin complex through binding phosphorylated TP motifs (Kõivomägi et al., 2013; McGrath et al., 2013). Thus to further define the role of p9 in the M-phase supershift, we mutated all TP motifs in Myt1406-548 and Wee1A1-199 to SPs to eliminate the phospho-adaptor function of p9 and determined the effects on the gel mobility shifts induced by p9-Cdk1 and MEE. While the all SP mutation retarded heterogenous shifts of Myt1406-548 induced by p9-Cdk1, it did not affect the MEE-induced quantitative shift of Myt1406-548 or Wee1A1-199 (Figure 12D). These results lend further support to the conclusion that p9-enhanced multisite phosphorylation by Cdk1/CycB complex does not play a physiologically important role in the M-phase supershift.
Previous studies demonstrated that depletion of p9 from interphase-arrested Xenopus egg extracts inhibited the M-phase supershift of APC3 and delayed the M-phase supershift of Cdc25C, Myt1, and Wee1 induced by exogenously added Cdc2-AF/CycB, respectively (Patra and Dunphy, 1998). Since our results showed clearly that Cdk1 is not required for MEE-induced supershifts of the five key mitotic regulators, it is possible that p9 binds certain non-Cdk1 factors that positively regulate the M-phase supershift. To explore this possibility, we absorbed MEE with GST-p9 twice and determined the effects on both the Cdk1 level and the MEE-induced supershift of Cdc25C9-375. While the first round of absorption with GST-p9 already removed most of the Cdk1 in MEE, the second round of absorption had little effect on the level of Cdk1 (Figure 12E). In contrast, while the first-round absorption slightly retarded the supershift of Cdc25C9-375 in early time points, the second-round absorption dramatically inhibited the supershift of Cdc25C9-375 (Figure 12F). These results support interaction of p9 with non-Cdk1 factors that regulate the M-phase supershift.
The four major mitotic kinases are not required for the M-phase supershift in OA-induced Xenopus oocyte maturation
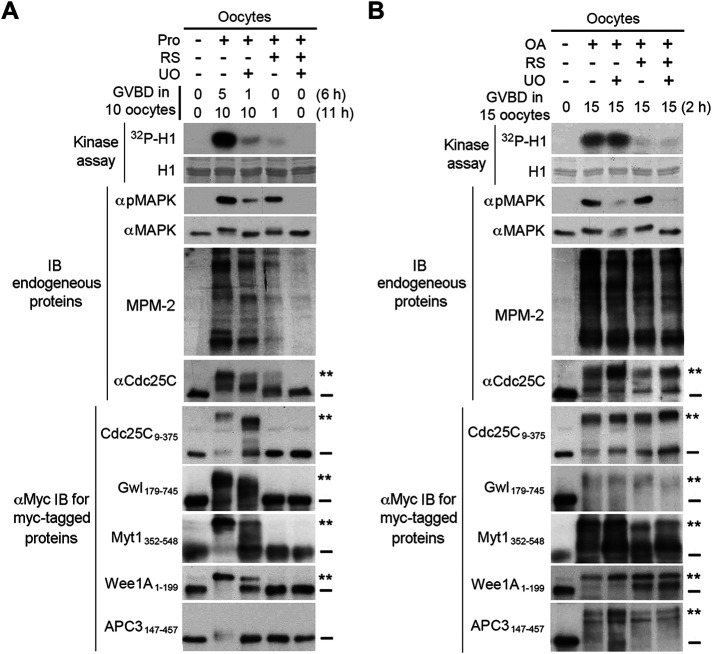
To address the physiological relevance of the above findings on the kinase involvement in the high stoichiometry comprehensive phosphorylation of the supershift domain, we determined the role of Cdk1, Plx1, MAPK, and RSK2 in the M-phase supershift in Xenopus oocytes. For this objective, we ectopically expressed the five supershift fragments in Xenopus oocytes by mRNA injection and induced oocyte maturation by either progesterone stimulation or OA injection (Schorderet-Slatkine, 1972; Goris et al., 1989) with or without several hours of preincubation with the Cdk1/2 inhibitor RS and/or the MEK inhibitor UO126. As determined in our pilot studies, inhibition of Cdk1 or MAPK activation in OA-injected oocytes also led to inhibition of activation-associated slight gel mobility shift of Plx1 (Kumagai and Dunphy, 1996; Qian et al., 1998) or RSK2 (Bhatt and Ferrell, 2000), respectively (Supplemental Figure S14), consistent with results in previous studies (Karaiskou et al., 1998; Bhatt and Ferrell, 2000; Gross et al., 2000). M-phase entry was monitored by germinal vesicle breakdown (GVBD) and/or a burst of the phosphoproteins recognized by MPM-2, which was previously shown to consistently correlate with both MPF activation and GVBD in Xenopus oocytes (Kuang et al., 1989). The M-phase supershift was monitored by gel mobility shifts of both endogenous Cdc25C and ectopically expressed five supershift fragments. Since progesterone-induced oocyte maturation is known to depend on Cdk1 and MAPK activities for M-phase entry and M-phase arrest, respectively (Kosako et al., 1994; Meijer et al., 1997; Maller et al., 2001; Wang et al., 2007), results from progesterone-stimulated oocytes would indicate validity of our experimental system and effectiveness of kinase inhibition. In contrast with progesterone-induced oocyte maturation, OA-induced oocyte maturation did not depend on Cdk1 or MAPK activity, as determined in our pilot studies. Separation of M-phase entry from the four major mitotic kinase activities in OA-injected oocytes would allow unambiguous determination of their roles in the M-phase supershift.
In progesterone-stimulated oocytes, inhibition of Cdk1 activity correlated with inhibition of both M-phase entry, judging from 90% inhibition of GVBD and much reduced MPM-2 reactivity, and the M-phase supershift, judging from both endogenous Cdc25C and the ectopically expressed supershift fragments. Differing from Cdk1 inhibition, inhibition of MAPK activation delayed the M-phase entry, as indicated by GVBD scores, and prevented stable M-phase arrest, judging from declined H1 kinase activity and MPM-2 reactivity. The compromised M-phase arrest correlated with partial or complete reversal of the M-phase supershift of Cdc25C and the five supershift fragments (Figure 13A). In OA-injected oocytes, however, dramatic inhibition of Cdk1 activity and/or MAPK activation did not block GVBD induction, the burst of the MPM-2 reactivity or the M-phase supershift of endogenous Cdc25C and the five supershift fragments (Figure 13B). These results support the physiological relevance of the finding that the four major mitotic kinases are not required for MEE-induced supershifts. These results also provide an example in which the major mitotic kinase activities become dispensable for M-phase entry in the presence of phosphatase inhibition, whereas the M-phase supershift still tightly associates with M-phase entry. This contrast suggests that the extraordinary type of mitotic phosphorylation that produces the M-phase supershift of the five key mitotic regulators may also promote the cellular changes that characterize M-phase entry.
FIGURE 13:
The four major mitotic kinases are not required for the M-phase supershift in OA-induced Xenopus oocyte maturation. (A) Xenopus oocytes were injected with mRNA for each of the five myc-tagged supershift fragments and cultured for 6–8 h. These injected oocytes were further cultured in the presence of indicated kinase inhibitors for 2–4 h and then either stimulated with progesterone (Prog) (A) or injected with OA (B) in the continued presence of the kinase inhibitors. GVBD was scored at the indicated h. Proteins extracts of lastly collected oocytes were assayed for H1 kinase activity by 32P incorporation and immunoblotted with indicated antibodies.
