Abstract
Background:
While reduced exposure to prescription opioids may decrease risks, including overdose and opioid use disorder, abrupt tapering or discontinuation may pose new risks.
Objectives:
To examine potentially unsafe tapering and discontinuation among dosage changes in opioid prescriptions dispensed to US patients on high-dose long-term opioid therapy.
Design:
Longitudinal observational study of adults (≥18 years) on stable high-dose (≥50 oral morphine milligram equivalents [MME] daily dosage) long-term opioid therapy during a 180-day baseline and a 360-day follow-up using all-payer pharmaceutical claims data, 2017–2019.
Measures:
Dosage tapering, increases, and/or stability during follow-up; sustained dosage stability, reductions, or discontinuation at the end of follow-up; and tapering rate. Patients could experience more than one outcome during follow-up.
Results:
Among 595,078 patients receiving high-dose long-term opioid therapy in the sample, 26.7% experienced sustained dosage reductions and 9.3% experienced discontinuation. Among patients experiencing tapering, 62.0% experienced maximum taper rates between > 10–40% reductions per month and 36.1% experienced monthly rates ≥ 40%. Among patients with mean baseline daily dosages ≥ 150 MME, 47.7% experienced a maximum taper rate ≥ 40% per month. Relative to baseline, 19.7% of patients experiencing tapering had long-term dosage reductions ≥ 40% per month at the end of follow-up.
Implications:
Dosage changes for patients on high-dose long-term opioid therapy may warrant special attention, particularly over shorter intervals, to understand how potentially sudden tapering and discontinuation can be reduced while emphasizing patient safety and shared decision-making. Rapid discontinuation of opioids can increase risk of adverse outcomes including opioid withdrawal.
Keywords: Prescription opioids, Tapering, Long-term opioid therapy, Discontinuation
1. Introduction
Prescription opioid dispensing rates among the US population have decreased from a peak of 81.2 opioid prescriptions per 100 persons in 2012 to 51.4 opioid prescriptions per 100 persons in 2018 (Centers for Disease Control and Prevention, 2019). While reducing exposure to prescription opioids may reduce risks, including overdose and opioid use disorder, abrupt tapering or sudden discontinuation may pose new risks (Dowell et al., 2016). The Centers for Disease Control and Prevention’s Guideline for Prescribing Opioids for Chronic Pain (CDC Guideline) recommends tapers slow enough to minimize symptoms and signs of opioid withdrawal when reducing or discontinuing opioids (Dowell et al., 2016). The HHS Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long-Term Opioid Analgesics (i.e., HHS Guide) (US Department of Health and Human Services, 2019) notes that rapid tapering or sudden discontinuation of opioids among physically dependent patients can increase risks including acute withdrawal symptoms, serious psychological distress, suicidal ideation, and also references the U.S. Food and Drug Administration safety announcement that patients may use other substances, including illicit opioids, to try to manage pain or withdrawal symptoms (U.S. Food and Drug Administration (FDA), 2019). These guidelines emphasize the importance of careful consideration of risk and benefits through a shared decision-making process with the patient when considering dosage reductions.
Prior research has examined tapering among patients with commercial or Medicare Advantage insurance through 2018 (Fenton et al., 2019, 2021). Several studies have focused on dosage discontinuation among patients on long-term opioid therapy across a variety of payer types (Hallvik et al., 2021; Hayes et al., 2021; Minegishi et al., 2020; Neprash et al., 2021; Quinn et al., 2021; Stein et al., 2021). However, limited information exists regarding recent changes in both dosage tapering and discontinuation of opioid prescriptions dispensed to patients on high-dose long-term opioid therapy, across payer sources. This study quantifies potentially unsafe tapering and/or discontinuation by examining dosage changes in opioid prescriptions dispensed to patients on high-dose long-term opioid therapy from 2017 through 2019 using a large all-payer dataset. This study builds on the existing literature by examining more recent years of data, focusing on the period following the release of the CDC Guideline in 2016, which has been shown to be associated with changes in opioid prescribing practices in the United States (Bohnert et al., 2018; Mazurenko et al., 2021). Additionally, this study uses an all-payer sample, and is the first to examine patients on long-term opioid therapy for a 12-month follow-up period and utilize tapering thresholds of larger magnitudes based on the HHS Guide. The results of this study can help inform thoughtful, measured, and collaborative changes to opioid prescribing as part of a comprehensive approach to improve patient safety and prevent opioid-related harms.
2. Methods
2.1. Data
This retrospective cohort study used data from the IQVIA Longitudinal Prescription (LRx) retail pharmacy database from 2017 to 2019. The LRx database covers 92% of all retail pharmacy prescriptions in the United States. Opioids obtained through mail order or dispensed directly by clinicians, including methadone dispensed by opioid treatment programs, are excluded. We calculated oral morphine milligram equivalents (MME) using published conversion factors from the CDC (National Center for Injury Prevention and Control, 2018 version). All prescriptions in the LRx database with corresponding MME conversion factors had data available on days’ supply, dispense date, and quantity, allowing us to determine patients’ total daily dosage of opioids.
2.2. Cohort
Fig. 1 shows the cohort selection and associated outcome measures. The population of interest was patients receiving long-term opioid therapy in 2017 and 2018. We define the cohort of patients on long-term opioid therapy starting in 2017 to focus this study on the years following the release of the 2016 CDC Guideline (US Department of Health and Human Services, 2019). We identified adults (age ≥ 18 years) with oral and transdermal opioid prescriptions dispensed between 2017 and 2018 in the United States. We excluded individuals with prescriptions from clinicians with oncology, palliative care, or hematology specialties during the study period or within a 365-day lookback period prior to their first prescription. Cold and cough products, buprenorphine formulations typically used to treat opioid use disorder, and prescriptions with possibly erroneous daily quantities in solution (<1 unit per day) or 72-hour patch form (>1 unit every three days) were excluded.
