Abstract
Only listeriolysin O (LLO)-producing strains of Listeria monocytogenes generate protective immunity in mice. Based on the findings that endogenous gamma interferon (IFN-γ) production was induced only by such strains and that purified LLO could induce IFN-γ from NK cells, we have postulated that LLO may play a pivotal role in the induction of Th1-type protective T cells, which are highly dependent on IFN-γ. In this study, mice were immunized with L. monocytogenes ATCC 15313, an LLO-nonproducing avirulent strain, along with LLO encapsulated in liposome (LLO-liposome). LLO-liposome was highly potent in the induction of various cytokines, including IFN-γ. Immunization of mice with either LLO-liposome or the viable strain ATCC 15313 alone did not induce protection against challenge infection. In contrast, the combination of LLO-nonproducing bacteria plus LLO-liposome induced a significant level of protective immunity mediated mainly by Th1-type cells capable of producing a large amount of IFN-γ in an antigen-specific manner. The protection afforded by the combination was not dependent on LLO-specific cytotoxic T cells. These results support the idea that the inability of an LLO-nonproducing avirulent strain or killed bacteria to induce the generation of protective T cells is due not to the lack of a central T-cell epitope(s) but to the lack of ability to induce the production of endogenous cytokine during the early stage of immunization; the results also suggest that an appropriate use of LLO at least in an animal model may be effective in the induction of antigen-specific Th1-dependent protective immunity to various kinds of intracellular parasitic bacteria.
Listeria monocytogenes is a gram-positive, facultative intracellular bacterial pathogen which multiplies mainly in the liver and spleen of the mouse (27). L. monocytogenes is able to survive inside macrophages by escaping into the cytoplasm from the phagosome (6, 22, 39, 40) by virtue of a 58-kDa listeriolysin O (LLO) (2, 15, 35) and several enzymes encoded by genes located adjacent to the hly gene coding for LLO (23, 36, 38, 47).
LLO, the major virulence factor of L. monocytogenes, shows a pH-dependent hemolytic activity (8), and this activity is blocked by oxidation (13, 33) or small amounts of cholesterol (19). The ability to produce LLO is unique to virulent strains of L. monocytogenes (7), and the LLO-producing, virulent strain has been shown to induce the expression of various cytokines in the infected host (12, 37), such as tumor necrosis factor alpha (TNF-α), interleukin-1α (IL-1α), IL-6, and gamma interferon (IFN-γ). These inflammatory cytokines produced during the early stage of infection with L. monocytogenes are believed to be necessary for the nonspecific protection of mice (10, 11, 29). Though inflammatory cytokines may contribute to the protection to some extent, nonspecific protection never results in the complete clearance of replicating bacteria, and the generation of antigen-specific, T-cell-mediated immunity is required for the complete resolution and establishment of long-lived acquired protection. Using various Listeria strains with different LLO production abilities, it has been shown that the generation of cell-mediated acquired immunity in mice is achieved after infection with LLO-producing, virulent strains but not with the LLO-nonproducing [LLO(−)] strain or killed bacteria (1, 26, 32).
In previous studies, we demonstrated that there was a significant difference in the ability to induce endogenous inflammatory cytokines between viable LLO-producing bacteria and viable LLO(−) bacteria or killed bacteria (26, 49). While several cytokines were equally expressed, IL-1α and IFN-γ could be induced only by viable LLO-producing bacteria. Using purified LLO, we confirmed that the expression of IL-1α and IFN-γ was induced by LLO itself in vitro (31, 45, 52). These findings suggested that the induction of protective T cells is highly dependent on the endogenous cytokines induced by LLO liberated from LLO-producing bacteria. Strongly supportive evidence for the requirement of endogenous IFN-γ is our recent finding that the functional blockade of initially produced IFN-γ by neutralizing antibodies resulted in abolishment of the generation of protective T cells in vivo (51).
Based on these findings, we have postulated that protective immunity may be generated in mice immunized with an LLO(−) strain plus purified LLO. However, an intravenous injection of LLO is lethal within 4 to 5 min, as reported over two decades ago (19), probably because of its effect on the heart (18).
In the present study, we used purified LLO molecules encapsulated into liposome (LLO-liposome) to deliver LLO to the liver and spleen, the site of the immune response, thus avoiding the cardiotoxic effects of LLO. Using LLO-liposome, we examined whether the combination of LLO and an LLO(−) strain could induce the cell-mediated acquired immunity to L. monocytogenes in mice.
MATERIALS AND METHODS
Experimental animals.
Male C3H/He mice (Charles River Japan, Atsugi, Japan), raised and maintained in a specific-pathogen-free environment, were used for experiments at 8 to 10 weeks of age.
Bacterial strain and LLO purification.