DISCUSSION
Protein phosphorylation plays important roles in both growth factor triggered signal transduction and cell cycle progression. However, only protein phosphorylation during M-phase induction produces a sudden and substantial increase in the apparent molecular weight of numerous proteins of varied biological functions, hinting the activation of a unique protein phosphorylation mechanism during M-phase induction. This mechanism has been generally thought to be the Cdk1-catalyzed phosphorylation of multiple S/TP motifs in numerous substrate proteins; however, this view is challenged by multiple lines of paradoxical observations in previous studies. To solve this conundrum, we made a comprehensive analysis of the protein phosphorylation that produces the M-phase supershift of Xenopus Cdc25C, Gwl, APC3, Myt1, and Wee1A in MEE. Our results demonstrate that their M-phase supershifts are each due to simultaneous phosphorylation of a considerable portion of S/T/Y residues, termed the high stoichiometry comprehensive phosphorylation, in a long intrinsically disordered region that is enriched in both S/T residues and S/TP motifs, termed the supershift domain. The high stoichiometry comprehensive phosphorylation of the supershift domain generates the supershift through additive numerous small shifts from phosphorylation of diverse sites. Although the four major mitotic kinases in MEE, Cdk1, Plx1, MAPK, and RSK2 are each able to phosphorylate the five mitotic regulators, they are neither sufficient to induce the M-phase supershift by themselves nor required for MEE to induce the M-phase supershift. Further, although high levels of the Cdk1/CycB complex that contains high stoichiometry levels of the Cks protein p9 are able to induce readily detectable or substantial gel mobility shifts of the five mitotic regulators as observed in previous studies, this mechanism of protein phosphorylation is unlikely to play physiologically important roles in the M-phase supershift. Finally, inhibition of the four major mitotic kinase activities in OA-injected oocytes inhibited neither M-phase entry nor the M-phase supershift. Together, these findings indicate that the M-phase supershift is produced by a previously unrecognized category of mitotic phosphorylation that likely plays important roles in M-phase induction.
Promiscuous versus highly efficient nonspecific protein phosphorylations
In currently understood protein phosphorylation, specific protein kinases phosphorylate specific phosphoacceptor sites in substrate proteins due to structural complementarity (Alexander et al., 2011; Kettenbach et al., 2011). Although site-specific protein kinases may phosphorylate nonideal sites due to promiscuous effects, these promiscuous phosphorylations occur more slowly and reach lower stoichiometries than site-specific phosphorylations (Kanshin et al., 2015). As a result, promiscuous phosphorylations require higher kinase and substrate concentrations and longer phosphorylation time than site-specific phosphorylations to produce significant outcomes. In contrast with currently understood promiscuous phosphorylations, the site-nonspecific phosphorylation that produces the M-phase supershift of the five mitotic regulators occurred more quickly and reached higher levels and stoichiometries than the site-specific phosphorylations catalyzed by the four major mitotic kinases (Figure 8, B–F). Rather than requiring high kinase activities, MEE could be diluted up to 16- to 32-fold without losing the ability to induce the M-phase supershift (Supplemental Figure S10C; Figure 12B). The extremely high efficiency of the site-nonspecific phosphorylation that produces the M-phase supershift sets it apart from currently understood promiscuous protein phosphorylations.
A previously unrecognized category of mitotic phosphorylation
Current understanding of mitotic phosphorylation revolves around phosphorylation of S/TP motifs by the proline-directed kinase Cdk1/CycB, phosphorylation of acidophilic motifs by the acidophilic kinase Plk1, and phosphorylation of basophilic motifs by basophilic kinases AURKA&B (Dephoure et al., 2008; Olsen et al., 2010). However, although the M-phase supershifts of the five key mitotic regulators associate with robust phosphorylation of multiple S/TP motifs, acidophilic motifs, and basophilic motifs in the supershift domain (Figures 4 and 5), inhibition or depletion of the major proline-directed, acidophilic, and basophilic kinases Cdk1, MAPK, Plx1, and RSK2 in MEE or inhibition of their activities in OA-injected Xenopus oocytes did not inhibit the M-phase supershift (Figures 10, 11, and 13). While the site-specific phosphorylation of Cdc25C by Cdk1, MAPK or RSK2 is a local event that persists after substrate fragmentation (Wang et al., 2007, 2010), we provide evidence that the supershift-producing phosphorylation of Cdc25C and Gwl is an integrated process that is sensitive to truncations of particular regions in the supershift domain (Supplemental Figure S3), indicating involvement of certain cis-operating elements that regulate the supershift-producing phosphorylation of the entire supershift domain. Further, while biochemical fractionation of MEE by consecutive chromatography methods identified Cdk1, MAPK, Plx1, and RSK2 as the major Cdc25C phosphorylating kinases, the Cdc25C supershift-producing activity always got lost during MEE fractionation (Wang et al., 2007; Wang et al., 2010), indicating involvement of multiple soluble factors in producing the M-phase supershift. Together, these findings indicate that the protein phosphorylation that produces the M-phase supershift is fundamentally different from currently understood site-specific phosphorylations catalyzed by the specific mitotic kinases.
In addition to the above-described site-specific phosphorylations by specific mitotic kinases, the Cdk1/CycB complex that associates with stoichiometric levels of the Xenopus CKS protein p9 has been shown to induce substantial gel mobility shifts of multiple key mitotic regulators (Patra and Dunphy, 1998; Patra et al., 1999), suggesting a unique ability of the trimeric Cdk1 complex to catalyze site-nonspecific phosphorylations. Since at least a portion of the Cdk1/CycB complex in M-phase cells associates with p9 (Patra and Dunphy, 1996), p9-enhanced multisite phosphorylation catalyzed by Cdk1/CycB complex seems to be a legitimate explanation for the site-nonspecific phosphorylations that produce the M-phase supershift. However, neither Cdk1 inhibition/depletion nor mutation of the p9 binding sites in the supershift domain of Myt1 and Wee1A eliminated the M-phase supershift induced by MEE (Figures 10B, 11B, and 12D). In contrast with MEE, very high kinase concentrations and lengthy phosphorylation times were required for the trimeric Cdk1 complex to induce substantial gel mobility shifts of the supershift fragments of Cdc25C and Wee1A (Figure 12, A and C). Further, immunodepletion of p9 from interphase Xenopus egg extracts only moderately delayed the M-phase supershift of Cdc25C, Myt1, and Wee1 in previous studies (Patra and Dunphy, 1998). Our results suggest that this could possibly be accounted for by interaction of p9 with certain non-Cdk1 proteins that positively regulate the M-phase supershift (Figure 12, E and F). These results all argue against the possibility that p9-enhanced multisite phosphorylation catalyzed by Cdk1/CycB complex plays a physiologically important role in the M-phase supershift.
A new angle to think about multiple key questions in mitotic regulation
Previously observed tight association of the M-phase supershift of Cdc25C, Gwl, Myt1, and Wee1A with robust activation of Cdc25C and Gwl and robust inactivation of Myt1 and Wee1 already indicates critical roles of the supershift-producing phosphorylations of these mitotic regulators in regulating their activities during M-phase induction. On the other hand, Cdc25C was also activated by phosphorylation with Cdk1, Plx1, MAPK, or RSK2 (Izumi and Maller, 1993; Kumagai and Dunphy, 1996; Wang et al., 2007, 2010), and Gwl was also activated by phosphorylation with purified Cdk1 or Cdk2 (Blake-Hodek et al., 2012). Conversely, Wee1 was also inactivated by phosphorylation with Cdk1 (Mueller et al., 1995a), and Myt1 was also inactivated by phosphorylation with RSK2 or Plx1 (Palmer et al., 1998; Inoue and Sagata, 2005). So it seems that both the site-specific and the supershift-producing phosphorylations regulate these key mitotic regulators during M-phase induction. But why does bimodal regulation exist? Functional redundancies or different spatial/temporal contributions? Since artificially increasing the activity of Cdk1, Plx1, MAPK, or RSK in Xenopus oocytes by RNA or protein injection is each able to induce oocyte maturation after a long delay (Haccard et al., 1995; Qian et al., 1999; Gross et al., 2001; Wang et al., 2007), it is possible that the site-specific phosphorylation of these mitotic regulators generates above-threshold levels of Cdk1 and Gwl activities that are required for M-phase commitment, whereas the supershift-producing phosphorylation of these mitotic regulators is responsible for quickly generating sufficiently high levels of Cdk1 and Gwl activities that drive M-phase entry. This hypothesis is certainly worth testing in a separate study.
