Abstract
Most Klebsiella pneumoniae clinical isolates are fully encapsulated and adhere in vitro to intestinal cell lines with an aggregative pattern. In this study, the influence of the capsule on interactions with epithelial cells was investigated by creating an isogenic mutant defective in the synthesis of the capsule. Determination of the uronic acid content of bacterial extracts confirmed that the mutant did not produce capsular polysaccharides whereas, with the wild-type strain, the level of encapsulation was growth phase dependent and reached a maximum during the lag and early log phases. Assays performed with different epithelial cell lines, Int-407, A-549, and HEp-2, showed that the capsule-defective mutant demonstrated greater adhesion than did the wild-type strain and that the aggregative pattern was maintained, indicating that the capsule was not related to the adhesion phenotype. In contrast, when the mucus-producing HT-29-MTX cells were used, the encapsulated wild-type strain adhered more strongly than did the capsule-defective mutant. No invasion properties were observed with any of the capsular phenotypes or cell lines used. The K. pneumoniae adhesin CF29K was detected by Western blot analysis and enzyme-linked immunosorbent assay on the surface of transconjugants obtained after transfer of a conjugative plasmid harboring the CF29K-encoding genes into both the wild-type and the capsule-defective strains. The amounts of adhesin detected were greater in the capsule-defective background strain than in the wild-type encapsulated strain and were associated with an increase in the level of adhesion to Caco-2 cells. Moreover, RNA slot blot experiments showed that transcription of the adhesin-encoding gene was markedly increased in the capsule-defective mutant compared to the wild-type encapsulated background. These results suggest (i) that the capsule plays an active role during the initial steps of the pathogenesis by interacting with mucus-producing cells but is subsequently not required for the adhesin-related interaction with the epithelial cell surface and (ii) that the expression of the adhesin is modulated by the presence of a capsule at a transcriptional level.
Klebsiella pneumoniae is a paradigm of opportunistic bacteria among gram-negative bacilli responsible for nosocomial infections. Extended-spectrum β-lactamase production, an event probably linked to a growing use of cephalosporins as therapeutic agents, was first described in this species. However, antibiotic selection pressure is not sufficient to explain the persistence and spread of opportunistic strains. Previous epidemiological studies showed that nosocomial infections due to K. pneumoniae are preceded by colonization of the gastrointestinal tract (7, 21). Contamination of different sites of the body from this reservoir is likely to occur via exogenous or endogenous processes.
The colonization of mucous membranes by bacteria is linked to an adhesion process involving specific adhesins on the bacterial surface (16). Several pili involved in either adhesion to bladder epithelial and tracheal ciliated cells (type 1 pili) or adhesion to kidney epithelium through the type V collagen (type 3 pili) have been described in K. pneumoniae isolates (12, 13, 33). To reproduce the interactions between K. pneumoniae and the intestinal mucus, Caco-2 and Intestine-407 cell lines have been used in in vitro adhesion assays (6, 9). Three adhesion phenotypes have been described to date: a diffuse pattern of adhesion where bacteria spread all over the cell surface, an aggregative phenotype in which bacteria clump onto the cells, and a localized pattern associated with microcolonies (15, 20). A nonfimbrial adhesin named CF29K, as well as a fimbrial one, KPF28, have been shown to be responsible for the diffuse adhesion phenotype (6, 9). No such adhesins have been characterized in K. pneumoniae strains showing either aggregative or localized phenotypes. Electron microscopic observations revealed that bacteria expressing aggregative adhesion are surrounded by a capsule material which is probably involved in bacterium-eucaryotic cell and bacterium-bacterium interactions (15).
Little is known about the involvement of capsular polysaccharides in bacterial interactions with mucosal surfaces. The presence of a capsule was shown to reduce the adhesion of enterotoxigenic Escherichia coli to pig intestinal epithelial cells (27) and of Neisseria meningitidis to both mucosal and Chang cells (29, 35, 36). Similar results were obtained when comparing a wild-type Haemophilus influenzae type b isolate with its capsule-deficient mutant in adhesion assays with Chang epithelial cells (31). It was recently demonstrated that the capsule of H. influenzae type b inhibits adhesion by masking the bacterial fibrils and therefore impairing the bacterium-eucaryotic cell receptor recognition (30). Thus, in at least some cases, capsules may reduce adherence rather than mediating it.
Since most clinical and environmental isolates of K. pneumoniae are fully encapsulated, it is likely that the first contacts with human mucosal surfaces occur via bacterial capsular polysaccharides. The following experiments were undertaken to demonstrate whether the capsule was involved in the interactions of K. pneumoniae with epithelial cells. We created a capsule-defective mutant of a K. pneumoniae aggregative isolate, taking advantage of the recent description by Arakawa et al. of the genomic organisation of the 29-kb region responsible for the K. pneumoniae capsular polysaccharide synthesis (2). The nucleotide sequence analysis revealed 19 open reading frames (ORF), some of which were highly conserved among the different capsular phenotypes. In this paper we describe the mutant construction by allelic exchange techniques and present evidence of the role of capsule in the interactions with several epithelial cell lines. We also discuss CF29K adhesin expression at the cell surface according to the level of bacterial encapsulation.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Bacterial strains were stored at −20°C in Luria-Bertani (LB) broth (Difco, Paris, France) containing 15% glycerol and were cultivated on LB agar supplemented with the appropriate antibiotics at the following concentrations: ampicillin, 50 μg/ml; kanamycin, 20 μg/ml; or tetracycline, 20 μg/ml. Bacterial growth curves were obtained with cultures in LB broth; fresh medium was inoculated (1:50) with an overnight culture of the K. pneumoniae strain and was incubated at 37°C in a shaker for 8 h. Bacterial growth was monitored by measuring the optical density at 620 nm, and the numbers of CFU were quantified by plating serial dilutions of the suspension onto LB agar plates. For quantification of capsular polysaccharides, K. pneumoniae strains were cultivated in D.W. medium supplemented with 0.1% Casamino Acids, 200 μg of MgCl2 per ml, 20 μg of CaCl2 per ml, 1 μg of ZnCl2 per ml, and 4 μg of FeCl3 per ml (10). Cultures were performed by inoculating fresh D.W. medium with an overnight culture broth (1:50) and incubated at 37°C for 8 h.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| K. pneumoniae | ||
| LM21 | Wild type; K35 | This study |
| LM21 Rifr | K. pneumoniae LM21, Rifr | This study |
| LM21(cps) | K. pneumoniae LM21, Rifr, Knr, capsule-defective mutant | This study |
| CF504 | Clinical isolate carrying cf29K | 6 |
| CH067 | Transconjugant from LM21, Rifr and CF504 | This study |
| CH009 | Transconjugant from LM21(cps) and CF504 | This study |
| E. coli | ||
| CF604 | Transconjugant harboring pCFF504 | 6 |
| JM109 | Cloning strain | Promega |
| CJ236 | dam cloning strain | 17 |
| BW20767 | pir+ strain used for conjugative transfer | 24 |
| BW21038 | Cloning strain, lacI lacZα Cmrpir+ | 24 |
| Plasmids | ||
| pUC18 | Apr | Boehringer |
| pUC4k | Knr; source of Knr cassette | Pharmacia |
| pLD55 | Suicide vector, derivative of pBluescript II (SK+), Tetr | 24 |
| pS1-3 | pUC18 plus 2,560 bp of cps region | This study |
| pS1-3Δ | pS1-3 with 509-bp deletion | This study |
| pS1-3ΔKn | pS1-3Δ plus Knr | This study |
| pSFB1-3ΔKn | pLD55 containing orf1–3ΔKn | This study |
Cell cultures and adhesion assays.
