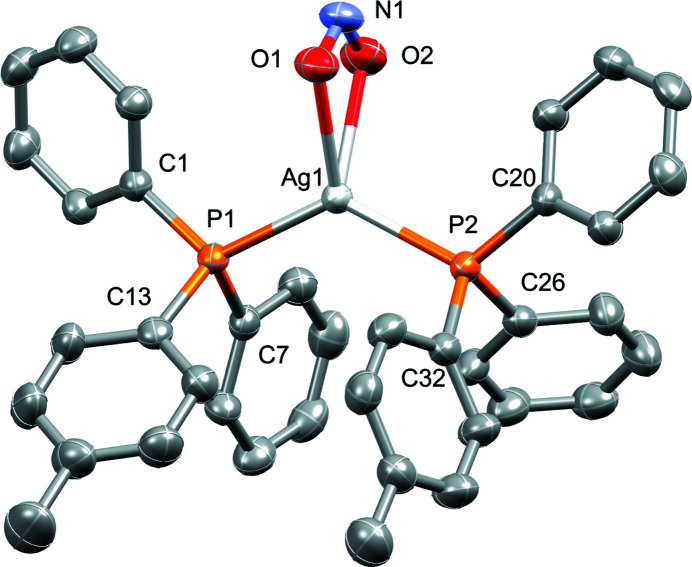
The title silver(I) diphenyl-p-tolylphosphine complex crystallizes with one complete molecule in the asymmetric unit that features a bidentate nitrito, as well as two diphenyl-p-tolylphosphine ligands coordinated to a AgI center.
Keywords: silver(I) complex, diphenyl-p-tolylphosphine, nitrite, crystal structure
Abstract
The title AgI complex, [Ag(NO2)(C19H17P)2], reveals a distorted pseudo-trigonal–planar shape around the AgI atom geometry resulting from the coordination of two phosphine ligands, as well as a nitrito-O,O′ ligand coordinating to the silver(I) atom through the oxygen atoms; in this description, the two oxygen atoms are assumed to occupy one position, forming an acute O—Ag—O angle of 51.44 (9)°. The plane resulting from the NO2 coordination to Ag is nearly perpendicular to the plane from the coordination of the phosphine-P atoms to Ag [dihedral angle = 86.43 (9)°].
Structure description
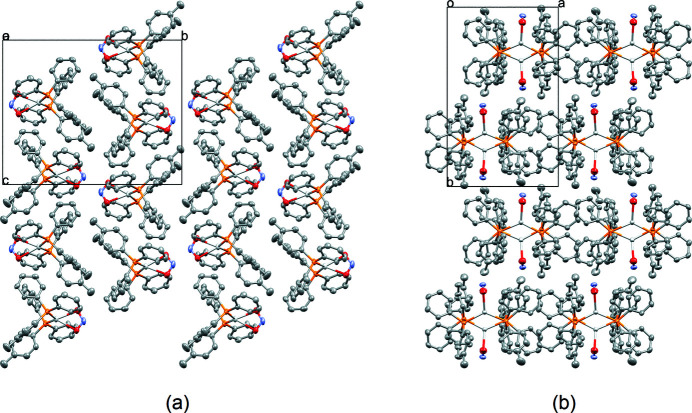
The molecular structure of the title compound is shown in Fig. 1 ▸. The complex crystallizes in the monoclinic space group P21/c with Z = 4. The asymmetric unit contains one complete silver complex molecule, featuring an AgI atom, two diphenyl-p-tolylphosphine ligands, and one NO2 coordinating in a bidentate fashion. Near-identical Ag—P bond lengths are observed [Ag1—P1 = 2.4209 (7) Å and Ag1—P2 = 2.4251 (8) Å]. The nitrito ligand is similarly coordinating in a near symmetric fashion (Ag1—O1 = 2.422 (2), Ag1—O2 = 2.415 (2), N1—O1 = 1.253 (4) and N1–O2 = 1.255 (4) Å). As seen in Fig. 1 ▸, the four-coordinate silver(I) atom essentially exhibits a pseudo trigonal–planar shape with the three coordinating ligands, with bond angles P1—Ag1—P2 [129.51 (3)°], P1—Ag1—O1 [116.23 (7)°], P1—Ag1—O2 [111.09 (7)°], P2—Ag1—O1 [110.79 (7)°], P2—Ag1—O2 [111.96 (7)°], and O1—Ag1—O2 [51.44 (9)°]; in this description, the two oxygen atoms are assumed to occupy one position. The plane Pl1 defined by Ag1, O1, O2 and N1 crosses the plane Pl2 defined by P1, P2 and Ag1 at an angle of 86.43 (9)°. The ipso-carbon atoms of each of the phosphine ligands overlap in a near-eclipsed fashion when viewed down the P1—Ag1—P2 plane Pl2. Corresponding torsion angles are Ag1—P1—C1—C2 = −23.4 (3)°, Ag1—P1—C7—C8 = −51.9 (3)°, Ag1—P1—C13—C14 = 147.8 (3)°, Ag1—P2—C20—C21 = −29.0 (3)°, Ag1—P2—C26—C27 = 133.3 (3) and Ag1—P2—C32—C33 = 132.3 (3)°. The complex packs in three dimensions as layers of molecules, leaving thin corrugated channels in between the inorganic layers when viewed along the a axis (Fig. 2 ▸).
