ABSTRACT
A 56-year-old man with a history of gallbladder carcinoma, hypothyroidism and hypertension was examined by us after developing marked visual loss in his left eye. A left ischaemic type of central retinal vein occlusion (CRVO) with macular oedema was diagnosed. Three months later, a non-ischaemic type of CRVO with no macular oedema developed in his right eye. While the left eye received five intravitreal ranibizumab injections and panretinal photocoagulation, the right central retinal vein occlusion improved spontaneously without any treatment. Ten months after his first visit we noticed optociliary shunt vessel formation in the right eye and neovascularisation of the optic disc in the left eye. Fluorescein angiography and optical coherence tomography angiography were performed at the same visit. The place of fluorescein angiography and optical coherence tomography angiography in distinguishing the optociliary shunt vessel from neovascularisation of the optic disc is discussed.
KEYWORDS: Central retinal vein occlusion, fluorescein angiography, neovascularization of the disc, optical coherence tomography angiography, optociliary shunt vessel, panretinal photocoagulation, ranibizumab
A 56-year-old man with a history of gallbladder carcinoma, hypothyroidism and hypertension was examined by us after developing marked visual loss in his left eye (OS) a week previously during the pandemic quarantine in April 2020. His best-corrected visual acuity (BCVA) was 20/20 in the right eye (OD) and 20/400 OS. He was diagnosed with left ischaemic central retinal vein occlusion (CRVO) and macular oedema. There was only some venous tortuosity at the right fundus. After three intravitreal ranibizumab injections, the macular oedema resolved in his left eye. In addition to intravitreal therapy, panretinal laser photocoagulation was also performed after each intravitreal ranibizumab injection due to the ischaemic nature of the venous occlusion. In July 2020, a mild non-ischaemic type of CRVO without any sign of macular oedema was noticed in his right eye during the follow-up. The right eye was managed conservatively due to the absence of macular oedema and the left eye received two more ranibizumab injections. In February 2021, his BCVA was 20/20 OD and 20/200 OS. His intraocular pressure was 14 mmHg with Goldmann applanation tonometry in both eyes (OU). Slit-lamp examination was unremarkable without any evidence of rubeosis iridis OU. Fundus examination revealed unusual vessels on the optic discs in both eyes (Figures 1a,d and 2a,e). We performed fundus fluorescein angiography (FA) and optical coherence tomography angiography (OCTA) on the same day. The right optic disc exhibited no fluorescein leakage (Figures 1b,c and 2b,c) whereas FA showed progressive profuse leakage from the left optic disc (Figures 1e,f and 2f,g). Radial peripapillary capillary (RPC) slabs of the OCTA images from the right eye delineated looped vessels that were larger than the radial peripapillary capillaries (Figure 2d). However, optic disc OCTA images of the left eye showed multiple new vessels with terminal loops and irregular proliferation of small-calibre vessels at the optic nerve head on vitreous and RPC slabs (Figure 2h). We concluded that the vessels on the right optic disc were optociliary shunt vessels (OSVs) and the vessels on the left disc were due to neovascularisation of the optic disc (NVD). The left eye was further treated with additional laser photocoagulation and two more intravitreal ranibizumab injections; thereafter, the optic disc neovascularisation regressed.
Figure 1.
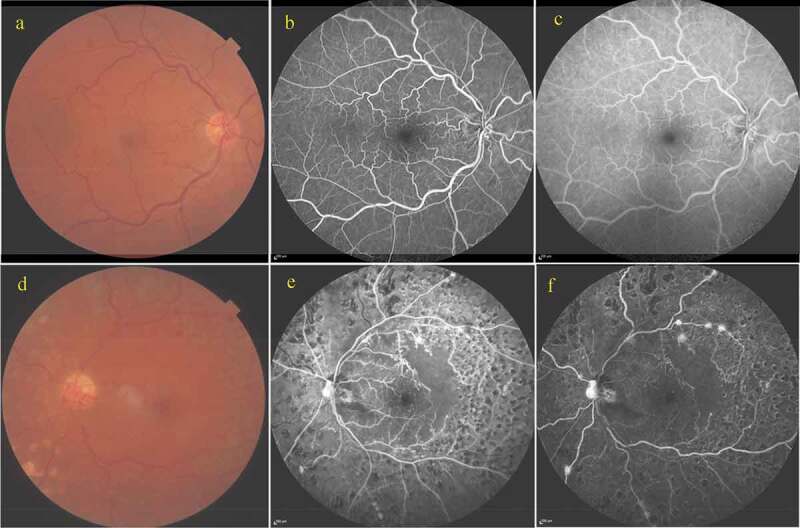
Right eye: (a) Colour fundus photograph demonstrating venous tortuosity and irregular vessels at the optic disc; (b, c) Early and late phases, respectively of fluorescein angiography showing shunt vessels without showing any leakage.