Fig. 1.
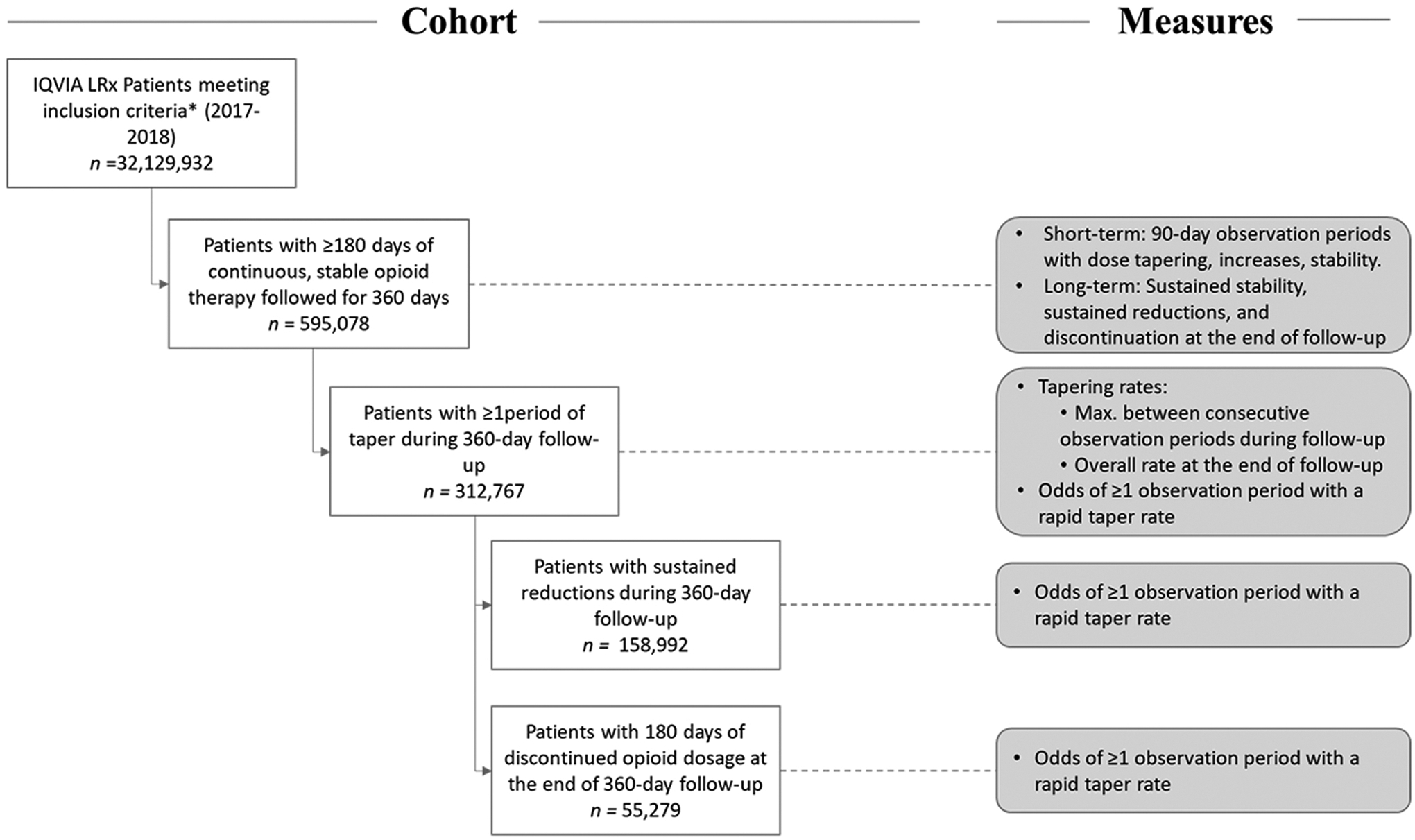
Study populations and associated measures of interest. High-dose, long-term opioid therapy is operationalized as patients with a mean baseline daily dosage of ≥ 50 MME and ≥ 90% proportion of days with non-zero opioid dosage during a 180-day baseline period. *Adults (age ≥ 18 years) with oral and transdermal opioid prescriptions dispensed between 2017 and 2018 in the United States, excluding (1) patients with a history of prescriptions from oncology, palliative care, and hematology specialties, and (2) prescriptions for cold and cough products, buprenorphine formulations typically used to treat opioid use disorder, and prescriptions with possibly erroneous daily quantities were excluded.
We defined the study cohort as patients with: 1) continuous high-dose opioid therapy, identified as those with a mean baseline daily dosage of ≥ 50 MME and ≥ 90% proportion of days with non-zero opioid dosage during a 180-day baseline period in 2017 and 2018; and 2) stable opioid therapy, identified as those with monthly mean MME that varied < 10% from the average monthly baseline dosage in order to ensure a clean baseline period with no tapering events, consistent with earlier approaches(Fenton et al., 2019). Using 2017–2019 data, we identified any opioid prescriptions dispensed to patients during a follow-up period of 360 days after baseline. During follow-up, outcomes were tracked across ten 90-day observation periods with 60 days of overlap between consecutive periods (i.e., 10 sets of averaged month triads across 12 follow-up months; Appendix-A1).
2.3. Measures
2.3.1. Identifying tapering
Absent clearly defined indicators within our data, we based our algorithm for identifying tapering events on the general approach used by Fenton et al. (2019) with a few modifications to the tapering threshold, definition, and observation periods (Appendix-A2). These modifications helped increase the sensitivity of the algorithm in identifying tapers between 10% and 15% over shorter durations under 6 months. We defined dosage tapers in patients as a ≥ 10% reduction in mean daily opioid dosage: 1) from mean dosage during baseline compared to any 90-day observation period during follow-up and 2) between any two consecutive observation periods, starting at baseline. The first criterion helped identify dosage reductions initiated at any point during follow-up as compared to baseline dosage and prevented the inclusion of patients who only experienced decreases from any increased dosages during follow-up. The second criterion ensured we captured patients with at least a 10% reduction in dosage between any two consecutive periods, preventing the inclusion of patients with small dosage changes that eventually accumulated to 10% only over the duration of follow-up. We selected a relatively conservative 90-day observation period to account for minor daily or monthly fluctuations in dispensed prescriptions that could arise from a variety of circumstances.
