Abstract
Insects have evolved highly diverse genetic sex-determination mechanisms and a relatively balanced male to female sex ratio is generally expected. However, selection may shift the optimal sex ratio while meiotic drive and endosymbiont manipulation can result in sex ratio distortion (SRD). Recent advances in sex chromosome genomics and CRISPR/Cas9-mediated genome editing brought significant insights into the molecular regulators of sex determination in an increasing number of insects and provided new ways to engineer SRD. We review these advances and discuss both naturally occurring and engineered SRD in the context of the Anthropocene. We emphasize SRD-mediated biological control of insects to help improve One Health, sustain agriculture, and conserve endangered species.
Introduction
Sex of an insect is determined by the chromosome complement it inherits from its parents. The chromosome systems that underly genetic sex-determination are quite diverse among insect species (reviewed in Bachtrog et al., 2014; Beukeboom and Perrin, 2014; Biedler and Tu, 2016). Flies and mosquitoes are among the many insects that evolved the XX/XY sex chromosome system, where the heterogametic (XY) individuals are males and the homogametic (XX) individuals are females. Lepidopterans such as the silkworm, Bombyx mori, evolved the ZZ/ZW sex chromosome system where ZZ males are the homogametic sex while heterogametic ZW individuals are females. Hemizygous sex chromosome systems are also found including XX/XO in several insect orders, where the males are hemizygous as they only have one X chromosome (XO); and ZZ/ZO in some lepidopteran and tricopteran species, where the females are hemizygous (ZO). Although species within an insect order tend to share the same type of sex chromosome system, variations can occur within an order or even a family. In the aforementioned systems, the sex of an offspring is determined by the genotype of the gamete of the heterogametic or hemigametic parent. In Hymenopteran and Thysanopteran insects, however, sex is instead determined by the haplodiploidy of the individual, where fertilized diploid eggs (2n) develop to females while unfertilized haploid eggs (n) develop as males (reviewed in Beukeboom and Perrin, 2014; Biedler and Tu, 2016).
In contrast to the apparent plasticity and diversity of the sex-determining chromosome systems, two highly conserved transcription factors doublesex (dsx) and fruitless (fru) control the development of sexual dimorphism in all insects studied thus far (Herpin and Schartl, 2015; Biedler and Tu, 2016; Hopkins and Kopp, 2021). Sex-specific isoforms of the DSX and FRU proteins, which result from sex-specific splicing of their primary RNA transcripts, program sexual differentiation (Figure 1). Therefore, sex determination is a process in which the primary sex-specific signals, which reflect the sex-specific chromosome composition of the early embryo, are transduced in a signal cascade to modulate sex-specific splicing of the RNA transcripts of dsx and fru. Figure 1 presents two simplified models using dsx as an example. In the vinegar fly Drosophila melanogaster, the double dosage of the X chromosome in the females (XX) initiates the transcription of the sex lethal (sxl) gene in the early embryo, leading to the production of the primary signal which is the SXL protein (Figure 1A). The presence of SXL leads to the production of a functional protein isoform of transformer (TRA), which in turn enables female-specific splicing of the primary RNA transcripts of dsx and fru. In the male (XY) embryo, the single X chromosome fails to initiate the production of the primary signal SXL, leading to the default male-specific splicing of dsx and fru transcripts and hence the production of male-specific DSX and FRU protein isoforms. In the Mediterranean fruit fly Ceratitis capitata, however, the default sex is female (Figure 1B). The primary signal, a male-determining factor (M factor) MoY (Meccariello et al., 2019), resides on the Y chromosome. The presence of the M factor in the early male (XY) embryos results in male-specific splicing of the tra pre-mRNA, leading to the production of a non-functional TRA protein and subsequently, male-specific splicing of dsx and fru. The lack of the Y chromosome in females (XX) enables the TRA protein complex to catalyze the female-specific splicing of dsx and fru. The TRA intermediate evolved much faster than DSX and FRU and TRA is not found in all insects (reviewed in Biedler and Tu, 2016). Recent advances have brought significant molecular insights into the diverse mechanisms of sex determination in an increasing number of insects (e.g., Kiuchi et al., 2014; Hall et al., 2015, 2016; Crisione et al., 2016; Krzywinska et al., 2016, 2021; Sharma et al., 2017; Meccariello et al., 2019; Qi et al., 2019; Wexler et al., 2019; Aryan et al., 2020; Liu et al., 2020; Zou et al., 2020; Lutrat et al., 2021; Zhuo et al., 2021).
Figure 1.
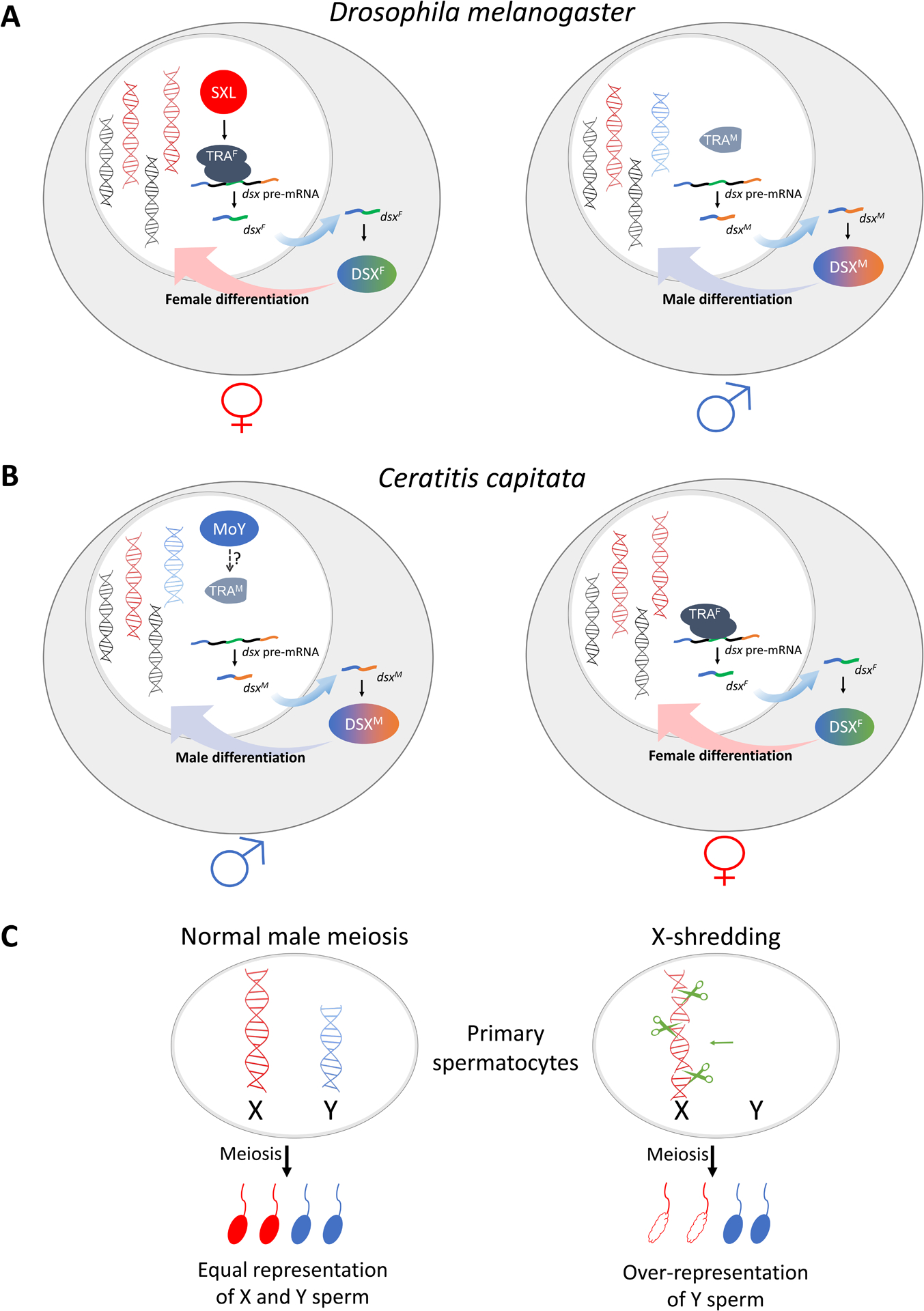