Left eye: (e) Colour fundus photograph demonstrating an irregular vascular network at the optic disc and laser photocoagulation scars in the mid-periphery; (f, g) Early and late phases, respectively of fluorescein angiography showing hyperfluorescence at the optic disc, hyperfluorescent spots at the supero-temporal arcus and infero-nasal mid-periphery and ischaemic areas at 360° of the mid-peripheral retina.
Figure 2.
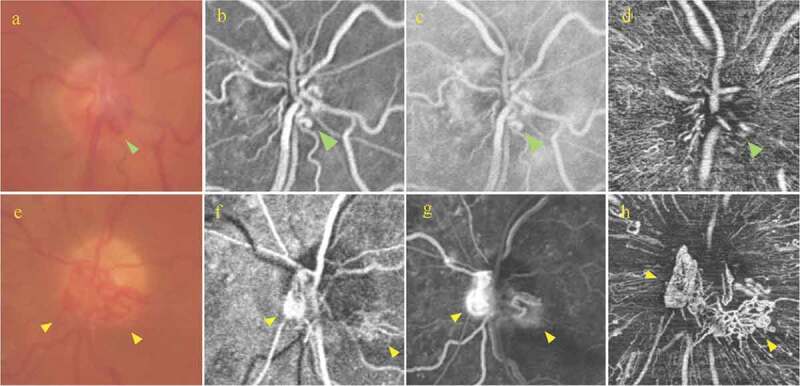
Right eye: (a) Magnified colour optic disc photograph demonstrating irregular vascular loops at the optic disc (green arrow); (b, c) Magnified early and late fluorescein angiography, respectively showing that the vessels at the optic disc show no leakage (green arrow); (d) Radial peripapillary capillary (RPC) slab of optical coherence tomography angiography (OCTA) showing an irregular looking vascular network (green arrow).
Left eye: (e) Magnified colour optic disc photograph demonstrating vascular loops located at the nasal and inferior optic disc quadrants (yellow arrows); (f, g) Early and late phases of fluorescein angiography showing the presence of progressive leakage from the optic disc (yellow arrows); (h) RPC slab of OCTA showing the presence of multiple irregular and looped vessels in a mesh-like structure (yellow arrows).
Discussion
CRVO can cause severe vision loss and is the second most common retinal vascular disease after diabetic retinopathy.1 Hypercoagulable states are among the causes of CRVO.2 Trousseau3 was the first to mention the presence of increased hypercoagulability in cancer patients in 1865 and described Trousseau syndrome, an acquired blood clotting disorder that results in migratory thrombophlebitis secondary to existing cancer.4 CRVO may result from a paraneoplastic process where dynamic interactions among the tumour cells, blood coagulation system, vascular endothelium, leucocytes, and platelets contribute to the pathogenesis.5 In several reports, the association between malignancies and CRVO has been discussed.5–7 In the present case, sequential, bilateral CRVO was diagnosed in a man who had a history of treated gallbladder carcinoma and systemic hypertension.
