ABSTRACT
Background:
Infertility is a global health issue. The variation in the prevalence of unexplained infertility is attributed to the choice of investigation. There remains a knowledge gap on the impact of minimal and mild endometriosis on treatment outcomes following intrauterine insemination (IUI).
Aim:
The aim of this study was to compare treatment outcomes following ovarian stimulation (OS) and intrauterine insemination (IUI) between minimal and mild endometriosis versus unexplained infertility.
Settings and Design:
A retrospective analysis of women undergoing OS with intrauterine insemination during the year 20142020 in the Department of Reproductive Medicine and Surgery, Christian Medical College, Vellore, was considered for the study.
Materials and Methods:
Women with minimal and mild endometriosis or unexplained infertility diagnosed by diagnostic hysterolaparoscopy were included for the analysis. Univariate and multivariate analysis was done. The primary outcome was live birth rate (LBR) per cycle. The secondary outcomes measured were clinical pregnancy rate (CPR) , cumulative LBR (CLBR) per women, cumulative CPR (CCPR) per women and miscarriage rate.
Statistical Analysis Used:
The baseline parameters were compared using a t-test for continuous data, and categorical data were compared using the Chi-square/Fisher's exact test as appropriate. The outcomes were assessed using logistic regression analysis and expressed as odds ratio (OR) with 95% confidence intervals (CI).
Results:
There were no significant differences in CPR per cycle (14.28% vs. 18.8%, OR: 0.71; 95% CI: 0.401.28) and LBR per cycle (14.28% vs. 16.6%, OR: 0.84; 95% CI: 0.461.51) between the endometriosis and unexplained infertility groups. The cumulative LBR per woman and CCPR per woman also did not show any significant difference in between the two groups.
Conclusion:
The current study did not find any significant differences in cumulative LBR and CPR following OS-IUI in women with minimal or mild endometriosis and unexplained infertility.
KEYWORDS: Diagnostic hysterolaparoscopy, unexplained infertility, intrauterine insemination, minimal and mild endometriosis, ovarian stimulation
INTRODUCTION
Infertility is considered a global health issue which adversely impacts an individual as well as the society.[1,2] One in six couples are infertile in the reproductive age group, and amongst them, 22%30% are unexplained.[3,4,5] A diagnosis of unexplained infertility is reached when the standard fertility work-up does not reveal any obvious abnormality and is considered a ’diagnosis of exclusion’.[6]
The variation in the prevalence of unexplained infertility may be attributed to the choice of investigation performed during a fertility work-up. An overdiagnosis of unexplained infertility remains a possibility when hysterosalpingography (HSG) or hystero contrast sonography (HyCoSy) is chosen for assessing the tubal patency due to the likelihood of missing early endometriosis and peritubal adhesions.[7] It has been suggested that the clinician should make all the efforts to provide a specific diagnosis for a couple undergoing fertility work-up and avoid categorising them under unexplained infertility.[7] Other authors have debated whether identifying pelvic abnormalities such as early endometriosis within the broader diagnosis of unexplained infertility helps in the treatment-related decision.[8]
It is worth considering whether detection of early endometriosis during a diagnostic hysterolaparoscopy (DHL) impacts the outcome of subsequent fertility treatment such as ovarian stimulation (OS) with intrauterine insemination (IUI). Any information on the impact of early endometriosis on the subsequent treatment outcome would be of prognostic value while planning OS with IUI. In addition, if the presence of early endometriosis does not impact the treatment outcome, then it supports the argument for simplifying fertility work-up by avoiding DHL for tubal assessment.[8] Earlier studies which compared IUI outcomes in women with unexplained infertility and endometriosis reported conflicting results. In a retrospective study by Werbrouck et al., investigators reported comparable cumulative pregnancy rates following four cycles of OS-IUI in unexplained infertility versus early endometriosis.[9] In contrast, other studies reported significantly higher pregnancy rates following OS-IUI in women with unexplained infertility when compared to those with endometriosis.[10,11]
Apart from the conflicting data, there is also a paucity of studies which have studied the impact of early endometriosis on live birth following OS with IUI. In order to fill the knowledge gap, we planned a study to compare the LBR following OS and IUI in women with minimal or mild endometriosis versus unexplained infertility.