Simplified models of the sex-determination pathways in the vinegar fly Drosophila melanogaster (A) and the Mediterranean fruit fly Ceratitis capitata (B), and meiotic sex ratio distortion (C). A) In D. melanogaster, embryos that inherit two X chromosomes (depicted as red DNA molecules) express the primary signal Sex-lethal (SXL) which effects the female-specific splicing of the transformer (tra) transcript, leading to the production of a functional TRA protein (TRAF). The TRAF protein complex enables the female-specific splicing of the doublesex pre-mRNA, leading to the production of the female DSX protein isoform (DSXF) which programs female differentiation (left). In contrast, embryos with a single X chromosome do not express a functional SXL, resulting in a truncated non-functional TRA (TRAM) and subsequently the default male-specific splicing of dsx. This leads to the production of DSXM, which programs male differentiation (right). The Y chromosome (depicted as a blue DNA molecule) does not directly participate in sex-determination. B) In C. capitata, a dominant male-determining factor, Maleness-on-the-Y (MoY) resides on the Y chromosome (in blue). Expression of MoY somehow induces male-specific splicing of the tra pre-mRNA, leading to the production of truncated and non-functional TRA proteins (TRAM). The lack of a functional TRA results in male DSXM isoform and male differentiation (left). Embryos that do not inherit the Y chromosome (or MoY) produce a functional TRA protein (TRAF) complex by default, which leads to the production of DSXF and female differentiation (right). See Meccariello et al. (2019) and Primo et al. (2020) for details and for the concept of an autoregulatory loop. C) Normal spermatogenesis of a heterogametic male with XY chromosomes will produce X- or Y-bearing sperms in equal proportion (left). In a hypothetical Y-linked X-shredder system (right), either natural or engineered, double-stranded DNA breaks along the X chromosome could lead to non-functional X-bearing sperms without affecting the Y chromosome-bearing sperms. As a result, sex ratio distortion will occur in the progeny of an affected heterogametic male (XY).
A relatively balanced male to female sex ratio is expected for most insects. However, selection may shift the optimal sex ratios and meiotic drive and microbial manipulation can result in sex ratio distortion (SRD). In the following sections, we will review both naturally occurring and engineered SRD in the context of developing biological control of insects to help improve One Health, sustain agriculture, and conserve endangered species.
Naturally occurring meiotic drives can lead to sex ratio distortion (SRD) by biased production or transmission of gametes.
Gene drive refers to the phenomena in which one of the homologous chromosome pair, or an allele on one of the homologous chromosome pair, is transmitted to the next generation at a frequency greater than the expected 50% Mendelian segregation. If this bias results from a bias in the representation of gametes of a certain chromosome or genotype during meiosis (Figure 1C), then it is a meiotic drive. If this bias involves the sex chromosomes during meiosis in the heterogametic sex (e.g., during spermatogenesis in XY males), SRD will ensue. Meiotic and sex-linked drives have been discussed in a number of reviews including Jaenike 2001 and Courret 2019. We will highlight a few examples emphasizing the concept and recent advances (Table 1). Meiotic drives typically involve two loci: a drive allele/locus and a drive-sensitive allele targeted by the drive locus (Lyttle, 1991). For example, the D. simulans Winters drive is comprised of the Distorter on the X (Dox) that targets Y-chromosome repeats and kills the Y-bearing sperm, leading to a female sex ratio bias (Tao et al., 2007a; Tao et al., 2007b). Two autosomal suppressor alleles were found that counteract the drive (Tao et al., 2001, 2007a) and suppress Dox via RNA interference (Lin et al., 2018). The intragenomic arms race between an SRD drive and its suppressor and resistance alleles may have resulted in cryptic SRDs that are derived from multiple waves of SRD invasion followed by suppression and/or resistance (e.g., Muirhead et al., 2021). In Aedes aegypti mosquitoes a Y-linked distorter locus (D) is thought to disrupt the formation of the X-bearing sperm by causing X-chromosome breakage mainly at one of four sites during male meiosis I (Craig et al., 1960; Newton et al., 1976). Thus, D increases its own transmission and causes male-biased sex distortion (Figure 1C). This male bias is especially attractive in the context of controlling mosquito-borne infectious diseases as only female mosquitoes bite and transmit disease-causing pathogens. Suppressor alleles, resistant X chromosomes, and distortion enhancers associated with the D drive have been reported in various laboratory strains and wild populations (Wood 1976, Suguna et al. 1977; Wood and Ouda 1987; Owusu-Daaku et al. 1997). The Ae. aegypti Y chromosome is also called the M chromosome and it is similar to the X chromosome (or the m chromosome) except for its male-determining locus M (Matthews et al., 2018). Strictly speaking, these M- and m-bearing chromosomes are homomorphic sex-determining chromosomes.
Table 1. Examples of Natural and Engineered Sex Ratio Distortion.
Additional examples can be found in Hurst and Jiggins (2000), Jaenike (2001), and Kageyama et al. (2012). Some sex-determination factors listed are examples of good targets for SRD.
