ABSTRACT
46,XX male sex reversal syndrome is a rare genetic cause of male infertility. We report on two new cases of this syndrome in men presenting with hypogonadism and infertility. Cytogenetic and molecular analysis was performed in both patients. An extensive review of the literature for 46,XX male sex reversal syndrome cases related to infertility was also performed to fully characterise this syndrome. Genetic analyses showed translocation of the SRY on Xp chromosome and complete absence of all Azoospermia factor (AZF) genetic regions. All patients included in the review presented hypergonadotropic hypogonadism. Small testes were the most common clinical characteristic present in 90.2% of the patients, followed by small penis (31.8%), gynecomastia (26.8%) and poor hair distribution (15.4%). The presence of the SRY was identified in 130/154 (84.4%) patients: in 98.5% of cases, it was translocated on the Xp chromosome and in 1.5% on an autosome. All patients were azoospermic, due to the lack of AZF genetic regions. Males with normal phenotype and primary hypogonadism should be properly evaluated by the physicians and must be referred for cytogenetic and molecular analysis to exclude or confirm 46,XX male sex reversal syndrome. More cases of this syndrome with SRY translocated on an autosome are needed to identify if these patients have different characteristics than those with SRY translocated on Xp chromosome. Whole genome analysis of these patients is required to elucidate the genetic differences which are responsible for the phenotypic variability of the syndrome.
KEYWORDS: 46, XX male, infertility, sex reversal, SRY gene
INTRODUCTION
46,XX male sex reversal syndrome or de la Chapelle syndrome, first described by A. de la Chapelle et al. in 1964, is a rare genetic syndrome occurring in about 1/20.000–25.000 newborn males.[1,2] In 2006, 46,XX male sex reversal syndrome was renamed as 46,XX testicular disorder of sex development (DSD) by the ’Chicago Consensus’.[3]
There are three clinical phenotypes associated with the 46,XX syndrome: Males with normal phenotype, males with genital ambiguities and males who are hermaphrodites.[4] The majority of men have normal external genitalia, but 10%–15% of XX males show various degrees of hypospadias.[5] A normal male phenotype mainly depends on the detection of the SRY (Sex determining Region Y) gene, since it is a well-known fact that SRY directs the male sex-determination pathway.[6] SRY is normally located on the short arm of the Y chromosome (Yp11.3) and encodes a testes determining factor which induces the differentiation of the bipotential primitive gonad into testis.[7,8]
It has been reported that in about 80%–90% of the cases, the SRY is present, while the remaining 10%–20% are SRY-negative.[7,8] SRY is the main gene regulating the testes determination cascade and depending on the SRY detection, 46,XX males can be divided into two distinct groups: SRY-positive group which includes those who carry the SRY gene and SRY-negative group, where the SRY is absent.[7,8] In 46,XX SRY-positive patients, SRY is usually translocated on the short arm of chromosome X, due to an unequal Yp to Xp chromosomal interchange occurring during paternal meiosis.[7,8] In rare cases, SRY is translocated on an autosome.[9,10,11]
46,XX SRY-positive males, usually have a normal male phenotype at birth and the diagnosis is placed after puberty, usually due to infertility problems.[7,12] The main clinical features of the syndrome are hypergonadotropic (primary) hypogonadism, testis hypoplasia, gynecomastia, short stature, pelvic cyst and infertility due to azoospermia.[7] However, SRY-positive patients may present with genital ambiguities or hermaphroditism.[13] The reason for this discrepancy is currently unknown.
In the current study, we present two new cases of 46,XX sex reversal syndrome and provide a comprehensive update of 46,XX male sex reversal syndrome cases related to infertility in order to fully characterize the clinical and genetic features of this syndrome.
CASE REPORT
Case 1
A 39-year-old male was referred for karyotypic analysis due to primary hypogonadism and infertility. His height was 171 cm and his weight 70 kg. He had a normal male phenotype with normal development of secondary male characteristics, reduced libido, mild gynecomastia (grade 1) and small testes inside the scrotum. Testicular ultrasound and pelvic magnetic resonance imaging revealed bilateral hypotrophic testes (2 cm in diameter) with several small calcifications. Semen analysis unveiled azoospermia. The patient had no history of surgical procedure, mumps or exposure to chemical or toxic agents that could explain hypogonadism and his family medical history was negative for infertility. He was on testosterone treatment, not on a regular basis, since adulthood, because of primary hypogonadism: increased follicle-stimulating hormone (FSH) levels (30.2 mIU/ml, normal range: 1–13 mIU/ml), increased luteinizing hormone (LH) levels (31.4 mIU/ml, normal range: 1–9 mIU/ml), and reduced total testosterone (TT) levels (2.34 ng/ml, normal range 3–12 ngr/ml), decreased libido and mild gynecomastia.
At the time of examination, his endocrinological testing showed primary hypogonadism: increased FSH levels (47 mIU/ml, normal range: 1–13 mIU/ml), increased LH levels (28.5 mIU/ml, normal range: 1–9 mIU/ml) and normal TT levels (6 ng/ml, normal range 3–12 ng/ml), since he was on testosterone treatment. Estradiol (E2) and Prolactin (PRL) levels were 18.2 pg/ml (normal range 10–40 pg/ml) and 22.84 ng/ml (normal range 4–23 ng/ml) respectively. Thyroid function tests, complete blood count and blood biochemistry were normal.
Case 2
A 39-year-old male was referred for karyotypic analysis due to primary hypogonadism and infertility. His height was 169 cm and his weight 69 kg. He had a normal male phenotype and the only phenotypic finding was small testes inside the scrotum (testicular volume 6 ml). His hormone values displayed primary hypogonadism: Increased FSH levels (43 mIU/ml, normal range: 1–13 mIU/ml), increased LH levels (18.2 mIU/ml, normal range: 1–9 mIU/ml) and reduced TT levels (1.57 ng/ml, normal range 3–12 ng/ml). PRL levels were 10.4 ng/ml (normal range 4–23 ng/ml), while Estradiol (E2) levels were not reported. Thyroid function tests, complete blood count and blood biochemistry were normal.
METHODS
Cytogenetic and molecular analyses
Conventional cytogenetic analysis was performed on phytohaemagglutinin-stimulated peripheral blood lymphocytes by GTG banding. Twenty metaphases were fully analyzed and the karyotypes were described according to the International System for Human Cytogenetic Nomenclature 2020.[14]
Following DNA extraction, quantitative fluorescent polymerase chain reaction (QF-PCR, Devyser compact v3 Kit, CE-IVD) analysis was performed to evaluate the sex chromosome constitution and test for the presence or absence of SRY.
Fluorescent in situ hybridisation (FISH) analysis was performed on metaphase preparations, using the Vysis CEP X (DXZ1) Spectrum Green Probe (Abbott, Abbott Park, IL) and the locus specific LSI SRY Spectrum Orange probe (Abbott, Abbott Park, IL), according to the manufacturers’ instructions.
A sequence tagged site based multiplex PCR analysis was used for the detection of the SRY and Azoospermia factor (AZF) region microdeletions. The specific protocol has been proposed by the European Academy of Andrology and the European Molecular Genetics Quality Network.[15]
The Helsinki Declaration (1975) complied with the survey. Both patients were informed and gave their written consent for anonymous and voluntary participation.
Literature review
Search strategy for the literature review was designed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. PubMed, Google Scholar and Research Gate databases were searched for studies regarding ’46,XX male DSD’ in March 2022. The keywords “46,XX male infertility”, “46,XX male sex reversal”, 46,XX male DSD” and “46,XX male SRY” were used and inclusion criteria, such as English language, human studies, adult males (19+ years old), were defined in order to select the most relevant publications. Moreover, references of similar review articles were used so as to search for additional studies.
Search results were screened based on study titles and abstracts. Articles concerning familial cases, abnormal male genitalia or phenotype, as well as other disorders, were excluded. The selected papers were assessed based on the full-text in order to choose all the relevant publications to be included in the analysis.