Figure 1.
Perspective view of the molecular structure of the title compound showing displacement ellipsoids at the 50% probability level. Hydrogen atoms are omitted for clarity.
Figure 2.
Packing diagrams as viewed along the (a) a and (b) c axes. Hydrogen atoms are omitted for clarity.
Synthesis and crystallization
Diphenyl-p-tolylphosphine (1 mmol) was dissolved in acetonitrile (10 ml). Silver nitrite (1 mmol) was dissolved in acetonitrile (5 ml). The diphenyl-p-tolylphosphine solution (10 ml) was added to the silver nitrite solution (5 ml), to give a 2:1 molar ratio reaction. The mixture was heated under reflux for 2 h after which the solution was left to crystallize.
Refinement
For full experimental details including crystal data, data collection and structure refinement details, refer to Table 1 ▸.
Table 1. Experimental details.
| Crystal data | |
| Chemical formula | [Ag(NO2)(C19H17P)2] |
| M r | 706.47 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 150 |
| a, b, c (Å) | 11.8709 (2), 18.6292 (2), 15.4003 (2) |
| β (°) | 103.055 (1) |
| V (Å3) | 3317.68 (8) |
| Z | 4 |
| Radiation type | Cu Kα |
| μ (mm−1) | 6.05 |
| Crystal size (mm) | 0.24 × 0.13 × 0.10 |
| Data collection | |
| Diffractometer | XtaLAB Synergy R, DW system, HyPix |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2022 ▸) |
| T min, T max | 0.188, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 41716, 7030, 6535 |
| R int | 0.049 |
| (sin θ/λ)max (Å−1) | 0.637 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.040, 0.104, 1.07 |
| No. of reflections | 7030 |
| No. of parameters | 399 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.68, −0.82 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314622007714/tk4082sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314622007714/tk4082Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314622007714/tk4082Isup3.cdx
CCDC reference: 2193913
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We would like to greatly acknowledge the National Research Foundation (NRF, SA), University of Pretoria and the University of Johannesburg for funding provided.