| CLASSIFICATION | DESCRIPTION | SPECIES | REFERENCES | DOI |
|---|---|---|---|---|
| MEIOTIC | Male-linked Distorter | Aedes aegypti | Craig et al., 1960; Newton et al., 1976 | 10.1126/science.132.3443.1887; 10.1007/BF00055473 |
| MEIOTIC | X-linked distorter disrupts maturation of Y-sperm | Drosophila simulans | Tao et al. 2007a; Muirhead and Presgraves, 2021 | 10.1371/journal.pbio.0050293; 10.1038/s41559-021-01543-8. |
| MEIOTIC | X-shredding by Cas9 | Anopheles gambiae | Galizi et al., 2016 | 10.1038/srep31139 |
| MEIOTIC | X-shredding by Cas9 | Drosophila melanogaster | Fasulo et al., 2020 | 10.1371/journal.pgen.1008647 |
| MEIOTIC | X-shredding by Cas9 | Ceratitis capitata | Meccariello et al., 2021 | 10.1186/s12915-021-01010-7 |
| MEIOTIC, POSTZYGOTIC | X-poisoning by Cas9 knockout of haploinsufficient genes | Drosophila melanogaster | Fasulo et al., 2020 | 10.1371/journal.pgen.1008647 |
| ENDOSYMBIONT-INDUCED | Meiotic drive and Feminization mediated by Wolbachia | Eurema mandarina | Kern et al., 2015; Kageyama et al., 2017 | 10.1098/rsbl.2015.0095; 10.1002/evl3.28 |
| ENDOSYMBIONT-INDUCED | Hamiltonella manipulaiton of fertilization | Bemisia tabaci | Shan et al., 2019 | 10.1098/rspb.2019.1677 |
| ENDOSYMBIONT-INDUCED | Sex ratio distortion by inhibition of fertilization | Trialeurodes vaporariorum | Wang et al., 2020 | 10.1038/s41396-020-0717-0 |
| ENDOSYMBIONT-INDUCED | Temperature-sensitive effects on sex allocation | Pezothrips kellyanus | Katlav et al., 2022 | 10.1038/s41437-022-00505-5 |
| ENDOSYMBIONT-INDUCED | Male killing Wolbachia | Drosophila bifasciata | Harumoto et al., 2018 | 10.1098/rspb.2017.2167 |
| ENDOSYMBIONT-INDUCED | Male-killing Spiroplasma: mis-regulating dosage compensation/sex determination | Drosophila melanogaster | Cheng et al., 2016; Harumoto et al., 2016 | 10.1016/j.cub.2016.03.050; 10.1038/ncomms12781 |
| ENDOSYMBIONT-INDUCED | A male-killing Wolbachia: interacts with sex determination and dosage compensation | Ostrinia scapulalis | Sugimoto and Ishikawa, 2012; Sugimoto et al., 2015; Fukui et al., 2015 | 10.1098/rsbl.2011.1114; 10.1016/j.ibmb.2015.10.001; 10.1371/journal.ppat.1005048 |
| ENDOSYMBIONT-INDUCED | Male-killing Wolbachia targeting the masculinizing gene | Ostrinia furnacalis | Fukui et al., 2015 | 10.1371/journal.ppat.1005048 |
| ENDOSYMBIONT-INDUCED | Potential male-killing by Wolbachia | Anastrepha fraterculus | Conte et al., 2019 | 10.1186/s12866-019-1652-y |
| SEX DETERMINATION | Female-to-male conversion by expression of Nix | Aedes aegypti | Hall et al., 2015; Aryan et al., 2020 | 10.1126/science.aaa2850; 10.1073/pnas.2001132117 |
| SEX DETERMINATION | Female-to-male conversion by expression of Nix | Aedes albopictus | Liu et al., 2020; Lutrat et al., 2021 | 10.1016/j.ibmb.2019.103311; 10.1101/2021.07.28.454191 |
| SEX DETERMINATION | Male-to-female conversion by disruption of Mdmd | Musca domestica | Sharma et al., 2017 | 10.1126/science.aam5498 |
| SEX DETERMINATION | Maternal knockdown of tra results in all-male offspring | Blattella germanica | Wexler et al., 2019 | 10.7554/eLife.47490 |
| SEX DETERMINATION | Knockdown of Masc results in male-specific lethality 1 | Bombyx mori | Kiuchi et al., 2014 | 10.1038/nature13315 |
| SEX DETERMINATION | Female-specific lethality by expression of Guy1 1 | Anopheles stephensi | Criscione et al., 2016; Qi et al., 2019 | 10.7554/eLife.19281; 10.7554/eLife.43570 |
| SEX DTERMINATION | Female-specific lethality by expression of Yob and fle 1 | Anopheles gambiae | Krzywinska et al., 2018; Krzywinska et al., 2021 | 10.1186/s13071-018-3211-z; 10.1016/j.cub.2020.12.014 |
| SEX DETERMINATION | Female-specific lethality by depletion of Nlfmd in embryos 1 | Nilaparvata lugens | Zhuo et al., 2021 | 10.1126/sciadv.abf9237 |
| SEX DETERMINATION | Gene drive targeting transformer | Ceratitis capitata | Carrami et al., 2018 | 10.1073/pnas.1713825115 |
| SEX DETERMINATION | Knockdown of MoY feminizes males; ectopic expression masculinizes females | Ceratitis capitata | Meccariello et al., 2019 | 10.1126/science.aax1318 |
| SEX DETERMINATION | Gene drive targeting female doublesex | Anopheles gambiae | Kyrou et al., 2018, Simoni et al., 2020 | 10.1038/nbt.4245; 10.1038/s41587-020-0508-1 |
| SEX DETERMINATION | Female-specific lethality by Cas9 knockout of transformer 2 | Bombyx mori | Zhang et al., 2018 | 10.1073/pnas.1810945115L |
In these cases, sex-specific lethality is caused by mis-regulation of dosage compensation.
Sex ratio can be skewed by maternally inherited bacterial endosymbionts.
Bacterial endosymbionts such as Wolbachia, Rickettsia, Cardinium and Spiroplasma can infect and manipulate the germline of insects that harbor them (reviewed in Werren et al., 2008; Ma et al., 2014; Hurst and Frost, 2015, Landmann, 2019). They are normally transmitted through the maternal germline. Wolbachia is thought to infect more than half of all arthropod species (Weinert et al., 2015) and is well known for its induction of cytoplasmic incompatibility (CI) and CI-related applications in pest control (e.g. Laven, 1967; Dobson et al., 2002; Zheng et al., 2019). Wolbachia can cause SRD by feminizing or killing males, inducing parthenogenesis, and regulating sex allocation. For example, Wolbachia can cause damage in the dosage compensated X chromosome that is only found in males to confer male-specific lethality in Drosophila (Harumoto et al., 2018). Wolbachia can also feminize and kill males by mis-regulating the factors in the sex determination pathway to shift dsx splicing (Sugimoto and Ishikawa, 2012). Wolbachia in female Eurema mandarina butterflies (genotype ZW) prevents the production of Z-bearing oocytes during oogenesis and initiates female development in ZO individuals, resulting in all-female offspring through a dual-acting meiotic drive (Kern et al., 2015; Kageyama et al., 2017). Wolbachia and other intracellular symbionts can also mediate higher fertilization rates leading to a female bias in haplodiploid species (Wang et al., 2020; Bagheri et al., 2021).
Engineering SRD through sex conversion and sex-specific lethality.
Altering or perturbing factors involved in sex-determination (Figure 1A and 1B) could either result in sex conversion, or sex-specific lethality, or infertile intersex, depending on the relative position of the factor in the sex-determination pathway (i.e., top master switch such as SXL or MoY versus bottom effector such as DSX) and whether or not sex chromosome dosage compensation is required (reviewed in Bideler and Tu, 2016; Scott, 2021). Table 1 lists a number of candidates that can be manipulated to cause SRD in diverse insect species. We will only highlight a few recent examples in which SRDs were demonstrated over many generations when the factors were stably inherited as transgenes. Stable expression of a transgenic copy of Nix, a master switch for male determination in Ae. aegypti and Ae. albopictus (Hall et al., 2015), converted genetic females into fertile males and resulted in a clear SRD (Aryan et al., 2020; Lurat et al., 2021). On the other hand, stable germline transformation of a Y-linked primary signal Guy1 resulted in female-specific lethality in An. stephensi (Criscione et al., 2016). Unlike Ae. aegypti, X chromosome dosage compensation is needed in An. stephensi (Jiang et al., 2015) and Guy1 regulates dosage compensation by increasing the transcription of genes on the X chromosome in XY males (Qi et al., 2019). Transgenic expression of Guy1 in XX females result in abnormally high transcription of X-linkedgenes and hence lethality (Qi et al., 2019). Targeting the sex-specific exon (or its splicing signal) of dsx, a gene at the bottom of the sex-determination pathway, could also result in SRD. A gene drive was developed in An. gambiae that disrupts the formation of female dsxF transcript while leaving the male dsxM unaffected (Kyrou et al., 2018, see below for details), resulting in sterile intersex XX individuals without affecting the development or fertility of XY males. In addition to genetic manipulations mentioned above, SRD can also be achieved by silencing genes in the sex-determination pathway using interfering RNA (e.g., Pane et al., 2002; Whyard et al., 2015; Meccariello et al., 2019; Taracena et al., 2019). Other sex-specific phenomena can also be used to engineer SRD (e.g., Fu et al., 2010; Kandul et al., 2020; Li et al., 2021).
CRISPR/Cas9 technology expands ways to engineer SRD.