RESULTS
Cytogenetic and molecular analyses
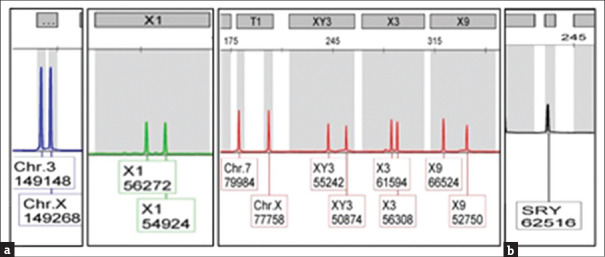
Karyotypic analysis showed that both patients had a 46,XX karyotype in all 20 metaphases analysed [Figure 1]. QF-PCR confirmed the presence of 2 X chromosomes as well as of the SRY [Figure 2]. FISH confirmed the presence of SRY translocated on the short (p) arm of one X chromosome [Figure 3]. In addition, multiplex PCR analysis verified the presence of SRY and revealed the complete absence of all AZF genetic regions.
Figure 1.
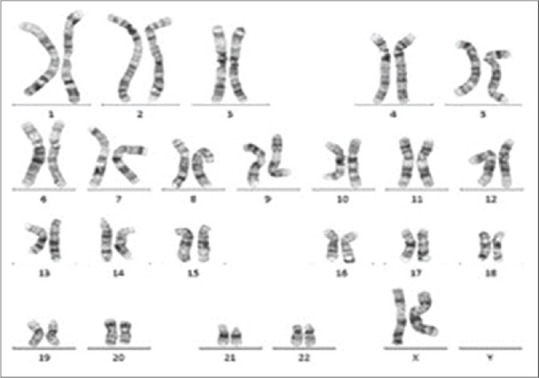
A 46,XX karyotype
Figure 2.
QF-PCR (a) confirmed the presence of 2 X chromosomes and (b) revealed the presence of SRY gene. QR-PCR = Quantitative fluorescent polymerase chain reaction
Figure 3.
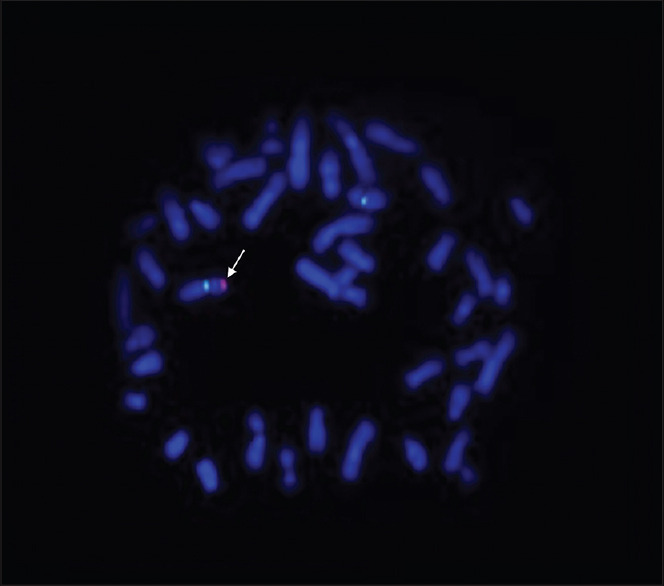
FISH analysis showed 2 green signals (X centromere) and 1 red signal (SRY gene - arrow) onto an X chromosome. FISH = Fluorescent in situ hybridization
Literature research results
The database search according to the set inclusion criteria identified 320 papers. After the removal of duplicates (n = 90), 230 papers were screened based on study titles and abstracts and 170 papers were excluded because of irrelevant topic and wrong study population (women, children and abnormal genitalia). From the 60 potentially eligible papers, another 4 were excluded because the 46,XX karyotype was already known. Literature search led finally to the identification of 56 papers [Figure 4] which described 178 patients with 46,XX male sex reversal syndrome and infertility.
Figure 4.
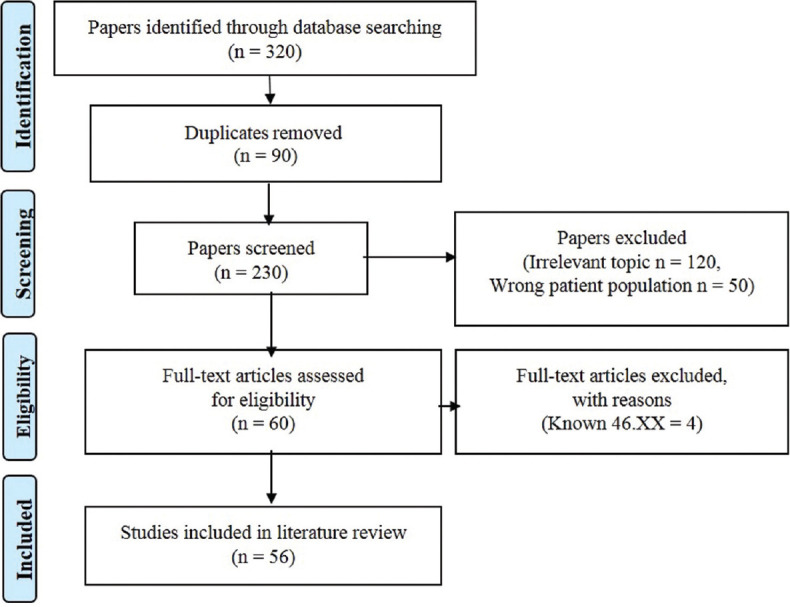
PRISMA flowchart for the included studies in the literature review. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Table 1 summarizes the clinical data, hormone profile and SRY presence/location of all 46,XX males described in the literature, including our patient (n = 180).
Table 1.