M-phase entry consists of abrupt and complete dismantling of various interphase cellular structures, inactivation of various interphase cellular processes, and condensing interphase chromatin into mitotic chromosomes (Gottesfeld and Forbes, 1997; Dangi and Shapiro, 2005; Guttinger et al., 2009; Chatel and Fahrenkrog, 2011; Giunta and Jackson, 2011; Fielding and Royle, 2013; Champion et al., 2017; Jones et al., 2019). Because the switchlike M-phase entry is concomitant with abrupt activation of Cdk1/CycB, which has been thought to catalyze most of the mitotic phosphorylation, a widely accepted model is that the protein phosphorylation catalyzed by robustly activated Cdk1/CycB directly drives M-phase entry (Morgan, 2007; Alberts et al., 2015). However, Cdk1/CycB is a typical protein kinase that has a normal range of abundance and activity, which makes it hard to understand how this single protein kinase is capable of catalyzing most of the mitotic phosphorylation with a switchlike kinetics. Further, landmark events of mitotic entry, such as chromosome condensation, nuclear lamina depolymerization, and centrosome separation, occurred in mammalian cell lines without Cdk1 activity if G2-phase cells were treated with the PP1/PP2A inhibitor OA or fostriecin (Guo et al., 1995; Gowdy et al., 1998). In interphase-arrested Xenopus egg extracts that were deprived of both mitotic cyclins and Cdk1/2 proteins, nuclear envelope breakdown and chromosome condensation were induced by the PP1/PP2A inhibitor MC (Izumi and Maller, 1995). So there has to be a Cdk1-independent mechanism that promotes M-phase entry. Thus far, the M-phase supershift is the most reliable biochemical indicator for M-phase entry. This point is strengthened further by our results that the M-phase supershift associates with M-phase entry in OA-injected oocytes even in the absence of significant Cdk1, Plx1, MAPK, and RSK2 activities (Figure 13B). Since the M-phase supershift occurs not only on multiple key mitotic regulators but also on numerous other proteins of varied biological functions, an interesting possibility is that the supershift-producing phosphorylation may also promote the cellular changes that characterize M-phase entry. Consistent with this possibility, the five additional M-phase supershift proteins examined in this study, i.e., anillin, GTSE1, INCENP, Ki-67, and NuMA-1, are all associated with subcellular structures that dramatically change during M-phase induction. Further, in a limited literature search, we found numerous supershift proteins that participate in nuclear envelope breakdown (Macaulay et al., 1995; Favreau et al., 1996; Laurell et al., 2011; Patel et al., 2014; Chen et al., 2018), chromosome condensation (Hirano et al., 1997; Abe et al., 2011; Kagami et al., 2017), and reorganization of cytoskeletons (Hosoya et al., 1993; Masson and Kreis, 1995; Ookata et al., 1997; Shiina and Tsukita, 1999; Yamashiro et al., 2001; Kim et al., 2017; Tipton et al., 2017).
Although Cdk1 activity is not required for the M-phase supershift or M-phase entry in the presence of phosphatase inhibition, Cdk1 activity is always required for both the M-phase supershift and M-phase entry under physiological conditions. Since the phosphatase inhibition cannot induce Cdk1-catalyzed phosphorylations without Cdk1 activity, the most plausible explanation for this contrast is that Cdk1 plays a key role in inhibiting an M-phase inhibitory protein phosphatase that prohibits the phosphorylation mechanism that produces the M-phase supershift and promotes M-phase entry. From the phosphatase inhibitors used in previous studies (Izumi and Maller, 1995), the putative M-phase inhibitory protein phosphatase seems to be PP2A or a closely related Ser/Thr phosphatase. Interestingly, INH, originally discovered as an activity that inhibits spontaneous activation of MPF in G2-arrested Xenopus oocytes, was identified to be a form of PP2A (Lee et al., 1991). More recent studies showed that PP2A-B55δ activity is specifically inhibited in mitosis through a Gwl-dependent pathway and that its removal accelerates M-phase entry (Mochida et al., 2009). So, it is possible that PP2A-B55δ is the putative M-phase inhibitory protein phosphatase that inhibits the activation of the phosphorylation mechanism that produces the M-phase supershift and induces M-phase entry. If such is the case, this will expand the biological function of PP2A-B55δ from simply removing Cdk1-catalyzed phosphorylations to more important regulatory roles upstream.
In summary, findings in this study break new ground in mitotic phosphorylation and provide a new angle to think about multiple key questions in mitotic regulation.
MATERIALS AND METHODS
Request a protocol through Bio-protocol.
Preparations of Xenopus oocyte and egg extracts
The classical MPF extraction buffer EB (Wu and Gerhart, 1980) consists of 80 mM β-glycerophosphate, 20 mM EGTA, and 15 mM MgCl2, pH 7.3. For large-scale preparations of MEE, unfertilized eggs were obtained and extracted as previously described (Kuang and Ashorn, 1993); the extraction buffer was an equal volume of cold EB supplemented with 20 mM NaF, 5 mM dithiothreitol (DTT), 1 mM ATP-γ-S (Roche), 1 μM OA (Calbiochem, 495604), and 10 μg/ml each of leupeptin, chymostatin, and pepstatin (Roche). For large-scale preparations of IOE, stage V and VI oocytes were obtained through collagenase treatment of surgically removed ovarian tissues and extracted as previously described (Kuang and Ashorn, 1993); the extraction buffer was an equal volume of cold EB supplemented with 5 mM DTT and 10 μg/ml each of leupeptin, chymostatin, and pepstatin. Both MEE and IOE were stored at −80°C until use. CSF extract was prepared as previously described (Murray et al., 1989) and used only once after being stored at −80°C.
Construction of expression plasmids for recombinant proteins
pCS2+MT- and pCS2+HA-based expression plasmids were constructed to express various myc-tagged or HA-tagged recombinant proteins by TNT. The pCS2+MT-based expression plasmids were also used to produce mRNAs by in vitro transcription. pGEX-based expression plasmids were constructed to express various GST-tagged recombinant proteins in BL21 Escherichia coli. While a small portion of these plasmids were produced or described in our previous studies, most of them were newly constructed in this study via PCR amplification of desired coding regions using verified cDNA constructs as templates and subcloning of the amplified cDNA into one of the above-described vectors.
To summarize the source cDNAs for the five key mitotic regulators examined in this study, all expression constructs for Xenopus Cdc25C proteins were derived from a pGEX-based expression construct for GST-tagged Cdc25C9-550 (Perdiguero et al., 2003), which was kindly provided by T. Hunt and A. Nebreda and verified to encode the NM_001088449.1 sequence in the NCBI database. All site-specific phosphodefective and phosphomimetic mutations in Cdc25C proteins were performed by site-directed mutagenesis of the parental constructs (Takara, R050A). All expression constructs for Xenopus Gwl proteins were derived from a pMalC2-based expression construct for MBP-tagged Gwl (Yu et al., 2006), which was kindly provided by M. Goldberg and verified to encode the NM_001091160.1 sequence in the NCBI database. All expression constructs for Xenopus APC3 proteins were derived from a pcDNA3.1-based cDNA construct, which was purchased from GenScript and verified to encode the NM_001092099.1 sequence in the NCBI database. All expression constructs for Xenopus Myt1 proteins were derived from a pCMV-based cDNA construct, which was obtained from Invitrogen (IMAGE clone; ID 6865778) and verified to encode the NM_001086466.1 sequence in the NCBI database. The all SP mutant form of myc-tagged Myt1406-548 was produced by site-directed mutagenesis of the parental constructs. All expression constructs for Xenopus Wee1A proteins were derived from a pET-based expression construct for his-tagged Xenopus Wee1A (Mueller et al., 1995a), which was kindly provided by J. Ferrell and verified to encode the U13962.1 sequence in the NCBI database. The all SP mutant form of Wee1A1-199 and all DE mutant forms of the five supershift fragments were produced by DNA synthesis of the coding cDNAs (Beijing Ruibio Biotech Co., Ltd), followed by subcloning of the coding cDNAs into the pCS2+MT vector.
To summarize the source cDNAs for the five nonsupershift proteins and the four major mitotic kinases examined in this study, the expression plasmid for myc-tagged human Pin1 was derived from a bacterial expression construct for GST-tagged Pin1 (Stukenberg and Kirschner, 2001), which was kindly provided by P. T. Stukenberg and verified to encode the U49070.1 sequence in the NCBI database. The expression plasmid for myc-tagged sea urchin cyclin B ∆90 was derived from a bacterial expression construct for GST-tagged sea urchin cyclin B ∆90 in our previous studies (Wu et al., 2010) and verified to encode the Y00608.1 sequence in the NCBI database. The expression constructs for Xenopus MEK1 and MAPK were derived from pMalC2-based bacterial expression vectors for MBP-tagged MEK1 and MAPK (Rouse et al., 1994; Palmer et al., 1998), which were kindly provided by A. Nebreda and verified to encode the NM_001086830.1 and NM_001090079.1 sequences in the NCBI database. The expression plasmid for HA-tagged CA-MEK1 was described in our previous studies (Wang et al., 2007). The expression plasmids for myc-tagged Xenopus Plk1 (Plx1) and HA-tagged constitutively active Plx1 were derived from baculovirus expression vectors for his-tagged Plx1 proteins (Kumagai and Dunphy, 1996), which were kindly provided by W. Dunphy and verified to encode the U58205.1 sequence in the NCBI database. The expression plasmid for HA-tagged Xenopus RSK2 was constructed from a pcDNA3.1-based cDNA, which was obtained from Invitrogen (IMAGE clone; ID 3401402) and verified to encode the AF165162.1 sequence in the NCBI database. The expression constructs for myc-tagged and GST-tagged Xenopus p9 were derived from a baculovirus expression vector for His-tagged p9 (Patra and Dunphy, 1996), which was kindly provided by P. T. Stukenberg and verified to encode the NM_001088329.1 sequence in the NCBI database. The expression plasmid for myc-tagged human Cdk1 was constructed from a human Cdc2 cDNA in our lab inventory and verified to encode the NM_001786.5 sequence in the NCBI database. The expression plasmid for myc-tagged human Cdk1 T161E (Dissmeyer et al., 2007) was produced by site-directed mutagenesis.