A hemolytic and virulent strain of L. monocytogenes, EGD (serovar 1/2a), was used. The 50% lethal dose of this strain was approximately 4.87 log10 CFU for ICR mice (49). L. monocytogenes ATCC 15313, which has been shown to be an hly-defective LLO(−) strain (32), was used as the avirulent strain. Heat-killed L. monocytogenes (HKLM) was prepared by heating viable L. monocytogenes EGD at 74°C for 90 min (44). LLO was prepared by the procedure described previously (45, 52). Briefly, an overnight bacterial culture in 10 ml of brain heart infusion broth (Eiken Chemical Co., Ltd., Tokyo, Japan) was grown in 3 liters of fresh broth for about 18 h at 37°C with shaking. A cell-free supernatant was obtained by centrifugation at 11,000 × g for 30 min at 4°C followed by filtration through a 0.45-μm-pore-size Millipore filter unit (Millipore Corp., Bedford, Mass.). The sterilized supernatant was concentrated by centrifugation at 11,000 × g after addition of ammonium sulfate to give a final concentration of 60%. The concentrated crude supernatant (crude LLO) was then applied to a DEAE-Sephacel column (Pharmacia, Uppsala, Sweden) and eluted with a 0 to 0.3 M NaCl gradient. Several fractions showing high levels of hemolytic activity were pooled and subjected to gel filtration on a Sephadex G-100 column (Pharmacia). Active fractions were pooled and stored at −20°C until used. The purity was confirmed by a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described before (32, 45).
Preparation of liposome.
Multilamellar liposomes were prepared as described elsewhere (50). In brief, 58.72 mg of phosphatidylcholine (Wako Pure Chemicals Co., Osaka, Japan), 8.75 mg of dehexadesyl phosphate, and 24.75 mg of cholesterol (Wako) were dissolved in chloroform in a round-bottom flask. The chloroform phase was removed by low-vacuum rotation evaporation. The lipid film was dispersed in 4 ml of phosphate-buffered saline (PBS-liposome) or crude LLO solution (500 μg/ml) (LLO-liposome) by gentle rotation. The liposomes were extruded through polycarbonate membranes (Advantec Toyo Roshi Co. Ltd., Japan) with a pore size of 0.8 μm. Nonencapsulated LLO was removed by centrifugation at 10,000 × g. The liposomes were composed of phosphatidylcholine-dehexadesyl phosphate-cholesterol at a molar ratio of 5:1:4.
Immunoblotting.
After SDS-PAGE, LLO proteins were transferred electrophoretically from the gel to a nitrocellulose sheet by the method of Towbin et al. (41). After transfer, the sheet was blocked by soaking in 0.5% (wt/vol) bovine serum albumin-containing PBS-Tween (PBS [pH 7.8] supplemented with 0.05% [vol/vol] Tween 20) and then washed three times with PBS-Tween. The sheet was incubated with anti-LLO monoclonal antibody 7D10E12 prepared in our laboratory at room temperature for 90 min and then washed. The reactivity of an antibody probe to LLO was detected by incubating the sheet with peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; Zymed Laboratories, Inc., South San Francisco, Calif.) at room temperature for 90 min. After three washings with PBS-Tween, the sheet was soaked in a solution of 4-chloro-1-naphthol (40 mg of substrate in 100 ml of 50 mM Tris-HCl [pH 7.5] to which 35 μl of 30% [vol/vol] H2O2 had been added).
Preparation of cells.
Peritoneal exudate cells (PEC) were recovered from mice 3 days after intraperitoneal injection of 2 ml of 3% thioglycolate medium (Eiken). The PEC were washed with Hanks’ balanced salt solution (HBSS) and suspended in culture medium consisting of RPMI 1640 (Nikken Bio Medical Laboratory, Kyoto, Japan) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco-BRL, Life Technologies, Inc., Auckland, New Zealand) and 5 μg of gentamicin per ml. PEC were cultivated in six-well culture plates (Costar, Cambridge, Mass.) for 1.5 h at 37°C in 5% CO2; then nonadherent cells were removed by washing with warmed HBSS, and adherent cells were used as macrophages. Spleens were aseptically removed from mice and teased between two sterile glass slides. After treatment with 0.83% ammonium chloride in 0.17 mM Tris-HCl (pH 7.6) to lyse contaminating erythrocytes, spleen cells were washed with HBSS and suspended in culture medium. Bone marrow-derived cells were harvested from the femur and placed in culture medium supplemented with 30% L929 conditioned medium as the source of granulocyte-macrophage colony-stimulating factor at 37°C in 5% CO2. After 7 days of culture, nonadherent cells were removed by washing with warmed HBSS, and adherent cells were used as bone marrow macrophages (BMMΦ).
LY labeling of lysosomes.
Washed peritoneal macrophages were suspended to 106/ml in culture medium and plated onto glass coverslips which had been placed in six-well plates (Costar). The lysosomal compartment of macrophages was labeled by incubating cells for 3 h in 2 ml RPMI 1640 containing lucifer yellow lithium salt (LY; 250 μg/ml; Sigma Chemical Co., St. Louis). The cells were washed to remove extracellular LY, and fresh culture medium was added to each well. After a further hour of incubation, LY was pinocytosed and LY-containing pinosomes were fused with lysosomes (4, 48).