Clinical data, hormone profile and sex determining region Y of 180 patients
| References | Age (years) | Weight (kg) | Height (cm) | FSH (mIU/mL) | LH (mIU/mL) | TT (ng/mL) | E2 (pg/mL) | PRL (ng/mL) | HD | GM | PS | TV | SRY presence/location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valetto et al., 2005[16] | 35 | 48 | 152 | 23.9 | 17.7 | 3.06 | NK | 7.13 | N | − | N | NK | - |
| Rigola et al., 2002[17] | 33 | NK | NK | NK | NK | NK | NK | NK | N | − | N | NK | +/Xp |
| Dauwerse et al., 2006[9] | 61 | NK | 171 | 13 | 10 | 3.25 | 31.3 | NK | NK | − | N | S | +/16q |
| Ryan and Akbar, 2013[18] | 40 | NK | NK | 1 | NK | NK | 10 | NK | P | − | S | NK | - |
| Wegner and Nürnberger, 1983[19] | 35 | 81 | 167 | 23.7 | 37.1 | 6.30 | NK | 3.8 | N | − | N | NK | +/Xp |
| Pais and Vasudevan, 1977[20] | 29 | 82 | 170 | 53 | 45 | 2.67 | NK | NK | N | + | S | NK | +/Xp |
| Zakharia and Krauss, 1990[21] | 28 | 65 | 165 | 72 | 61 | 2.40 | NK | 16.3 | NK | + | N | NK | NK |
| Pepene et al., 2008[22] | 28 | 65 | 167 | 43.9 | 25.3 | 3.33 | NK | NK | N | + | N | S | +/Xp |
| Hado et al., 2003[23] | 76 | NK | 157 | 27.8 | 21 | 2.59 | NK | NK | N | + | NK | NK | +/Xp |
| Xiao et al., 2013[24] | 27 | NK | 170 | 47 | 18.7 | 1.80 | NK | 14.6 | NK | NK | S | NK | - |
| Butler et al., 1983[25] | 31 | 72 | 169 | 51 | NK | 4.77 | NK | NK | N | + | N | NK | +/Xp |
| Yencilek and Baykal, 2005[26] | 26 | 72 | 165 | 45.6 | 48.9 | 2.70 | NK | 9.4 | N | − | S | NK | NK |
| Mustafa and Mehmet, 2010[27] | 30 | 75 | 170 | NK | 40.7 | 2.11 | 16.6 | 8.5 | N | + | N | NK | - |
| Matthews et al., 1983[28] | 27 | 68 | 166 | 46 | 19 | 2.82 | 33 | 9.87 | P | − | NK | NK | +/Xp |
| Tomomasa et al., 1999[29] | 25 | 55 | 177 | 19.7 | 10.3 | 4.28 | NK | NK | N | − | NK | S | +/Xp |
| Chernykh et al., 2009[30] | 37 | 74 | 160 | 26.9 | 13.5 | 2.90 | NK | NK | P | + | NK | NK | +/Xp |
| Castiñeyra et al., 2002[31] | 28 | NK | 180 | 50 | 16 | 3.00 | 28 | 14 | N | + | NK | NK | +/Xp |
| Castiñeyra et al., 2002[31] | 35 | NK | 170 | 3.5 | 6.2 | 7.00 | 38 | 3.4 | N | NK | NK | S | +/Xp |
| Castiñeyra et al., 2002[31] | 28 | NK | 160 | 21 | 5.2 | 1.40 | 19 | 8.1 | P | NK | NK | NK | +/Xp |
| Castiñeyra et al., 2002[31] | 39 | NK | 174 | 6.7 | 4.2 | 5.60 | 30 | 6.2 | P | NK | NK | NK | +/Xp |
| Castiñeyra et al., 2002[31] | 24 | NK | 172 | 45 | 40 | 3.00 | 20 | 5.4 | N | NK | NK | NK | +/Xp |
| Chiang et al., 2013[32] | 33 | NK | NK | 46.5 | 17.6 | 2.03 | NK | 27.05 | NK | NK | NK | NK | +/Xp |
| Chiang et al., 2013[32] | 34 | NK | NK | 54.3 | 19.6 | 2.17 | NK | 8.15 | NK | NK | NK | NK | +/Xp |
| Chiang et al., 2013[32] | 52 | NK | NK | 64.3 | 20.2 | 1.44 | NK | 16.08 | NK | NK | S | NK | - |
| Rajender et al., 2006[33] | 34 | 64 | 156 | 25.8 | 15.8 | 5.8 | NK | NK | N | − | N | NK | - |
| Tan and Khalid, 1993[34] | 32 | NK | 176 | 21 | 34 | 2.63 | 25 | NK | N | + | S | NK | NK |
| Mićić et al., 1983[35] | 25 | 63 | 171 | 31 | 18 | 3.19 | 47 | 6.8 | P | − | NK | NK | NK |
| Wu et al., 2014[36] | NK | NK | 165 | 35.5 | 13.8 | 1.95 | 30.5 | 4.6 | NK | NK | NK | NK | +/Xp |
| Wu et al., 2014[36] | NK | NK | 162 | 29.2 | 12.9 | 1.55 | 19.1 | 3.6 | NK | NK | NK | NK | +/Xp |
| Wu et al., 2014[36] | NK | NK | 164 | 45.9 | 25.1 | 2.56 | 26.7 | 7.8 | NK | NK | NK | NK | +/Xp |
| Wu et al., 2014[36] | NK | NK | 167 | 33.7 | 22.3 | 2.41 | 29.1 | 10.9 | NK | NK | NK | NK | +/Xp |
| Wu et al., 2014[36] | NK | NK | 165 | 31.4 | 19.6 | 2.01 | 22.1 | 7.8 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 163 | 93.6 | 19.4 | 3.08 | 33 | 17.9 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 163 | 24.7 | 14.4 | 2.77 | 42 | 18.5 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 162 | NK | NK | 1.29 | NK | NK | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 161 | 81.6 | 27.7 | 1.37 | 19.8 | 22.9 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 158 | 13.1 | 3.61 | 2.44 | 34 | 9.67 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 162 | 54.7 | 19.4 | 1.72 | 27 | 10.08 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 162 | 37.1 | 16.5 | 3.19 | 28 | 9.88 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 161 | 43 | 33.9 | 2.16 | 22 | 7.28 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 160 | 72 | 34.6 | 3.36 | 19.8 | 10 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 160 | 49 | 26.8 | 1.80 | 19.8 | 15.8 | NK | NK | NK | NK | +/Xp |
| Gao et al., 2013[37] | NK | NK | 161 | 87.7 | 31.4 | 5.21 | 30.5 | 49.6 | NK | NK | NK | NK | +/Xp |
| Fuse et al., 1991[38] | 30 | 90 | 172 | 47 | 60 | 1.60 | NK | NK | N | − | NK | NK | +/Xp |
| Majzoub et al., 2017[39] | 40 | 84 | 175 | 38 | 12 | 3.35 | 29 | 13.6 | N | − | N | NK | +/Xp |
| Majzoub et al., 2017[39] | 31 | NK | NK | 14 | 6 | 1.29 | 25 | 3.2 | N | − | N | NK | +/Xp |
| Majzoub et al., 2017[39] | 35 | NK | NK | 10 | 23 | 0.74 | 5.7 | NK | P | + | N | NK | +/Xp |
| Majzoub et al., 2017[39] | 29 | 77 | 181 | 28 | 15 | 0.74 | NK | NK | N | − | N | S | - |
| Majzoub et al., 2017[39] | 39 | 74 | 160 | 13.4 | 12 | 2.46 | 19 | NK | N | − | N | NK | +/Xp |
| Majzoub et al., 2017[39] | 32 | 86 | 170 | 29.7 | 16.9 | 0.95 | NK | 12 | N | + | N | NK | +/Xp |
| Queralt et al., 2008[10] | 31 | 58 | 170 | 62.2 | 25.8 | 3.23 | 17 | NK | N | − | NK | NK | +/1q |
| Ahsan et al., 1998[40] | 24 | NK | 165 | 35 | 21 | 1.8 | NK | NK | P | + | N | S | NK |
| Yamamoto et al., 1995[41] | 32 | 45 | 161 | 48.9 | 20.4 | 4.15 | NK | 12.3 | N | − | N | S | +/NK |
| Jellad et al., 2016[42] | 34 | 70 | 168 | 15.3 | 6.7 | 1.98 | NK | 5.3 | N | + | S | N | - |
| Rizvi, 2008[43] | 33 | 93 | 178 | 46.1 | 23.0 | 2.07 | NK | NK | NK | NK | N | NK | +/Xp |
| Bouayed Abdelmoula et al., 2003[13] | 32 | 64 | 172 | 18.0 | 10.1 | 5.00 | NK | NK | N | − | S | S | +/Xp |
| Casas-Vargas et al., 2019[44] | 40 | 59.7 | 156 | NK | NK | 6.10 | 32.46 | NK | N | − | NK | S | - |
| Vetro et al., 2011[45] | 47 | NK | NK | 39.5 | 14.4 | 1.00 | NK | NK | NK | + | NK | S | - |
| Vetro et al., 2011[45] | 46 | NK | NK | 39.5 | 14.4 | 1.00 | NK | NK | NK | NK | NK | S | - |
| Vetro et al., 2015[46] | 30 | NK | NK | NK | NK | NK | NK | NK | NK | + | NK | NK | - |
| Vetro et al., 2015[46] | 41 | NK | 171 | NK | NK | NK | NK | NK | NK | − | N | S | - |
| Pastor Guzmán et al., 2011[47] | 20 | 76 | 169 | 27.9 | 16.5 | 2.3 | 24.8 | 24.5 | N | − | N | S | +/Xp |
| Gunes et al., 2013[48] | 30 | 70 | 155 | 37.88 | 18.96 | 0.51 | 17.57 | 17.54 | P | + | N | S | +/Xp |
| Gunes et al., 2013[48] | 16 | 65 | 152 | 41.05 | 14.55 | 2.16 | 32 | 24.11 | N | − | N | S | +/Xp |