In vitro transcription-linked translation
In vitro transcription-linked translation (TNT) from pSC2+MT or pCS2+HA based plasmids was performed by using TnT Quick Coupled Transcription/Translation System (L2080) or TnT Coupled Transcription/Translation System (L4600) obtained from Promega Corporation following the instruction manual. The TNT products were either used freshly or stored at −80°C until use.
Expression and purification of GST-tagged proteins
GST-tagged proteins were all produced in BL21 E. coli from pGEX-based plasmids, and the protein expression was induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside at 22°C for 5 h when fresh bacterial cultures grew to A600 of 0.6–0.8 at 37°C. From induced cultures, cells were harvested by centrifugation, washed with cold phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4), and lysed in PBS supplemented with 250 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 3 mM DTT, 0.5% Triton X-100, and 1 mg/ml lysozyme (lysis buffer) at 4°C for 30 min. On average, 3 ml of lysis buffer were used to lyse pelleted cells from a 100-ml culture. After cell lysates were sonicated and centrifuged at 13,000 × g at 4°C for 15 min, SNs were collected and mixed with 1/20 vol of glutathione sepharose (GenScript) on rotation at 4°C overnight. After beads were pelleted, they were washed three times with a wash buffer consisting of 50 mM Tris-HCl, pH 8.0, 500 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5% Triton X-100, and 0.5% Tween-20 and rinsed three times with PBS. The average concentration of immobilized GST-tagged proteins ranged from 0.3 to 3 µg/µl. The beads were stored in PBS at 4°C for up to a week before being used for phosphorylation reactions.
Preparation of recombinant kinases for phosphorylation reactions
The active Cdk1/CycB/p9 complex, CA-Plx1, and the MAPK/RSK2 mix were freshly prepared from TNT-produced proteins as described in Supplemental Figure S9, A, D, and G. Phosphorylation buffer consisted of 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 5 mM β-glycerophosphate, 1 µM MC (Cayman Chemical Company, 10007188), 1 mM DTT, and 1× protease inhibitor cocktail (Roche). Note that the addition of exogenous ATP did not affect phosphorylation efficiencies of TNT-produced kinases. Our pilot studies indicated that some batches of TNT system contained significant levels of endogenous PKA activity, which phosphorylated Cdc25C at the RSK2 sites. To solve this complication, we routinely added 100 µM PKA inhibitor, which was purchased from BACHEM (H-3222), to TNT-produced MAPK/RSK2 mix before phosphorylation reactions.
To prepare p9-Cdk1, 50 µl GST-p9 beads were incubated with 500 µl MEE (excess amounts) on rotation at 4°C for 2 h, and pelleted beads were washed three times with Tris-buffered saline (TBS) (50 mM Tris-HCl, pH 7.5, and 150 mM NaCl) supplemented with 0.1% Triton X-100 and two times with TBS. Washed beads were eluted with 100 µl TBS supplemented with 10 mM reduced glutathione (Sigma, 3541), 1 mM DTT, and protease inhibitor cocktail at 22°C for 15 min, and SNs were collected for use as p9-Cdk1. Note that the H1 kinase activity of p9-Cdk1 varied from 0.75 to 2 times of the H1 kinase activity of MEE, and that the p9-Cdk1 samples could be stored at 4°C for 0–5 d without much loss of activity.
Phosphorylation reactions
It should be emphasized that all phosphorylation reactions contained equivalent concentrations of either OA or MC. As described above, MEE and TNT-produced kinases already contained 1 µM OA or MC. For parallel phosphorylations with IOE and MEE, 1 µM OA (Calbiochem) was freshly added to IOE. For phosphorylations with serially diluted MEE, MEE was freshly diluted with EB supplemented with 1 mM ATP, 1 mM DTT, 1 µM OA, and protease inhibitor cocktail. To use purified p9-Cdk1 for phosphorylation, p9-Cdk1 was freshly supplemented with 10 mM MgCl2, 5 mM β-glycerophosphate, 1 mM ATP, and 1 µM MC. For phosphorylations with serially diluted p9-Cdk1, p9-Cdk1 was freshly diluted with TBS supplemented with 10 mM MgCl2, 5 mM β-glycerophosphate, 1 mM ATP, 1 µM MC, and protease inhibitor cocktail.
Phosphorylation of TNT-produced substrate proteins was typically carried out by mixing 1 µl substrate sample with 2 µl kinase sample (IOE, MEE, TNT kinases, or p9-Cdk1), followed by incubation at 22°C for indicated lengths of time. The phosphorylation reaction was stopped by the addition of 25–50 µl 1.5× SDS–PAGE sample buffer, and 5- to 10-µl aliquots from each set of samples were taken for immunoblotting. Phosphorylation of calf thymus histone H1 (MilliporeSigma, 382150) was carried out by mixing 1 µl of 1 mg/ml histone H1 with 2 µl MEE or Cdk1 sample, followed by incubation at 22°C for indicated lengths of time and termination of the phosphorylation by the addition of 25 µl 1.5× SDS–PAGE sample buffer. From each set of the samples, 5-µl aliquots were immunoblotted with anti-pH1 antibodies to detect H1 phosphorylation, whereas the remaining portions were processed in parallel to indicate even loading of histone H1 by Ponceau S staining. Phosphorylation of GST-tagged proteins immobilized onto glutathione sepharose was typically carried out by mixing 3–5 µl substrate beads with 6–10 µl kinase sample, followed by incubation at 22°C for indicated lengths of time. After the substrate beads were washed three times with cold TBS supplemented with 350 mM NaCl, 0.5% Triton X-100, and 0.5% Tween-20, bound proteins were eluted with 10 µl 1.5× SDS–PAGE sample buffer. All of the eluates were used for immunoblotting. Determination of Plx1 phosphorylation sites in Cdc25C by 32P incorporation was performed as described in previous studies along with mapping the Cdk1 and MAPK phosphorylation sites in Cdc25C (Wang et al., 2007).
Microinjection, maturation of Xenopus oocytes, and radioactive H1 kinase assay
In vitro transcription of capped mRNAs from the pCS2+MT based plasmids was performed by using the mMESSAGE mMACHINE high yield capped RNA transcription kit (ThermoFisher, AM1340). Stage VI Xenopus oocytes were manually defolliculated from surgically removed ovarian tissues in Modified Bath’s Solution (MBS) and each was injected with 2–3 ng of indicated mRNA in 10–20 nl of H2O. Several hours (4–8) after mRNA injection, oocytes were cultured in the presence of 3 µM progesterone for indicated lengths of time. Harvested oocytes were homogenized with 6 µl/oocyte EB supplemented with 1 mM DTT, 1 mM ATP, and protease inhibitor cocktail, and oocyte homogenates were centrifuged at 16,000 × g at 4°C for 3 min. The translucent layer between lipid cap and yolk platelets was collected for immunoblotting.