full crystallographic data
Crystal data
| [Ag(NO2)(C19H17P)2] | F(000) = 1448 |
| Mr = 706.47 | Dx = 1.414 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54184 Å |
| a = 11.8709 (2) Å | Cell parameters from 29792 reflections |
| b = 18.6292 (2) Å | θ = 3.8–78.9° |
| c = 15.4003 (2) Å | µ = 6.05 mm−1 |
| β = 103.055 (1)° | T = 150 K |
| V = 3317.68 (8) Å3 | Block, colourless |
| Z = 4 | 0.24 × 0.13 × 0.10 mm |
Data collection
| XtaLAB Synergy R, DW system, HyPix diffractometer | 7030 independent reflections |
| Radiation source: Rotating-anode X-ray tube, Rigaku (Cu) X-ray Source | 6535 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.049 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 79.2°, θmin = 3.8° |
| ω scans | h = −14→15 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2022) | k = −23→23 |
| Tmin = 0.188, Tmax = 1.000 | l = −18→19 |
| 41716 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.040 | H-atom parameters constrained |
| wR(F2) = 0.104 | w = 1/[σ2(Fo2) + (0.0425P)2 + 5.6399P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max = 0.002 |
| 7030 reflections | Δρmax = 0.68 e Å−3 |
| 399 parameters | Δρmin = −0.82 e Å−3 |
| 0 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Ag1 | 0.33615 (2) | 0.79867 (2) | 0.56752 (2) | 0.03178 (8) | |
| P1 | 0.52794 (6) | 0.74875 (4) | 0.61894 (5) | 0.02991 (16) | |
| P2 | 0.14991 (6) | 0.73885 (4) | 0.53633 (6) | 0.03298 (17) | |
| O1 | 0.3244 (2) | 0.91204 (13) | 0.48903 (17) | 0.0459 (6) | |
| O2 | 0.3250 (2) | 0.91839 (14) | 0.62523 (18) | 0.0506 (6) | |
| N1 | 0.3202 (3) | 0.95191 (15) | 0.5538 (2) | 0.0492 (8) | |
| C7 | 0.5331 (3) | 0.69367 (17) | 0.7177 (2) | 0.0325 (6) | |
| C1 | 0.6466 (3) | 0.81224 (16) | 0.6530 (2) | 0.0321 (6) | |
| C25 | −0.0805 (3) | 0.77034 (18) | 0.4467 (2) | 0.0380 (7) | |
| H25 | −0.0857 | 0.7224 | 0.4253 | 0.046* | |
| C20 | 0.0223 (3) | 0.79524 (16) | 0.5000 (2) | 0.0334 (6) | |
| C26 | 0.1234 (3) | 0.69196 (16) | 0.6342 (2) | 0.0355 (7) | |
| C13 | 0.5720 (3) | 0.68644 (16) | 0.5419 (2) | 0.0337 (6) | |
| C6 | 0.7500 (3) | 0.79432 (18) | 0.7117 (2) | 0.0385 (7) | |
| H6 | 0.7610 | 0.7473 | 0.7360 | 0.046* | |
| C24 | −0.1757 (3) | 0.8154 (2) | 0.4248 (2) | 0.0443 (8) | |
| H24 | −0.2462 | 0.7980 | 0.3888 | 0.053* | |
| C32 | 0.1398 (3) | 0.66934 (17) | 0.4515 (2) | 0.0360 (7) | |
| C2 | 0.6317 (3) | 0.88145 (17) | 0.6186 (2) | 0.0352 (7) | |
| H2 | 0.5610 | 0.8943 | 0.5790 | 0.042* | |
| C8 | 0.4942 (3) | 0.7238 (2) | 0.7887 (2) | 0.0413 (7) | |
| H8 | 0.4726 | 0.7729 | 0.7870 | 0.050* | |
| C37 | 0.1822 (3) | 0.68542 (19) | 0.3764 (2) | 0.0425 (8) | |