MATERIALS AND METHODS
A retrospective cohort design was used for the current study, and it was conducted in the Department of Reproductive Medicine, Christian Medical College, Vellore, which provides tertiary-level infertility care. The study was approved by the Institutional Review Board, IRBMin. No. 14081. The study period was between January 2014 and December 2020. All procedures followed were in accordance with ethical standards laid down in the Helsinki Declaration (2013). Consent was taken for use of anonymised data for educational and research purposes. All the women who underwent OS with IUI and had been previously diagnosed with either unexplained infertility or minimal/mild endometriosis during DHL and chromopertubation, performed at our hospital, were included in the study. A diagnosis of minimal and mild endometriosis was made based on revised ASRM classification.[12] Surgical intervention like ablation and adhesiolysis was done for women with minimal and mild endometriosis according to individual specialist's discretion. The following exclusion criteria were applied: (i) moderate and severe endometriosis and (ii) unexplained infertility diagnosed following HSG.
OS was performed either with oral ovulogens alone, gonadotropins or a combination of both. The oral ovulogen included administration of clomiphene citrate (CC) 100 mg/day or letrozole 2.5 mg/day from day 2 to day 6 of menstrual cycle. Gonadotropin stimulation was carried out using 75 IU of human menopausal gonadotropin (HMG) (Menopur, Ferring Pharmaceuticals, Mumbai, India), administered intramuscularly from the 5th day of menstrual cycle and continued daily. The combined regimen included administration of letrozole 2.5 mg/CC 100 mg from the 2nd day to the 6th day of the menstrual cycle, along with 75 IU HMG intramuscularly on alternative days from the 5th day. Serial transvaginal ultrasonography was continued and follicular size was monitored until the mean follicle diameter reached ≥17 mm. Serum or urine luteinising hormone (LH) levels were measured. The IUI was scheduled 24 h after a positive urine or serum LH (>15 mIU/L). In case of a negative urine or serum LH level, ovulatory trigger was administered with urinary human chorionic gonadotropin (5000 IU) (Choragon, Ferring Pharmaceuticals, Mumbai, India) or recombinant human chorionic gonadotropin (250 mcg) (Ovitrelle, Merck Serono, Middlesex, UK). The IUI was scheduled approximately 3436 h after the trigger. In case, more than three follicles measuring greater than 17 mm size in both the ovaries were noted, the IUI treatment cycle was cancelled, and abstinence advised.
Semen collection was done by masturbation after 27 days of sexual abstinence. Semen analysis was performed following the WHO guidelines.[13] After liquefaction, semen sample prepared by a density gradient method using PureSperm (Nidacon, International AB, Gothenburg, Sweden) and the total motile fraction was calculated. The samples were centrifuged just before insemination, and the pellets were resuspended in a volume of 0.5 mL HEPES buffer. A single intrauterine insemination of 0.3 ml was carried out using a soft IUI catheter (Manish Medi Innovation, Bengaluru, Karnataka, India). Following the procedure, all women were given luteal support with vaginal progesterone 200 mg twice daily for a period of 20 days.
Pregnancy was tested using a urine pregnancy kit after 20 days post insemination. Women who had conceived an ultrasound were performed after 2 weeks for confirmation of clinical pregnancy. Women who tested negative for pregnancy were offered a total of up to three IUI cycles. Women with a confirmed clinical intrauterine pregnancy were referred and followed up for further antenatal care and delivery at obstetric unit of their choice. Data regarding baseline demographics and IUI cycle characteristic were obtained from clinical case records and department database. The treatment outcomes for each cycle were followed up by individual contact, electronic communication and phone interviews.