Cell lines included Intestine-407 (Int-407) cells (ATCC CCL6) derived from human embryonic jejunum and ileum, Caco-2 cells derived from a human colon carcinoma, HEp-2 cells (ATCC CCL23) derived from a human laryngeal carcinoma, A-549 cells (ATCC CCL185) derived from a human lung carcinoma, and HT-29-Rev MTX 10−6, a mucus-secreting cell line derived from a human colon carcinoma (19). Both Caco-2 and HT-29-Rev MTX 10−6 cells were kindly provided by A. Zweibaum (Unité de Recherche sur la Différenciation Cellulaire Intestinale, INSERM U178, Villejuif, France).
Both Int-407 and HEp-2 cells were maintained in Eagle minimal essential medium (Eagle MEM) (Seromed, Poly Labo, Strasbourg, France) supplemented with 10% heat-inactivated fetal calf serum (Seromed), 1% l-glutamine, 1% nonessential amino acids, 10,000 U of penicillin per ml, 10 μg of streptomycin per ml, and 25 μg of amphotericin B per ml in an atmosphere of 5% CO2. A-549 cells were cultivated under the same conditions, except that Ham’s F-12 minimal medium (Seromed) was used instead of Eagle MEM. Caco-2 and HT-29-Rev MTX 10−6 cells were cultivated as described above, but minimal medium was Dulbecco MEM (Seromed) supplemented with 20 or 10% fetal calf serum under an atmosphere of 10% CO2.
Adhesion to the different cell lines was assayed in the presence of d-mannose to block type 1 pilus-mediated adherence as described previously (15), but to relate adhesion to specific physiological states, samples of bacteria collected at different points of the growth curve were incubated with epithelial cells for a shorter time, i.e., 1 h instead of 3 h. Confluent monolayers (6 × 105 epithelial cells) in tissue culture plate wells were rinsed twice with 1 ml of phosphate-buffered saline (PBS; pH 7.4). For each assay, a suspension of 109 viable bacteria in the appropriate medium containing 2% d-mannose was inoculated onto the cells and allowed to adhere at 37°C in a 10% CO2 atmosphere. After incubation, each well was rinsed three times with PBS. Adherent bacteria were released by addition of 0.5% Triton X-100 (Sigma) and quantified by plating appropriate dilutions on LB agar plates. Adhesion was expressed as the number of CFU adherent to eucaryotic cells per monolayer.
For qualitative adhesion experiments, assays were performed as described above, except that eucaryotic cells were seeded onto glass coverslips. After being washed, the cells were fixed with methanol, stained with 20% Giemsa, and examined under a light microscope.
Invasion assays.
Confluent monolayers of the different cell lines were rinsed twice with 1 ml of PBS. Bacteria (109) were suspended in the appropriate minimal medium containing 2% d-mannose and added to the cells. After 1 h of incubation at 37°C, the cell monolayers were rinsed four times with 1 ml of PBS, and gentamicin was added to each well at 100 μg/ml. After 3 h of incubation, four washes were performed with PBS and intracellular bacteria were released by addition of 1 ml of 0.5% Triton X-100. Appropriate dilutions of the resulting suspension were plated on LB agar to quantify intracellular bacteria.
Extraction and quantitation of capsular polysaccharides.
Capsular polysaccharides were extracted according to the method previously described by Domenico et al. (10). Samples (500 μl) of bacterial cultures were removed at various times and mixed with 100 μl of 1% Zwittergent 3-14 detergent (Calbiochem, Meudon, France) in 100 mM citric acid (pH 2.0). This mixture was incubated at 50°C for 20 min. After it was centrifuged for 5 min at 14,000 rpm in a no. 5415C Eppendorf centrifuge, 300 μl of the supernatant was transferred to a new tube and absolute ethanol was added to a final concentration of 80%. The mixture was placed at 4°C for 20 min. After centrifugation (14,000 rpm), the supernatant was decanted and the pellet was dissolved in 200 μl of distilled water.
Polysaccharides were then quantitated by measuring the amount of uronic acid (4). A 1,200-μl volume of 0.0125 M tetraborate in concentrated H2SO4 was added to 200 μl of the sample to be tested. The mixture was vigorously vortexed and heated in a boiling-water bath for 5 min. The mixture was cooled, and 20 μl of 0.15% 3-hydroxydiphenol (Sigma-Aldrich Chimie, L’Isle d’Abeau, France) in 0.5% NaOH was added. The tubes were shaken, and absorbance measurements were made at 520 nm. The uronic acid concentration in each sample was determined from a standard curve of d-mannuronic acid (Sigma-Aldrich Chimie). In parallel, serial dilutions of the bacterial culture were plated to determine the number of CFU. The uronic acid content was expressed in nanograms per 106 CFU.
Construction of a capsule-defective mutant.