| H37 | 0.2095 | 0.7325 | 0.3690 | 0.051* | |
| C31 | 0.2138 (3) | 0.65361 (19) | 0.6870 (3) | 0.0456 (8) | |
| H31 | 0.2859 | 0.6510 | 0.6702 | 0.055* | |
| C5 | 0.8371 (3) | 0.8450 (2) | 0.7349 (3) | 0.0461 (8) | |
| H5 | 0.9076 | 0.8326 | 0.7750 | 0.055* | |
| C18 | 0.4881 (3) | 0.64259 (19) | 0.4912 (3) | 0.0440 (8) | |
| H18 | 0.4096 | 0.6485 | 0.4941 | 0.053* | |
| C10 | 0.5184 (3) | 0.6103 (2) | 0.8646 (2) | 0.0459 (8) | |
| H10 | 0.5119 | 0.5816 | 0.9143 | 0.055* | |
| C12 | 0.5662 (3) | 0.62220 (17) | 0.7224 (2) | 0.0374 (7) | |
| H12 | 0.5942 | 0.6014 | 0.6750 | 0.045* | |
| C14 | 0.6851 (3) | 0.6784 (2) | 0.5339 (3) | 0.0474 (8) | |
| H14 | 0.7437 | 0.7089 | 0.5665 | 0.057* | |
| C11 | 0.5589 (3) | 0.5806 (2) | 0.7959 (2) | 0.0447 (8) | |
| H11 | 0.5819 | 0.5317 | 0.7985 | 0.054* | |
| C4 | 0.8218 (3) | 0.9132 (2) | 0.7003 (2) | 0.0453 (8) | |
| H4 | 0.8817 | 0.9478 | 0.7167 | 0.054* | |
| C3 | 0.7192 (3) | 0.93168 (19) | 0.6415 (2) | 0.0445 (8) | |
| H3 | 0.7090 | 0.9787 | 0.6171 | 0.053* | |
| C23 | −0.1685 (3) | 0.8854 (2) | 0.4552 (3) | 0.0457 (8) | |
| H23 | −0.2335 | 0.9164 | 0.4398 | 0.055* | |
| C21 | 0.0295 (3) | 0.86579 (18) | 0.5299 (3) | 0.0447 (8) | |
| H21 | 0.1000 | 0.8837 | 0.5653 | 0.054* | |
| C27 | 0.0206 (3) | 0.6970 (2) | 0.6615 (3) | 0.0491 (9) | |
| H27 | −0.0422 | 0.7235 | 0.6271 | 0.059* | |
| C29 | 0.0976 (4) | 0.6233 (2) | 0.7899 (3) | 0.0515 (9) | |
| H29 | 0.0878 | 0.5992 | 0.8421 | 0.062* | |
| C35 | 0.1463 (3) | 0.5644 (2) | 0.3217 (3) | 0.0482 (8) | |
| C9 | 0.4873 (3) | 0.6817 (2) | 0.8616 (2) | 0.0492 (9) | |
| H9 | 0.4609 | 0.7023 | 0.9098 | 0.059* | |
| C22 | −0.0663 (3) | 0.9098 (2) | 0.5080 (3) | 0.0518 (9) | |
| H22 | −0.0615 | 0.9577 | 0.5297 | 0.062* | |
| C36 | 0.1850 (3) | 0.6340 (2) | 0.3126 (3) | 0.0465 (8) | |
| H36 | 0.2137 | 0.6462 | 0.2617 | 0.056* | |
| C30 | 0.2004 (3) | 0.6192 (2) | 0.7633 (3) | 0.0520 (9) | |
| H30 | 0.2628 | 0.5924 | 0.7978 | 0.062* | |
| C17 | 0.5172 (4) | 0.5902 (2) | 0.4363 (3) | 0.0523 (9) | |
| H17 | 0.4585 | 0.5601 | 0.4028 | 0.063* | |
| C33 | 0.0991 (3) | 0.60034 (19) | 0.4599 (3) | 0.0488 (9) | |
| H33 | 0.0689 | 0.5882 | 0.5101 | 0.059* | |
| C15 | 0.7132 (4) | 0.6261 (2) | 0.4787 (3) | 0.0580 (11) | |
| H15 | 0.7914 | 0.6210 | 0.4744 | 0.070* | |
| C16 | 0.6305 (4) | 0.5809 (2) | 0.4296 (3) | 0.0542 (10) | |
| C28 | 0.0095 (4) | 0.6631 (2) | 0.7393 (3) | 0.0579 (10) | |
| H28 | −0.0611 | 0.6676 | 0.7581 | 0.069* | |
| C34 | 0.1023 (4) | 0.5492 (2) | 0.3954 (3) | 0.0568 (10) | |
| H34 | 0.0735 | 0.5025 | 0.4019 | 0.068* | |
| C38 | 0.1539 (4) | 0.5072 (3) | 0.2557 (3) | 0.0684 (12) | |
| H38A | 0.0815 | 0.5056 | 0.2100 | 0.103* | |