Outcomes
The primary outcome was live birth rate (LBR) per cycle defined as live birth ≥24 weeks of gestation. The secondary outcomes measured were clinical pregnancy rate (CPR) defined by presence of an intra- or extrauterine gestational sac on ultrasonography and miscarriage rate per cycle, defined as loss of pregnancy <24 weeks of gestation. The secondary outcomes measured were clinical pregnancy rate (CPR) , cumulative LBR (CLBR) per women, cumulative CPR (CCPR) per women and miscarriage rate.
Statistical analysis plan
Summary data were presented as mean (standard deviation) and median (interquartile range) for continuous variables and categorical variables as numbers and percentages. The characteristics of patients with endometriosis and unexplained infertility were compared using a t-test for continuous data, and categorical data were compared using Chi-square/Fisher's exact test as appropriate. Outcomes associated with endometriosis and unexplained infertility were assessed using logistic regression analysis and expressed as odds ratio (OR) with 95% confidence intervals (CIs). Statistical significance was defined as P < 0.05. All analyses were performed using SPSS v25 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0.Armonk, NY: IBM Corp.).
RESULTS
A total 1716 IUI cycles were performed during the study period, out of which 1221 cycles were excluded on basis of exclusion criteria [Figure 1]. Out of total 495 initiated IUI treatment cycles, 92 were cancelled for various reasons such as poor response, hyperstimulation and poor semen quality [Figure 1]. Finally, 152 cycles in the endometriosis group and 251 cycles in the unexplained infertility group were included for analysis. The outcomes for about 12 cycles in the endometriosis group and 28 cycles in the unexplained infertility group could not be traced and were excluded as loss to follow-up. Therefore, the final analysis included 140 cycles in women with endometriosis (101 women) and 223 cycles in women with unexplained infertility (171 women cycles).
Figure 1.
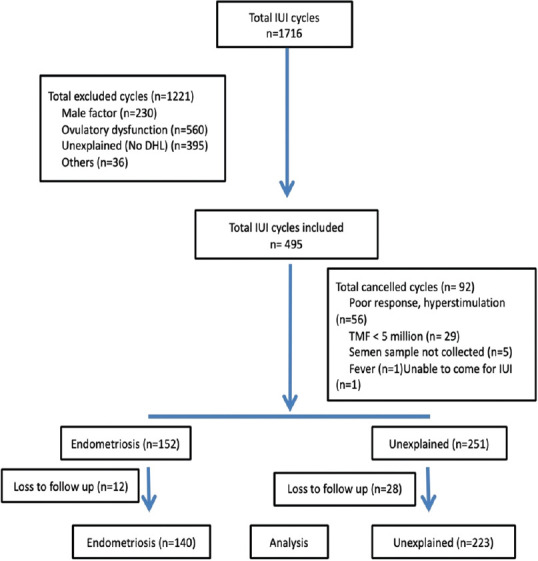
Algorithm
The baseline clinical characteristics are summarised in Table 1. We found no significant difference in mean female age, paternal age and total motile fraction in between the two groups. However, there was significantly higher mean basal metabolic index (BMI) in the unexplained group (P 0.006). The mean duration of infertility (P 0.004) and the mean DHL to IUI time (P < 0.001) were also significantly higher in the unexplained infertility group in comparison to the minimal/mild endometriosis group. Table 2 summarises treatment characteristics, and no significant difference was found between the two groups.
Table 1.