An engineered X-shredder was developed in the malaria mosquito An. gambiae that uses an endonuclease I-PpoI to target ribosomal DNA repeats exclusive to the X chromosome to induce X chromosome breakage during spermatogenesis (Galizi et al., 2014). This results in the reduction of X-bearing sperm and >95% male progeny. Releasing this X-shredder mosquito strain successfully suppressed a cage population, confirming its potential for genetic control. Unlike the D-locus in Ae. aegypti which is Y-linked and thus favors its own transmission at the expense of the X chromosome, the I-PpoI X-shredder is on an autosome and is transmitted at a 50% probability. Attempts to engineer a more powerful Y-linked sex ratio distorter have not been successful presumably due to inactivation of the Y-linked distorter transgene during meiosis (Turner et al., 2007; Alcalay et al., 2019; Taxiarchi et al., 2019). As an alternative, the I-PpoI X-shredder in An. gambiae was integrated into a CRISPR/Cas9-based gene drive (Simoni et al., 2020) that targets the female doublesex (dsx) isoform as previously described (Kyrou et al., 2018). This new strain produces male-only progeny and eventually crashed cage populations in only 10–14 generations with a starting gene drive frequency as low as 2.5%, demonstrating the potential for large-scale applications (Simoni et al., 2020). Large cage trials that incorporated mosquito ecology showed further promise as the dsx gene drive suppressed the mosquito population within a year without selecting for resistance to the drive (Hammond et al., 2021).
An X-shedder was also developed using an RNA-guided CRISPR/Cas9 nuclease to target the X chromosome in An. gambiae (Galizi et al., 2016). The use of a programmable CRISPR/Cas9 nuclease has facilitated the development of X-shredders in other insect species including D. melanogaster (Fasulo et al., 2020) and C. capitata (Meccariello et al., 2021). All that are required are guide RNAs that target X-specific sequences/repeats and a male germline promoter that directs the expression of the Cas9 nuclease at the appropriate stage during meiosis. A bioinformatic pipeline was developed to identify X-specific sequences for shredding (Papathanos and Windbichler, 2018). Single cell RNA sequencing could enable discovery of appropriate promoters (reviewed in Compton et al, 2020).
Discussion: Insect SRD in the Anthropocene
We discuss insect SRDs from three main perspectives in the context of the Anthropocene. First, we consider whether or not climate change may impact the sex ratio of wild insect populations. It is not yet clear whether climate change will introduce instability to the otherwise relatively stable sex-determination pathways in some insects. It is also not clear how climate change may affect the naturally occurring SRDs including those mediated by endosymbiotic bacteria. However, sex-biased heat-tolerance has been shown in diverse insects which may lead to shifting sex ratios inresponse to climate change (Edmands, 2021). Temperature can also affect female fertilization and alter the sex ratio in Hymenoptera parasitic wasps (Moiroux, 2014). One study demonstrated that longer drought periods caused by a delay in the timing of summer rainfall in the Mediterranean region led to a female sex ratio bias in the acorn Weevil (Bonal et al., 2015). Endosymbiotic manipulation of sex ratio in Pezothrips kellyanus Thrips is influenced by abiotic factors such as temperature (Katlav et al., 2022). It has also been suggested that temperature may differentially impact male and female body size, mortality, protandry, and population-level sex ratios of wild bees (Slominski et al., 2019).
Second, engineered SRDs have shown great promise in controlling insect pests of agricultural and medical importance as discussed in previous sections. These efforts are critical to help sustain agriculture and improve human health. However, a less discussed but perhaps equally important application of SRDs relates to conservation of wildlife or endangered species that are under the threat of insect-borne infectious diseases (NAS, 2016). For example, only 20 of the 46 recorded forest bird species in Hawaii are still extant in the wild (Fortini et al., 2015). Many of these species are threatened by avian malaria, transmitted by the Culex quinquefasciatus mosquito. The presence of avian malaria constrains these birds to a narrow range of habitats that are unsuitable for the mosquito (Samuel et al., 2011). Climate change is expected to broaden the distribution of C. quinquefasciatus, thereby further shrink the habitat range for susceptible bird species (Samuel et al., 2011; Benning et al., 2002; Ahumada et al., 2009; Fortini et al., 2015). Integrating SRD-mediated mosquito population suppression strategies into the long-term conservation efforts would allow Hawaiian forest birds to reclaim lost habitat. New genomic resources (https://www.ncbi.nlm.nih.gov/assembly/GCF_015732765.1) and newly established genome-editing methods (Feng et al., 2021; Purusothaman et al., 2021) for C. quinquefasciatus provide the foundation for SRD development.
Finally, the potential environmental impacts of the engineered SRD control strategies should be critically evaluated in the context of current methods. Current insect control methods rely heavily on insecticides and increasing resistance has significantly reduced their effectiveness. Some widely used insecticides such as neonicotinoids, which were previously thought to be safe and highly target-specific (reviewed in Jeschke and Naun, 2008), have been shown to have potential health risks (Thompson et al., 2020), can impact non-target invertebrates and insectivorous birds (Pisa et al., 2015; Hallman et al., 2014), and potentially contribute to the decline of honeybees (Rundlöf et al., 2015). Genetic control strategies are species-specific as they all require mating and thus a direct impact on non-target species is not anticipated. However, some of the SRD-mediated methods, especially the ones involving gene drives, are very powerful and could potentially eliminate a pest species from a large region, which may have ecological consequences. There are ongoing efforts to fine-tune, confine or neutralize the power of some of the gene drive systems (Noble et al., 2019; Li et al., 2020; Kandul et al., 2019; Li et al., 2021; Vella et al., 2017; Taxiarchi et al., 2021). It is unlikely that any one control measure will be the ‘silver bullet’. Integration or selective implementation of a set of control measures most appropriate to the specific goals and the social and environmental context will likely be most effective. Therefore, continued development of a diverse range of genetic control methods for insect pests are important for promoting sustainable agriculture, improving One Health, and preserving wildlife.
Acknowledgement
We thank James K. Biedler for helpful comments. This work was supported by grants AI157491, AI154871, AI123338 and AI121284 from the National Institutes of Health and by the Virginia Experimental Station.