| H38B | 0.1668 | 0.4607 | 0.2860 | 0.103* | |
| H38C | 0.2183 | 0.5177 | 0.2275 | 0.103* | |
| C19 | 0.6634 (5) | 0.5233 (3) | 0.3706 (4) | 0.0806 (16) | |
| H19A | 0.6400 | 0.4763 | 0.3889 | 0.121* | |
| H19B | 0.7473 | 0.5239 | 0.3763 | 0.121* | |
| H19C | 0.6242 | 0.5325 | 0.3085 | 0.121* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Ag1 | 0.02537 (12) | 0.02500 (12) | 0.04510 (14) | −0.00004 (7) | 0.00826 (9) | 0.00223 (8) |
| P1 | 0.0263 (3) | 0.0251 (3) | 0.0388 (4) | 0.0012 (3) | 0.0084 (3) | 0.0051 (3) |
| P2 | 0.0250 (3) | 0.0251 (4) | 0.0489 (5) | −0.0007 (3) | 0.0084 (3) | 0.0014 (3) |
| O1 | 0.0571 (15) | 0.0328 (12) | 0.0506 (14) | 0.0036 (11) | 0.0179 (12) | 0.0019 (10) |
| O2 | 0.0616 (16) | 0.0403 (14) | 0.0527 (15) | −0.0017 (12) | 0.0191 (12) | −0.0120 (12) |
| N1 | 0.0524 (18) | 0.0248 (14) | 0.071 (2) | 0.0032 (12) | 0.0154 (15) | −0.0042 (14) |
| C7 | 0.0261 (14) | 0.0343 (16) | 0.0376 (16) | −0.0021 (11) | 0.0080 (12) | 0.0028 (12) |
| C1 | 0.0288 (14) | 0.0299 (15) | 0.0390 (16) | 0.0013 (12) | 0.0104 (12) | 0.0028 (12) |
| C25 | 0.0315 (16) | 0.0320 (16) | 0.0496 (19) | 0.0001 (12) | 0.0075 (13) | −0.0020 (14) |
| C20 | 0.0252 (14) | 0.0295 (15) | 0.0462 (17) | −0.0008 (11) | 0.0098 (12) | 0.0019 (12) |
| C26 | 0.0319 (15) | 0.0267 (14) | 0.0482 (18) | −0.0009 (12) | 0.0097 (13) | −0.0024 (13) |
| C13 | 0.0364 (16) | 0.0284 (14) | 0.0373 (16) | 0.0020 (12) | 0.0105 (13) | 0.0086 (12) |
| C6 | 0.0302 (16) | 0.0354 (17) | 0.0488 (19) | 0.0031 (12) | 0.0064 (13) | 0.0082 (14) |
| C24 | 0.0332 (17) | 0.051 (2) | 0.0455 (19) | 0.0061 (15) | 0.0027 (14) | 0.0003 (16) |
| C32 | 0.0291 (15) | 0.0294 (15) | 0.0499 (18) | 0.0013 (12) | 0.0094 (13) | 0.0004 (13) |
| C2 | 0.0336 (16) | 0.0323 (15) | 0.0381 (16) | 0.0010 (12) | 0.0049 (12) | 0.0034 (12) |
| C8 | 0.0414 (18) | 0.0389 (17) | 0.0440 (18) | −0.0020 (14) | 0.0107 (14) | −0.0029 (14) |
| C37 | 0.0407 (18) | 0.0345 (17) | 0.052 (2) | −0.0064 (14) | 0.0102 (15) | 0.0017 (15) |
| C31 | 0.0332 (17) | 0.0369 (17) | 0.068 (2) | −0.0016 (14) | 0.0136 (16) | 0.0122 (16) |
| C5 | 0.0319 (16) | 0.053 (2) | 0.051 (2) | −0.0049 (15) | 0.0037 (14) | 0.0041 (16) |
| C18 | 0.0378 (18) | 0.0388 (18) | 0.056 (2) | 0.0015 (14) | 0.0121 (15) | −0.0036 (15) |
| C10 | 0.0444 (19) | 0.054 (2) | 0.0378 (18) | −0.0127 (16) | 0.0067 (14) | 0.0094 (15) |
| C12 | 0.0369 (16) | 0.0339 (16) | 0.0428 (17) | 0.0064 (13) | 0.0119 (13) | 0.0091 (13) |
| C14 | 0.0400 (19) | 0.046 (2) | 0.063 (2) | −0.0086 (15) | 0.0252 (17) | −0.0084 (17) |
| C11 | 0.0467 (19) | 0.0419 (19) | 0.0436 (19) | −0.0007 (15) | 0.0064 (15) | 0.0128 (15) |