Baseline characteristics of the two cohorts (minimal and mild endometriosis vs. unexplained infertility)
| Parameters | Endometriosis (n=152) | Unexplained (n=251) | P |
|---|---|---|---|
| Female age* (years) | 29.6±3.4 | 29.6±3.6 | 0.88 |
| Female BMI* (kg/m2) | 24.1±3.7 | 25.3±4.6 | 0.006§ |
| Paternal age* (years) | 34.0±4.0 | 34.0±4.5 | 0.91 |
| Primary infertility | 122 (80.3) | 188 (74.9) | 0.23 |
| Secondary infertility | 30 (19.7) | 63 (25.1) | |
| Duration of subfertility (years)† | 4 (3-8) | 5 (4-8) | 0.004§ |
| TMF†,‡ | 20 (10-33) | 22 (12-31) | 0.58 |
| DHL to IUI time (months)† | 6 (3-10) | 12 (6-25) | <0.001§ |
*Presented as mean, SD, † Presented as median, IQR, ‡ Total motile fraction assessed at the time of IUI, § Statistically significant. BMI=Body mass index, DHL=Diagnostic hysterolaparoscopy, IUI=Intrauterine insemination, SD=Standard deviation, IQR=Interquartile range, TMF=Total motile fraction
Table 2.
Treatment characteristics of the ovarian stimulation with intrauterine insemination treatment cycle for woman with minimal and mild endometriosis versus unexplained infertility
| Endometriosis (n=152) | Unexplained (n=251) | P | |
|---|---|---|---|
| Protocol type | |||
| Clomiphene/letrozole | 1 (0.7) | 1 (0.4) | |
| Clomiphene/letrozole+gonadotropins | 11 (7.2) | 14 (5.6) | 0.52 |
| Gonadotrophins | 139 (91.4) | 236 (94) | |
| Natural cycle | 1 (0.7) | 0 | |
| Number of gonadotropin doses administered* | 7.0±2.7 | 6.7±2.6 | 0.24 |
| Follicle number >15 mm* | 1.9±0.6 | 2.0±0.5 | 0.14 |
| Follicle number ≥17 mm* | 1.3±0.7 | 1.2±0.5 | 0.56 |
| rhCG trigger | 112 (73.7) | 179 (71.3) | 0.607 |
| Endometrial thickness (mm)* | 9.43±2.102 | 9.12±2.161 | 0.166 |
*Presented as mean, SD. rhCG=Recombinant human chorionic gonadotropin, SD=Standard deviation
Out of total 272 women, 169 (62.1%) women underwent first cycle of IUI and did not turn up for subsequent cycles. Similarly, 75 (27.6%) women had undergone 2 cycles of IUI and 28 (10.2%) women completed all three cycles of IUI in the current study.
Outcome analysis
There were no significant differences in CPR per cycle (14.28% vs. 18.8%, OR: 0.71; 95% CI: 0.401.28) and LBR per cycle (14.28% vs. 16.6%, OR: 0.84; 95% CI: 0.461.51) between the endometriosis and unexplained infertility groups. The miscarriage rate (0% vs. 11.9%) and the multiple pregnancy rate (5% vs. 16.6%, OR: 0.26; 95% CI: 0.032.30) were not significantly different as well [Table 3].
Table 3.
Comparison of treatment outcomes (per cycle) following ovarian stimulation and intrauterine insemination in minimal and mild endometriosis versus unexplained infertility
| Endometriosis (n=140), n (%) | Unexplained (n=223), n (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Clinical pregnancy* | 20/140 (14.28) | 42/223 (18.8) | 0.71 (0.40-1.28) | 0.26 |
| Live birth* | 20/140 (14.28) | 37/223 (16.6) | 0.84 (0.46-1.51) | 0.56 |
| Miscarriage† | 0/20 | 5/42 (11.9) | - | - |
| Ectopic pregnancy† | 1/20 (5) | 1/42 (2.4) | 2.16 (0.13-36.37) | 0.59 |
| Multiple pregnancy† | 1/20 (5) | 7/42 (16.6) | 0.26 (0.03-2.30) | 0.23 |
*Calculated per intrauterine insemination done, † Calculated per clinical pregnancy. OR=Odds ratio, CI=Confidence interval
The CLBR per woman (19.8% vs. 21.6%, OR: 0.91; 95% CI: 0.501.66) and CCPR per woman (19.8% vs. 24.6%; OR: 0.81; 95% CI: 0.44-1.45) did not show any significant difference in women with minimal/mild endometriosis compared with unexplained infertility [Table 4].