Eight patient-level outcome measures were defined across three categories: short-term events (during follow-up), long-term outcomes (at the end of follow-up), and tapering rate. All dosages were measured in daily MME.
2.3.2. Short-term events
Short-term events were defined for each observation period; it was possible for a single patient to experience multiple short-term events across successive periods, including tapering, stability, and increases, during follow-up. We used percentage changes in daily opioid dosage at any of the ten 90-day follow-up observation periods from the baseline to identify dosage tapering (≥10% decrease), increases (≥10% increase), and/or stability (any change <10%).
2.3.3. Long-term outcomes
Long-term outcomes helped capture dosage changes, beyond short-term events, over the duration of follow-up. For example, a patient could experience all three short-term events during follow-up, but still have an overall reduction compared to their baseline dosage. We quantified three longer-term measures: sustained dosage stability was defined as patients who had all ten 90-day observation periods during follow-up consecutively meet the short-term stability criteria relative to the previous period; sustained dosage reduction was defined as patients who had no 90-day observation periods of increases during follow-up and at least one observation period with tapering relative to the previous period; and discontinuation was defined as any consecutive 180-day period during follow-up with zero-MME daily opioid dosage. Some patients could experience both sustained dosage reductions and dosage discontinuation.
2.3.4. Tapering rate
We determined the taper rate, or velocity, using the approach detailed in Fenton et al. (2019), which relies on the mean starting and ending dosages at any two time points, and the corresponding duration of taper. Rates were expressed as percent change in daily opioid dosage per month. Two additional measures were calculated among patients who experienced dosage tapering in at least one observation period to capture the rate at which reductions occurred (Appendix-B). The first quantified the short-term, maximum taper rate during follow-up by comparing the beginning and ending mean daily dosages in consecutive observation periods, starting at baseline. This allowed us to group patients based on their fastest, and potentially most unsafe, taper rate. The second measure quantified the long-term, overall dosage reduction rate across all 12 follow-up months, calculated by comparing the mean daily dosage during baseline with that of the final 90-day observation period (spanning months 10–12). Among patients who were discontinued or experienced at least 90 consecutive days with no opioid dosage, the overall dosage reduction rate compared the mean baseline daily dosage with the first full 90-day observation period with zero daily dosage.
Based on the HHS Guide (US Department of Health and Human Services, 2019) and the CDC Guideline (Dowell et al., 2016) dosage reductions exceeding 10% per week (i.e., 34.4% per month since each subsequent dosage is a 10% reduction relative to the previous week’s dosage) are considered to put patients at a higher risk for adverse events such as withdrawal, worsening of pain, and serious psychological distress. As in previous studies (Fenton et al., 2019), rapid taper rates were conservatively defined to be a dosage reduction of ≥ 40% month relative to the previous observation period (for the maximum taper rate) or baseline (for the overall dosage reduction rate). A dichotomous variable was created to identify patients with rapid taper rates, i.e., at least one 90-day observation period showing a maximum taper of ≥ 40% month.
2.4. Analyses
We calculated descriptive statistics, stratified by patients’ mean baseline daily dosages, for study population demographics, short-term events, and long-term outcomes. We reported frequency distributions of patients based on maximum taper and overall dosage reduction rates. Finally, we used logistic regression models to determine the odds of patients experiencing at least one 90-day observation period with a rapid taper (≥40% month) in subgroups of patients with: 1) any dosage tapering during follow-up (at least one observation period with tapering), 2) sustained reductions during follow-up (no observation periods with dosage increases and at least one with tapering), and 3) discontinued dosage at the end of follow-up (180 days with no dosage). We also conducted sensitivity analyses of the short-term events and long-term outcomes using a reduced baseline period of 90 days to identify patients with long-term opioid therapy.
We included the following covariates: patient sex, age-group, most common payer type (across all dispensed prescriptions during the study period), and mean baseline daily dosage. We report adjusted odds ratios (aORs) and 95% confidence intervals (CIs). We performed all analyses in SAS 9.3 (SAS Institute, Cary NC).
3. Results
Between 2017 and 2018, 595,078 adult patients on long-term opioid therapy were identified in the final study population (Table 1). Most patients were aged 35–64 years (67.1%) or ≥ 65 years (30.0%); 51.6% were female; and Medicare (46.4%) and third-party (39.0%) were the most frequent payer types. Most patients had mean baseline daily dosages < 150 MME: 44.8% of patients had dosages 50–89 MME and 25.7% had dosages 90–149 MME. Over one-in-four patients had substantially high mean baseline daily dosages of ≥ 150 MME (29.5%), among whom a larger proportion were male (52.0%).
Table 1.