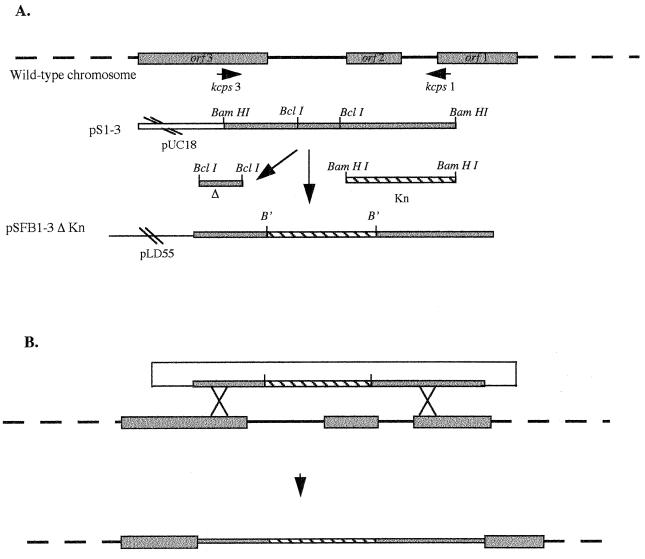
The genomic organization of a serotype K2 K. pneumoniae isolate cps region responsible for capsular polysaccharide synthesis has been determined by Arakawa et al. (2). Only some parts of this 29-kb region are highly conserved among the different K serotypes as demonstrated by hybridization assays and PCR amplifications (2, 14). To create a capsule-defective mutant of K. pneumoniae LM21 (serotype K35), a 2,560-bp fragment was amplified from a highly conserved region including a putative promoter sequence of a polycistronic operon by using the following primers designed from the published K. pneumoniae Chedid (serotype K2) isolate sequence: kcps1, 5′-GCCGGATCCCCGCGAAAGCATTAA-3′; and kcps3, 5′-GCCGGATCCGTTAGATTTTCGGCA-3′. Chromosomal DNA isolated from the wild-type K. pneumoniae LM21 was subjected to 30 cycles of 1 min of denaturation at 94°C, 1 min of annealing at 55°C, and 4 min of extension at 68°C. The resulting 2,560-bp PCR product was cloned into the BamHI site of pUC18 (Boehringer Mannheim, Meylan, France), resulting in plasmid pS1-3. This plasmid was then deleted from 509 bp internal to the cloned fragment and comprising the ς54-dependent promoter region located just upstream of the first ORF (ORF3) of a polycistronic structure by digesting with BclI and self-ligating to form pS1-3Δ. An aph3′ kanamycin resistance-encoding cassette (Pharmacia Biotech, Saclay, France), including a translation termination, digested by BamHI was inserted into the BclI site of plasmid pS1-3Δ. The resulting plasmid, pS1-3ΔKn, was then digested with BamHI. The cloned fragment was subcloned into the BamHI site of the π-dependent suicide vector pLD55, a lambda pir vector (24), to form plasmid pSFB1-3ΔKn and was transformed into the host strain, Escherichia coli BW21038. Plasmid pSFB1-3ΔKn was introduced by electroporation in E. coli BW20767, a host used for maintenance and conjugative transfer of oriR6k oriRP4 plasmids. pSFB1-3ΔKn was then introduced into K. pneumoniae LM21Rif by mating experiments carried out by patch mating. The resulting colonies were streaked out onto counterselection agar plates containing tetracycline (20 μg/ml) and kanamycin (20 μg/ml). The transconjugants resulting from a double recombination event were screened for the loss of tetracycline resistance.
Southern hybridization.
Chromosomal DNA was prepared from wild-type K. pneumoniae LM21 and from mutant LM21(cps) by the method described by Sambrook et al. (28). The chromosomal DNA was digested with appropriate restriction endonucleases and separated by agarose gel electrophoresis (0.7% agarose). The probes used contained either the kanamycin cassette or an internal fragment from the published orf3 K2 capsule sequence (2). The DNA probes were labelled with [α-32P]dATP by using the random-primed DNA-labelling kit (Boehringer Mannheim), and hybridization experiments were performed under high-stringency conditions with Rapid-hyb buffer (Amersham France, Les Ulis, France) as recommended by the manufacturer.
Mating experiments between K. pneumoniae strains.
Conjugation experiments were carried out as previously described (6). An R plasmid harboring the CF29K adhesin-encoding gene together with the extended-spectrum β-lactamase-encoding gene was transferred from the wild-type K. pneumoniae CF504 to K. pneumoniae LM21 Rifr and to K. pneumoniae LM21(cps). Transconjugants were selected on Mueller-Hinton agar (Sanofi-Pasteur, Marnes la Coquette, France) containing rifampin (300 μg/ml) and ceftazidime (2 μg/ml).
Bacterial surface protein analysis.
Bacterial surface proteins were extracted as previously described (6) by heating at 60°C for 20 min with gentle agitation. After centrifugation at 10,000 × g for 20 min, the supernatant was brought to pH 4.0. The precipitated proteins were collected by centrifugation and resuspended in PBS. Extracted proteins (15 μg for each sample) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with 12% (wt/vol) acrylamide for the separation gel (18). Western immunoblotting conditions for the adsorbed anti-CF29K antiserum were reported previously (6).
Quantitative analysis of the CF29K adhesin by ELISA.
The amount of CF29K adhesin expressed from the same plasmid-encoded gene in the different strain backgrounds was estimated by an enzyme-linked immunosorbent assay (ELISA) with optimal concentrations of anti-CF29K polyclonal antibody (6) and anti-CS31A biotinylated rabbit immunoglobulin G recognizing the CF29K adhesin and provided by J. P. Girardeau (Laboratoire de Microbiologie, Institut National de la Recherche Agronomique, Theix, France). ELISA microtiter plates (ICN Biomedicals, Orsay, France) were coated overnight with anti-CF29K antibodies. After nonspecific binding sites were blocked by addition of 1% bovine serum albumin in PBS, whole bacteria were added to the wells and incubated for 2 h at 37°C. After several washes with 0.1% Tween 20 in PBS, biotin-labelled anti-CS31A antibodies were added in excess and a streptavidin-alkaline phosphatase complex (Bio-Rad, Ivry Sur Seine, France) was added. For signal development, the wells were incubated with p-nitrophenylphosphate (Sigma-Aldrich) and the optical density was determined at a wavelength of 405 nm.
RNA slot blot analysis.