Table 4.
Comparison of treatment outcomes (per woman) following ovarian stimulation and intrauterine insemination in minimal and mild endometriosis versus unexplained infertility
| Endometriosis (n=101), n (%) | Unexplained (n=171), n (%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Live birth | 20/101 (19.8) | 37/171 (21.6) | 0.91 (0.50-1.66) | 0.84 |
| Clinical pregnancy | 20/101 (19.8) | 42/171 (24.6) | 0.81 (0.44-1.45) | 0.45 |
OR=Odds ratio, CI=Confidence interval
We adjusted for important clinical confounding factors such as female age, BMI and duration of infertility both expressed per cycle and per women. We found no significant difference in LBR per cycle (aOR: 0.78; 95% CI: 0.431.42)) [Table 5a] and CLBR (aOR: 0.78; 95% CI: 0.411.50) [Table 5b]. Similarly, the CPR per cycle (aOR: 0.66; 95% CI: 0.361.18) and the CCPR (aOR: 0.64; 95% CI: 0.341.12) were also found to be comparable between the two groups [Table 5b].
Table 5a.
Multivariate logistic regression analysis for live birth rate and clinical pregnancy rate (per cycle)
| Study group | No live birth (n=306), n (%) | Live birth (n=57), n (%) | Unadjusted OR (95%CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|---|
| Unexplained Infertility | 186 (60.8) | 37 (64.9) | Reference | |||
| Endometriosis | 120 (39.2) | 20 (35.1) | 0.84 (0.46-1.51) | 0.56 | 0.78 (0.43-1.42) | 0.42 |
|
| ||||||
| Study group | No clinical pregnancy (n=301), n (%) | Clinical pregnancy (n=62), n (%) | Unadjusted OR (95%CI) | P | Adjusted OR (95%CI) | P |
|
| ||||||
| Unexplained Infertility | 181 (60.1) | 42 (67.7) | Reference | |||
| Endometriosis | 120 (39.9) | 20 (32.3) | 0.71 (0.40-1.28) | 0.26 | 0.66 (0.36-1.18) | 0.16 |
Adjusted for female age, BMI and duration of infertility in the logistic regression analysis. OR=Odds ratio, CI=Confidence interval, BMI- Body mass index
Table 5b.
Multivariate logistic regression analysis for live birth rate and clinical pregnancy rate (per woman)
| Study group | No live birth (n=215), n (%) | Live birth (n=57), n (%) | Unadjusted OR 95% CI | P | Adjusted OR (95%CI) | P |
|---|---|---|---|---|---|---|
| Unexplained Infertility | 134 (62.3) | 37 (64.9) | Reference | |||
| Endometriosis | 81 (37.7) | 20 (35.1) | 0.91 (0.50-1.66) | 0.84 | 0.78 (0.41-1.5) | 0.46 |
|
| ||||||
| Study group | No clinical pregnancy (n=210), n (%) | Clinical pregnancy (n=62), n (%) | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|
| ||||||
| Unexplained Infertility | 129 (61.4) | 42 (67.7) | Reference | |||
| Endometriosis | 81 (38.6) | 20 (32.3) | 0.81 (0.44-1.45) | 0.45 | 0.64 (0.34-1.12) | 0.17 |
Adjusted for female age, BMI and duration of infertility in the logistic regression analysis. OR=Odds ratio, CI=Confidence interval, BMI- Body mass index
We further conducted a subgroup analysis to assess whether surgical intervention by ablation or adhesiolysis in the endometriosis group was associated with IUI outcomes. We found no significant difference in LBR in women who underwent surgical intervention versus no intervention group (7/49 [14.3%] vs. 13/103 [12.6%], OR: 1.2; 95% CI: 0.43.1; P 0.77).