| Bogdanet et al., 2020[49] | 33 | 92.4 | 180 | 43.1 | 4.4 | 1.93 | NK | 5.3 | NK | − | NK | S | +/Xp |
| Onrat et al., 2012[50] | 23 | NK | NK | 9.95 | 17.3 | 0.20 | NK | NK | N | − | N | S | +/NK |
| Jain et al., 2013[51] | 38 | 63 | 162 | 76.6 | 36.3 | 1.20 | NK | NK | N | + | N | NK | +/Xp |
| Yue et al., 2019[52] | 23 | 49 | 165 | NK | NK | NK | NK | NK | P | NK | NK | S | +/Xp |
| Yue et al., 2019[52] | 36 | 57 | 171 | 45 | 29.6 | 1.12 | 19.27 | 9.21 | N | NK | NK | S | +/Xp |
| Yue et al., 2019[52] | 28 | 66 | 160 | 18.8 | 14.1 | 1.36 | 33.36 | 12.64 | N | NK | NK | S | +/Xp |
| Yue et al., 2019[52] | 32 | 50 | 169 | 45.4 | 28.5 | 1.56 | 31.8 | 10.29 | P | NK | NK | S | +/Xp |
| Yue et al., 2019[52] | 26 | 80 | 175 | 21.2 | 14.3 | 1.87 | 22.63 | 6.91 | N | NK | NK | S | +/Xp |
| Yue et al., 2019[52] | 30 | 60 | 170 | 62.83 | 27.12 | 2.65 | 17.9 | 14.88 | N | NK | NK | S | +/Xp |
| Yue et al., 2019[52] | 23 | 56 | 165 | 40.05 | 22.11 | 0.64 | 13.6 | 14.88 | N | NK | NK | S | +/Xp |
| Yue et al., 2019[52] | 27 | 51 | 168 | 6.6 | 9.6 | 1.04 | 33.14 | 22.8 | N | NK | NK | S | - |
| Akinsal et al., 2017[53] | 26 | 63 | 170 | 58.1 | 42.2 | 1.83 | 52 | 5.6 | P | − | NK | S | +/Xp |
| Akinsal et al., 2017[53] | 31 | 72 | 167 | 36.0 | 16.8 | 3.76 | 57.9 | 3.5 | NK | − | NK | S | +/Xp |
| Akinsal et al., 2017[53] | 30 | 72 | 170 | 17.9 | 10.3 | 2.73 | 24.6 | 7.9 | NK | − | NK | S | +/Xp |
| Akinsal et al., 2017[53] | 39 | 74 | 161 | 37.5 | 16.3 | 0.97 | 24.5 | 6.9 | P | + | NK | S | +/Xp |
| Akinsal et al., 2017[53] | 40 | 81 | 168 | 36.8 | 9.8 | 2.9 | 53.8 | 5.6 | NK | − | NK | S | +/Xp |
| Akinsal et al., 2017[53] | 30 | 66 | 162 | 57.3 | 16.9 | 1.54 | 49.2 | 6.4 | NK | − | NK | S | +/Xp |
| Akinsal et al., 2017[53] | 28 | 68 | 165 | 50.5 | 11.3 | 1.03 | 39.1 | 9.0 | NK | − | NK | S | +/Xp |
| Akinsal et al., 2017[53] | 24 | 66 | 163 | 43.1 | 17.9 | 2.42 | 21.2 | 8.6 | P | − | NK | S | +/Xp |
| Ashfaq et al., 2021[54] | 30 | NK | NK | 26 | 16 | 2.02 | NK | NK | NK | NK | NK | S | - |
| Sreejith et al., 2019[55] | 29 | 75 | 170 | 35 | 19.9 | 3.2 | NK | 6.7 | N | − | N | S | +/Xp |
| Yiğman et al.,2021[56] | n=10 | NK | NK | n=10 | n=10 | n=10 | NK | n=10 | NK | NK | n=10 | n=10 | n=10 |
| Mean age | Median | Mean | Median | Mean | Mean | Mean | Mean | ||||||
| 12.2 | 7.6.9±4.0 | 2.6 | 0.33±3.0 | N | N | +/NK | |||||||
| 27.9±3.5 | |||||||||||||
| Akar et al., 2020[57] | 34 | 74 | 161 | 28 | 23 | 2.8 | 20.9 | NK | NK | + | S | S | +/Xp |
| Akar et al., 2020[57] | 27 | 74 | 168 | 51.1 | 33.2 | 1.5 | 15.1 | NK | NK | − | S | Right NK/left S | +/Xp |
| Akar et al., 2020[57] | 25 | 62 | 160 | 38 | 30 | 2.4 | 36.1 | NK | NK | − | S | S | +/Xp |
| Akar et al., 2020[57] | 32 | 68 | 169 | 28.3 | 17.1 | 1.9 | 39.9 | NK | NK | − | S | S | +/Xp |
| Akar et al., 2020[57] | 25 | 75 | 171 | 23.7 | 16.4 | 2 | 40.4 | NK | NK | − | N | S | +/Xp |
| Akar et al., 2020[57] | 27 | 96 | 175 | 20.6 | 9.6 | 2.8 | 40.6 | NK | NK | + | N | S | +/Xp |
| Akar et al., 2020[57] | 30 | 70 | 155 | 40 | 20.1 | 0.6 | 21.2 | NK | NK | + | N | S | +/Xp |
| Akar et al., 2020[57] | 22 | 95 | 172 | 35 | 17.1 | 2.3 | 30.6 | NK | NK | + | N | S | +/Xp |
| Akar et al., 2020[57] | 16 | 65 | 152 | 41.1 | 14.6 | 2.2 | 32 | NK | NK | − | S | S | +/Xp |
| Baziz et al., 2016[58] | 44 | NK | NK | 51 | 11.71 | NK | NK | NK | N | + | N | S | +/Xp |
| Terribile et al., 2019[59] | 36 | 74 | 165 | 24.7 | 9.4 | 2.7 | 14 | 12.2 | NK | + | N | S | +/Xp |
| Rajput et al.,2016[60] | 25 | 85 | 170 | 13.7 | 10.16 | 1.8 | NK | 6.32 | N | + | S | S | NK |
| Abusheikha et al.,2001[61] | 28 | NK | 171 | 50.7 | 15.6 | 3.17 | NK | 6.58 | N | − | N | S | - |
| Yabiku et al., 2018[62] | NK | NK | NK | ↑ | ↑ | ↓ | NK | NK | NK | NK | NK | S | +/NK |
| Lee et al., 2016[63] | 37 | NK | NK | NK | NK | NK | NK | NK | NK | NK | NK | S | NK |
| Lee et al., 2016[63] | 37 | NK | NK | 22 | 9.7 | 3.4 | 25 | NK | NK | NK | S | S | NK |
| Lee et al., 2016[63] | 36 | 59 | 165 | 42 | 18 | 1.9 | 13 | NK | NK | NK | S | S | NK |
| Lee et al., 2016[63] | 28 | 55 | 165 | 64 | 22 | 3.6 | NK | 7.8 | NK | NK | S | S | +/NK |
| Lee et al., 2016[63] | 31 | NK | NK | 42 | 14 | 2.2 | NK | NK | NK | NK | S | S | NK |
| Lee et al., 2016[63] | 30 | NK | NK | 16 | 4.5 | 5.1 | 2.2 | NK | NK | NK | NK | S | NK |
| Lee et al., 2016[63] | 33 | 65 | NK | NK | NK | NK | NK | NK | NK | NK | NK | S | NK |
| Lee et al., 2016[63] | 37 | 65 | 162 | 48 | 17 | 4.7 | NK | NK | NK | NK | S | S | NK |
| Lee et al., 2016[63] | 33 | 73 | 174 | 22.3 | 17.2 | 1.95 | NK | NK | NK | NK | NK | S | NK |
| Lee et al., 2016[63] | 38 | 62 | 160 | 45 | 18 | 3.5 | NK | 4.3 | NK | NK | NK | S | +/NK |
| Lee et al., 2016[63] | 28 | NK | NK | 29 | 13 | 1.8 | NK | NK | NK | NK | NK | S | NK |
| Lee et al., 2016[63] | 36 | 76 | 173 | 35 | 13 | 2.1 | NK | NK | NK | NK | S | S | +/NK |
| Lee et al., 2016[63] | 29 | 80 | 173 | 44 | 9.2 | 2.78 | 11 | 3.8 | NK | NK | NK | S | +/NK |
| Lee et al., 2016[63] | 29 | 72 | 163 | 34 | 5.9 | 1.3 | 8 | 8.8 | NK | NK | NK | +/NK | |
| Lee et al., 2016[63] | 41 | 60 | 163 | 48.8 | 30.7 | 1.27 | 9.9 | 7.6 | NK | NK | NK | S | +/NK |
| Lee et al., 2016[63] | 37 | 56 | 165 | 5.3 | 8.4 | 0.58 | 144.7 | 14.4 | NK | NK | NK | Right S/left NK | - |
| Lee et al., 2016[63] | 42 | 45 | 156 | 45.1 | 13.3 | 1.62 | NK | 8.1 | NK | NK | NK | S | - |
| Lee et al., 2016[63] | 36 | 78 | 172 | 27 | 19.1 | 0.47 | 17 | 2.9 | NK | NK | NK | S | - |
| Dada et al., 2002[64] | 32 | NK | 163 | 70 | 84 | NK | NK | NK | N | NK | N | S | - |
| Kaur et al., 2007[65] | 32 | NK | NK | 56.18 | 25.83 | NK | NK | N | NK | N | S | +/NK | |
| Mohammadpour Lashkari et al., 2017[66] n=47 |
NK | NK | NK | Median 37.7 |
Median 21.27 |
Median ↓2.77 |
NK | NK |
n=44 N n=2 P n=1 NK |
n=43− n=3 + n=1 NK |
NK |
n=29 S n=18 NK |
n=34 (+/Xp) n=1 (−) n=12 NK |
| El Salam et al., 2021[67] | 35 | NK | NK | 68.21 | 59.03 | 0.63 | 58 | NK | NK | + | S | S | - |
| Nguyen et al., 2017[68] | 33 | 58 | 169 | 46.16 | 16.8 | 3.23 | NK | NK | NK | NK | NK | S | - |
| Case 1 | 39 | 70 | 171 | 47 | 28.5 | 6.0 | 18.2 | 22.84 | N | + | NK | S | +/Xp |
| Case 2 | 39 | NK | NK | 43 | 18.2 | 1.57 | NK | 10.4 | N | N | N | S | +/Xp |
NK=Not known, HD=Hair distribution, GM=Gynecomastia, PS=Penis size, TV=Testes volume, FSH=Follicle-stimulating hormone, LH=Luteinizing hormone, TT=Testosterone, E2=Estradiol, PRL=Prolactin, SRY=Sex determining region Y, N=Normal, P=Poor, S=Small, +=yes, -=no, ↓=low, ↑=elevated
