Improved survival from childhood cancer has been achieved at the cost of long-term comorbidities that reduce quality of life. The chemotherapy agent cisplatin is essential for treatment of many paediatric and adolescent malignancies but in a large proportion of patients results in cisplatin-induced hearing loss, a debilitating and permanent late effect, with consequences for neurocognition, academic achievement, employment, and social and psychological outcomes.1,2
In The Lancet Oncology, we previously reported the results of the Children’s Oncology Group study ACCL0431, an international, randomised, controlled trial of sodium thiosulfate for prevention of cisplatin-induced hearing loss in children and adolescents.3 Participants were randomly assigned to sodium thiosulfate or observation in addition to their planned cisplatin-containing chemotherapy. Patients with any type or stage of cancer treated with cisplatin were eligible. In ACCL0431, we found compelling evidence for hearing protection from sodium thiosulfate, a finding replicated by the concurrent International Childhood Liver Tumours Strategy Group 6 trial (SIOPEL-6) in standard-risk (ie, localised) hepatoblastoma.4 As described in the initial ACCL0431 report, survival was a prespecified secondary outcome to assess for potential interference with chemotherapy efficacy by sodium thiosulfate. Among all participants in aggregate, there was no significant difference in overall survival by randomised group at a relatively early median follow-up of 3·5 years (IQR 3·0–4·5; relative hazard ratio 2·03 [95% CI 0·93–4·44]). However, a non-significant trend towards lower overall survival among patients treated with sodium thiosulfate than in the control group prompted an unplanned survival analysis using post-hoc stratification of participants by extent of disease (localised or disseminated [ie, tumour identified in sites distant from the primary tumour at original diagnosis]). Among patients deemed to have localised disease, there was no difference in 3-year overall survival for observation (89% [95% CI 74–96]) versus treatment with sodium thiosulfate (83% [66–92]; log rank p=0·88), but among those deemed to have disseminated disease, 3-year overall survival was significantly lower for treatment with sodium thiosulfate (45% [95% CI 23–65]) than for observation (84% [62–94]; relative hazard ratio 4·10 [95% CI 1·30–12·97], log rank p=0·0090; figure A).3 Due to differing eligibility criteria between the two sodium thiosulfate trials (ACCL0431 and SIOPEL-6), survival data for disseminated disease were available only from ACCL0431. These findings resulted in a safety concern for children with disseminated disease, currently precluding routine use of sodium thiosulfate in this population.5
Figure: Overall survival.
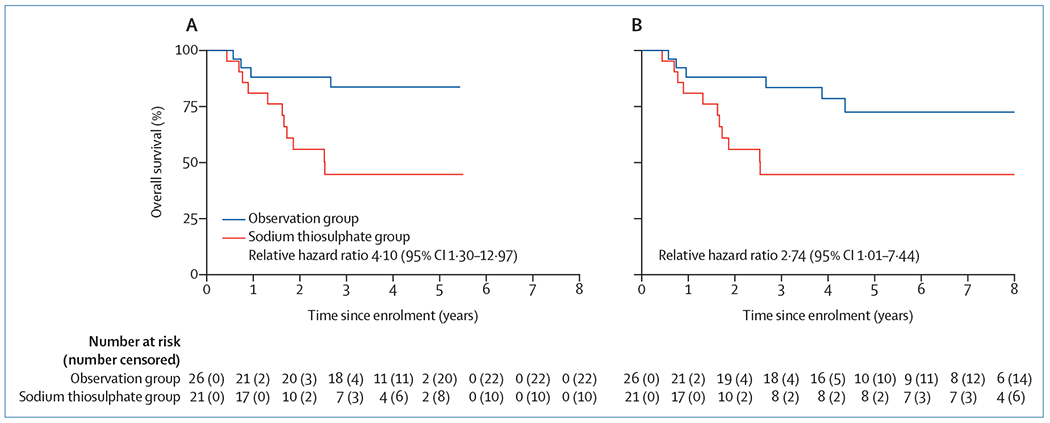
Overall survival for ACCL0431 participants with disseminated cancer randomly assigned to observation or treatment with sodium thiosulfate with median follow-up of 3.5 years (A; log rank p=0.0090) and with a median follow-up of 7·8 years (B; log rank p=0.040). In panel B, in the observation group at year 2, there is one patient whose classification was changed from at-risk to censored due to not providing written informed consent for continued use of data beyond the age of 18 years.