For kinase inhibition experiment, mRNA-injected oocytes were first incubated for 2–4 h in MBS that was supplemented with either or both of 50 µM UO126 (Calbiochem, 109511-58-2) and 50 µM RS or the drug vehicle DMSO and then either stimulated with 3 µM progesterone or injected with 5 nl of 100 µM OA (∼0.5 µM final). At 7 or 11 h after progesterone stimulation and 2–4 h after OA injection, GVBD was observed by white spot formation and confirmed by oocyte dissection in 5% trichloroacetic acid. For H1 kinase assay, harvested oocytes were homogenized with 3 µl/oocyte EB supplemented with 1 mM DTT, 0.1 mM ATP and protease inhibitor cocktail, and the homogenate was spun at 16,000 × g for 3 min at 4°C. After the translucent layer between the lipid cap and yolk platelets was recovered, a 3-µl sample was mixed with 12 µl EB supplemented with 1 mM DTT, 0.1 mM ATP, 4 µg histone H1, and 5 µCi [γ-32P]ATP (PerkinElmer, NEG002A), and the reaction mixture was incubated at 22°C for 15 min, followed by mixing with 10 µl 5× of SDS–PAGE sample buffer. Samples were separated by 12.5% SDS–PAGE and subjected to autoradiography. For immunoblotting, harvested oocytes were extracted as described above.
SDS–PAGE and immunoblotting
For regular SDS–PAGE, 5- to 10-µl sample aliquots were separated by 10% (30% acr:1% bis = 2.57:1) or 12.5% (30% acr:1% bis = 4:1) SDS–PAGE along with prestained molecular weight standards (MilliporeSigma, SDS7B2) calibrated as described in our previous studies (Kuang et al., 1991b). For Phos-tag SDS–PAGE, SDS–PAGE gel was prepared with 30 or 50 µM of Phos-tag (Wako Chemicals) added following the manufacturer’s instruction. After SDS–PAGE, proteins were transblotted to nitrocellulose membrane (Santa Cruz Biotechnology), stained with Ponceau S solution (MilliporeSigma), and immunoblotted with indicated antibodies.
MPM-2 ascites was produced in our previous studies (Wu et al., 2010). Anti-myc tag antibodies were obtained from Santa Cruz Technology (sc-40). Antibodies used to immunoblot phosphorylated histone H1 were obtained from Upstate Technology (06-597). Antibodies used to immunoblot total Plx1 and T210-phosphorylated Plx1 were obtained from Invitrogen (33-1700) and Cell Signaling Technology (5472), respectively. Antibodies used to immunoblot total MAPK and phosphorylated MAPK were obtained from Santa Cruz Biotechnology (sc-1647) and Cell Signaling Technology (9106), respectively. Antibodies used to immunoblot total RSK2 were obtained either from Santa Cruz Technology (sc-9986) or from Cell Signaling Technology (9355). Antibodies used to immunoblot S380-phosphorylated RSK2 were obtained Cell Signaling Technology (9335). Antibodies used to immunoblot total Cdk1 and pY15-phosphorylated Cdk1 were obtained from Santa Cruz Biotechnology (sc-54) and Cell Signal Technology (9111), respectively. The phosphospecific antibodies used to immunoblot SpTP or RXXpS/T motif were obtained from Cell Signaling Technology (5243 and 9614). In all of the immunoblotting performed in this study, horseradish peroxidase–conjugated goat anti-rabbit or goat anti-mouse IgG antibodies from Bio-Rad (1706515 and 1706516) were used as secondary antibodies, and immunoblots were developed by ECL.
Analysis of protein phosphorylation by mass spectrometry
GST-tagged five supershift fragments were produced and immobilized onto glutathione sepharose for phosphorylation by MEE for indicated lengths of time. The substrate beads were washed three times with cold PBS supplemented with 1% Triton X-100 and eluted with 2 vol of 1.5× SDS–PAGE sample buffer three times. Combined samples were then separated by SDS–PAGE and stained with Coomassie blue (MilliporeSigma).
For mass spectrometry, stained gels were sent to The Proteomics and Metabolomics Facility of MD Anderson Cancer Center for routine processing. Briefly, full-length super-shifted proteins were digested in gel slices with modified sequencing-grade trypsin and Lys-C (Promega), and recovered peptides were directly analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific) involving HCD fragmentation without phosphopeptide enrichment. Peptide spectrum matches (PSMs) were identified by searching obtained fragment spectra against manually entered protein sequences using the Mascot search engine (version 2.3; Matrix Science) via the Proteome Discoverer software program (version 1.4; Thermo Fisher Scientific). Note that output PSMs only contained unambiguously identified peptides. Also, whenever a single peak of peptide from LC resulted in multiple unambiguous PSMs, only the most predominant PSM was listed in the output dataset. From obtained PSM data sets, PSMs with high or medium significance were selected and combined for data analysis by a macro of Excel edited with the programming language of Visual Basic. The analysis generated sequence alignments of PSMs with individual spectra counts listed (Supplemental Tables S1–S5), determined numbers of different categories of PSMs (Supplemental Figure S4B), and also produced spectra counts for calculation of the phosphorylation identification frequencies for individual S/T/Y residues (Figure 5, A–E) and for total S/T/Y residues (Figure 5G). The phosphorylation identification frequency for a particular S/T/Y residue was calculated by dividing the total number of PSMs that contained this S/T/Y residue by the total number of PSMs that were phosphorylated at this residue. The phosphorylation identification frequency for total S/T, Y or S/T/Y residues was calculated by dividing the total number of S/T, Y or S/T/Y residues recovered by the total number of these residues phosphorylated.
Activation of the four major mitotic kinases in diluted IOE
To make IOE*, 50–100 stage VI oocytes were manually defolliculated from surgically removed ovarian tissues and homogenized with an equal volume of cold EB supplemented with 5 mM DTT and protease inhibitor cocktail (Roche). After oocyte homogenates were centrifuged at >400,000 × g at 4°C for 1 h, which presumably removed most of membrane vesicles and associated Myt1 protein (Mueller et al., 1995b), the translucent layer between lipid cap and yolk platelets was collected and stored at –80°C until use. To prepare myc-Cdc25C immunocomplex for activation of Cdk1 in IOE*, 5 µl TNT product of myc-Cdc25C was mixed with 1 µl anti-myc antibodies and 3–5 µl protein A/G beads on rotation at 22°C for 1 h or at 4°C overnight, and antibody beads were pelleted and washed three times with 200 µl EB. The obtained immunocomplex was phosphorylated with 10 µl MEE or IOE or mock-treated with control buffer EB at 22°C for 1 h, washed three times with cold EB, and rinsed once with 15 µl cold EB supplemented with 1 mM ATP, I mM DTT, and 1 µM MC. To activate the major mitotic kinases in IOE*, IOE* was 1:10-diluted with cold EB supplemented with 1 mM ATP, 1 mM DTT, 1 µM OA, and protease inhibitor cocktail, and a 120-µl sample was mixed with the above-described myc-Cdc25C immunocomplexes on rotation at 22°C for indicated lengths of time. At each of indicated time point, myc-Cdc25C beads were temporarily spun down, and a 20-µl aliquot was taken from SN to mix with 10 µl 5× SDS–PAGE sample buffer. From each set of collected samples, 15-µl aliquots were immunoblotted with indicated antibodies.
Kinase inhibition, kinase immunodepletion, and GST-p9 absorption of MEE
The Cdk inhibitor RS was obtained from LC Laboratories (R1234). The ERK inhibitor Vx-11e was obtained from ChemieTeK (896720). The RSK inhibitor SL0101 was obtained from either Toronto Research Chemicals (S560000) or MedChemExpress (HY-15237). The Plk1 inhibitor BI2536 was obtained from either Biovision (2730-5) or Selleck Chemicals (S1109). The pan kinase inhibitor staurosporine was obtained from MilliporeSigma (S4400). All kinase inhibitors were dissolved in DMSO to make high concentration stocks, which were stored in small aliquots at –80°C until use. TNT-kinases or MEE were preincubated with DMSO or indicated concentrations of indicated kinase inhibitors for 3–5 min on ice before being used to phosphorylate substrate proteins.
Anti-Xenopus MAPK antibodies and anti-Plx1 antibodies used in immunodepletion were produced by immunization of rabbits with recombinant proteins and affinity-purification of specific antibodies from the immune sera against the recombinant proteins. Anti-Cdk1 antibodies used in immunodepletion were purchased from AS ONE INTERNATIONAL (69-003). Anti-RSK2 antibodies used in immunodepletion were purchased from Santa Cruz Biotechnology (sc-9986). Negative control mouse and rabbit IgGs were purchased from MilliporeSigma (I8765 and I8140). To prepare antibody affinity beads for individual kinase depletion, 1.5 µg of anti-Cdk1 antibodies, 4 µg of anti-Xenopus MAPK antibodies, 6.5 µg of anti-Plx1 antibodies, 2.5 µg of anti-RSK2, which were each determined in pilot experiments, or equal amounts of control IgGs were each immobilized onto 4 µl protein A/G sepharose by incubation at 22°C for 1 h, respectively. To prepare antibody affinity beads for multiple kinase immunodepletions, anti-Plx1 and anti-MAPK antibodies were first immobilized onto 12 µl protein A/G sepharose at 4°C overnight due to their much bigger volumes. After these antibody beads were washed two times with 500 µl PBS, the anti-Cdk1 and anti-RSK2 antibodies were further immobilized onto these antibody beads by incubation at 4°C overnight. For immunodepletion, freshly prepared antibody beads were washed three times with 500 µl EB and incubated with 15 µl MEE at 4°C for 2 h. After beads were spun down, SNs were recovered for use as the control or corresponding kinase(s)-depleted MEE.