Total cellular RNAs were extracted from K. pneumoniae CH067 and CH009 grown at 37°C in LB broth. Briefly, cells from a 10-ml culture were suspended in 10 ml of protoplasting buffer (15 mM Tris HCl [pH 8.0], 0.45 M sucrose, 8 mM EDTA) containing 4 mg of lysozyme. The protoplasts were collected by centrifugation, resuspended in 0.5 ml of lysing buffer (10 mM Tris HCl [pH 8.0], 10 mM NaCl, 1 mM sodium citrate, 1.5% sodium dodecyl sulfate) containing 15 μl of diethylpyrocarbonate (Sigma), and incubated for 5 min at 37°C. After addition of 250 μl of saturated NaCl, the mixture was incubated for 10 min on ice and centrifuged. The supernatant containing total RNAs was precipitated by addition of ethanol. The RNA pellet was finally suspended in 100 μl of diethylpyrocarbonate-treated water. RNA concentrations were determined spectrophotometrically at 260 nm, and purity was evaluated by the ratio of absorption at 260 to 280 nm. RNA extracts (1 and 5 μg) were then spotted onto Immobilon N+ (Amersham, Les Ulis, France), and hybridization was performed with the Amersham rapid hybridization system and the following two DNA probes: an HpaI-EcoRV 0.8-kb fragment internal to cf29A, prepared by digestion of plasmid pCFF15 (8), and an rRNA-specific probe consisting of a 7.0-kb restriction fragment from plasmid pKK3535, carrying the entire rrnB operon of E. coli K-12 and the flanking sequences spanning the 16S, 23S, and 5S rRNA regions (5). The probes were radiolabelled with [α-32P]dATP by using the random-primed DNA-labelling kit (Boehringer Mannheim).
RESULTS
Construction of an isogenic capsule-defective mutant.
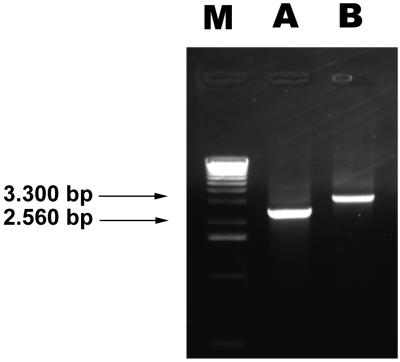
To construct a mutant impaired in capsule synthesis, a 2,560-bp DNA fragment from the capsule-encoding region of K. pneumoniae LM21 was amplified by PCR with primers complementary to regions of ORF1 and ORF3 (Fig. 1) of the Klebsiella cps region previously published by Arakawa et al. (2). The whole cps region includes 19 ORFs, 13 of them (ORF3 to ORF15) being part of a same transcriptional unit. Only a few ORFs have been assigned a function, mostly by homology to other species cps clusters; they are implicated in either the assembly of polysaccharides or their transport to the cell surface. Our strategy was based upon the high sequence homology observed between the different K serotypes in the 5′ end of the capsule-encoding region, allowing amplification of the DNA fragment whatever the serotype. An internal 509-bp fragment encompassing the potential promoter region of the 13-ORF polycistronic operon of the amplified DNA fragment was deleted and replaced by a kanamycin-encoding cassette, and the resulting sequence was cloned into the suicide vector, pLD55. The resulting recombinant plasmid, pSFB1-3ΔKn, harboring 1,200 and 850 bp of K. pneumoniae LM21 genomic DNA on the 5′ and 3′ regions, respectively, of the kanamycin cassette, was then introduced by conjugational transfer into K. pneumoniae LM21Rif, giving rise to 122 kanamycin-resistant clones. These colonies were streaked out onto agar plates containing both kanamycin and tetracycline to screen for the loss of the plasmid (Tets) and integration of the mutated copy into the cps operon (Knr). The occurrence of a double recombination event was checked by PCR with the same primers as the ones used in the initial amplification, i.e., kcps1 and kcps3. As shown in Fig. 2, a 2,560-bp DNA fragment was observed when genomic DNA from the wild-type K. pneumoniae LM21 was used as a template (Fig. 2, lane A) whereas a 3,300-bp fragment, corresponding to the 1,250 bp of the kanamycin resistance encoding-cassette and the 2,050 bp of the cps region, was amplified with genomic DNA from the mutant K. pneumoniae LM21(cps) (lane B). The occurrence of a double recombination event was further confirmed by southern hybridization experiments with genomic DNA from the mutant and DNA probes specific for both the cps region (a 600-bp DNA fragment) and the Knr-encoding cassette (data not shown).
FIG. 1.
Construction of the capsule-defective mutant K. pneumoniae LM21(cps). (A) A 2,560-bp DNA fragment was amplified from K. pneumoniae LM21 chromosomal DNA by PCR and ligated into the BamHI site of pUC18 to obtain pS1-3. An internal BclI 509-bp fragment was deleted and replaced by the Knr cassette, and the construct was cloned into the BamHI site of pLD55. B′, BamHI-BclI junctions. (B) Allelic exchange mutagenesis. After introducing pSFB1-3ΔKn into K. pneumoniae LM21, a single recombination event leads to the formation of a cointegrate (Knr Tetr) and a double recombination event leads to the mutated copy (Knr) after loss of the suicide vector (Tetr).
FIG. 2.
Electrophoretic analysis of the amplified products from the K. pneumoniae LM21 wild-type strain (lane A) and the isogenic mutant strain LM21(cps) (lane B) with primers kcps1 and kcps3. The 2,560- and 3,300-bp fragments (arrows) are indicated. Lane M contains the 1-kb molecular size ladder (Gibco BRL, Cergy-Pontoise, France).
Characterization of capsular polysaccharide production by the mutant.