The mean age ± standard deviation (SD) of the patients was 32.4 ± 8 years, mean weight ± SD was 68.8 ± 11.3 kg and mean height ± SD was 166.2 ± 6.3 cm. All had hypergonadotropic hypogonadism: mean FSH ± SD was 38.5 ± 15.8 mIU/ml, mean LH ± SD was 25.2 ± 18.2 mIU/ml and mean TT ± SD was 2 ± 0.5 ng/ml. Estradiol and PRL levels were normal (mean E2 ± SD was 27.2 ± 12.3 pg/ml and mean PRL ± SD was 12 ± 2 respectively). Poor hair distribution was present in 16/104 (15.4%) patients (76 patients had no data), gynecomastia was present in 30/112 (26.8%) cases (68 patients had no data), penis size was small in 21/66 (31.8%) patients (114 patients had no data) and testes volume was reduced in 101/112 (90.2%) patients (68 patients had no data). SRY was reported in 154 cases: it was present in 130/154 (84.4%) patients and absent in the remaining 24/154 (15.6%) patients. The SRY was translocated on the Xp chromosome in 128/130 (98.5%) cases and on an autosome in 2/130 (1.5%) cases, one on 1q and the other on 16q.
DISCUSSION
46,XX male sex reversal syndrome accounts for 2% of cases of male infertility.[13] Its incidence may be higher, because the majority of 46,XX males have a normal phenotype at birth and are usually diagnosed in adolescence or later during fertility evaluation.[7] Most cases occur sporadically and there is no association between XX male prevalence and paternal age. However, very few familial cases have also been reported.[2]
Diagnosis of 46,XX males is based on clinical phenotype, endocrinological testing, cytogenetic analysis and molecular analysis combining PCR and FISH. In addition, it is very important to exclude a misdiagnosis in men with a female karyotype as a result of allogeneic bone marrow transplantation from a female donor due to a previous hematological malignancy highlighting the need for a complete and extensive medical history.
Our patients with normal male phenotype and small testes volume were diagnosed during fertility investigation. Indeed, all patients in this review were discovered due to fertility problems (mean age 32.4 years old), otherwise they would have missed diagnosis. They all had normal male external genitalia and testes volume was reduced in 90.2% patients, followed by small penis size in 31.8% patients, gynecomastia in 26.8% patients and poor hair distribution in 15.4% patients.
The hormone profile of all patients, including ours, showed primary (hypergonadotropic) hypogonadism which arises from primary testicular failure and is characterized by low testosterone (hypogonadic) and elevated FSH and LH (hypergonadotropic). Testosterone levels are reported to be normal during adolescence, but decrease in adulthood.[69,70] Although their testicular biopsies in infancy have shown normal testes morphology and normal spermatogonia, in puberty, disappearance of spermatogonia and hyalinisation of the seminiferous tubules are observed later.[7,69]
Based on our patients and the review of the literature, hormone imbalance and testosterone deficiency are mainly responsible for the syndrome's phenotype including: small testes, gynecomastia, small penis and poor hair distribution. Reduced testicular volume was the most common clinical characteristic in 90.1% of the patients, followed by small penis, gynecomastia and poor hair distribution in 32.5%, 27% and 15.5% of the patients, respectively. It has been postulated that the addition of an X chromosome in 46,XX males, like in Klinefelter syndrome, results in hypoplastic testes and may be related to the DAX1.[52] DAX1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1) or NR0B1 gene is located on chromosome Xp21.3-p21.2 and it is widely expressed in the adrenals, hypothalamus, pituitary and testis.[52] Normal DAX1 levels play a critical role in testicular development and spermatogenesis; however, a double dose of DAX1 acts as an anti-testes gene.[52,71]
46,XX male patients have similar phenotype with Klinefelter syndrome patients, but are shorter. The mean height of all patients (166.2 ± 6.3 cm) was lower than the global average normal male height (171 cm) as defined by the WHO growth reference standards,[72] suggesting a potential role of Y chromosome genes in the control of male height.[2,57] Indeed, several papers have proposed a putative Y-specific growth gene involved in the determination of normal adult male height; however, its precise location remains unknown.[73,74] Moreover, short stature may be the consequence of the deletion of the Short Stature Homeobox gene included in the sex chromosome Pseudoautosomal Region 1 of Y chromosome.[57,73]
Molecular testing of our patients using PCR and FISH showed that they both were SRY-positive and the SRY was translocated on Xp, in line with most of the reported patients. In total, 84.4% of the patients were SRY-positive: in the majority (98.5%) of these patients the SRY was translocated on Xp and in only 1.5% of cases on an autosome and more specifically on 1q in one patient and 16q on another. These findings may justify the presence of normal external male genitalia in the patients. This wide phenotypic spectrum of 46,XX SRY-positive males may be related to the size of the translocated Y chromosomal segment, variable inactivation of SRY-carrying X chromosome, position effect or presence of another Y linked gene at the traslocated segment, which may be implicated in male sexual development.[13]
15.6% of patients in the study were 46,XX SRY-negative. Usually these patients present with ambiguous genitalia at birth; however, cases with normal male phenotypes have also been described, suggesting that complete masculinization can occur even in the absence of SRY or other Y-chromosome sequences.[12,16,61] The underlying genetic mechanism for the development of male phenotype in most of SRY-negative 46,XX males remains unexplained. It has been suggested that mutations in autosomal or X-linked genes located downstream of SRY in the sex determining pathway (SOX9, RSPO1, SOX3, DAX1, SOX10, WT1, WNT4 and SF1) may exist.[9,10,16,18,27] Rarely, hidden gonadal mosaicism of the SRY has also been suggested to be the reason for the male phenotype in 46,XX males.[16,27,75]
All 46,XX males are infertile due to azoospermia, attributed to the absence of the AZF region which is essential for spermatogenesis.[76] The AZF region is located on the long arm of the Y chromosome (Yq11) and contains the three AZF regions (AZFa, AZFb and AZFc).[76] Consequently, the desire for fatherhood in 46,XX males could be fulfilled through artificial insemination with sperm donation or child adoption.