Characteristics of patients 18 years and older on long-term opioid therapy, by average daily baseline dosage (MME), 2017–2018. Column percentages reported unless otherwise indicated.
| Characteristics | Overall, N (%) | Mean Baseline Daily Dosage, N (%) | ||
|---|---|---|---|---|
| 595,078 (100%) | 50–89 MME | 90–149 MME | ≥ 150 MME | |
| 266,765 (44.8%a) | 153,069 (25.7%a) | 175,244 (29.5%a) | ||
| Demographics | ||||
| Sex | ||||
| Female | 307,145 (51.6%) | 143,748 (53.9%) | 79,266 (51.8%) | 84,131 (48.0%) |
| Male | 287,933 (48.4%) | 123,017 (46.1%) | 73,803 (48.2%) | 91,113 (52.0%) |
| Age Group | ||||
| 18–34 | 17,149 (2.9%) | 7704 (2.9%) | 4664 (3.1%) | 4781 (2.7%) |
| 35–64 | 399,443 (67.1%) | 172,226 (64.6%) | 103,533 (67.6%) | 123,684 (70.6%) |
| 65 + | 178,486 (30.0%) | 86,835 (32.6%) | 44,872 (29.3%) | 46,779 (26.7%) |
| Payer | ||||
| Medicaid | 69,154 (11.6%) | 33,391 (12.5%) | 18,274 (11.9%) | 17,489 (10.0%) |
| Medicare | 276,000 (46.4%) | 123,103 (46.2%) | 71,111 (46.5%) | 81,786 (46.7%) |
| Third Party | 232,265 (39.0%) | 104,534 (39.2%) | 59,118 (38.6%) | 68,613 (39.2%) |
| Self-Pay | 15,798 (2.7%) | 4940 (1.9%) | 4059 (2.7%) | 6799 (3.9%) |
| Otherb | 1861 (0.3%) | 797 (0.3%) | 507 (0.3%) | 557 (0.3%) |
| Patient Outcomes | ||||
| Short-term eventsc | ||||
| Dosage tapering | 312,767 (52.6%) | 132,002 (49.5%) | 81,314 (53.1%) | 99,451 (56.8%) |
| Dosage stability | 510,227 (85.7%) | 232,464 (87.1%) | 130,654 (85.4%) | 147,109 (84.0%) |
| Dosage increases | 97,654 (16.4%) | 49,653 (18.6%) | 25,454 (16.6%) | 22,547 (12.9%) |
| Long-term outcomesd | ||||
| 12-month sustained reductions | 158,992 (26.7%) | 63,071 (23.6%) | 41,858 (27.4%) | 54,063 (30.9%) |
| 12-month sustained stable dosages | 229,727 (38.6%) | 106,521 (39.9%) | 58,389 (38.2%) | 64,817 (37.0%) |
| Discontinued Prescriptions (180 days with no dosage) | 55,279 (9.3%) | 25,185 (9.4%) | 14,575 (9.5%) | 15,519 (8.9%) |
Row percentages.
Patients with multiple, equally common payer types across all prescriptions dispensed during the study period.
Percentage changes in daily opioid dosage during follow-up in any of the ten 90-day follow-up observation periods from the baseline period.
Changes in daily opioid dosage across the 360-day follow-up period.
Data source: IQVIA Longitudinal Prescription (LRx) retail pharmacy database, 2017–2019.
Table 1 also shows short-term events and long-term outcomes among patients during follow-up. Approximately half the study population experienced at least one period of dosage tapering (52.6%). Most people experienced dosage stability (85.7%) during at least one period in follow-up. Among patients with mean baseline daily dosage ≥ 150 MME, a higher proportion of patients experienced tapering (56.8%) while slightly lower proportions of patients experienced stability (84.0%) relative to those with lower baseline dosages. A much smaller proportion of patients experienced a dosage increase (16.4%), with the largest percentage of patients with increases observed among those with baseline dosages of 50–89 MME (18.6%). Among patients receiving baseline dosages ≥ 150 MME, 12.9% had a dosage increase at any point. In sensitivity analyses examining dosage changes with a shorter baseline period of 90-days, a slightly higher proportion of patients experienced tapering (59.9%) and dosage increases (20.6%), and a lower proportion experienced dosage stability (81.4%) (Appendix-C).
About 1-in-4 patients (26.7%) experienced sustained dosage reductions over follow-up. The proportion of patients with sustained dosage reductions increased with mean baseline daily dosage: 23.6% of patients with 50–89 MME baseline daily dosage, 27.4% of patients with dosages between 90 and 149 MME, and 30.9% of patients with dosages ≥ 150 MME. In the overall sample, 38.6% of patients experienced stable dosages compared to baseline through follow-up. Overall, 9.3% experienced dosage discontinuation of at least 180 days, with a slightly lower proportion of patients with ≥ 150 MME discontinued (8.9%). Sensitivity analyses using a baseline period of 90-days found a slightly higher discontinuation rate (12.3%).
Among patients experiencing at least one 90-day period of dosage tapering, 36.1% experienced rapid maximum taper rates of ≥ 40% reductions per month (Table 2). Among this population, the subset of patients with mean baseline daily dosages ≥ 150 MME, close to half (47.7%), experienced a maximum taper rate that was rapid, compared to 45.1% among those with dosages between 90 and 149 MME, and 21.9% among those with dosages between 50 and 89 MME. By overall dosage reduction (baseline to final observation period), across all categories of baseline daily dosage, 72.1% of all patients with dosage tapering had rates between < 40% per month and 19.7% had rates ≥ 40% per month. Among patients with a mean baseline daily dosage ≥ 150 MME, 21.8% experienced overall dosage reduction rates of ≥ 40% per month. Some patients (8.2%) experienced no overall dosage reductions at the end of follow-up (i.e., experienced dosage increases compared to baseline).
Table 2.