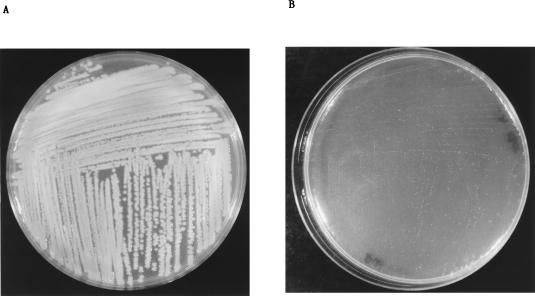
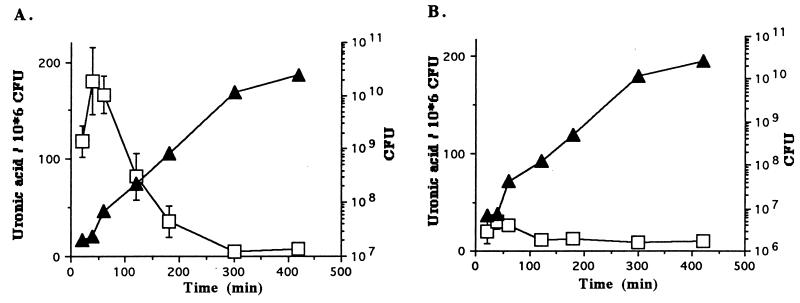
As shown in Fig. 3, the colonies obtained after overnight culture of the K. pneumoniae mutant on agar plates were quite different from the ones obtained with wild-type K. pneumoniae LM21; they were much smaller and did not exhibit the smooth phenotype characteristic of surface polysaccharide-producing colonies. Since capsule synthesis is known to be dependent on the physiological state (23), specific quantitation of capsular polysaccharides was performed with both the wild-type and mutant strains at different times of the growth curve. With the wild-type K. pneumoniae strain (Fig. 4A), the amount of uronic acid reached a maximum during the lag and the early log phases (180.2 ng of uronic acid/106 CFU after 40 min of incubation) and then constantly declined. After 300 min, the level of capsule remained minimal (5.5 ng of uronic acid/106 CFU). Similar experiments performed with the mutant strain showed that the level of uronic acid remained low (below 30 ng/106 CFU) at all points of the growth curve (Fig. 4B), indicating that this mutant was in fact defective in the synthesis of capsular polysaccharides.
FIG. 3.
Aspects of the colonies of K. pneumoniae LM21 (A) and its capsule-defective mutant strain LM21(cps) (B) after overnight culture at 37°C on D.W. agar plates.
FIG. 4.
Plots of capsule quantities related to the growth phase of wild-type K. pneumoniae LM21 (A) and mutant K. pneumoniae LM21(cps) (B). Growth curves were determined by measuring the number of CFU (solid triangles). Capsule was quantified by measuring the quantity of uronic acid per 106 CFU (open squares). Values for uronic acid contents are the mean of measurements made in triplicate.
Influence of the capsule on the interactions of K. pneumoniae with epithelial cells.
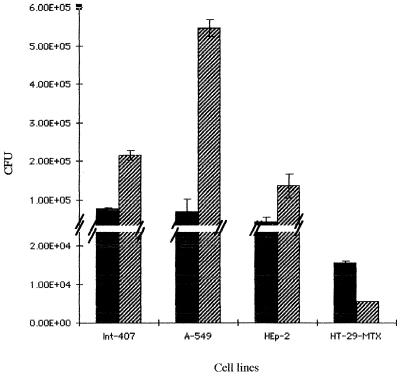
Previous adhesion experiments performed with samples of the wild-type K. pneumoniae LM21 removed at various time of the growth curve revealed that the level of adhesion to Int-407 cells was inversely proportional to the level of capsule detected; i.e., adhesion was minimal during the lag and early log phases and maximal with bacterial samples taken during the late exponential and stationary phases (data not shown). For these reasons and because we wished to compare the behavior of the wild-type capsule-producing strain with its capsule-defective mutant, we performed adhesion assays with bacteria collected during the early log phase (2 h postinoculation) and incubated for 1 h only with eucaryotic cells. The results obtained with the different cell lines, namely, Int-407, A-549, and HEp-2, cells are presented in Fig. 5. For all cell lines tested, the capsule-defective mutant adhered more than the wild-type strain; the greatest difference was observed with the type II pneumocyte-like cell line, A-549 (the level of adhesion being eight times higher for the mutant strain than for the wild-type strain). Microscopic observations after Giemsa staining showed bacteria from the mutant strain adhering to the epithelial cells via an aggregative phenotype, identical to the one observed with the wild-type strain (data not shown).
FIG. 5.
Adherence assays performed with Int-407, A-549, HEp-2 and HT-29-MTX 10−6 cell lines with the wild-type K. pneumoniae LM21 (solid bars) and the capsule-defective mutant LM21(cps) (hatched bars). The results are expressed in CFU per monolayer. The data are the mean of measurements made in triplicate.
Interactions with mucus-producing cells were investigated with HT-29-Rev MTX 10−6 differentiated cells. After 2 h of culture, bacteria were added to the confluent monolayer containing an average of 5 × 105 cells. Determination of the number of CFU associated with the cells indicated that the encapsulated wild-type strain adhered to these mucus-producing cells to a higher level (three times higher) than did the capsule-deficient strain, in contrast to the results obtained with the previous cell lines tested (Fig. 5).
To determine if the loss of capsule production would favor an invasion phenotype, gentamicin resistance assays were performed with both the capsule-defective mutant and the wild-type strain, using Int-407, HEp-2, and A-549 cells. Intracellular bacteria were detected with neither strain after 3 h of incubation in a gentamicin-containing medium.
Influence of the capsule on the expression of K. pneumoniae adhesin at the bacterial cell surface.
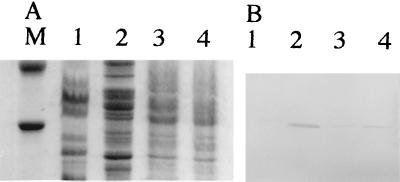
To determine the influence of encapsulation on the adhesin-mediated interaction with epithelial cells, we introduced the self-transmissible pCFF504 plasmid harboring the genes coding for a known adhesin from K. pneumoniae, the CF29K adhesin (6), into both the K. pneumoniae LM21 wild type and its capsule-defective mutant. Adhesion to Caco-2 cells was at least three times higher with transconjugants obtained with the capsule-defective mutant, CH009, as recipient than with the mutant itself, LM21(cps) (4.70 × 103 ± 0.83 × 103 and 1.35 × 103 ± 0.15 × 103 CFU, respectively), whereas no difference was observed between the wild-type strain, LM21, and its transconjugant, CH067, (2.24 × 102 ± 0.23 × 102 and 2.80 × 102 ± 0.18 × 102, respectively). Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of similar amounts of total bacterial surface protein extracts of the two types of transconjugants revealed that only transconjugants obtained with the mutant strain as recipient produced large amounts of the CF29K adhesin as detected by immunoblotting with specific anti-CF29K antibodies (Fig. 6B, lane 4). Plasmid pCFF504 had been previously introduced into an E. coli K-12 recipient strain (6), known to produce low levels of extracellular polysaccharides. The surface extract analysis revealed similar results, i.e., a higher quantity of CF29K protein detected with this transconjugant than with the K. pneumoniae CF504 donor wild-type strain (Fig. 6, lanes 1 and 2).