GST and GST-p9 were expressed and purified as described above and affinity absorbed onto glutathione sepharose at protein concentrations of 2–3 µg/µl. For GST-p9 absorption of MEE, 15 µl MEE were mixed with 5 µl immobilized GST or GST-p9 on rotation at 4°C for 1.5 h. After beads were pelleted, SNs were absorbed again with fresh GST or GST-p9 beads at 4°C for 1.5 h. From both rounds of absorption, proteins in the SNs and proteins eluted from washed beads were immunoblotted with anti-Cdk1 and anti-Plx1 antibodies. The SNs were also used to phosphorylate myc-Cdc25C9-375.
Supplementary Material
Acknowledgments
This work was supported by a DOD/PCRP grant (W81XWH-09-1-0274) and an Institutional Research Grant awarded to J. Kuang, grants from NIH (CA174798, P50 CA140388 and P30 CA016672) and from CPRIT (RP150179 and RP150282) awarded to S-H Lin, and a grant from Natural Science Foundation of Hunan province (2020JJ5478) awarded to T. Tan. The Proteomics and Metabolomics Facility was supported in part by CPRIT (RP130397) and NIH (1S10OD012304-01 and P30CA016672). The DNA Analysis Facility of The University of Texas Anderson Cancer Center was supported by NIH/NCI (P30CA016672).
Abbreviations used:
- EB
the classical MPF extraction buffer
- GVBD
germinal vesicle breakdown
- IOE
interphase-arrested immature Xenopus oocyte extract
- IOE*
400,000 × g cytosolic extract from manually defolliculated stage VI Xenopus oocytes
- IP
immunoprepitates
- MC
microcystin
- MEE
M-phase arrested/stabilized Xenopus egg extract
- MPM-2
mitotic protein monoclonal 2
- OA
okadaic acid
- PBS
phosphate-buffered saline
- PSM
peptide sequence match
- SN
supernatant
- TBS
tris-buffered saline
- TNT
in vitro transcription coupled translation.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E22-04-0118) on August 17, 2022.
REFERENCES
- Abe S, Nagasaka K, Hirayama Y, Kozuka-Hata H, Oyama M, Aoyagi Y, Obuse C, Hirota T (2011). The initial phase of chromosome condensation requires Cdk1-mediated phosphorylation of the CAP-D3 subunit of condensin II. Genes Dev 25, 863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P (2015). The cell cycle. In: Molecular Biology of the Cell, New York: Garland Science. [Google Scholar]
- Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, et al. (2011). Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci Signal 4, ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt RR, Ferrell JE Jr (2000). Cloning and characterization of Xenopus Rsk2, the predominant p90 Rsk isozyme in oocytes and eggs. J Biol Chem 275, 32983–32990. [DOI] [PubMed] [Google Scholar]
- Blake-Hodek KA, Williams BC, Zhao Y, Castilho PV, Chen W, Mao Y, Yamamoto TM, Goldberg ML (2012). Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol Cell Biol 32, 1337–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher RN, Holman PS, Fattaey A (1997). Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J Biol Chem 272, 22300–22306. [DOI] [PubMed] [Google Scholar]
- Brunet A, Pagès G, Pouysségur J (1994). Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene 9, 3379–3387. [PubMed] [Google Scholar]
- Casado-Vela J, Ruiz EJ, Nebreda AR, Casal JI (2007). A combination of neutral loss and targeted product ion scanning with two enzymatic digestions facilitates the comprehensive mapping of phosphorylation sites. Proteomics 7, 2522–2529. [DOI] [PubMed] [Google Scholar]
- Champion L, Linder MI, Kutay U (2017). Cellular reorganization during mitotic entry. Trends Cell Biol 27, 26–41. [DOI] [PubMed] [Google Scholar]
- Chatel G, Fahrenkrog B (2011). Nucleoporins: leaving the nuclear pore complex for a successful mitosis. Cell Signal 23, 1555–1562. [DOI] [PubMed] [Google Scholar]
- Chen F, Jiao XF, Zhang JY, Wu D, Ding ZM, Wang YS, Miao YL, Huo LJ (2018). Nucleoporin35 is a novel microtubule associated protein functioning in oocyte meiotic spindle architecture. Exp Cell Res 371, 435–443. [DOI] [PubMed] [Google Scholar]
- Collavin L, Monte M, Verardo R, Pfleger C, Schneider C (2000). Cell-cycle regulation of the p53-inducible gene B99. FEBS Lett 481, 57–62. [DOI] [PubMed] [Google Scholar]
- Cyert MS, Scherson T, Kirschner MW (1988). Monoclonal antibodies specific for thiophosphorylated proteins recognize Xenopus MPF. Dev Biol 129, 209–216. [DOI] [PubMed] [Google Scholar]
- Dangi S, Shapiro P (2005). Cdc2-mediated inhibition of epidermal growth factor activation of the extracellular signal-regulated kinase pathway during mitosis. J Biol Chem 280, 24524–24531. [DOI] [PubMed] [Google Scholar]
- Daub H, Olsen JV, Bairlein M, Gnad F, Oppermann FS, Korner R, Greff Z, Keri G, Stemmann O, Mann M (2008). Kinase-selective enrichment enables quantitative phosphoproteomics of the kinome across the cell cycle. Mol Cell 31, 438–448. [DOI] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN (1983). Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA 80, 2926–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Gould KL, Gygi SP, Kellogg DR (2013). Mapping and analysis of phosphorylation sites: a quick guide for cell biologists. Mol Biol Cell 24, 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP (2008). A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA 105, 10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissmeyer N, Nowack MK, Pusch S, Stals H, Inzé D, Grini PE, Schnittger A (2007). T-loop phosphorylation of Arabidopsis CDKA;1 is required for its function and can be partially substituted by an aspartate residue. Plant Cell 19, 972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Newport JW (1988). Mitosis-inducing factors are present in a latent form during interpgase in the Xenopus embryo. J Cell Biol 106, 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endl E, Gerdes J (2000). Posttranslational modifications of the KI-67 protein coincide with two major checkpoints during mitosis. J Cell Physiol 182, 371–380. [DOI] [PubMed] [Google Scholar]
- Favreau C, Worman HJ, Wozniak RW, Frappier T, Courvalin JC (1996). Cell cycle-dependent phosphorylation of nucleoporins and nuclear pore membrane protein Gp210. Biochemistry 35, 8035–8044. [DOI] [PubMed] [Google Scholar]
- Fielding AB, Royle SJ (2013). Mitotic inhibition of clathrin-mediated endocytosis. Cell Mol Life Sci 70, 3423–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimitsu K, Grimaldi M, Yamano H (2016). Cyclin-dependent kinase 1-dependent activation of APC/C ubiquitin ligase. Science 352, 1121–1124. [DOI] [PubMed] [Google Scholar]
- Gabrielli BG, Clark JM, McCormack AK, Ellem KA (1997). Hyperphosphorylation of the N-terminal domain of Cdc25 regulates activity toward cyclin B1/Cdc2 but not cyclin A/Cdk2. J Biol Chem 272, 28607–28614. [DOI] [PubMed] [Google Scholar]
- Gautier J, Solomon MJ, Booher RN, Bazan JF, Kirschner MW (1991). cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell 67, 197–211. [DOI] [PubMed] [Google Scholar]
- Georgi AB, Stukenberg PT, Kirschner MW (2002). Timing of events in mitosis. Curr Biol 12, 105–114. [DOI] [PubMed] [Google Scholar]
- Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T (2010). The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330, 1673–1677. [DOI] [PubMed] [Google Scholar]
- Giunta S, Jackson SP (2011). Give me a break, but not in mitosis: the mitotic DNA damage response marks DNA double-strand breaks with early signaling events. Cell Cycle 10, 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J, Hermann J, Hendrix P, Ozon R, Merlevede W (1989). Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett 245, 91–94. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Nishida E, Shimanuki M, Toda T, Imai Y, Yamamoto M (1993). Schizosaccharomyces pombe Spk1 is a tyrosine-phosphorylated protein functionally related to Xenopus mitogen-activated protein kinase. Mol Cell Biol 13, 6427–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesfeld JM, Forbes DJ (1997). Mitotic repression of the transcriptional machinery. Trends Biochem Sci 22, 197–202. [DOI] [PubMed] [Google Scholar]
- Gowdy PM, Anderson HJ, Roberge M (1998). Entry into mitosis without Cdc2 kinase activation. J Cell Sci 111, 3401–3410. [DOI] [PubMed] [Google Scholar]
- Gropengiesser J, Varadarajan BT, Stephanowitz H, Krause E (2009). The relative influence of phosphorylation and methylation on responsiveness of peptides to MALDI and ESI mass spectrometry. J Mass Spectrom 44, 821–831. [DOI] [PubMed] [Google Scholar]