Maximum and overall monthly tapering and dosage reduction rates by average daily baseline dosage (MME) among patients with at least one 90-day observation period of tapering during 360-day follow-up.
| Rate Characteristic | Mean Baseline Daily Dosage (MME) | Rate, N (%) | |||
|---|---|---|---|---|---|
| No dosage reductiona | 0–10% per month | > 10–40% per month | ≥ 40% per month | ||
| Max. Taper (consecutive observation periods)b | 50–89 | – | 1449 (1.1%) | 98,092 (74.3%) | 28,843 (21.9%) |
| 90–149 | – | 494 (0.6%) | 44,126 (54.3%) | 36,694 (45.1%) | |
| ≥ 150 | – | 299 (0.3%) | 51,750 (52.0%) | 47,402 (47.7%) | |
| Total | – | 2242 (0.7%) | 193,968 (62.0%) | 112,939 (36.1%) | |
| Overall Dosage Reduction (baseline to final 90-day observation period) | 50–89 | 12,956 (9.8%) | 64,961 (49.2%) | 30,746 (23.3%) | 23,339 (17.7%) |
| 90–149 | 6414 (7.9%) | 42,386 (52.1%) | 16,004 (19.7%) | 16,510 (20.3%) | |
| ≥ 150 | 6383 (6.4%) | 54,235 (54.5%) | 17,183 (17.3%) | 21,650 (21.8%) | |
| Total | 25,753 (8.2%) | 161,582 (51.7%) | 63,933 (20.4%) | 61,499 (19.7%) | |
Maximum taper rate comparisons between consecutive 90-day observation periods were only calculated when a taper was identified during that period. Overall dosage reduction rates with no reductions at the end of follow-up indicate the mean daily dosage in the final 90-day observation period exceeded the mean baseline daily dosage.
Denominator (N = 309,149) includes 3618 missing values for patients where all reductions meeting the taper threshold (10%) occurred starting at levels < 50 MME. Rates were not calculated due to concerns around accuracy. Data source: IQVIA Longitudinal Prescription (LRx) retail pharmacy database, 2017–2019.
Table 3 shows regression results for the characteristics of patients with any dosage tapering associated with experiencing a rapid taper (maximum taper rate ≥40% month). Rapid tapers were far less likely among older patients 35–64 years (aOR 0.52; 95% CI 0.500–0.541, p < .0001) and ≥ 65 years (0.47; 0.448–0.487, p < .0001) compared to patients aged 18–34 years. Relative to patients with third party insurance, patients with Medicaid (1.12; 1.096–1.150, p < .0001), Medicare (1.04; 1.017–1.053, p < .0001), and self-pay (1.52; 1.456–1.590, p < .0001) were each more likely to experience rapid tapers. Compared to patients with mean baseline daily dosages of 90–149 MME, those with dosages ≥ 150 MME were slightly more likely to experience a rapid taper (1.09; 1.073–1.114, p < .0001), while those with dosages of 50–89 MME were less likely to experience a rapid taper (0.34; 0.334–0.347, p < .0001). Characteristics of patients with sustained reductions were similar, with a few exceptions. Among patients experiencing sustained reductions, there was no significant difference in rapid tapers between patients with baseline dosages ≥ 150 MME and those with 50–89 MME. On the other hand, among patients with discontinued dosages at the end of follow-up, having Medicaid payer type (relative to third party insurance) was associated with a slight decrease in the odds of experiencing a rapid taper (0.91; 0.849–0.975, p = .008). Among patients discontinued, a mean baseline daily dosage ≥ 150 MME had substantially higher odds of being associated with rapid tapers (39.06; 26.024–58.620, p < .0001 respectively).
Table 3.
Characteristics of patients experiencing rapid taper rates of ≥ 40% daily opioid dosage reduction per month (aOR – Adjusted Odds Ratio).
| Characteristic | Patients with any dosage tapering, aOR (95% CI) N = 312,767 | p Value | Patients with sustained reductions, aOR (95% CI) N = 158,992 | p Value | Patients discontinued at the end of follow-up, aOR (95% CI) N = 55,279 | p Value |
|---|---|---|---|---|---|---|
| Sex (ref. Male) | ||||||
| Female | 0.91 (0.900–0.927) | < 0.0001 | 0.88 (0.860–0.897) | < 0.0001 | 1.03 (0.983–1.079) | 0.219 |
| Age Group (ref. 18–34) | ||||||
| 35–64 | 0.52 (0.500–0.541) | < 0.0001 | 0.39 (0.371–0.412) | < 0.0001 | 0.55 (0.502–0.610) | < 0.0001 |
| 65 + | 0.47 (0.448–0.487) | < 0.0001 | 0.36 (0.343–0.383) | < 0.0001 | 0.56 (0.503–0.621) | < 0.0001 |
| Payer (ref. Third Party) | ||||||
| Medicaid | 1.12 (1.096–1.150) | < 0.0001 | 1.16 (1.122–1.201) | < 0.0001 | 0.91 (0.849–0.975) | 0.008 |
| Medicare | 1.04 (1.017–1.053) | < 0.0001 | 1.04 (1.015–1.065) | 0.0014 | 1.06 (0.999–1.114) | 0.056 |
| Self-Pay | 1.52 (1.456–1.590) | < 0.0001 | 1.53 (1.435–1.626) | < 0.0001 | 0.90 (0.779–1.040) | 0.153 |
| Othera | 1.58 (1.406–1.778) | < 0.0001 | 1.85 (1.579–2.174) | < 0.0001 | 1.03 (0.756–1.397) | 0.862 |
| Baseline MME (ref. 90–149) | ||||||
| 50–89 | 0.34 (0.334–0.347) | < 0.0001 | 0.32 (0.314–0.331) | < 0.0001 | 0.07 (0.068–0.079) | < 0.0001 |
| ≥ 150 | 1.09 (1.073–1.114) | < 0.0001 | 1.00 (0.970–1.022) | 0.742 | 39.07 (26.029–58.629) | < 0.0001 |
Patients with multiple, equally common payer types across all prescriptions dispensed during the study period.
Data source: IQVIA Longitudinal Prescription (LRx) retail pharmacy database, 2017–2019.