FIG. 6.
SDS-PAGE (A) and Western blot (B) of bacterial surface proteins from K. pneumoniae CF504 (lane 1), E. coli transconjugant CF604 (lane 2), K. pneumoniae CH067 (lane 3), and K. pneumoniae CH009 (lane 4). Molecular weight marker positions are shown for carbonic anhydrase (30,000) and ovalbumin (43,000).
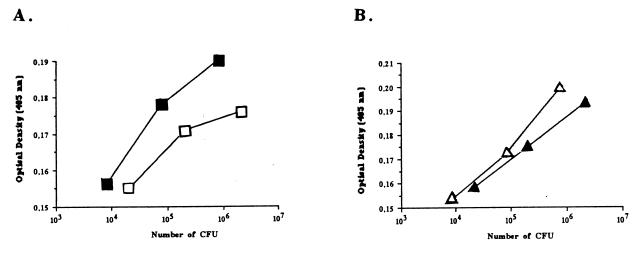
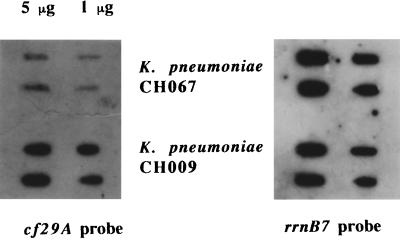
To quantify the differences observed in the previous immunoblot experiments, an ELISA was performed with antibodies raised against the CF29K protein and whole bacteria. Regardless of the number of bacteria used in the assay, the binding curves showed that the amounts of CF29K adhesin detected were greater with both the capsule-defective mutant and the E. coli K-12 backgrounds compared to the corresponding encapsulated wild-type K. pneumoniae strains, respectively LM21 and CF504 (Fig. 7). Finally, cf29A transcription was examined in both the wild-type and the capsule-defective transconjugants by probing RNA extracts with a cf29A-specific probe. As shown in Fig. 8, cf29A expression was strongly increased in the capsule-defective mutant compared to the fully encapsulated wild type.
FIG. 7.
Anti-CF29K antibody-binding analysis of the whole bacteria: K. pneumoniae CH067 and CH009 (A) and K. pneumoniae CF504 with its transconjugant E. coli CF604 (B). The abilities of the bacteria from different backgrounds to bind the anti-CF29K antibodies were measured by ELISA. Microtiter plates precoated with anti-CF29K antibodies were incubated with different quantities of the whole K. pneumoniae CH067 (open squares), K. pneumoniae CH009 (solid squares), K. pneumoniae CF504 (solid triangles), or E. coli CF604 (open triangles). To detect bound bacteria, wells were incubated with biotinylated anti-CS31A antiserum; streptavidin-alkaline phosphatase and an appropriate substrate were added, and the optical density at 405 nm was monitored. The optical density measured at 405 nm from the negative control (no bacteria added) was equal to 0.130.
FIG. 8.
Regulation of cf29A transcription by the presence of capsule. For the RNA slot-blot analysis: 1 or 5 μg of RNA isolated from K. pneumoniae CH067 (encapsulated) and CH009 (nonencapsulated) was hybridized with a cf29A-specific probe (left). The same blot was probed with an rrnB7 probe as a standard for the total amount loaded onto the membrane (right).
DISCUSSION
A mutant defective in the synthesis of capsule was created by allelic exchange by using a lambda pir-dependent vector in a K. pneumoniae strain. Based on the genomic sequence encoding the capsule of a K2 serotype K. pneumoniae isolate (2), a DNA fragment was amplified from the K. pneumoniae LM21 wild-type strain serotype K35 in a conserved capsule-encoding region. This fragment was deleted from an internal sequence encompassing the putative promoter sequence of a polycistronic operon involved in both capsule polysaccharide assembly and transport to the cell surface. An aph3′ cassette was inserted in this deleted fragment, and a double-recombination event was screened after introducing this construction in a λ-pir-dependent vector. To our knowledge, this is the first report of the use of a λ-pir system in bacteria belonging to the genus Klebsiella. The resulting modification in capsular polysaccharide expression at the cell surface was appreciable by streaking out the mutant on an agar plate. Because K. pneumoniae serotype K35 capsular polysaccharides include acidic saccharides such as uronic acids (11), the level of encapsulation was estimated by quantifying uronic acids. We demonstrated that the mutant strain had at least sevenfold less capsular material than the wild type and that the capsule levels observed in the mutant strain were similar to background levels observed with other nonencapsulated K. pneumoniae strains (14, 25).
We noted that the level of encapsulation of the K. pneumoniae wild-type strain was affected by the growth phase. This level was maximal 40 min after the beginning of growth and decreased during the exponential and stationary phases. These results are in agreement with a previous study showing that a K1 K. pneumoniae strain produced maximum levels of capsular polysaccharides at low growth rate, i.e., when bacteria are dividing slowly (23). Moreover, Whitfield et al. (38) demonstrated that the synthesis of E. coli K1 capsule occurred 10 min after a temperature upshift and no further increase occurred after 45 min. Since the biosynthesis of capsule in K. pneumoniae is controlled by a two-component regulatory system (1, 22), it is likely that encapsulation is modulated by the environmental milieu and can shift very quickly.
In a previous study, we showed that the adhesion of a K. pneumoniae aggregative isolate to Int-407 cells was maximal at the end of the exponential phase and during the stationary phase (15). Similar results were obtained with the wild-type K. pneumoniae LM21 in the present study. Thus, the expression of the adhesion phenotype is inversely correlated with the level of encapsulation, suggesting that during infection, the loss of capsule favors the process of adhesion to epithelial cells.