Due to data limitations imposed by the ACCL0431 study design, explanations for the observed overall survival difference among children with disseminated disease have remained speculative.6 One leading hypothesis has been that the difference did not actually represent lower overall survival in the sodium thiosulfate-treated group due to chemotherapy interference, but rather a spuriously increased overall survival in the observation group that was an artifact of short follow-up. With follow-up longer than 3 years, published overall survival rates for patients with disseminated cancers in ACCL0431 are, in fact, about half that noted in the ACCL0431 observation group and very similar to the sodium thiosulfate group (overall survival of approximately 39–46%).7–9 Moreover, an increasing proportion of all-cause mortality now occurs 5–10 years after diagnosis.10 With these data in mind, initial ACCL0431 overall survival results for the sodium thiosulfate group were similar to what might be expected but for the observation group were unrealistically high. Under this hypothesis, with longer follow-up, overall survival in the observation group might be expected to decrease disproportionately and reach equivalence with the sodium thiosulfate group. Such a scenario would have important clinical implications and suggest that chemotherapy interference by sodium thiosulfate was unlikely to account for the difference in overall survival observed at an early timepoint and that use of sodium thiosulfate could be considered for otoprotection without regards to cancer stage.
We tested this hypothesis by querying study sites for updated survival on all ACCL0431 participants (125 participants: 61 in the observation group and 64 in the sodium thiosulfate group) as of Dec 31, 2019. We used statistical methods as described in the initial publication.3 With median follow-up now extended to 7·8 years (IQR 4·4–8·5), there were an additional eight deaths (five patients with localised disease and three patients with disseminated disease). For those classified as having disseminated disease, all three deaths occurred in the observation group; no additional deaths were reported in the sodium thiosulfate group. Among those classified as having localised disease, 6-year overall survival remained stable and equivalent for the observation group (84% [95% CI 68–92]) versus the sodium thiosulfate group (80% [63–90]; log rank p=0·67). For those classified as having disseminated disease, overall survival decreased somewhat in the observation group, as expected, but the group remained at significantly reduced risk of death (relative hazard ratio for sodium thiosulfate vs observation was 2·74 [95% CI 1.01-7·44, log rank p=0·040; figure B]). 6-year overall survival was 73% (95% CI 48–87) for the observation group versus 45% (23–65) for the sodium thiosulfate group. In the observation group, four additional deaths would have been necessary for 6-year overall survival to be below 49%.
We conclude that the significant survival difference initially observed for participants of ACCL0431 treated with sodium thiosulfate deemed to have had disseminated disease persists and was not merely an artifact of short follow-up, as hypothesised. As discussed elsewhere,6 a potential alternative explanation is unbalanced randomisation of participants for disease-specific prognostic factors not measured in the ACCL0431 trial, but this hypothesis is not testable with the existing ACCL0431 dataset. Therefore, future research designed specifically to explore safety of sodium thiosulfate use or alternative approaches to otoprotection is warranted to address the risk for severe ototoxicity also facing children with disseminated tumours.
Acknowledgments
Funding supporting the ACCL0431 study was provided by the National Institutes of Health (NIH) through the National Cancer Institute Community Oncology Research Program (NCORP; grant UG1CA189955), the National Cancer Institute Clinical Trials Network (NCTN) Statistics and Data Center (grant U10CA180899), and by St Baldrick’s Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
We declare no competing interests.
Contributor Information
Etan Orgel, Cancer and Blood Disease Institute, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA; Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
David R Freyer, Cancer and Blood Disease Institute, Children’s Hospital Los Angeles, Los Angeles, CA 90027, USA; Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA; Department of Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA; Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Mark D Krailo, Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Doojduen Villaluna, Children’s Oncology Group, Monrovia, CA, USA.
Adam Esbenshade, Monroe Carell Jr, Children’s Hospital at Vanderbilt, Nashville, TN, USA; Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA.
Lillian Sung, Hematology/Oncology, Hospital for Sick Children, Toronto, ON, Canada.
References
- 1.Moke DJ, Luo C, Millstein J, et al. Prevalence and risk factors for cisplatin-induced hearing loss in children, adolescents, and young adults: a multi-institutional North American cohort study. Lancet Child Adolesc Health 2021; 5: 274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics 2007; 120: e1229–36. [DOI] [PubMed] [Google Scholar]
- 3.Freyer DR, Chen L, Krailo MD, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017; 18: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 2018; 378: 2376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freyer DR, Brock PR, Chang KW, et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline. Lancet Child Adolesc Health 2020; 4: 141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minasian LM, Frazier AL, Sung L, et al. Prevention of cisplatin-induced hearing loss in children: Informing the design of future clinical trials. Cancer Med 2018; 7: 2951–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coughlan D, Gianferante M, Lynch CF, Stevens JL, Harlan LC. Treatment and survival of childhood neuroblastoma: evidence from a population-based study in the United States. Pediatr Hematol Oncol 2017; 34: 320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dufour C, Beaugrand A, Pizer B, et al. Metastatic medulloblastoma in childhood: Chang’s classification revisited. Int J Surg Oncol 2012; 2012: 245385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers RL, Rowland JR, Krailo M, Chen Z, Katzenstein HM, Malogolowkin MH. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2009; 53: 1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol 2009; 27: 2328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]