Testosterone replacement therapy is widely used in men with hypogonadism, aiming to improve quality of life, sense of well-being, sexual function, muscle strength and bone mineral density.[77] However, despite its beneficial effects, men receiving testosterone therapy should be occasionally monitored, due to possible correlations with prostate and breast cancers, cardiovascular diseases and systemic sleep apnea.[77]
CONCLUSIONS
The present report offers clinical information on two 46,XX males identified through screening for infertility and compares the findings with those reported in the literature. Although it does not provide any new additional information with regards to this rare genetic syndrome, it emphasises the necessity to take this condition into consideration when testing men with normal phenotype and infertility problems. A detailed and concise patient medical history is the first crucial step in the evaluation of the patients who must be referred for karyotypic analysis and in cases of a 46,XX karyotype they must be further tested with molecular techniques for the detection of the SRY and AZF. More cases of this syndrome with SRY translocated on autosomes are needed to identify if these patients have different characteristics from those with SRY translocated on Xp chromosome. Moreover, whole genome analysis of these patients is required to unveil the genetic differences which are responsible for the wide phenotypic spectrum of the 46,XX male sex reversal syndrome. In addition, more SRY-negative patients are needed to be tested, because the exact genetic control of human sex determination is not fully understood and the investigation of the effects of other genes involved is necessary. Testosterone administration is the main therapeutic strategy, but recurrent monitoring of the patients is recommended because of possible risk of breast and testis cancer. Management of such cases is multidirectional and collaboration of several specialists is required. Genetic counseling should be offered to all 46,XX males in order to help them understand the aetiology of their infertility and recognise that they have no possibility of having a child naturally.
Statement of ethics
The Helsinki Declaration (1975) complied with the survey. All participants in the study were informed and gave their written consent for anonymous and voluntary participation.
Authors’ contributions
EK conceived and designed the study; SZ, LZ, KP, TT, KC and HT performed the experiments; EK and HT performed literature search and analyzed the data; RD and KS provided the clinical data; EK and HT wrote the manuscript; KM, EK, AM contributed to manuscript review and editing. All authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Data availability statement
Please note that all data of the study are held in our premises in accordance of the provisions of the applicable legislation and may be accessible by you on a codified basis upon request.
REFERENCES
- 1.Delachapelle A, Hortling H, Niemi M, Wennstroem J. XX sex chromosomes in a human male. First case. Acta Med Scand. 1964;175:L41225–8. doi: 10.1111/j.0954-6820.1964.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 2.de la Chapelle A. The etiology of maleness in XX men. Hum Genet. 1981;58:105–16. doi: 10.1007/BF00284157. [DOI] [PubMed] [Google Scholar]
- 3.Hughes IA, Houk C, Ahmed SF, Lee PA Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology Consensus Group. Consensus statement on management of intersex disorders. J Pediatr Urol. 2006;2:148–62. doi: 10.1016/j.jpurol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 4.de la Chapelle A. The Y-chromosomal and autosomal testis-determining genes. Development. 1987;101 Suppl:33–8. [PubMed] [Google Scholar]
- 5.López M, Torres L, Méndez JP, Cervantes A, Pérez-Palacios G, Erickson RP, et al. Clinical traits and molecular findings in 46,XX males. Clin Genet. 1995;48:29–34. doi: 10.1111/j.1399-0004.1995.tb04050.x. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–4. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 7.Ergun-Longmire B, Vinci G, Alonso L, Matthew S, Tansil S, Lin-Su K, et al. Clinical, hormonal and cytogenetic evaluation of 46,XX males and review of the literature. J Pediatr Endocrinol Metab. 2005;18:739–48. doi: 10.1515/jpem.2005.18.8.739. [DOI] [PubMed] [Google Scholar]
- 8.Vorona E, Zitzmann M, Gromoll J, Schüring AN, Nieschlag E. Clinical, endocrinological, and epigenetic features of the 46,XX male syndrome, compared with 47, XXY Klinefelter patients. J Clin Endocrinol Metab. 2007;92:3458–65. doi: 10.1210/jc.2007-0447. [DOI] [PubMed] [Google Scholar]
- 9.Dauwerse JG, Hansson KB, Brouwers AA, Peters DJ, Breuning MH. An XX male with the sex-determining region Y gene inserted in the long arm of chromosome 16. Fertil Steril. 2006;86:5.e1–5. doi: 10.1016/j.fertnstert.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 10.Queralt R, Madrigal I, Vallecillos MA, Morales C, Ballescá JL, Oliva R, et al. Atypical XX male with the SRY gene located at the long arm of chromosome 1 and a 1qter microdeletion. Am J Med Genet A. 2008;146A:1335–40. doi: 10.1002/ajmg.a.32284. [DOI] [PubMed] [Google Scholar]
- 11.Chien SC, Li YC, Ho M, Hsu PC, Teng RH, Lin WD, et al. Rare rearrangements: A “jumping satellite” in one family and autosomal location of the SRY gene in an XX male. Am J Med Genet A. 2009;149A:2775–81. doi: 10.1002/ajmg.a.32958. [DOI] [PubMed] [Google Scholar]
- 12.Zenteno JC, López M, Vera C, Méndez JP, Kofman-Alfaro S. Two SRY-negative XX male brothers without genital ambiguity. Hum Genet. 1997;100:606–10. doi: 10.1007/s004390050561. [DOI] [PubMed] [Google Scholar]
- 13.Bouayed Abdelmoula N, Portnoi MF, Keskes L, Recan D, Bahloul A, Boudawara T, et al. Skewed X-chromosome inactivation pattern in SRY positive XX maleness: A case report and review of literature. Ann Genet. 2003;46:11–8. doi: 10.1016/s0003-3995(03)00011-x. [DOI] [PubMed] [Google Scholar]
- 14.McGowan-Jordan J, Hastings RJ, Moore S, editors. An International System for Human Cytogenomic Nomenclature. Basel: Karger; 2020. [DOI] [PubMed] [Google Scholar]
- 15.Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of y-chromosomal microdeletions. State of the art 2004. Int J Androl. 2004;27:240–9. doi: 10.1111/j.1365-2605.2004.00495.x. [DOI] [PubMed] [Google Scholar]
- 16.Valetto A, Bertini V, Rapalini E, Simi P. A 46,XX SRY-negative man with complete virilization and infertility as the main anomaly. Fertil Steril. 2005;83:216–9. doi: 10.1016/j.fertnstert.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 17.Rigola MA, Carrera M, Ribas I, Egozcue J, Miró R, Fuster C. A comparative genomic hybridization study in a 46,XX male. Fertil Steril. 2002;78:186–8. doi: 10.1016/s0015-0282(02)03165-5. [DOI] [PubMed] [Google Scholar]