We demonstrated by performing adhesion assays with the isogenic capsule-defective mutant and intestinal cell lines, laryngeal cells, and epithelial cells derived from the lungs that this strain adhered more efficiently than the encapsulated K. pneumoniae strain. The greatest adhesion was obtained with the A-549 cells derived from a human lung carcinoma. Since K. pneumoniae is a pathogen frequently involved in pneumonia, this type of cell could be the preferential target of this organism’s adhesins. The adhesion of the capsule-defective mutant was higher in all cell lines used, and the aggregative adhesion pattern was similar in all cases. This result demonstrated that the capsule itself was not responsible for the aggregative phenotype but in fact impaired adhesion to these cell lines. Several other examples of polysaccharide capsules disrupting bacterial interactions with epithelial cells have been reported: adhesion experiments with enterotoxigenic E. coli demonstrated that capsule inhibits the recognition of pig intestinal cells by K99 pili (27) and the presence of the group B streptococcus capsule attenuated the adherence of this bacteria to A-549 cells (32). Likewise, adhesion of Neisseria meningitidis to human epithelial cells was enhanced by the loss of the polysialic acid capsule (29). When the adhesion abilities of Haemophilus influenzae type b strain and its isogenic capsule-negative mutant were compared, it was found that the mutant strain demonstrated a greater adhesion to Chang epithelial cells (31).
Unlike the results obtained with the epithelial cell lines Int-407, A-549, and HEp-2, adhesion experiments performed with the mucus-producing HT-29-Rev MTX 10−6 cells revealed that the capsule-defective mutant adheres less than strongly the wild-type encapsulated strain. Although the difference was not very great, it was reproducible and would indicate that the presence of mucus components on the cell surface favors the establishment of interaction with polysaccharide-surrounded bacteria.
Introduction of an adhesin-encoding gene by conjugation into both the wild-type encapsulated strain and its capsule-defective mutant induced higher levels of adhesion to Caco-2 cells, but only with the latter transconjugant. Two independent possible explanations concerning the higher adhesion level observed with bacteria expressing little capsular polysaccharide material can be proposed. Because of the absence of capsule, the adhesin protein would be much more accessible at the cell surface. The masking of adherence factors by capsular polysaccharides has been previously observed with other bacterial species. The presence of a capsule in N. meningitidis isolates is known to reduce the Opa- and Opc-mediated adhesion to epithelial and endothelial cells, respectively (34, 36). Likewise, fibril-mediated adhesion to epithelial cells is inhibited by the presence of a capsule in H. influenzae type b (30). The second explanation is that the adhesin is overexpressed when synthesis of capsule is turned off. Western blot analysis of surface extracts from wild-type K. pneumoniae LM21 transformed with pCFF504 versus the capsule-defective corresponding transconjugant showed that the latter strain expressed a large amount of the adhesin whereas the CF29K protein was not detectable in the encapsulated strain. Darfeuille-Michaud et al. previously demonstrated that this adhesin was overexpressed in an E. coli background producing low levels of extracellular polysaccharide compared to the K. pneumoniae encapsulated parental strain (6). We verified this phenomenon by performing an ELISA in which whole bacteria were tested for the surface accessibility of the CF29K adhesin. Moreover, RNA analysis demonstrated that CF29K messengers were produced in larger quantities in the nonencapsulated background. Therefore, it is likely that the adhesin transcription is downregulated by a high level of bacterial encapsulation. This capsule-adhesin coregulation would correspond to a synchronized expression of the two surface components, which are both probably necessary but at different steps during the physiopathological process.
Since adhesion to A-549, Int-407, and HEp-2 cells was enhanced in the capsule-defective mutant, we were interested in knowing whether loss of the capsule also resulted in invasion. Indeed, it has been demonstrated in N. meningitidis that loss of the capsule was correlated with invasion properties (35, 37). However, in invasion assays performed with both the wild-type K. pneumoniae and its capsule-defective mutant, no invasion properties was observed in any of the cell lines tested. Since Oelschlaeger and Tall (26) recently described the ability of K. pneumoniae to invade cultured human epithelial cells in vitro under similar experimental conditions, it is likely that this property is strain related.
Taken together, these results indicate that (i) the presence of capsular polysaccharides at the K. pneumoniae cell surface partially inhibits adhesion to epithelial cells unless they produce mucus and (ii) the expression of a known adhesin at the bacterial cell surface is largely diminished when capsule is synthesized. We can speculate that there are steps during the mucosal surface colonization process where the capsule plays an active role by interacting with the mucus layer whereas its presence is a disadvantage when bacteria come in contact with the underlying epithelial cells. The expression of the two bacterial surface factors would be closely synchronized and influenced by external factors. It has been recently demonstrated with Salmonella typhi that the cell surface-associated polysaccharide (antigen Vi) prevents the secretion of both invasion proteins and flagellin and that this control occurs at both transcriptional and posttranscriptional levels and is environment dependent (3). Further studies are required to determine the genetic organization involved in the K. pneumoniae coordination of adhesin and capsule expression.
ACKNOWLEDGMENTS
We thank Jean-Pierre Girardeau, Dennis Hansen, Arlette Tridon, and Philip Domenico for giving relevant advice. We are grateful to Alain Zweibaum for providing mucus-producing HT-29-MTX cells, Michel Artur for providing plasmid pKK3535, and Kristin Swihart for critical reading of the manuscript.