OSVs are collateral vessels on the optic disc that enable bypass of retinal venous blood from an occluded central retinal vein through to the choroidal circulation.8 OSVs are thought to develop as a protective mechanism for the reperfusion of an ischaemic retina.9 It has been reported that OSVs can be seen in 30–61% of eyes with CRVO.8,10,11 However, NVD is a rare finding (6%) in CRVO eyes.12 NVD can be a consequence of the pathological production of vascular endothelial growth factor from the ischaemic retina.11 Hayreh et al. reported that NVD was detected in only three out of 673 non-ischaemic CRVO eyes in their prospective study and suggested that the cumulative probability of NVD occurrence was 6% within the first 6 months from onset and 10% after 12 months in eyes with ischaemic CRVO.12
In the present case, we observed OSVs in the right eye and NVD in the left eye as a consequence of bilateral CRVO. Distinguishing the OSVs from NVD is of paramount importance in order to select the appropriate treatment and estimate the prognosis. Singh et al. reported that OSVs are characterised by small calibre, sinusoidal vessels that can be best observed on RPC slabs of the optic disc OCTA.13 They also showed that OSVs are larger than RPCs and have a smaller calibre than the retinal veins. Moreover, they mentioned that NVD is a mesh of irregular new vessels that arise from the venous circulation and tend to penetrate the internal limiting membrane. In cross-sectional OCTA images, NVD appears on a plane rising above the retinal surface and shows abnormally present flow signal. In contrast to NVD, OSVs exhibit no abnormal signal and are not visible at the anterior surface of the optic disc on OCTA images.13
In a very recent study on CRVO, the authors argued that the development of OSVs might be related to the site of the occlusion.8 In milder forms of CRVO, if the occlusion site in the optic nerve occurs downstream of the central retinal vein, a greater number of tributaries within the optic nerve are available to form collateral circulation and thereby OSVs. However, in severe CRVO, where the site of occlusion is closer to the lamina cribrosa, namely upstream to the central retinal vein, fewer collateral vessels develop in the optic nerve.8 Consistent with this suggestion, OSVs developed in the right eye with the non-ischaemic type of CRVO in our case. Hayreh et al. found out that NVD occurred only in eyes with ischaemic type of central and hemi-central retinal vein occlusion.12 In the present case, angiographically documented severe retinal ischaemia led to the formation of NVD in the left eye, which required vigorous therapy.
We present a patient with bilateral CRVO who developed OSVs in one eye and NVD in the other eye. A correct differentiation between NVD and OSD should be reached by the physician to pursue a proper treatment strategy.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Declaration of interest statement
No potential conflict of interest was reported by the author(s).
References
- 1.Cugati S, Wang JJ, Knudtson MD, et al. Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology. 2007;114:520–524. doi: 10.1016/j.ophtha.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 2.Lahey JM, Kearney JJ, Tunc M.. Hypercoagulable states and central retinal vein occlusion. Curr Opin Pulm Med. 2003;9:385–392. doi: 10.1097/00063198-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 3.A T Phlegmasia alba dolens. Lectures on clinical medicine, delivered at the Hotel-Dieu, Paris 1865;5:281–332. [Google Scholar]
- 4.Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112:853–862. doi: 10.1172/JCI18882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madanagopalan VG, Paneer Selvam V, Sarath Sivan NV, Govindaraju NV. Central retinal vein occlusion in a patient with breast carcinoma. GMS Ophthalmol Cases. 2009;9:Doc04. doi: 10.3205/oc000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonderska M, Ciszewska J, Drobecka-Brydak E. Central retinal vein occlusion in the tumor of colon and kidney–difficulties in diagnosis. Klin Oczna. 2005;107:700–702. [PubMed] [Google Scholar]
- 7.Kim MS, Cho JH, Byun SJ, Oh CM, Park KH, Park SJ. Increased risk of cancer in patients with retinal vein occlusion: a 12-year nationwide cohort study. Br J Ophthalmol. 2021;105:1705–1710. doi: 10.1136/bjophthalmol-2020-316947. [DOI] [PubMed] [Google Scholar]
- 8.Kanai M, Sakimoto S, Hara C, et al. The caliber of optociliary shunt vessels is associated with macular blood flow and visual acuity in central retinal vein occlusion. Ophthalmol Sci. 2021. Ahead on print. doi: 10.1016/j.xops.2021.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller JJ, Mason JO 3rd, White MF Jr., McGwin G Jr., Emond TL, Feist RM. Retinochoroidal collateral veins protect against anterior segment neovascularization after central retinal vein occlusion. Arch Ophthalmol. 2003;121:332–336. doi: 10.1001/archopht.121.3.332. [DOI] [PubMed] [Google Scholar]
- 10.Giuffre G, Palumbo C, Randazzo-Papa G. Optociliary veins and central retinal vein occlusion. Br J Ophthalmol. 1993;77:774–777. doi: 10.1136/bjo.77.12.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg DV, Wahle AE, Ip MS, et al. Score study report 12: development of venous collaterals in the score study. Retina. 2013;33:287–295. DOI 10.1097/IAE.0b013e318263d106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayreh SS, Zimmerman MB. Ocular neovascularization associated with central and hemicentral retinal vein occlusion. Retina. 2012;32:1553–1565. doi: 10.1097/IAE.0b013e318246912c. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Agarwal A, Mahajan S, et al. Morphological differences between optic disc collaterals and neovascularization on optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol. 2017;255:753–759. doi: 10.1007/s00417-016-3565-x. [DOI] [PubMed] [Google Scholar]