- 18.Ryan NA, Akbar S. A case report of an incidental finding of a 46,XX, SRY-negative male with masculine phenotype during standard fertility workup with review of the literature and proposed immediate and long-term management guidance. Fertil Steril. 2013;99:1273–6. doi: 10.1016/j.fertnstert.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Wegner RD, Nürnberger F. Clinical, cytological, and biochemical investigations in a case of an XX male. Andrologia. 1983;15:253–8. doi: 10.1111/j.1439-0272.1983.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 20.Pais VM, Vasudevan P. Infertility in an XX male. J Urol. 1977;118:690–1. doi: 10.1016/s0022-5347(17)58160-4. [DOI] [PubMed] [Google Scholar]
- 21.Zakharia G, Krauss DJ. Sex reversal syndrome (XX male) Urology. 1990;36:322–4. doi: 10.1016/0090-4295(90)80238-i. [DOI] [PubMed] [Google Scholar]
- 22.Pepene CE, Coman I, Mihu D, Militaru M, Duncea I. Infertility in a new 46,XX male with positive SRY confirmed by fluorescence in situ hybridization: A case report. Clin Exp Obstet Gynecol. 2008;35:299–300. [PubMed] [Google Scholar]
- 23.Hado HS, Helmy SW, Klemm K, Miller P, Elhadd TA. XX male: A rare cause of short stature, infertility, gynaecomastia and carcinoma of the breast. Int J Clin Pract. 2003;57:844–5. [PubMed] [Google Scholar]
- 24.Xiao B, Ji X, Xing Y, Chen YW, Tao J. A rare case of 46,XX SRY-negative male with approximately 74-kb duplication in a region upstream of SOX9. Eur J Med Genet. 2013;56:695–8. doi: 10.1016/j.ejmg.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Butler MG, Walzak MP, Sanger WG, Todd CT. A possible etiology of the infertile 46XX male subject. J Urol. 1983;130:154–6. doi: 10.1016/s0022-5347(17)51010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yencilek F, Baykal C. 46 XX male syndrome: A case report. Clin Exp Obstet Gynecol. 2005;32:263–4. [PubMed] [Google Scholar]
- 27.Mustafa O, Mehmet E. A 46,XX SRY – Negative man with infertility, and co-existing with chronic autoimmune thyroiditis. Gynecol Endocrinol. 2010;26:413–5. doi: 10.3109/09513591003632225. [DOI] [PubMed] [Google Scholar]
- 28.Matthews CD, Ford J, Kirby C. The XX male. Clinical and theoretical aspects. Clin Reprod Fertil. 1983;2:207–15. [PubMed] [Google Scholar]
- 29.Tomomasa H, Adachi Y, Iwabuchi M, Tohyama Y, Yotsukura M, Oshio S, et al. XX-male syndrome bearing the sex-determining region Y. Arch Androl. 1999;42:89–96. doi: 10.1080/014850199262922. [DOI] [PubMed] [Google Scholar]
- 30.Chernykh VB, Kurilo LF, Shilova NV, Zolotukhina TV, Ryzhkova OP, Bliznetz EA, et al. Hidden X chromosomal mosaicism in a 46,XX male. Sex Dev. 2009;3:183–7. doi: 10.1159/000228718. [DOI] [PubMed] [Google Scholar]
- 31.Castiñeyra G, Copelli S, Levalle O. 46,XX male: Clinical, hormonal/genetic findings. Arch Androl. 2002;48:251–7. doi: 10.1080/01485010290031556. [DOI] [PubMed] [Google Scholar]
- 32.Chiang HS, Wu YN, Wu CC, Hwang JL. Cytogenic and molecular analyses of 46,XX male syndrome with clinical comparison to other groups with testicular azoospermia of genetic origin. J Formos Med Assoc. 2013;112:72–8. doi: 10.1016/j.jfma.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Rajender S, Rajani V, Gupta NJ, Chakravarty B, Singh L, Thangaraj K. SRY-negative 46,XX male with normal genitals, complete masculinization and infertility. Mol Hum Reprod. 2006;12:341–6. doi: 10.1093/molehr/gal030. [DOI] [PubMed] [Google Scholar]
- 34.Tan TT, Khalid BA. Primary infertility in a phenotypic male with 46XX chromosomal constitution. Postgrad Med J. 1993;69:315–7. doi: 10.1136/pgmj.69.810.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mićić S, Mićić M, Ozerović B. An example of a 46,XX infertile man and his permanent tooth sizes. Int J Fertil. 1983;28:165–8. [PubMed] [Google Scholar]
- 36.Wu QY, Li N, Li WW, Li TF, Zhang C, Cui YX, et al. Clinical, molecular and cytogenetic analysis of 46,XX testicular disorder of sex development with SRY-positive. BMC Urol. 2014;14:70. doi: 10.1186/1471-2490-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao X, Chen G, Huang J, Bai Q, Zhao N, Shao M, et al. Clinical, cytogenetic, and molecular analysis with 46,XX male sex reversal syndrome: Case reports. J Assist Reprod Genet. 2013;30:431–5. doi: 10.1007/s10815-013-9939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuse H, Satomi S, Kazama T, Katayama T, Nagabuchi S, Tamura T, et al. DNA hybridization study using Y-specific probes in an XX-male. Andrologia. 1991;23:237–9. doi: 10.1111/j.1439-0272.1991.tb02547.x. [DOI] [PubMed] [Google Scholar]
- 39.Majzoub A, Arafa M, Starks C, Elbardisi H, Al Said S, Sabanegh E. 46 XX karyotype during male fertility evaluation; case series and literature review. Asian J Androl. 2017;19:168–72. doi: 10.4103/1008-682X.181224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahsan T, Saleem M, Ahmed A, Muzaffar M. 46 XX male: A case of sex reversal syndrome. J Pak Med Assoc. 1998;48:19–20. [PubMed] [Google Scholar]
- 41.Yamamoto M, Yokoi K, Katsuno S, Hibi H, Miyake K. A case of sex reversal syndrome with sex-determining region (XX male) Nagoya J Med Sci. 1995;58:111–5. [PubMed] [Google Scholar]
- 42.Jellad S, Basly M, Bougrine F, Chibani M, Rachdi R. SRY – Negative 46,XX male with complete virilization and infertility: A case report. Tunis Med. 2016;94:637. [PubMed] [Google Scholar]
- 43.Rizvi AA. 46,XX man with SRY gene translocation: Cytogenetic characteristics, clinical features and management. Am J Med Sci. 2008;335:307–9. doi: 10.1097/MAJ.0b013e31811ec1b4. [DOI] [PubMed] [Google Scholar]
- 44.Casas-Vargas A, Galvis J, Blanco J, Rengifo L, Usaquén W, Velasco H. Male patient 46,XX SRY-negative and unambiguous genitalia: A case report Biomedica. 2019;39:622–30. doi: 10.7705/biomedica.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vetro A, Ciccone R, Giorda R, Patricelli MG, Della Mina E, Forlino A, et al. XX males SRY negative: A confirmed cause of infertility. J Med Genet. 2011;48:710–2. doi: 10.1136/jmedgenet-2011-100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vetro A, Dehghani MR, Kraoua L, Giorda R, Beri S, Cardarelli L, et al. Testis development in the absence of SRY: Chromosomal rearrangements at SOX9 and SOX3. Eur J Hum Genet. 2015;23:1025–32. doi: 10.1038/ejhg.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pastor Guzmán JM, Pastor Navarro H, Quintanilla Mata ML, Carrión López P, Martínez Ruíz J, Martínez Sanchiz C, et al. 46,XX T testicular disorder of sex development. Case report. Arch Esp Urol. 2011;64:468–73. [PubMed] [Google Scholar]
- 48.Gunes S, Asci R, Okten G, Atac F, Onat OE, Ogur G, et al. Two males with SRY-positive 46,XX testicular disorder of sex development. Syst Biol Reprod Med. 2013;59:42–7. doi: 10.3109/19396368.2012.731624. [DOI] [PubMed] [Google Scholar]