References
- Adelman ZN, Tu Z. Control of Mosquito-Borne Infectious Diseases: Sex and Gene Drive. Trends in Parasitology. 2016;32(3):219–29. doi: 10.1016/j.pt.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahumada JA, Samuel MD, Duffy DC, Dobson AP, Hobbelen PHF. Modeling the epidemiology of avian malaria and pox in Hawaii. In: Pratt TK, Atkinson CT, Banko PC, Jacobi J, Woodworth BL editors. Conservation Biology of Hawaiian Forest Birds. New Haven: Yale University Press; 2009. pp. 331–355. [Google Scholar]
- Alcalay Y, Fuchs S, Galizi R, Bernardini F, Haghighat-Khah RE, Rusch DB, Adrion JR, Hahn MW, Tortosa P, Rotenberry R, Papathanos PA. The Potential for a Released Autosomal X-Shredder Becoming a Driving-Y Chromosome and Invasively Suppressing Wild Populations of Malaria Mosquitoes. Frontiers in Bioengineering and Biotechnology. 2021;9. doi: 10.3389/fbioe.2021.752253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryan A, Anderson MAE, Biedler JK, Qi Y, Overcash JM, Naumenko AN, Sharakhova MV, Mao C, Adelman ZN, Tu Z. Nix alone is sufficient to convert female Aedes aegypti into fertile males and myo-sex is needed for male flight. Proc Natl Acad Sci U S A. 2020;117(30):17702–9. Epub 2020/07/15. doi: 10.1073/pnas.2001132117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtrog D, Mank JE, Peichel CL, Kirkpatrick M, Otto SP, Ashman T-L, Hahn MW, Kitano J, Mayrose I, Ming R, Perrin N, Ross L, Valenzuela N, Vamosi JC, Tree of Sex C. Sex determination: why so many ways of doing it? PLoS Biol. 2014;12(7):e1001899–e. doi: 10.1371/journal.pbio.1001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri Z, Talebi AA, Asgari S, Mehrabadi M. Wolbachia promotes successful sex with siblings in the parasitoid Habrobracon hebetor. Pest Management Science. 2022;78(1):362–8. doi: 10.1002/ps.6649. [DOI] [PubMed] [Google Scholar]
- Benning TL, LaPointe D, Atkinson CT, Vitousek PM. Interactions of climate change with biological invasions and land use in the Hawaiian Islands: Modeling the fate of endemic birds using a geographic information system. Proceedings of the National Academy of Sciences. 2002;99(22):14246. doi: 10.1073/pnas.162372399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukeboom LW, Perrin N. The Evolution of Sex Determination. Oxford: Oxford University Press; 2014. 240 p. [Google Scholar]
- Biedler JK, Tu Z. Chapter Two - Sex Determination in Mosquitoes. In: Raikhel AS, editor. Advances in Insect Physiology: Academic Press; 2016. p. 37–66. [Google Scholar]
- Blacquière T, Smagghe G, van Gestel CAM, Mommaerts V. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology. 2012;21(4):973–92. doi: 10.1007/s10646-012-0863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonal R, Hernández M, Espelta JM, Muñoz A, Aparicio JM. Unexpected consequences of a drier world: evidence that delay in late summer rains biases the population sex ratio of an insect. Royal Society open science. 2015;2(9):150198-. doi: 10.1098/rsos.150198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A, Shriner I, Yang T, Liu J, Antoshechkin I, Marshall JM, Perry MW, Akbari OS. Engineered reproductively isolated species drive reversible population replacement. Nature Communications. 2021;12(1):3281. doi: 10.1038/s41467-021-23531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrami EM, Eckermann KN, Ahmed HMM, Sánchez CHM, Dippel S, Marshall JM, Wimmer EA. Consequences of resistance evolution in a Cas9-based sex conversion-suppression gene drive for insect pest management. Proceedings of the National Academy of Sciences. 2018;115(24):6189. doi: 10.1073/pnas.1713825115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Kuppanda N, Aldrich JC, Akbari OS, Ferree PM. Male-Killing Spiroplasma Alters Behavior of the Dosage Compensation Complex during Drosophila melanogaster Embryogenesis. Curr Biol. 2016;26(10):1339–45. Epub 2016/05/11. doi: 10.1016/j.cub.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible C, Board on Life S, Division on E, Life S, National Academies of Sciences E, Medicine. Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with PublicValues. Washington (DC): National Academies Press (US). Copyright 2016 by the National Academies of Sciences. All rights reserved.; 2016. [PubMed] [Google Scholar]
- Conte CA, Segura DF, Milla FH, Augustinos A, Cladera JL, Bourtzis K, Lanzavecchia SB. Wolbachia infection in Argentinean populations of Anastrepha fraterculus sp1: preliminary evidence of sex ratio distortion by one of two strains. BMC Microbiol. 2019;19(Suppl 1):289. Epub 2019/12/25. doi: 10.1186/s12866-019-1652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton A, Sharakhov IV, Tu Z. Recent advances and future perspectives in vector-omics. Curr Opin Insect Sci. 2020;40:94–103. Epub 2020/07/11. doi: 10.1016/j.cois.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courret C, Chang C-H, Wei KHC, Montchamp-Moreau C, Larracuente AM. Meiotic drive mechanisms: lessons from Drosophila. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1913):20191430. doi: 10.1098/rspb.2019.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig GB, Hickey WA, VandeHey RC. An Inherited Male-Producing Factor in Aedes aegypti. Science. 1960;132(3443):1887–9. doi: 10.1126/science.132.3443.1887. [DOI] [PubMed] [Google Scholar]
- Criscione F, Qi Y, Tu Z. GUY1 confers complete female lethality and is a strong candidate for a male-determining factor in Anopheles stephensi. eLife. 2016;5:e19281. doi: 10.7554/eLife.19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SL, Fox CW, Jiggins FM. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc Biol Sci. 2002;269(1490):437–45. doi: 10.1098/rspb.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmands S Sex Ratios in a Warming World: Thermal Effects on Sex-Biased Survival, Sex Determination, and Sex Reversal. J Hered. 2021;112(2):155–64. Epub 2021/02/16. doi: 10.1093/jhered/esab006. [DOI] [PubMed] [Google Scholar]
- Fasulo B, Meccariello A, Morgan M, Borufka C, Papathanos PA, Windbichler N. A fly model establishes distinct mechanisms for synthetic CRISPR/Cas9 sex distorters. PLoS Genet. 2020;16(3):e1008647. Epub 2020/03/14. doi: 10.1371/journal.pgen.1008647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, López Del Amo V, Mameli E, Lee M, Bishop AL, Perrimon N, Gantz VM. Optimized CRISPR tools and site-directed transgenesis towards gene drive development in Culex quinquefasciatus mosquitoes. Nature Communications. 2021;12(1):2960. doi: 10.1038/s41467-021-23239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini LB, Vorsino AE, Amidon FA, Paxton EH, Jacobi JD. Large-Scale Range Collapse of Hawaiian Forest Birds under Climate Change and the Need 21st Century Conservation Options. PLoS One. 2015;10(10):e0140389. doi: 10.1371/journal.pone.0140389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Lees RS, Nimmo D, Aw D, Jin L, Gray P, Berendonk TU, White-Cooper H, Scaife S, Kim Phuc H, Marinotti O, Jasinskiene N, James AA, Alphey L. Female-specific flightless phenotype for mosquito control. Proceedings of the National Academy of Sciences. 2010;107(10):4550. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Kawamoto M, Shoji K, Kiuchi T, Sugano S, Shimada T, Suzuki Y, Katsuma S. The Endosymbiotic Bacterium Wolbachia Selectively Kills Male Hosts by Targeting the Masculinizing Gene. PLOS Pathogens. 2015;11(7):e1005048. doi: 10.1371/journal.ppat.1005048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizi R, Doyle LA, Menichelli M, Bernardini F, Deredec A, Burt A, Stoddard BL, Windbichler N, Crisanti A. A synthetic sex ratio distortion system for the control of the human malaria mosquito. Nature Communications. 