- Gross SD, Lewellyn AL, Maller JL (2001). A constitutively active form of the protein kinase p90Rsk1 is sufficient to trigger the G2/M transition in Xenopus oocytes. J Biol Chem 276, 46099–46103. [DOI] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL (2000). The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr Biol 10, 430–438. [DOI] [PubMed] [Google Scholar]
- Guo XW, Th’ng JP, Swank RA, Anderson HJ, Tudan C, Bradbury EM, Roberge M (1995). Chromosome condensation induced by fostriecin does not require p34cdc2 kinase activity and histone H1 hyperphosphorylation, but is associated with enhanced histone H2A and H3 phosphorylation. EMBO J 14, 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttinger S, Laurell E, Kutay U (2009). Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol 10, 178–191. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL (1995). Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol 168, 677–682. [DOI] [PubMed] [Google Scholar]
- Hara M, Abe Y, Tanaka T, Yamamoto T, Okumura E, Kishimoto T (2012). Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat Commun 3, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Kobayashi R, Hirano M (1997). Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell 89, 511–521. [DOI] [PubMed] [Google Scholar]
- Honda R, Korner R, Nigg EA (2003). Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell 14, 3325–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M (2012). PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res 40, D261–D270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya N, Hosoya H, Yamashiro S, Mohri H, Matsumura F (1993). Localization of caldesmon and its dephosphorylation during cell division. J Cell Biol 121, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D, Sagata N (2005). The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J 24, 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Maller JL (1993). Elimination of cdc2 phosphorylation sites in the cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell 4, 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Maller JL (1995). Phosphorylation and activation of the Xenopus Cdc25 phosphatase in the absence of Cdc2 and Cdk2 kinase activity. Mol Biol Cell 6, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MC, Zha J, Humphries MJ (2019). Connections between the cell cycle, cell adhesion and the cytoskeleton. Philos Trans R Soc Lond B Biol Sci 374, 20180227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami Y, Ono M, Yoshida K (2017). Plk1 phosphorylation of CAP-H2 triggers chromosome condensation by condensin II at the early phase of mitosis. Sci Rep 7, 5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanshin E, Bergeron-Sandoval LP, Isik SS, Thibault P, Michnick SW (2015). A cell-signaling network temporally resolves specific versus promiscuous phosphorylation. Cell Rep 10, 1202–1214. [DOI] [PubMed] [Google Scholar]
- Karaiskou A, Cayla X, Haccard O, Jessus C, Ozon R (1998). MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp Cell Res 244, 491–500. [DOI] [PubMed] [Google Scholar]
- Kettenbach AN, Schweppe DK, Faherty BK, Pechenick D, Pletnev AA, Gerber SA (2011). Quantitative phosphoproteomics identifies substrates and functional modules of Aurora and Polo-like kinase activities in mitotic cells. Sci Signal 4, rs5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Johnson JM, Lera RF, Brahma S, Burkard ME (2017). Anillin phosphorylation controls timely membrane association and successful cytokinesis. PLoS Genet 13, e1006511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Song EJ, Lee KJ, Ferrell JE Jr (2005). Multisite M-phase phosphorylation of Xenopus Wee1A. Mol Cell Biol 25, 10580–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Peters JM, Tugendreich S, Rolfe M, Hieter P, Kirschner MW (1995). A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279–288. [DOI] [PubMed] [Google Scholar]
- Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T (2006). Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics 5, 749–757. [DOI] [PubMed] [Google Scholar]
- Kõivomägi M, Ord M, Iofik A, Valk E, Venta R, Faustova I, Kivi R, Balog ER, Rubin SM, Loog M (2013). Multisite phosphorylation networks as signal processors for Cdk1. Nat Struct Mol Biol 20, 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Gotoh Y, Nishida E (1994). Requirement for the MAP kinase kinase/MAP kinase cascade in Xenopus oocyte maturation. EMBO J 13, 2131–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosako H, Nishida E, Gotoh Y (1993). cDNA cloning of MAP kinase kinase reveals kinase cascade pathways in yeasts to vertebrates. EMBO J 12, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft C, Herzog F, Gieffers C, Mechtler K, Hagting A, Pines J, Peters JM (2003). Mitotic regulation of the human anaphase-promoting complex by phosphorylation. EMBO J 22, 6598–6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Ashorn CL (1993). At least two kinases phosphorylate the MPM-2 epitope during Xenopus oocyte maturation. J Cell Biol 123, 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Ashorn CL, Gonzalez-Kuyvenhoven M, Penkala JE (1994). cdc25 is one of the MPM-2 antigens involved in the activation of maturation-promoting factor. Mol Biol Cell 5, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Penkala JE, Ashorn CL, Wright DA, Saunders GF, Rao PN (1991a). Multiple forms of maturation-promoting factor in unfertilized Xenopus eggs. Proc Natl Acad Sci USA 88, 11530–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Penkala JE, Wright DA, Saunders GF, Rao PN (1991b). A novel M phase-specific H1 kinase recognized by the mitosis-specific monoclonal antibody MPM-2. Dev Biol 144, 54–64. [DOI] [PubMed] [Google Scholar]
- Kuang J, Zhao J, Wright DA, Saunders GF, Rao PN (1989). Mitosis-specific monoclonal antibody MPM-2 inhibits Xenopus oocyte maturation and depletes maturation-promoting activity. Proc Natl Acad Sci USA 86, 4982–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG (1991). The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell free system. Cell 64, 903–914. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG (1992). Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell 70, 139–151. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG (1996). Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science 273, 1377–1380. [DOI] [PubMed] [Google Scholar]
- Laurell E, Beck K, Krupina K, Theerthagiri G, Bodenmiller B, Horvath P, Aebersold R, Antonin W, Kutay U (2011). Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell. 144, 539–550. [DOI] [PubMed] [Google Scholar]
- Lee CR, Park YH, Kim YR, Peterkofsky A, Seok YJ (2013). Phosphorylation-dependent mobility shift of proteins on SDS-PAGE is due to decreased binding of SDS. Bull Korean Chem Soc 34, 2063–2066. [Google Scholar]
- Lee CR, Park YH, Min H, Kim YR, Seok YJ (2019). Determination of protein phosphorylation by polyacrylamide gel electrophoresis. J Microbiol 57, 93–100. [DOI] [PubMed] [Google Scholar]
- Lee TH, Solomon MJ, Mumby MC, Kirschner MW (1991). INH, a negative regulator of MPF, is a form of protein phosphatase 2A. Cell 64, 415–423. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Maller JL (1985). Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol 101, 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaulay C, Meier E, Forbes DJ (1995). Differential mitotic phosphorylation of proteins of the nuclear pore complex. J Biol Chem 270, 254–262. [DOI] [PubMed] [Google Scholar]
- Maller JL, Schwab MS, Roberts BT, Gross SD, Taieb FE, Tunquist BJ (2001). The pathway of MAP kinase mediation of CSF arrest in Xenopus oocytes. Biol Cell 93, 27–33. [DOI] [PubMed] [Google Scholar]
- Masson D, Kreis TE (1995). Binding of E-MAP-115 to microtubules is regulated by cell cycle-dependent phosphorylation. J Cell Biol 131, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath DA, Balog ER, Koivomagi M, Lucena R, Mai MV, Hirschi A, Kellogg DR, Loog M, Rubin SM (2013). Cks confers specificity to phosphorylation-dependent CDK signaling pathways. Nat Struct Mol Biol 20, 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux J (1997). Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. [DOI] [PubMed] [Google Scholar]
- Mochida S, Ikeo S, Gannon J, Hunt T (2009). Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J 28, 2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Maslen SL, Skehel M, Hunt T (2010). Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330, 1670–1673. [DOI] [PubMed] [Google Scholar]