DISCUSSION
The current study found no significant difference in per cycle LBR or CLBR following OS and IUI in women with minimal/mild endometriosis versus unexplained. The miscarriage and multiple pregnancy rates were comparable between both the groups. In comparison with unexplained infertility, the findings of the current study suggest no impact of early endometriosis on the OS and IUI treatment outcomes.
In an earlier retrospective cohort study by Werbrouck et al., (n = 259 cycles, 107 women) the authors investigated the effectiveness of OS and IUI in women with laparoscopy treated minimal and mild endometriosis versus unexplained infertility.[9] The authors reported no significant difference in CPR as well as CCPR in women with minimal (21% and 70.2%) or mild endometriosis (18.9% and 68.2%) versus women diagnosed with unexplained infertility (20.5% and 66.5%) which is broadly in agreement with the current study.[9] Similarly, in another retrospective cohort study, which included 260 women also reported no significant difference in the pregnancy rates between women with different stages of endometriosis (10.7%) and unexplained infertility (17.9%) following OS-IUI.[14]
In contrast, a retrospective study evaluating the prognostic factors affecting OS and IUI results (811 cycles) reported a significantly lower pregnancy rates in endometriosis group (6.5%) compared to unexplained infertility (15%) which is not in agreement with the current study findings.[10] The reason for the contradictory finding could be due to differences in study population, stimulation protocol and treatment cycle numbers. A prospective cohort study reported significantly lower pregnancy rate in women with minimal and mild endometriosis (16.3%) versus the unexplained group (33.3%) which is contradictory to current study results.[15] The difference in results could be due to the inclusion of only primary cycle, performance of two IUIs within the same treatment cycle as well as differences in sample size.
In the Cochrane review, the authors concluded that laparoscopic ablation or excision of endometriosis lesion probably increases spontaneous pregnancy rate compared to diagnostic laparoscopy alone based on pooled results from three randomized controlled trials.[16] While studies have explored the impact of laparoscopic treatment of early endometriosis on natural conception, there is paucity of data on the benefit of surgical intervention on IUI treatment outcomes.[16,17] The current study found the LBR comparable within women who underwent surgical intervention versus no intervention in the sub population of women with endometriosis. However, these findings should be interpreted with caution and considered only for hypothesis generation.
The current study is one of the few studies which has reported live birth outcome. The results of the study also suggest that technological advances and improvement in clinical skills may allow clinicians to rely lesser on invasive techniques for evaluation of infertility, especially since final treatment outcomes are comparable. The included control population of unexplained infertility was homogenous due to inclusion of only women who underwent DHL for tubal assessment. Excluding those women who were diagnosed with unexplained infertility following HSG and other tubal evaluation methods eliminated the possibility of including participants with early endometriosis, thus increasing the validity of the results. The study sample size was modest. Limitations include retrospective design and data from a single centre and the power of the current study after post hoc analysis was calculated to be 20%.
Furthermore, only limited number of participants completed three treatment cycles which could introduce some attrition bias. In addition, a prolonged study period of 7 years may have introduced an unintentional bias in the observations due to different clinicians and laboratory staff; although these variations do reflect a real life scenario. The stimulation protocol for OS-IUI however has remained fairly unchanged during the study period.
CONCLUSION
The current study did not find any significant differences in CLBR and CPR following OS-IUI in women with minimal or mild endometriosis and unexplained infertility. The current findings suggest no impact of early endometriosis on OS and IUI treatment outcomes. Less invasive tubal assessment methods such as HSG or HyCoSy should be considered to simplify the fertility work-up. There is a need for larger prospective studies to validate the current study findings. In addition, the impact of excisional surgeries on the IUI treatment outcomes should also be explored to clearly establish the role of DHL in the fertility work-up.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr Mohan S Kamath is a deputy editor with the JHRS, but he has had no role in the blinded peer-review or editorial process pertaining to this manuscript.
Data availability statement
Data shall be available from the corresponding author upon specific request.
Acknowledgement
We thank all the couples who underwent treatment in our department.