2014;5(1):3977. doi: 10.1038/ncomms4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizi R, Hammond A, Kyrou K, Taxiarchi C, Bernardini F, O’Loughlin SM, Papathanos P-A, Nolan T, Windbichler N, Crisanti A. A CRISPR-Cas9 sex-ratio distortion system for genetic control. Scientific Reports. 2016;6(1):31139. doi: 10.1038/srep31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AB, Basu S, Jiang X, Qi Y, Timoshevskiy VA, Biedler JK, Sharakhova MV, Elahi R, Anderson MAE, Chen X-G, Sharakhov IV, Adelman ZN, Tu Z. A male-determining factor in the mosquito Aedes aegypti. Science. 2015;348(6240):1268. doi: 10.1126/science.aaa2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AB, Papathanos P-A, Sharma A, Cheng C, Akbari OS, Assour L, Bergman NH, Cagnetti A, Crisanti A, Dottorini T, Fiorentini E, Galizi R, Hnath J, Jiang X, Koren S, Nolan T, Radune D, Sharakhova MV, Steele A, Timoshevskiy VA, Windbichler N, Zhang S, Hahn MW, Phillippy AM, Emrich SJ, Sharakhov IV, Tu ZJ, Besansky NJ. Radical remodeling of the Y chromosome in a recent radiation of malaria mosquitoes. Proceedings of the National Academy of Sciences. 2016;113(15):E2114. doi: 10.1073/pnas.1525164113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann CA, Foppen RP, van Turnhout CA, de Kroon H, Jongejans E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature. 2014;511(7509):341–3. Epub 2014/07/18. doi: 10.1038/nature13531. [DOI] [PubMed] [Google Scholar]
- Hammond A, Pollegioni P, Persampieri T, North A, Minuz R, Trusso A, Bucci A, Kyrou K, Morianou I, Simoni A, Nolan T, Müller R, Crisanti A. Gene-drive suppression of mosquito populations in large cages as a bridge between lab and field. Nature Communications. 2021;12(1):4589. doi: 10.1038/s41467-021-24790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto T, Anbutsu H, Lemaitre B, Fukatsu T. Male-killing symbiont damages host’s dosage-compensated sex chromosome to induce embryonic apoptosis. Nature communications. 2016;7:12781-. doi: 10.1038/ncomms12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harumoto T, Fukatsu T, Lemaitre B. Common and unique strategies of male killing evolved in two distinct Drosophila symbionts. Proceedings of the Royal Society B: Biological Sciences. 2018;285(1875):20172167. doi: 10.1098/rspb.2017.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpin A, Schartl M. Plasticity of gene-regulatory networks controlling sex determination: of masters, slaves, usual suspects, newcomers, and usurpators. EMBO Rep. 2015;16(10):1260–74. Epub 2015/09/12. doi: 10.15252/embr.201540667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins BR, Kopp A. Evolution of sexual development and sexual dimorphism in insects. Current Opinion in Genetics & Development. 2021;69:129–39. doi: 10.1016/j.gde.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst GD, Frost CL. Reproductive parasitism: maternally inherited symbionts in a biparental world. Cold Spring Harb Perspect Biol. 2015;7(5). Epub 2015/05/03. doi: 10.1101/cshperspect.a017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst GDD, Jiggins FM. Male-Killing Bacteria in Insects: Mechanisms, Incidence, and Implications. Emerging Infectious Disease journal. 2000;6(4):329. doi: 10.3201/eid0604.000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J Sex Chromosome Meiotic Drive. Annual Review of Ecology and Systematics. 2001;32(1):25–49. doi: 10.1146/annurev.ecolsys.32.081501.113958. [DOI] [Google Scholar]
- Jeschke P, Nauen R. Neonicotinoids—from zero to hero in insecticide chemistry. Pest Management Science. 2008;64(11):1084–98. doi: 10.1002/ps.1631. [DOI] [PubMed] [Google Scholar]
- Jiang X, Biedler JK, Qi Y, Hall AB, Tu Z. Complete Dosage Compensation in Anopheles stephensi and the Evolution of Sex-Biased Genes in Mosquitoes. Genome Biology and Evolution. 2015;7(7):1914–24. doi: 10.1093/gbe/evv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama D, Narita S, Watanabe M. Insect Sex Determination Manipulated by Their Endosymbionts: Incidences, Mechanisms and Implications. Insects. 2012;3(1). doi: 10.3390/insects3010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama D, Ohno M, Sasaki T, Yoshido A, Konagaya T, Jouraku A, Kuwazaki S, Kanamori H, Katayose Y, Narita S, Miyata M, Riegler M, Sahara K. Feminizing Wolbachia endosymbiont disrupts maternal sex chromosome inheritance in a butterfly species. Evolution Letters. 2017;1(5):232–44. doi: 10.1002/evl3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandul NP, Liu J, Hsu AD, Hay BA, Akbari OS. A drug-inducible sex-separation technique for insects. Nature Communications. 2020;11(1):2106. doi: 10.1038/s41467-020-16020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandul NP, Liu J, Sanchez CHM, Wu SL, Marshall JM, Akbari OS. Transforming insect population control with precision guided sterile males with demonstration in flies. Nature Communications. 2019;10(1):84. doi: 10.1038/s41467-018-07964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlav A, Nguyen DT, Morrow JL, Spooner-Hart RN, Riegler M. Endosymbionts moderate constrained sex allocation in a haplodiploid thrips species in a temperature-sensitive way. Heredity. 2022. doi: 10.1038/s41437-022-00505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern P, Cook JM, Kageyama D, Riegler M. Double trouble: combined action of meiotic drive and Wolbachia feminization in Eurema butterflies. Biology Letters. 2015;11(5):20150095. doi: 10.1098/rsbl.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler KE, Vosshall LB, Matthews BJ. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 2015;11(1):51–60. Epub 2015/03/31. doi: 10.1016/j.celrep.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi T, Koga H, Kawamoto M, Shoji K, Sakai H, Arai Y, Ishihara G, Kawaoka S, Sugano S, Shimada T, Suzuki Y, Suzuki MG, Katsuma S. A single female-specific piRNA is the primary determiner of sex in the silkworm. Nature. 2014;509(7502):633–6. doi: 10.1038/nature13315. [DOI] [PubMed] [Google Scholar]
- Krzywinska E, Dennison Nathan J, Lycett Gareth J, Krzywinski J. A maleness gene in the malaria mosquitoAnopheles gambiae. Science. 2016;353(6294):67–9. doi: 10.1126/science.aaf5605. [DOI] [PubMed] [Google Scholar]
- Krzywinska E, Ferretti L, Li J, Li JC, Chen CH, Krzywinski J. femaleless Controls Sex Determination and Dosage Compensation Pathways in Females of Anopheles Mosquitoes. Curr Biol. 2021;31(5):1084–91.e4. Epub 2021/01/09. doi: 10.1016/j.cub.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinska E, Krzywinski J. Effects of stable ectopic expression of the primary sex determination gene Yob in the mosquito Anopheles gambiae. Parasites & Vectors. 2018;11(2):648. doi: 10.1186/s13071-018-3211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, Crisanti A. A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nature biotechnology. 2018;36(11):1062–6. Epub 2018/09/24. doi: 10.1038/nbt.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F The Wolbachia Endosymbionts. Microbiol Spectr. 2019;7(2). Epub 2019/04/07. doi: 10.1128/microbiolspec.BAI-0018-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven H Eradication of Culex pipiens fatigans through Cytoplasmic Incompatibility. Nature. 1967;216(5113):383–4. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Li M, Yang T, Bui M, Gamez S, Wise T, Kandul NP, Liu J, Alcantara L, Lee H, Edula JR, Raban R, Zhan Y, Wang Y, DeBeaubien N, Chen J, Sánchez CHM, Bennett JB, Antoshechkin I, Montell C, Marshall JM, Akbari OS. Suppressing mosquito populations with precision guided sterile males. Nature Communications. 