4. Discussion
4.1. Principal findings
Among patients on high-dose long-term opioid therapy, 52.6% had dosage tapering during at least one of the observation periods, across all categories of mean baseline daily dosage. These decreases are consistent with recent temporal trends demonstrating a reduction in dispensed opioid prescriptions (Centers for Disease Control and Prevention, 2019). Over one-third of all patients with tapers during at least one observation period had taper rates exceeding 40% per month at some point during follow-up despite exhibiting stability during baseline; among the subset of patients with baseline dosages ≥ 150 MME close to half experienced such rapid reductions (47.7%). Throughout follow-up, almost 1-in-5 patients experienced rapid dosage tapering rates. The proportion of patients with overall rapid dosage tapering rates was highest among patients tapered from substantially high dosages ≥ 150 MME (21.8%). Even using a conservative definition of discontinuation of opioid therapy spanning six months with zero dosage, we found close to 1-in-10 patients on stable, high-dose long-term opioid therapy experienced discontinuation. The likelihood of any rapid taper across all patients experiencing a taper was higher among patients with high baseline dosages (≥150 MME), and substantially so among those experiencing discontinuation at the end of follow-up. The likelihood of a rapid taper relative to patients experiencing sustained reductions with baseline dosages between 90 and 149 MME was lower among those with dosages between 50 and 89 MME, but not significantly different from those with dosages ≥ 150 MME. Among both patients with any dosage tapering as well as those with sustained reductions, the likelihood of a rapid taper was also higher among Medicaid and Medicare patients, as well as those whose prescriptions were primarily self-paid.
4.2. Interpretation
These findings indicate that over 1-in-2 patients on long-term opioid therapy experienced a dosage taper. Many of these patients, particularly those with substantially high, yet consistently stable baseline dosages, experienced tapering and dosage reductions at rates exceeding recommendations (US Department of Health and Human Services, 2019). Research using national commercial claims data from 2011 to 2017 found a high prevalence of abrupt discontinuation without tapering among privately insured (80%) and Medicare Advantage enrollees (88%) (Bao et al., 2021). The effect of sudden discontinuation and sharp dosage changes among patients on long-term opioid therapy is still not well understood, underscoring the importance of careful dosage changes that consider individualized patient factors and values (Mackey et al., 2020). Recent research has found increases in risk of adverse events including overdose and mental health crises during post-tapering periods (Agnoli et al., 2021). Another study found that patients with sustained dosage discontinuation were almost half as likely to have experienced an overdose as counterparts without discontinuation (Glanz et al., 2019). However, the same study also found that dosage variability was associated with an increased risk of opioid overdose, further highlighting the importance of patient buy-in (Dowell and Haegerich, 2017) and minimizing dosage variability prior to discontinuing long-term opioid therapy.
These results suggest that clinicians may be reacting to heightened awareness (Knight et al., 2017) of mitigating opioid prescribing risks in different ways. Findings that patients with self-pay insurance status had an increased likelihood of rapid tapers could reflect that prescribers are concerned about the potential for multiple provider episodes and might view cash payments as an indicator of potential opioid misuse (Cepeda et al., 2014; McDonald and Carlson, 2013). Additionally, cost barriers (Craig and Strassels, 2010) could result in uninsured patients not being able to access opioids needed to manage their pain. Further research could investigate whether changes in insurance type are associated with dosage changes for patients on long-term opioid therapy. Given sustained dosage reductions, the lack of a significant difference in the likelihood of rapid tapering between patients with baseline dosages 90–149 MME and those with ≥ 150 MME possibly could be reflective of clinicians, health systems, and/or payers interpreting guideline recommendations to avoid increasing dosage above 90 MME (Dowell et al., 2016) as a directive to reduce higher dosages below 90 MME (Dowell et al., 2019).
Studies examining tapering and discontinuation have used differing methodological approaches, each of which involves inherent tradeoffs. Further, the lack of standard definitions for long-term opioid therapy (Karmali et al., 2020), tapering, and dosage discontinuation makes it difficult to compare findings across studies. Nonetheless, many recent studies looking at similar outcomes among patients on long-term opioid therapy have found recent increases in dosage tapering. Fenton et al. (2019) examined patients with commercial or Medicare Advantage insurance on stable, long-term opioid therapy and found that the annual percentage undergoing dosage tapering increased from 12.7% to 23.1% between 2008 and 2017, with 26.5% of patients undergoing tapering experiencing a maximum dose reduction rate exceeding 40% per month. Further study of the same population from 2008 to 2018 found that among patients who initiated tapers 69.8% had a relative dose reduction ≥ 15% sustained at a follow-up of 16 months and 14.2% had discontinued opioids (Fenton et al., 2021). The algorithm used in these studies was designed to capture tapers of 15% or more relative to baseline and was sensitive enough to record tapers of magnitudes as low as 2.5% that may have accumulated to 15% over a six-month period. Our study modified this algorithm to identify tapers of larger magnitudes during initial follow-up that may have been missed by earlier algorithms. The modified algorithm, coupled with using data spanning all-payers, helps explain our results showing a larger prevalence of tapering and rapid dosage reductions. Other studies have similarly found increases in prevalence of discontinuation among patients on long-term opioid therapy. A recent study found that a substantially high proportion (72%) of patients across all-payers initiated on long-term opioid therapy of at least 90 MME between 2017 and 2018 experienced rapid dosage discontinuation, defined using an empirical algorithm based on meeting specific dosage thresholds at specific times before discontinuation (Stein et al., 2021). Our approach relied on estimating rapidity of dosage changes using a rate-based method (Fenton et al., 2019), and our findings suggest that discontinuation with rapid dosage changes is comparatively less frequent among patients receiving stable dosages for at least 180 days. Other studies examining Medicare beneficiaries (Neprash et al., 2021) and Veterans Health Administration (Minegishi et al., 2020) populations have also found increases in discontinuations in recent years. It is especially noteworthy that multiple studies have found a high prevalence of abrupt discontinuation among individuals discontinued on long-term opioid therapy (Hallvik et al., 2021; Neprash et al., 2021).