| C4 | 0.0398 (18) | 0.0428 (19) | 0.053 (2) | −0.0134 (15) | 0.0101 (15) | −0.0008 (16) |
| C3 | 0.0465 (19) | 0.0351 (17) | 0.050 (2) | −0.0054 (14) | 0.0082 (15) | 0.0064 (15) |
| C23 | 0.0373 (18) | 0.0406 (18) | 0.059 (2) | 0.0137 (14) | 0.0114 (15) | 0.0070 (16) |
| C21 | 0.0310 (16) | 0.0308 (16) | 0.071 (2) | −0.0017 (13) | 0.0083 (15) | −0.0048 (15) |
| C27 | 0.0391 (19) | 0.050 (2) | 0.062 (2) | 0.0066 (16) | 0.0194 (17) | 0.0084 (17) |
| C29 | 0.068 (3) | 0.0368 (18) | 0.054 (2) | −0.0029 (17) | 0.0217 (19) | 0.0043 (16) |
| C35 | 0.0433 (19) | 0.0421 (19) | 0.061 (2) | 0.0021 (15) | 0.0152 (17) | −0.0068 (17) |
| C9 | 0.049 (2) | 0.064 (2) | 0.0366 (18) | −0.0079 (18) | 0.0137 (15) | −0.0050 (16) |
| C22 | 0.044 (2) | 0.0314 (17) | 0.081 (3) | 0.0053 (15) | 0.0172 (19) | −0.0036 (17) |
| C36 | 0.0430 (19) | 0.052 (2) | 0.048 (2) | −0.0034 (16) | 0.0178 (16) | 0.0013 (16) |
| C30 | 0.047 (2) | 0.0399 (19) | 0.067 (2) | −0.0023 (16) | 0.0076 (18) | 0.0153 (17) |
| C17 | 0.056 (2) | 0.046 (2) | 0.057 (2) | −0.0090 (17) | 0.0154 (18) | −0.0139 (17) |
| C33 | 0.058 (2) | 0.0320 (17) | 0.062 (2) | −0.0083 (16) | 0.0264 (18) | −0.0040 (16) |
| C15 | 0.052 (2) | 0.056 (2) | 0.078 (3) | −0.0100 (19) | 0.040 (2) | −0.016 (2) |
| C16 | 0.069 (3) | 0.044 (2) | 0.059 (2) | −0.0045 (19) | 0.033 (2) | −0.0089 (17) |
| C28 | 0.057 (2) | 0.056 (2) | 0.071 (3) | 0.0122 (19) | 0.036 (2) | 0.012 (2) |
| C34 | 0.069 (3) | 0.0321 (18) | 0.076 (3) | −0.0061 (17) | 0.032 (2) | −0.0040 (18) |
| C38 | 0.071 (3) | 0.065 (3) | 0.076 (3) | 0.001 (2) | 0.030 (2) | −0.014 (2) |
| C19 | 0.094 (4) | 0.072 (3) | 0.090 (4) | −0.013 (3) | 0.051 (3) | −0.037 (3) |
Geometric parameters (Å, º)
| Ag1—P1 | 2.4209 (7) | C32—C37 | 1.395 (5) |
| Ag1—P2 | 2.4251 (8) | C32—C33 | 1.390 (5) |
| Ag1—O1 | 2.422 (2) | C2—C3 | 1.383 (5) |
| Ag1—O2 | 2.415 (2) | C8—C9 | 1.386 (5) |
| P1—C7 | 1.825 (3) | C37—C36 | 1.377 (5) |
| P1—C1 | 1.823 (3) | C31—C30 | 1.380 (5) |
| P1—C13 | 1.820 (3) | C5—C4 | 1.373 (5) |
| P2—C20 | 1.824 (3) | C18—C17 | 1.385 (5) |
| P2—C26 | 1.830 (3) | C10—C11 | 1.373 (5) |
| P2—C32 | 1.824 (3) | C10—C9 | 1.380 (6) |
| O1—N1 | 1.253 (4) | C12—C11 | 1.389 (5) |
| O2—N1 | 1.255 (4) | C14—C15 | 1.382 (5) |
| C7—C8 | 1.396 (5) | C4—C3 | 1.387 (5) |
| C7—C12 | 1.386 (4) | C23—C22 | 1.377 (5) |
| C1—C6 | 1.391 (4) | C21—C22 | 1.380 (5) |
| C1—C2 | 1.390 (4) | C27—C28 | 1.386 (6) |
| C25—C20 | 1.388 (4) | C29—C30 | 1.374 (6) |
| C25—C24 | 1.387 (5) | C29—C28 | 1.374 (6) |
| C20—C21 | 1.389 (4) | C35—C36 | 1.394 (5) |
| C26—C31 | 1.388 (5) | C35—C34 | 1.381 (6) |
| C26—C27 | 1.381 (5) | C35—C38 | 1.489 (6) |