REFERENCES
- 1.Allen P, Hart C, Saunders J. Isolation from Klebsiella and characterization of two rcs genes that activate colanic acid capsular biosynthesis in Escherichia coli. J Gen Microbiol. 1987;133:331–340. doi: 10.1099/00221287-133-2-331. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 1995;177:1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey P S, Popoff M Y. The RcsB-RscC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. doi: 10.1046/j.1365-2958.1998.00976.x. [DOI] [PubMed] [Google Scholar]
- 4.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 5.Brosius J, Ullrich A, Raker M A, Gray A, Dull T J, Gutell R R, Noller H F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981;6:112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- 6.Darfeuille-Michaud A, Jallat C, Aubel D, Sirot D, Rich C, Sirot J, Joly B. R-plasmid-encoded adhesive factor in Klebsiella pneumoniae strains responsible for human nosocomial infections. Infect Immun. 1992;60:44–45. doi: 10.1128/iai.60.1.44-55.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Champs C, Sauvant M P, Chanal C, Sirot D, Gazui N, Malhuret R, Baguet J C, Sirot J. Prospective survey of colonization and infection caused by expended-spectrum-β-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J Clin Microbiol. 1989;27:2887–2890. doi: 10.1128/jcm.27.12.2887-2890.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Martino P, Bertin Y, Girardeau J P, Livrelli V, Joly B, Darfeuille-Michaud A. Molecular characterization and adhesive properties of CF29K, an adhesin of Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immun. 1995;63:4336–4344. doi: 10.1128/iai.63.11.4336-4344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Martino P, Livrelli V, Sirot D, Joly B, Darfeuille-Michaud A. A new fimbrial antigen harbored by CAZ-5/SHV-4-producing Klebsiella pneumoniae strains involved in nosocomial infections. Infect Immun. 1996;64:2266–2273. doi: 10.1128/iai.64.6.2266-2273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domenico P, Schwartz S, Cunha B A. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutton G G S, Lim A V S. Structural investigation of the capsular polysaccharide of Klebsiella serotype K35. Carbohydr Res. 1985;145:67–80. doi: 10.1016/s0008-6215(00)90413-0. [DOI] [PubMed] [Google Scholar]
- 12.Fader R C, Avots-Avotin A E, Davies C P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979;25:729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fader R C, Gondesen K, Tolley B, Ritchie D G, Moller P. Evidence that in vitro adherence of Klebsiella pneumoniae to ciliated hamster tracheal cells is mediated by type 1 fimbriae. Infect Immun. 1988;56:3011–3013. doi: 10.1128/iai.56.11.3011-3013.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre-Bonte, S. 1998. Unpublished data.
- 15.Favre-Bonte S, Darfeuille-Michaud A, Forestier C. Aggregative adherence of Klebsiella pneumoniae to the human Intestine-407 cell line. Infect Immun. 1995;63:1318–1328. doi: 10.1128/iai.63.4.1318-1328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finlay B B, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53:210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forestier C, Welch R A. Identification of RTX toxin target cell specificity domains by use of hybrid genes. Infect Immun. 1991;59:4212–4220. doi: 10.1128/iai.59.11.4212-4220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29-MTX human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990;50:6334–6343. [PubMed] [Google Scholar]
- 20.Livrelli V, Champs C D, Martino P D, Darfeuille-Michaud A, Joly B. Adhesive properties, antibiotic resistance and pathogenicity of Klebsiella, Enterobacter, and Serratia clinical isolates. J Clin Microbiol. 1996;34:1963–1969. doi: 10.1128/jcm.34.8.1963-1969.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markowitz S M, Veazey J M, Macrino F L, Mayhall C G, Lamb V A. Sequential outbreaks of infection due to Klebsiella pneumoniae in a neonatal intensive care unit: implication of a conjugative R plasmid. J Infect Dis. 1980;142:106–112. doi: 10.1093/infdis/142.1.106. [DOI] [PubMed] [Google Scholar]
- 22.McCallum K L, Whitfield C. The rcsA gene of Klebsiella pneumoniae O1:K20 is involved in expression of the serotype-specific K (capsular) antigen. Infect Immun. 1991;59:494–502. doi: 10.1128/iai.59.2.494-502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mengistu Y, Edwards C, Saunders J. Continuous culture studies on the synthesis of capsular polysaccharide by Klebsiella pneumoniae K1. J Appl Bacteriol. 1994;76:424–430. doi: 10.1111/j.1365-2672.1994.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 24.Metcalf W W, Jiang W, Daniels L L, Kim S, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 25.Nassif X, Fournier J-M, Arondel J, Sansonetti P J. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect Immun. 1989;57:546–552. doi: 10.1128/iai.57.2.546-552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oelschlaeger T, Tall B. Invasion of cultured human epithelial cells by Klebsiella pneumoniae isolated from the urinary tract. Infect Immun. 1997;65:2950–2958. doi: 10.1128/iai.65.7.2950-2958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Runnels P L, Moon H W. Capsule reduces adherence of enterotoxigenic Escherichia coli to isolated intestinal epithelial cells of pigs. Infect Immun. 1984;45:737–740. doi: 10.1128/iai.45.3.737-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Stephens D S, Spellman P A, Swartley J S. Effect of the (α2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J Infect Dis. 1993;167:475–479. doi: 10.1093/infdis/167.2.475. [DOI] [PubMed] [Google Scholar]
- 30.St. Geme J W, III, Cutter D. Influence of pili, fibrils, and capsule on in vitro adherence by Haemophilus influenzae type b. Mol Microbiol. 1996;21:21–31. doi: 10.1046/j.1365-2958.1996.6241331.x. [DOI] [PubMed] [Google Scholar]
- 31.St. Geme J W, III, Falkow S. Loss of capsule expression by Haemophilus influenzae type b results in enhanced adherence to and invasion of human cells. Infect Immun. 1991;59:1325–1333. doi: 10.1128/iai.59.4.1325-1333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura G S, Kuypers J M, Smith S, Raff H, Rubens C E. Adherence of group B Streptococci to cultured epithelial cells: roles of environmental factors and bacterial surface components. Infect Immun. 1994;62:2450–2458. doi: 10.1128/iai.62.6.2450-2458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarkkanen A M, Allen B L, Westerlund B, Holthofer H, Kuusela P, Risteli L, Clegg S, Korhonen T K. Type V collagen as the target for type-3 fimbriae, enterobacterial adherence organelles. Mol Microbiol. 1990;4:1353–1361. doi: 10.1111/j.1365-2958.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 34.Virji M, Kayhty H, Fergusson D J P, Alexandrescu C, Heckels J E, Moxon E R. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5:1831–1841. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 35.Virji M, Makepeace K, Fergusson D J P, Achtman M, Moxon E R. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and andothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 36.Virji M, Makepeace K, Fergusson D J P, Achtman M, Sarkari J, Moxon E R. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992;6:2785–2795. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 37.Virji M, Makepeace K, Peak I R A, Fergusson D J P, Jennings M P, Moxon E R. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol Microbiol. 1995;18:741–754. doi: 10.1111/j.1365-2958.1995.mmi_18040741.x. [DOI] [PubMed] [Google Scholar]
- 38.Whitfield C, Virm E R, Costerton J W, Troy F A. Protein synthesis is required for in vivo activation of polysialic acid capsule synthesis in Escherichia coli K1. J Bacteriol. 1984;159:321–328. doi: 10.1128/jb.159.1.321-328.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]