4.3. Strengths and limitations
Our study has several limitations. Tapering is generally difficult to identify based solely on prescription patterns, particularly in retrospective analyses utilizing pharmaceutical dispensing or claims data with no information on clinicians’ or patients’ decisions to taper. These findings are dependent on the definitions and algorithms used to identify tapering, stability, and discontinuation. Consequently, we were unable to identify tapering in certain patient populations, such as individuals with small dosage changes < 10% each month which might correspond to patients experiencing very slow tapers. Data do not include diagnoses, patient history of adverse events such as overdose, or outcomes. Therefore, we were unable to explore the risk-benefit ratio of tapering, adverse events associated with dose tapering, increases, and/or discontinuation, or tapering potentially initiated in response to adverse events related to opioids (Quinn et al., 2021; US Department of Health and Human Services, 2019). Given data limitations, we also cannot differentiate between patients that may be lost to follow-up due to switching to a minority of pharmacies not included in the IQVIA database versus those that lost due to death or other adverse events. Further, IQVIA prescription coverage may be lower in certain regions, such as those with higher numbers of health maintenance organizations (HMOs), which may dispense prescriptions through their own pharmacies(Tran et al., 2020). Despite this limitation, the IQVIA LRx data includes prescriptions dispensed by all insurance payers (including prescriptions paid for with cash), meaning there was minimal loss due to insurance transitions. Given the 180-day baseline period, we also cannot comment on possible differences in patients’ tapering needs that may be partly driven by overall duration of long-term opioid therapy. Finally, our definition could not accurately capture tapering rates among patients with daily opioid dosages < 50 MME. Thus, rate of taper among a few patients with baseline dosages ≥ 50 MME who continued to experience tapering below the 50 MME threshold may have been artificially large.
Nonetheless, this work has important strengths. Our study uses longitudinal data that includes prescriptions covered by all insurance types, meaning our study sample was not limited to a single insurance type or patients with continuous coverage. This is also the first study to examine patients on long-term opioid therapy over a 12-month follow-up period and utilize tapering definitions with larger thresholds specifically based on the HHS Guide (US Department of Health and Human Services, 2019). This allowed us to focus on clearly identifiable, rapid rates of tapers which may be unsafe for patients already on stable doses. Since physical dependence on opioids can develop relatively quickly on continuous opioid therapy, adverse events associated with rapid tapering can occur even among patients who have received opioids for shorter durations. Therefore, by using a relatively conservative baseline period of six months, we could capture potentially unsafe tapering prevalence among more patients on long-term opioid therapy than previously included. Our work builds off recently developed algorithms and our contributions can help improve the specificity of the algorithm, particularly in identifying tapers of larger magnitudes.
4.4. Implications for practice and conclusions
Almost 1-in-5 patients with tapers experienced overall dosage reductions outside of recommended rates and almost one-third experienced at least one period with a rapid taper. It is important that patients on long-term opioid therapy receive regular, careful, individualized attention to ensure less rapid and safer transitions between dosages. Opioid prescribing and tapering guidelines emphasize the importance of patient-centric care and incorporating individualized patient factors and values elicited during a shared decision-making process when identifying safe approaches to dosage changes (Berna et al., 2015; Dowell et al., 2019, 2016; US Department of Health and Human Services, 2019). Evidence-based clinical tools can help clinicians balance individualized pain care with potential risks of opioid use (Centers for Disease Control and Prevention, 2021); limiting the initiation of opioids to situations only where the benefits outweigh the risks can help minimize any future tapering needs. For patients already receiving opioids, it is important to incorporate risk-reduction strategies into patient-centered care, including careful assessments of both the benefits and risks of continuing opioids as well as those associated with tapering. Opportunities remain to improve guideline-concordant tapering, particularly over shorter intervals, with a focus on patient safety and shared-decision making.
Supplementary Material
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
CRediT authorship contribution statement
Nisha Nataraj led the study conceptualization and design, development of methodology, data curation and analysis, interpretation of results, manuscript drafting and editing, and approved the final manuscript as submitted. Andrea Strahan co-led the study conceptualization and design, and assisted with the development of methodology, data curation, interpretation of results, manuscript drafting and editing, and approved the final manuscript as submitted. Gery Guy supervised the study and assisted with study conceptualization and design, development of methodology, interpretation of results, manuscript reviewing and editing, and approved the final manuscript as submitted. Jan Losby supervised the study and assisted with study conceptualization and design, interpretation of results, manuscript reviewing and editing, and approved the final manuscript as submitted. Deborah Dowell assisted with the study conceptualization and design, development of methodology, interpretation of results, manuscript reviewing and editing, and approved the final manuscript as submitted.
Conflict of interest
No conflict declared.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.drugalcdep.2022.109392.
References
- Agnoli A, Xing G, Tancredi DJ, Magnan E, Jerant A, Fenton JJ, 2021. Association of dose tapering with overdose or mental health crisis among patients prescribed long-term opioids. JAMA 326 (5), 411–419. 10.1001/jama.2021.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Zhang H, Wen K, Johnson P, Jeng PJ, Witkin LR, Nicholson S, Reid MC, Schackman BR, 2021. Robust prescription monitoring programs and abrupt discontinuation of long-term opioid use. Am. J. Prev. Med 61 (4), 537–544. 10.1016/j.amepre.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna C, Kulich RJ, Rathmell JP, 2015. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin. Proc 90 (6), 828–842. 10.1016/j.mayocp.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Bohnert AS, Guy GP Jr., Losby JL, 2018. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann. Intern. Med 169 (6), 367–375. 10.7326/m18-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2019. Annual Surveillance Report of Drug-Related Risks and Outcomes —United States Surveillance Special Report Centers for Disease Control and Prevention. U.S. Department of Health and Human Services, Atlanta, GA. 〈https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf〉 (Accessed 24 Februaury 2022). [Google Scholar]
- Centers for Disease Control and Prevention, Opioid Prescribing Guideline Resources, 2021. 〈https://www.cdc.gov/drugoverdose/prescribing/resources.html〉. (Accessed 6 April 2021).