2021;12(1):5374. doi: 10.1038/s41467-021-25421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Yang T, Kandul NP, Bui M, Gamez S, Raban R, Bennett J, Sánchez CH, Lanzaro GC, Schmidt H, Lee Y, Marshall JM, Akbari OS. Development of a confinable gene drive system in the human disease vector Aedes aegypti. Elife. 2020;9. Epub 2020/01/22. doi: 10.7554/eLife.51701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-J, Hu F, Dubruille R, Vedanayagam J, Wen J, Smibert P, Loppin B, Lai EC. The hpRNA/RNAi Pathway Is Essential to Resolve Intragenomic Conflict in the Drosophila Male Germline. Developmental Cell. 2018;46(3):316–26.e5. doi: 10.1016/j.devcel.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jin B, Li X, Zhao Y, Gu J, Biedler JK, Tu ZJ, Chen X-G. Nix is a male-determining factor in the Asian tiger mosquito Aedes albopictus. Insect Biochemistry and Molecular Biology. 2020;118:103311. doi: 10.1016/j.ibmb.2019.103311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutrat C, Olmo RP, Baldet T, Bouyer J, Marois E. Transgenic expression of Nix; converts genetic females into males and allows automated sex sorting in Aedes albopictus. bioRxiv. 2021:2021.07.28.454191. doi: 10.1101/2021.07.28.454191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttle TW. Segregation distorters. Annu Rev Genet. 1991;25:511–57. Epub 1991/01/01. doi: 10.1146/annurev.ge.25.120191.002455. [DOI] [PubMed] [Google Scholar]
- Ma WJ, Vavre F, Beukeboom LW. Manipulation of arthropod sex determination by endosymbionts: diversity and molecular mechanisms. Sex Dev. 2014;8(1–3):59–73. Epub 2013/12/21. doi: 10.1159/000357024. [DOI] [PubMed] [Google Scholar]
- Matthews BJ, Dudchenko O, Kingan SB, Koren S, Antoshechkin I, Crawford JE, Glassford WJ, Herre M, Redmond SN, Rose NH, Weedall GD, Wu Y, Batra SS, Brito-Sierra CA, Buckingham SD, Campbell CL, Chan S, Cox E, Evans BR, Fansiri T, Filipović I, Fontaine A, Gloria-Soria A, Hall R, Joardar VS, Jones AK, Kay RGG, Kodali VK, Lee J, Lycett GJ, Mitchell SN, Muehling J, Murphy MR, Omer AD, Partridge FA, Peluso P, Aiden AP, Ramasamy V, Rašić G, Roy S, Saavedra-Rodriguez K, Sharan S, Sharma A, Smith ML, Turner J, Weakley AM, Zhao Z, Akbari OS, Black WC, Cao H, Darby AC, Hill CA, Johnston JS, Murphy TD, Raikhel AS, Sattelle DB, Sharakhov IV, White BJ, Zhao L, Aiden EL, Mann RS, Lambrechts L, Powell JR, Sharakhova MV, Tu Z, Robertson HM, McBride CS, Hastie AR, Korlach J, Neafsey DE, Phillippy AM, Vosshall LB. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature. 2018;563(7732):501–7. doi: 10.1038/s41586-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meccariello A, Krsticevic F, Colonna R, Del Corsano G, Fasulo B, Papathanos PA, Windbichler N. Engineered sex ratio distortion by X-shredding in the global agricultural pest Ceratitis capitata. BMC Biol. 2021;19(1):78. Epub 2021/04/18. doi: 10.1186/s12915-021-01010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meccariello A, Salvemini M, Primo P, Hall B, Koskinioti P, Dalíková M, Gravina A, Gucciardino Michela A, Forlenza F, Gregoriou M-E, Ippolito D, Monti Simona M, Petrella V, Perrotta Maryanna M, Schmeing S, Ruggiero A, Scolari F, Giordano E, Tsoumani Konstantina T, Marec F, Windbichler N, Arunkumar Kallare P, Bourtzis K, Mathiopoulos Kostas D, Ragoussis J, Vitagliano L, Tu Z, Papathanos Philippos A, Robinson Mark D, Saccone G. Maleness-on-the-Y (MoY) orchestrates male sex determination in major agricultural fruit fly pests. Science. 2019;365(6460):1457–60. doi: 10.1126/science.aax1318. [DOI] [PubMed] [Google Scholar]
- Moiroux J, Brodeur J, Boivin G. Sex ratio variations with temperature in an egg parasitoid: behavioural adjustment and physiological constraint. Animal Behaviour. 2014;91:61–6. doi: 10.1016/j.anbehav.2014.02.021. [DOI] [Google Scholar]
- Muirhead CA, Presgraves DC. Satellite DNA-mediated diversification of a sex-ratio meiotic drive gene family in Drosophila. Nature Ecology & Evolution. 2021;5(12):1604–12. doi: 10.1038/s41559-021-01543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton ILG, Rice DW. The Jekyll and Hyde Symbiont: Could Wolbachia Be a Nutritional Mutualist? J Bacteriol. 2020;202(4). Epub 2019/10/30. doi: 10.1128/jb.00589-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton ME, Wood RJ, Southern DI. A cytogenetic analysis of meiotic drive in the mosquito, Aedes aegypti (L.). Genetica. 1976;46(3):297–318. doi: 10.1007/BF00055473. [DOI] [Google Scholar]
- Noble C, Min J, Olejarz J, Buchthal J, Chavez A, Smidler AL, DeBenedictis EA, Church GM, Nowak MA, Esvelt KM. Daisy-chain gene drives for the alteration of local populations. Proceedings of the National Academy of Sciences. 2019;116(17):8275. doi: 10.1073/pnas.1716358116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Daaku K, Wood R, Butler R. Selected lines of Aedes aegypti with persistently distorted sex ratios. Heredity. 1997;79 (Pt 4):388–93. doi: 10.1038/hdy.1997.172. [DOI] [PubMed] [Google Scholar]
- Pane A, Salvemini M, Delli Bovi P, Polito C, Saccone G. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development. 2002;129(15):3715–25. Epub 2002/07/16. doi: 10.1242/dev.129.15.3715. [DOI] [PubMed] [Google Scholar]
- Papathanos PA, Windbichler N. Redkmer: An Assembly-Free Pipeline for the Identification of Abundant and Specific X-Chromosome Target Sequences for X-Shredding by CRISPR Endonucleases. The CRISPR Journal. 2018;1(1):88–98. doi: 10.1089/crispr.2017.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa LW, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Downs CA, Goulson D, Kreutzweiser DP, Krupke C, Liess M, McField M, Morrissey CA, Noome DA, Settele J, Simon-Delso N, Stark JD, Van der Sluijs JP, Van Dyck H, Wiemers M. Effects of neonicotinoids and fipronil on non-target invertebrates. Environmental Science and Pollution Research. 2015;22(1):68–102. doi: 10.1007/s11356-014-3471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primo P, Meccariello A, Inghilterra MG et al. Targeting the autosomal Ceratitis capitata transformer gene using Cas9 or dCas9 to masculinize XX individuals without inducing mutations. BMC Genet 21, 150 (2020). 10.1186/s12863-020-00941-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purusothaman D-K, Shackleford L, Anderson MAE, Harvey-Samuel T, Alphey L. CRISPR/Cas-9 mediated knock-in by homology dependent repair in the West Nile Virus vector Culex quinquefasciatus Say. Scientific Reports. 2021;11(1):14964. doi: 10.1038/s41598-021-94065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Wu Y, Saunders R, Chen X-G, Mao C, Biedler JK, Tu ZJ. Guy1, a Y-linked embryonic signal, regulates dosage compensation in Anopheles stephensi by increasing X gene expression. eLife. 2019;8:e43570. doi: 10.7554/eLife.43570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundlöf M, Andersson GKS, Bommarco R, Fries I, Hederström V, Herbertsson L, Jonsson O, Klatt BK, Pedersen TR, Yourstone J, Smith HG. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature. 2015;521(7550):77–80. doi: 10.1038/nature14420. [DOI] [PubMed] [Google Scholar]
- Samuel MD, Hobbelen PHF, Decastro F, Ahumada JA, Lapointe D, Atkinson CT, Woodworth BL, Hart PJ, Duffy DC. The dynamics, transmission, and population impacts of avian malaria in native hawaiian birds: A modeling approach. Ecological Applications. 2011;21(8):2960–73. doi: 10.1890/10-1311.1. [DOI] [Google Scholar]