REFERENCES
- 1.Thoma M, Fledderjohann J, Cox C, Kantum Adageba R, editors. In: Oxford Encyclopedia of Sexual and Reproductive Health. Oxford: Oxford University Press; 2021. Biological and social aspects of human infertility: A global perspective. [Google Scholar]
- 2.World Health Organization (WHO) International Classification of Diseases, 11th Revision (ICD-11) Geneva: WHO; 2018. [Google Scholar]
- 3.Gerrits T, Van Rooij F, Esho T, Ndegwa W, Goossens J, Bilajbegovic A, et al. Infertility in the Global South: Raising awareness and generating insights for policy and practice. Facts Views Vis Obgyn. 2017;9:39–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Kamath MS, Bhattacharya S. Demographics of infertility and management of unexplained infertility. Best Pract Res Clin Obstet Gynaecol. 2012;26:729–38. doi: 10.1016/j.bpobgyn.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Collins JA, Van Steirteghem A. Overall prognosis with current treatment of infertility. Hum Reprod Update. 2004;10:309–16. doi: 10.1093/humupd/dmh029. [DOI] [PubMed] [Google Scholar]
- 6.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female:A committee opinion. Fertil Steril. 2015;103:e44–50. doi: 10.1016/j.fertnstert.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Gleicher N, Barad D. Unexplained infertility: Does it really exist? Hum Reprod. 2006;21:1951–5. doi: 10.1093/humrep/del135. [DOI] [PubMed] [Google Scholar]
- 8.Evers JL. Female subfertility. Lancet. 2002;360:151–9. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 9.Werbrouck E, Spiessens C, Meuleman C, D’Hooghe T. No difference in cycle pregnancy rate and in cumulative live-birth rate between women with surgically treated minimal to mild endometriosis and women with unexplained infertility after controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril. 2006;86:566–71. doi: 10.1016/j.fertnstert.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 10.Nuojua-Huttunen S, Tomas C, Bloigu R, Tuomivaara L, Martikainen H. Intrauterine insemination treatment in subfertility: An analysis of factors affecting outcome. Hum Reprod. 1999;14:698–703. doi: 10.1093/humrep/14.3.698. [DOI] [PubMed] [Google Scholar]
- 11.Jeon YE, Jung JA, Kim HY, Seo SK, Cho S, Choi YS, et al. Predictive factors for pregnancy during the first four intrauterine insemination cycles using gonadotropin. Gynecol Endocrinol. 2013;29:834–8. doi: 10.3109/09513590.2013.808324. [DOI] [PubMed] [Google Scholar]
- 12.Canis M, Donnez JG, Guzick DS, Halme JK, Rock JA, Schenken RS, et al. (1997) Revised American Society for Reproductive Medicine Classification of Endometriosis. Fertility and Sterility. 1996;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 6. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 14.Göker EN, Ozçakir HT, Terek MC, Levi R, Adakan S, Tavmergen E. Controlled ovarian hyperstimulation and intrauterine insemination for infertility associated with endometriosis: A retrospective analysis. Arch Gynecol Obstet. 2002;266:21–4. doi: 10.1007/pl00007492. [DOI] [PubMed] [Google Scholar]
- 15.Omland AK, Tanbo T, Dale PO, Abyholm T. Artificial insemination by husband in unexplained infertility compared with infertility associated with peritoneal endometriosis. Hum Reprod. 1998;13:2602–5. doi: 10.1093/humrep/13.9.2602. [DOI] [PubMed] [Google Scholar]
- 16.Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JM. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. 2020;10:CD011031. doi: 10.1002/14651858.CD011031.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parazzini F. Ablation of lesions or no treatment in minimal-mild endometriosis in infertile women: A randomized trial. Gruppo Italiano per lo Studio dell’Endometriosi. Hum Reprod. 1999;14:1332–4. doi: 10.1093/humrep/14.5.1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data shall be available from the corresponding author upon specific request.