- Cepeda MS, Fife D, Kihm MA, Mastrogiovanni G, Yuan Y, 2014. Comparison of the risks of shopping behavior and opioid abuse between tapentadol and oxycodone and association of shopping behavior and opioid abuse. Clin. J. Pain 30 (12), 1051–1056. 10.1097/ajp.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig BM, Strassels SA, 2010. Out-of-pocket prices of opioid analgesics in the United States, 1999–2004. Pain Med 11 (2), 240–247. 10.1111/j.1526-4637.2009.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, 2017. Changing the conversation about opioid tapering. Ann. Intern. Med 167 (3), 208–209. 10.7326/m17-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm. Rep 65 (RR-1), 1–49. 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich T, Chou R, 2019. No shortcuts to safer opioid prescribing. New Engl. J. Med 380 (24), 2285–2287. 10.1056/nejmp1904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JJ, Agnoli AL, Xing G, Hang L, Altan AE, Tancredi DJ, Jerant A, Magnan E, 2019. Trends and rapidity of dose tapering among patients prescribed long-term opioid therapy, 2008–2017. JAMA Netw. Open 2 (11) 10.1001/jamanetworkopen.2019.16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JJ, Magnan EM, Agnoli AL, Henry SG, Xing G, Tancredi DJ, 2021. Longitudinal dose trajectory among patients tapering long-term opioids. Pain Med 22 (7), 1660–1668. 10.1093/pm/pnaa470. [DOI] [PubMed] [Google Scholar]
- Glanz JM, Binswanger IA, Shetterly SM, Narwaney KJ, Xu SJ Jno, 2019. Association between opioid dose variability and opioid overdose among adults prescribed long-term opioid therapy. JAMA Netw. Open 2 (4) 10.1001/jamanetworkopen.2019.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallvik SE, Johnston K, Geddes J, Leichtling G, Korthuis PT, Hartung DM, 2021. Identifying opioid dose reductions and discontinuation among patients with chronic opioid therapy. Pharmacoepidemiol. Drug Saf 30 (3), 395–399. 10.1002/pds.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CJ, Gressler LE, Hu B, Jones BL, Williams JS, Martin BC, 2021. Trajectories of opioid coverage after long-term opioid therapy initiation among a national cohort of US veterans. J. Pain Res 14, 1745 10.2147/JPR.S308196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali RN, Bush C, Raman SR, Campbell CI, Skinner AC, Roberts AW, 2020. Long-term opioid therapy definitions and predictors: a systematic review. Pharmacoepidemiol. Drug Saf 29 (3), 252–269. 10.1002/pds.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight KR, Kushel M, Chang JS, Zamora K, Ceasar R, Hurstak E, Miaskowski C, 2017. Opioid pharmacovigilance: a clinical-social history of the changes in opioid prescribing for patients with co-occurring chronic non-cancer pain and substance use. Soc. Sci. Med 186, 87–95. 10.1016/j.socscimed.2017.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey K, Anderson J, Bourne D, Chen E, Peterson K, 2020. Benefits and harms of long-term opioid dose reduction or discontinuation in patients with chronic pain: a rapid review. J. Gen. Intern. Med 35, 935–944. 10.1007/s11606-020-06253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurenko O, Blackburn J, Gupta S, Simon K, Harle CA, 2021. Long-term opioid therapy tapering: trends from 2014 to 2018 in a Midwestern State. Drug Alcohol Depend 228, 109108 10.1016/j.drugalcdep.2021.109108. [DOI] [PubMed] [Google Scholar]
- McDonald DC, Carlson KE, 2013. Estimating the prevalence of opioid diversion by “doctor shoppers” in the United States. PLoS One 8 (7), e69241. 10.1371/journal.pone.0069241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi T, Garrido MM, Stein M, Oliva EM, Frakt AB, 2020. Opioid discontinuation among patients receiving high-dose long-term opioid therapy in the veterans health administration. J. Gen. Intern. Med 35 (3), 903–909. 10.1007/s11606-020-06252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control, 2018. version. CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors Centers for Disease Control and Prevention; Atlanta, GA. Available upon request at 〈https://www.cdc.gov/opioids/data-resources/index.html〉 (Accessed 24 February 2022). [Google Scholar]
- Neprash HT, Gaye M, Barnett ML, 2021. Abrupt discontinuation of long-term opioid therapy among medicare beneficiaries, 2012–2017. J. Gen. Intern. Med 1–8. 10.1007/s11606-020-06402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Chang Z, Bair MJ, Rickert ME, Gibbons RD, Kroenke K, D’Onofrio BM, 2021. Associations of opioid prescription dose and discontinuation with risk of substance-related morbidity in long-term opioid therapy. Pain 10.1097/j.pain.0000000000002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Sherry TB, O’Neill B, Taylor EA, Sorbero M, 2021. Rapid discontinuation of chronic, high-dose opioid treatment for pain: prevalence and associated factors. J. Gen. Intern. Med 1–7. 10.1007/s11606-021-07119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Q, Warren JL, Barrett MJ, Annett D, Marth M, Cress RD, Deapen D, Glaser SL, Gomez SL, Schwartz SM, 2020. An evaluation of the utility of big data to supplement cancer treatment information: linkage between IQVIA pharmacy database and the surveillance, epidemiology, and end results program. JNCI Monogr 2020 (55), 72–81. 10.1093/jncimonographs/lgz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (FDA), 2019. FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering, FDA Drug Safety Communication 〈https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes〉 (Accessed 24 February 2022).
- US Department of Health and Human Services, 2019. HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long-term opioid analgesics Washington, D.C. 〈https://www.hhs.gov/opioids/sites/default/files/2019–10/Dosage_Reduction_Discontinuation.pdf〉 (Accessed 24 February 2022). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