- Shan H-W, Luan J-B, Liu Y-Q, Douglas AE, Liu S-S. The inherited bacterial symbiont Hamiltonella influences the sex ratio of an insect host. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1915):20191677. doi: 10.1098/rspb.2019.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Heinze Svenia D, Wu Y, Kohlbrenner T, Morilla I, Brunner C, Wimmer Ernst A, van de Zande L, Robinson Mark D, Beukeboom Leo W, Bopp D. Male sex in houseflies is determined by Mdmd, a paralog of the generic splice factor gene CWC22. Science. 2017;356(6338):642–5. doi: 10.1126/science.aam5498. [DOI] [PubMed] [Google Scholar]
- Simoni A, Hammond AM, Beaghton AK, Galizi R, Taxiarchi C, Kyrou K, Meacci D, Gribble M, Morselli G, Burt A, Nolan T, Crisanti A. A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nature Biotechnology. 2020;38(9):1054–60. doi: 10.1038/s41587-020-0508-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AH, Burkle LA. Solitary Bee Life History Traits and Sex Mediate Responses to Manipulated Seasonal Temperatures and Season Length. Frontiers in Ecology and Evolution. 2019;7. [Google Scholar]
- Sugimoto TN, Ishikawa Y. A male-killing Wolbachia carries a feminizing factor and is associated with degradation of the sex-determining system of its host. Biology Letters. 2012;8(3):412–5. doi: 10.1098/rsbl.2011.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto TN, Kayukawa T, Shinoda T, Ishikawa Y, Tsuchida T. Misdirection of dosage compensation underlies bidirectional sex-specific death in Wolbachia-infected Ostrinia scapulalis. Insect Biochem Mol Biol. 2015;66:72–6. Epub 2015/10/11. doi: 10.1016/j.ibmb.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Suguna SG, Wood RJ, Curtis CF, Whitelaw A, Kazmi SJ. Resistance to meiotic drive at the MD locus in an Indian wild population of Aedes aegypti. Genet Res. 1977;29(2):123–32. Epub 1977/04/01. doi: 10.1017/s0016672300017195. [DOI] [PubMed] [Google Scholar]
- Sweeny TL, Barr AR. Sex Ratio Distortion Caused by Meiotic Drive in a Mosquito, Culex pipiens L. Genetics. 1978;88(3):427–46. Epub 1978/03/01. doi: 10.1093/genetics/88.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Araripe L, Kingan SB, Ke Y, Xiao H, Hartl DL. A sex-ratio Meiotic Drive System in Drosophila simulans. II: An X-linked Distorter. PLoS Biol. 2007;5(11):e293. doi: 10.1371/journal.pbio.0050293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Hartl DL, Laurie CC. Sex-ratio segregation distortion associated with reproductive isolation in Drosophila. Proceedings of the National Academy of Sciences. 2001;98(23):13183. doi: 10.1073/pnas.231478798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Masly JP, Araripe L, Ke Y, Hartl DL. A sex-ratio Meiotic Drive System in Drosophila simulans. I: An Autosomal Suppressor. PLoS Biol. 2007;5(11):e292. doi: 10.1371/journal.pbio.0050292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taracena ML, Hunt CM, Benedict MQ, Pennington PM, Dotson EM. Downregulation of female doublesex expression by oral-mediated RNA interference reduces number and fitness of Anopheles gambiae adult females. Parasites & Vectors. 2019;12(1):170. doi: 10.1186/s13071-019-3437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxiarchi C, Beaghton A, Don NI, Kyrou K, Gribble M, Shittu D, Collins SP, Beisel CL, Galizi R, Crisanti A. A genetically encoded anti-CRISPR protein constrains gene drive spread and prevents population suppression. Nature Communications. 2021;12(1):3977. doi: 10.1038/s41467-021-24214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxiarchi C, Kranjc N, Kriezis A, Kyrou K, Bernardini F, Russell S, Nolan T, Crisanti A, Galizi R. High-resolution transcriptional profiling of Anopheles gambiae spermatogenesis reveals mechanisms of sex chromosome regulation. Scientific Reports. 2019;9(1):14841. doi: 10.1038/s41598-019-51181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Lehmler H-J, Kolpin DW, Hladik ML, Vargo JD, Schilling KE, LeFevre GH, Peeples TL, Poch MC, LaDuca LE, Cwiertny DM, Field RW. A critical review on the potential impacts of neonicotinoid insecticide use: current knowledge of environmental fate, toxicity, and implications for human health. Environmental Science: Processes & Impacts. 2020;22(6):1315–46. doi: 10.1039/C9EM00586B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM. Meiotic sex chromosome inactivation. Development. 2007;134(10):1823–31. Epub 2007/03/03. doi: 10.1242/dev.000018. [DOI] [PubMed] [Google Scholar]
- Vella MR, Gunning CE, Lloyd AL, Gould F. Evaluating strategies for reversing CRISPR-Cas9 gene drives. Scientific Reports. 2017;7(1):11038. doi: 10.1038/s41598-017-10633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-B, Ren F-R, Yao Y-L, Sun X, Walling LL, Li N-N, Bai B, Bao X-Y, Xu X-R, Luan J-B. Intracellular symbionts drive sex ratio in the whitefly by facilitating fertilization and provisioning of B vitamins. The ISME Journal. 2020;14(12):2923–35. doi: 10.1038/s41396-020-0717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial arthropods. Proceedings of the Royal Society B: Biological Sciences. 2015;282(1807):20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6(10):741–51. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Wexler J, Delaney EK, Belles X, Schal C, Wada-Katsumata A, Amicucci MJ, Kopp A. Hemimetabolous insects elucidate the origin of sexual development via alternative splicing. Elife. 2019;8. Epub 2019/09/04. doi: 10.7554/eLife.47490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyard S, Erdelyan CNG, Partridge AL, Singh AD, Beebe NW, Capina R. Silencing the buzz: a new approach to population suppression of mosquitoes by feeding larvae double-stranded RNAs. Parasites & Vectors. 2015;8(1):96. doi: 10.1186/s13071-015-0716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RJ. Between-family variation in sex ratio in the Trinidad (T-30) strain of Aedes aegypti (L.) indicating differences in sensitivity to the meiotic drive gene MD. Genetica. 1976;46(3):345–61. doi: 10.1007/BF00055477. [DOI] [Google Scholar]
- Wood RJ, Ouda NA. The genetic basis of resistance and sensitivity to the meiotic drive gene D in the mosquito Aedes aegypti L. Genetica. 1987;72(1):69–79. Epub 1987/05/15. doi: 10.1007/bf00126980. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Niu B, Ji D, Li M, Li K, James AA, Tan A, Huang Y. Silkworm genetic sexing through W chromosome-linked, targeted gene integration. Proceedings of the National Academy of Sciences. 2018;115(35):8752. doi: 10.1073/pnas.1810945115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Zhang D, Li Y, Yang C, Wu Y, Liang X, Liang Y, Pan X, Hu L, Sun Q, Wang X, Wei Y, Zhu J, Qian W, Yan Z, Parker AG, Gilles JRL, Bourtzis K, Bouyer J, Tang M, Zheng B, Yu J, Liu J, Zhuang J, Hu Z, Zhang M, Gong J-T, Hong X-Y, Zhang Z, Lin L, Liu Q, Hu Z, Wu Z, Baton LA, Hoffmann AA, Xi Z. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019;572(7767):56–61. doi: 10.1038/s41586-019-1407-9. [DOI] [PubMed] [Google Scholar]
- Zhuo JC, Zhang HH, Hu QL, Zhang JL, Lu JB, Li HJ, Xie YC, Wang WW, Zhang Y, Wang HQ, Huang HJ, Lu G, Chen JP, Li JM, Tu ZJ, Zhang CX. A feminizing switch in a hemimetabolous insect. Sci Adv. 2021;7(48):eabf9237. Epub 2021/11/27. doi: 10.1126/sciadv.abf9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Geuverink E, Beukeboom Leo W, Verhulst Eveline C, van de Zande L. A chimeric gene paternally instructs female sex determination in the haplodiploid wasp Nasonia. Science. 2020;370(6520):1115–8. doi: 10.1126/science.abb8949. [DOI] [PubMed] [Google Scholar]