- 49.Bogdanet D, Griffin TP, Bell M. A rare case of infertility: SRY positive 46,XX testicular disorder of sexual differentiation. Ir Med J. 2020;3:60. [PubMed] [Google Scholar]
- 50.Onrat ST, Söylemez Z, Elmas M. 46,XX, der(15), t(Y; 15)(q12;p11) karyotype in an azoospermic male. Indian J Hum Genet. 2012;18:241–5. doi: 10.4103/0971-6866.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jain M, Veeramohan V, Chaudhary I, Halder A. The sertoli cell only syndrome and glaucoma in a sex – Determining region Y (SRY) positive XX infertile male. J Clin Diagn Res. 2013;7:1457–9. doi: 10.7860/JCDR/2013/5186.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yue F, Zhang H, Xi Q, Jiang Y, Li L, Liu R, et al. Molecular cytogenetic analysis and genetic counseling: A case report of eight 46,XX males and a literature review. Mol Cytogenet. 2019;12:44. doi: 10.1186/s13039-019-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akinsal EC, Baydilli N, Demirtas A, Saatci C, Ekmekcioglu O. Ten cases with 46,XX testicular disorder of sex development: Single center experience. Int Braz J Urol. 2017;43:770–5. doi: 10.1590/S1677-5538.IBJU.2016.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashfaq S, Siddiqui A, Shafiq W, Azmat U. A rare presentation of disorder of sex development. Cureus. 2021;13:e12782. doi: 10.7759/cureus.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sreejith PS, Balakrishnan S, Sankar VH, Syamala R, Mohan R, Sundaram S, et al. Patient with disorders of sex development (DSD): A case report from a tertiary care hospital in Thiruvananthapuram, India. J Reprod Infertil. 2019;20:191–4. [PMC free article] [PubMed] [Google Scholar]
- 56.Yiğman M, Tangal S, Haliloğlu AH, Çağlar GS. Erectile function in SRY positive 46,XX males with normal phenotype. Cent European J Urol. 2021;74:95–8. doi: 10.5173/ceju.2021.0284.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akar OS, Gunes S, Abur U, Altundag E, Asci R, Onat OE, et al. Multiscale analysis of SRY-positive 46,XX testicular disorder of sex development: Presentation of nine cases. Andrologia. 2020;52:e13739. doi: 10.1111/and.13739. [DOI] [PubMed] [Google Scholar]
- 58.Baziz M, Hamouli-Said Z, Ratbi I, Habel M, Guaoua S, Sbiti A, et al. Cytogenetic investigation in a group of ten infertile men with non-obstructive azoospermia: First algerian 46,XX syndrome. Iran J Public Health. 2016;45:739–47. [PMC free article] [PubMed] [Google Scholar]
- 59.Terribile M, Stizzo M, Manfredi C, Quattrone C, Bottone F, Giordano DR, et al. 46,XX testicular disorder of sex development (DSD): A case report and systematic review. Medicina (Kaunas) 2019;55:371. doi: 10.3390/medicina55070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajput R, Jain D, Vohra S, Pathak V. De La chapelle syndrome: A rare case of male infertility. JAFES. 2016;31:151–5. [Google Scholar]
- 61.Abusheikha N, Lass A, Brinsden P. XX males without SRY gene and with infertility. Hum Reprod. 2001;16:717–8. doi: 10.1093/humrep/16.4.717. [DOI] [PubMed] [Google Scholar]
- 62.Yabiku RS, Guaragna MS, de Sousa LM, Fabbri-Scallet H, Mazzola TN, Piveta CS, et al. A search for disorders of sex development among infertile men. Sex Dev. 2018;12:275–80. doi: 10.1159/000493877. [DOI] [PubMed] [Google Scholar]
- 63.Lee BY, Lee SY, Lee YW, Kim SY, Kim JW, Ryu HM, et al. Three cases of rare SRY-negative 46,XX testicular disorder of sexual development with complete masculinization and a review of the literature. J Genet Med. 2016;13:78–88. [Google Scholar]
- 64.Dada R, Gupta NP, Kucheria K. AZF microdeletions associated with idiopathic and non-idiopathic cases with cryptorchidism and varicocele. Asian J Androl. 2002;4:259–63. [PubMed] [Google Scholar]
- 65.Kaur A, Sachdeva K, Mahajan S, Virk SP, Singh JR. An SRY-positive 46,XX male. Balkan J Med Genet. 2007;10:51–2. [Google Scholar]
- 66.Mohammadpour Lashkari F, Totonchi M, Zamanian MR, Mansouri Z, Sadighi Gilani MA, Sabbaghian M, et al. 46,XX males: A case series based on clinical and genetics evaluation. Andrologia. 2017;49:1–7. doi: 10.1111/and.12710. [doi: 10.1111/and.12710] [DOI] [PubMed] [Google Scholar]
- 67.El Salam MA, Ibrahim NH, Eskarous N. A rare case of male sex reversal syndrome (46,XX) with negative SRY gene: A disorder of sexual diferentiation (DSD) Afr J Urol. 2021;27:1–4. [Google Scholar]
- 68.Nguyen CT, Kien NT, Nhuan VT, Khoa ND, Vieng NC, Nguyen TB, et al. An infertility SRY-negative 46,XX male detected by quantitative fluorescent polymerase chain reaction. J Clin Case Rep. 2017;7:1013. [Google Scholar]
- 69.Abbas NE, Toublanc JE, Boucekkine C, Toublanc M, Affara NA, Job JC, et al. A possible common origin of “Y-negative” human XX males and XX true hermaphrodites. Hum Genet. 1990;84:356–60. doi: 10.1007/BF00196234. [DOI] [PubMed] [Google Scholar]
- 70.Velasco G, Savarese V, Sandorfi N, Jimenez SA, Jabbour S. 46,XX SRY-positive male syndrome presenting with primary hypogonadism in the setting of scleroderma. Endocr Pract. 2011;17:95–8. doi: 10.4158/EP10184.CR. [DOI] [PubMed] [Google Scholar]
- 71.Disorders of sex differentiation. In: Grumbach MM, Hughes IA, Conte FA, editors; Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. 10. Philadelphia: Saunders; 2003. P 842-62, 881-5, 961-2. [Google Scholar]
- 72.World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization; 2006. Multicentre Growth Reference Study Group. [Google Scholar]
- 73.Ellis JA, Stebbing M, Harrap SB. Significant population variation in adult male height associated with the Y chromosome and the aromatase gene. J Clin Endocrinol Metab. 2001;86:4147–50. doi: 10.1210/jcem.86.9.7875. [DOI] [PubMed] [Google Scholar]
- 74.Kirsch S, Weiss B, Kleiman S, Roberts K, Pryor J, Milunsky A, et al. Localisation of the Y chromosome stature gene to a 700 kb interval in close proximity to the centromere. J Med Genet. 2002;39:507–13. doi: 10.1136/jmg.39.7.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zenteno-Ruiz JC, Kofman-Alfaro S, Méndez JP. 46,XX sex reversal. Arch Med Res. 2001;32:559–66. doi: 10.1016/s0188-4409(01)00322-8. [DOI] [PubMed] [Google Scholar]
- 76.Vogt PH, Edelmann A, Kirsch S, Henegariu O, Hirschmann P, Kiesewetter F, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5:933–43. doi: 10.1093/hmg/5.7.933. [DOI] [PubMed] [Google Scholar]
- 77.Dohle GR, Arver S, Bettocchi C, Kliesch S, Punab M. EAU Guidelines on Male Hypogonadism, European Association of Urology. [Last accessed on 2022 Mar 31]. Available from: https://uroweborg/wp-content/uploads/EAU-guidelines-male-hypogonadism-2016-1.pdf.2016 .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please note that all data of the study are held in our premises in accordance of the provisions of the applicable legislation and may be accessible by you on a codified basis upon request.