- Monzo P, Gauthier NC, Keslair F, Loubat A, Field CM, Le Marchand-Brustel Y, Cormont M (2005). Clues to CD2-associated protein involvement in cytokinesis. Mol Biol Cell 16, 2891–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO (2007). In: The Cell Cycle: Principles of Control, London: New Science Press. [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG (1995a). Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell 6, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG (1995b). Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270, 86–90. [DOI] [PubMed] [Google Scholar]
- Murray AW, Kirschner MW (1989). Cyclin synthesis drives the early embryonic cell cycles. Nature 339, 275–280. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirshner MW (1989). The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339, 280–286. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Hunt T (1993). The c-mos proto-oncogene protein kinase turns on and maintains the activity of MAP kinase, but not MPF, in cell-free extracts of Xenopus oocytes and eggs. EMBO J 12, 1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg EA (1991). The substrates of the cdc2 kinase. Semin Cell Biol 2, 261–270. [PubMed] [Google Scholar]
- Oates ME, Romero P, Ishida T, Ghalwash M, Mizianty MJ, Xue B, Dosztanyi Z, Uversky VN, Obradovic Z, Kurgan L, et al. (2013). D(2)P(2): database of disordered protein predictions. Nucleic Acids Res 41, D508–D516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al. (2010). Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3, ra3. [DOI] [PubMed] [Google Scholar]
- Ookata K, Hisanaga S, Sugita M, Okuyama A, Murofushi H, Kitazawa H, Chari S, Bulinski JC, Kishimoto T (1997). MAP4 is the in vivo substrate for CDC2 kinase in HeLa cells: identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. Biochemistry 36, 15873–15883. [DOI] [PubMed] [Google Scholar]
- Palmer A, Gavin AC, Nebreda AR (1998). A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J 17, 5037–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JT, Bottrill A, Prosser SL, Jayaraman S, Straatman K, Fry AM, Shackleton S (2014). Mitotic phosphorylation of SUN1 loosens its connection with the nuclear lamina while the LINC complex remains intact. Nucleus 5, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D, Dunphy WG (1996). Xe-p9, a Xenopus Suc1/Cks homolog, has multiple essential roles in cell cycle control. Genes Dev 10, 1503–1515. [DOI] [PubMed] [Google Scholar]
- Patra D, Dunphy WG (1998). Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase- promoting complex at mitosis. Genes Dev 12, 2549–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D, Wang SX, Kumagai A, Dunphy WG (1999). The xenopus Suc1/Cks protein promotes the phosphorylation of G(2)/M regulators. J Biol Chem 274, 36839–36842. [DOI] [PubMed] [Google Scholar]
- Perdiguero E, Pillaire MJ, Bodart JF, Hennersdorf F, Frodin M, Duesbery NS, Alonso G, Nebreda AR (2003). Xp38gamma/SAPK3 promotes meiotic G(2)/M transition in Xenopus oocytes and activates Cdc25C. EMBO J 22, 5746–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, King RW, Hoog C, Kirschner MW (1996). Identification of BIME as a subunit of the anaphase-promoting complex. Science 274, 1199–1201. [DOI] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Li C, Maller JL (1998). Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol 18, 4262–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL (1999). Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol 19, 8625–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR (1994). A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78, 1027–1037. [DOI] [PubMed] [Google Scholar]
- Ruiz EJ, Hunt T, Nebreda AR (2008). Meiotic inactivation of Xenopus Myt1 by CDK/XRINGO, but not CDK/cyclin, via site-specific phosphorylation. Mol Cell 32, 210–220. [DOI] [PubMed] [Google Scholar]
- Schorderet-Slatkine S (1972). Action of progesterone and related steroids on oocyte maturation in Xenopus laevis. An in vitro study. Cell Differ 1, 179–189. [DOI] [PubMed] [Google Scholar]
- Shen M, Stukenberg PT, Kirschner MW, Lu KP (1998). The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev 12, 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiina N, Tsukita S (1999). Mutations at phosphorylation sites of Xenopus microtubule-associated protein 4 affect its microtubule-binding ability and chromosome movement during mitosis. Mol Biol Cell 10, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks CA, Fey EG, Vidair CA, Doxsey SJ (1995). Phosphorylation of NUMA occurs during nuclear breakdown and not mitotic spindle assembly. J Cell Sci 108, 3389–3396. [DOI] [PubMed] [Google Scholar]
- Stukenberg PT, Kirschner MW (2001). Pin1 acts catalytically to promote a conformational change in Cdc25. Mol Cell 7, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Stukenberg PT, Lustig KD, McGarry TJ, King RW, Kuang J, Kirschner MW (1997). Systematic identification of mitotic phosphoproteins. Curr Biol 7, 338–348. [DOI] [PubMed] [Google Scholar]
- Tang Z, Coleman TR, Dunphy WG (1993). Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J 12, 3427–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton AR, Wren JD, Daum JR, Siefert JC, Gorbsky GJ (2017). GTSE1 regulates spindle microtubule dynamics to control Aurora B kinase and Kif4A chromokinesin on chromosome arms. J Cell Biol 216, 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunnell NB, Poon AC, Kim SY, Ferrell JE Jr (2011). Ultrasensitivity in the regulation of Cdc25C by Cdk1. Mol Cell 41, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zon W, Ogink J, ter Riet B, Medema RH, te Riele H, Wolthuis RM (2010). The APC/C recruits cyclin B1-Cdk1-Cks in prometaphase before D box recognition to control mitotic exit. J Cell Biol 190, 587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S, Gharbi-Ayachi A, Raymond AA, Burgess A, Labbe JC, Labesse G, Monsarrat B, Lorca T, Castro A (2011). Characterization of the mechanisms controlling Greatwall activity. Mol Cell Biol 31, 2262–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S, Sundermann L, Labbe JC, Pintard L, Radulescu O, Castro A, Lorca T (2018). Cyclin A-cdk1-dependent phosphorylation of Bora is the triggering factor promoting mitotic entry. Dev Cell 45, 637–650.e637. [DOI] [PubMed] [Google Scholar]
- Voets E, Wolthuis RM (2010). MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 9, 3591–3601. [DOI] [PubMed] [Google Scholar]
- Wang R, He G, Nelman-Gonzalez M, Ashorn CL, Gallick GE, Stukenberg PT, Kirschner MW, Kuang J (2007). Regulation of Cdc25C by ERK-MAP kinases during the G2/M transition. Cell 128, 1119–1132. [DOI] [PubMed] [Google Scholar]
- Wang R, Jung SY, Wu CF, Qin J, Kobayashi R, Gallick GE, Kuang J (2010). Direct roles of the signaling kinase RSK2 in Cdc25C activation during Xenopus oocyte maturation. Proc Natl Acad Sci USA 107, 19885–19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T (1995). Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J 14, 1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Wang R, Liang Q, Liang J, Li W, Jung SY, Qin J, Lin SH, Kuang J (2010). Dissecting the M phase-specific phosphorylation of serine-proline or threonine-proline motifs. Mol Biol Cell 21, 1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Gerhart JC (1980). Partial purification and characterization of the maturation-promoting factor from eggs of Xenopus laevis. Dev Biol 79, 465–477. [DOI] [PubMed] [Google Scholar]
- Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, Xu J, Kuang J, Kirschner MW, Fischer G, et al. (1997). Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 278, 1957–1960. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Chern H, Yamakita Y, Matsumura F (2001). Mutant Caldesmon lacking cdc2 phosphorylation sites delays M-phase entry and inhibits cytokinesis. Mol Biol Cell 12, 239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhao Y, Li Z, Galas S, Goldberg ML (2006). Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 22, 83–91. [DOI] [PubMed] [Google Scholar]
- Yue J, Ferrell JE Jr (2004). Mos mediates the mitotic activation of p42 MAPK in Xenopus egg extracts. Curr Biol 14, 1581–1586. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Haccard O, Wang R, Yu J, Kuang J, Jessus C, Goldberg ML (2008). Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol Biol Cell 19, 1317–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.