| C13—C18 | 1.384 (5) | C17—C16 | 1.383 (6) |
| C13—C14 | 1.384 (5) | C33—C34 | 1.383 (5) |
| C6—C5 | 1.386 (5) | C15—C16 | 1.381 (6) |
| C24—C23 | 1.381 (5) | C16—C19 | 1.513 (6) |
| P1—Ag1—P2 | 129.51 (3) | C5—C6—C1 | 120.1 (3) |
| P1—Ag1—O1 | 116.23 (7) | C23—C24—C25 | 120.3 (3) |
| O1—Ag1—P2 | 110.79 (7) | C37—C32—P2 | 117.7 (2) |
| O2—Ag1—P1 | 111.09 (7) | C33—C32—P2 | 123.9 (3) |
| O2—Ag1—P2 | 111.96 (7) | C33—C32—C37 | 118.3 (3) |
| O2—Ag1—O1 | 51.44 (9) | C3—C2—C1 | 120.4 (3) |
| C7—P1—Ag1 | 109.92 (10) | C9—C8—C7 | 119.8 (3) |
| C1—P1—Ag1 | 116.95 (10) | C36—C37—C32 | 120.9 (3) |
| C1—P1—C7 | 104.28 (14) | C30—C31—C26 | 121.0 (3) |
| C13—P1—Ag1 | 114.79 (11) | C4—C5—C6 | 120.3 (3) |
| C13—P1—C7 | 103.00 (14) | C13—C18—C17 | 120.9 (3) |
| C13—P1—C1 | 106.52 (14) | C11—C10—C9 | 119.9 (3) |
| C20—P2—Ag1 | 116.91 (10) | C7—C12—C11 | 120.6 (3) |
| C20—P2—C26 | 104.02 (15) | C15—C14—C13 | 120.3 (4) |
| C26—P2—Ag1 | 112.02 (11) | C10—C11—C12 | 120.1 (3) |
| C32—P2—Ag1 | 112.17 (10) | C5—C4—C3 | 120.2 (3) |
| C32—P2—C20 | 105.88 (15) | C2—C3—C4 | 119.8 (3) |
| C32—P2—C26 | 104.80 (15) | C22—C23—C24 | 119.4 (3) |
| N1—O1—Ag1 | 97.3 (2) | C22—C21—C20 | 119.8 (3) |
| N1—O2—Ag1 | 97.59 (19) | C26—C27—C28 | 119.7 (4) |
| O1—N1—O2 | 113.6 (3) | C28—C29—C30 | 118.3 (4) |
| C8—C7—P1 | 118.2 (2) | C36—C35—C38 | 121.7 (4) |
| C12—C7—P1 | 122.7 (3) | C34—C35—C36 | 117.8 (3) |
| C12—C7—C8 | 119.0 (3) | C34—C35—C38 | 120.5 (4) |
| C6—C1—P1 | 122.8 (2) | C10—C9—C8 | 120.6 (3) |
| C2—C1—P1 | 117.9 (2) | C23—C22—C21 | 121.0 (3) |
| C2—C1—C6 | 119.2 (3) | C37—C36—C35 | 121.0 (3) |
| C24—C25—C20 | 120.1 (3) | C29—C30—C31 | 120.6 (4) |
| C25—C20—P2 | 123.2 (2) | C16—C17—C18 | 121.0 (4) |
| C25—C20—C21 | 119.4 (3) | C34—C33—C32 | 120.2 (4) |
| C21—C20—P2 | 117.3 (2) | C16—C15—C14 | 121.7 (4) |
| C31—C26—P2 | 118.3 (3) | C17—C16—C19 | 121.4 (4) |
| C27—C26—P2 | 123.1 (3) | C15—C16—C17 | 117.8 (4) |
| C27—C26—C31 | 118.4 (3) | C15—C16—C19 | 120.8 (4) |
| C18—C13—P1 | 117.9 (3) | C29—C28—C27 | 121.8 (4) |
| C14—C13—P1 | 123.7 (3) | C35—C34—C33 | 121.8 (4) |
| C14—C13—C18 | 118.3 (3) |
Funding Statement
Funding for this research was provided by: National Research Foundation (grant No. 138280).
References
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Rigaku OD (2022). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314622007714/tk4082sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314622007714/tk4082Isup2.hkl
Supporting information file. DOI: 10.1107/S2414314622007714/tk4082Isup3.cdx
CCDC reference: 2193913
Additional supporting information: crystallographic information; 3D view; checkCIF report