Immunization of mice.
Mice were immunized intravenously with 2 × 103 CFU of L. monocytogenes EGD, 2 × 107 CFU of L. monocytogenes ATCC 15313, 200 μl of LLO-liposome, or 2 × 107 CFU of L. monocytogenes ATCC 15313 mixed with 200 μl of LLO-liposome. Six days after the immunization, mice were challenged intravenously with 2 × 104 CFU of L. monocytogenes EGD; protective immunity was determined by enumeration of CFU in the spleen 2 days later. In some experiments, mice were immunized intravenously twice on days 0 and 2.
Total RNA extraction.
Cells were washed, and to the cell pellet was added 1 ml of solution D (4 M guanidinium isothiocyanate, 0.5% N-lauroylsarcosine, 25 mM sodium citrate, 0.05 mM 2-mercaptoethanol). Cells were disrupted by being passed through a 21-gauge needle. Subsequently, 0.1 ml of 2 M sodium acetate (pH 4), 1 ml of water-saturated phenol, and 0.2 ml of chloroform-isoamyl alcohol were added to the mixture, with thorough mixing by inversion after the addition of each reagent. The final suspension was vigorously spun in a vortex for 20 s and then cooled on ice for 15 min. Samples were centrifuged at 10,000 × g for 20 min at 4°C. After centrifugation, the aqueous phase containing RNA was transferred to a new tube, mixed with the same volume of isopropanol, and placed at −20°C overnight to precipitate RNA. After centrifugation at 10,000 × g for 20 min, the RNA pellet was dissolved in solution D and precipitated with the same volume of isopropanol at −20°C for 2 h. RNA was collected by centrifugation for 15 min at 4°C, washed once with 75% ethanol, vacuum dried, and dissolved in 20 μl of distilled water. The RNA concentration was measured by the A260, and the purity of the RNA was assessed on the basis of the A260/A280 ratio with a GeneQuant nucleic acid spectrophotometer (Pharmacia LKB Biochem Ltd., Cambridge, United Kingdom).
RT-PCR.
Production of cDNA by reverse transcription (RT) was done in the following way. Total RNA extracted (5 μg) was mixed with 4 μl of RT buffer, 2 μl of 0.1 M dithiothreitol, 0.5 μl of RNasin (Promega, Madison, Wis.), 1 μl of 10 mM deoxynucleoside triphosphates (Pharmacia), 2 μl of random primer (Pharmacia), 0.5 μl of reverse transcriptase (Gibco BRL, Life Technologies Inc., Gaithersburg, Md.), and distilled water to give a final volume of 20 μl. The mixture was incubated at 42°C for 60 min and then boiled at 95°C for 3 min. Samples were kept at −20°C until used. The PCR mixture consisted of 1 μl of sample cDNA, 5 μl of PCR amplification buffer, 2 μl of 25 mM MgCl2, 4 μl of 2.5 mM deoxynucleoside triphosphates, 0.3 μl of Taq DNA polymerase (5 U/μl; Promega), 2 μl of 20 mM primer, and double-distilled water to give a final volume of 50 μl. The sequences of oligonucleotide primers used were as follows: 5′-CTCTAGAGCACCATGCTACAGAC-3′ and 5′-TGGAATCCAGGGGAAACACTG-3′ for IL-1α, 5′-AGCGGCTGACTGAACTCAGATTGTAG-3′ and 5′-GTCACAGTTTTCAGCGTGATAGGG-3′ for IFN-γ, 5′-GGCAGGTCTACTTTGGAGTCATTGC-3′ and 5′-ACATTCGAGGCTCCAGTGAATTCGG-3′ for TNF-α, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ for β-actin, 5′-TGGAGTCACAGAAGGTGGCTAAG-3′ and 5′-TCTGACCACAGTGAGGAATGTCCAC-3′ for IL-6, and 5′-AACTGGCGTTGGAAGCACGG-3′ and 5′-GAACACATGCCCACTTGCTG-3′ for IL-12 (p40).
The predicted sizes of the amplified products for IL-1α, IFN-γ, TNF-α, β-actin, IL-6, and IL-12 (p40) were 288, 213, 309, 348, 130, and 368 bp, respectively. The primers were made by Kurabo Biomedicals (Osaka, Japan) according to our sequence design. The PCR was performed by a thermal cycler (TP Cycler-100; Toyobo, Osaka, Japan). The PCR program was one cycle of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. Samples were amplified by 25 to 31 cycles of PCR. The most appropriate number of amplification cycles for each cytokine was determined by a preliminary experiment. The reaction was terminated by incubation at 72°C for 1 min, and the products were kept at 4°C in the cycler. A 10-μl volume of PCR product was analyzed by agarose gel electrophoresis using a 1% low-melting-point agarose gel (Wako) in 1× Tris-acetate-EDTA buffer supplemented with 0.005% ethidium bromide. The bands were visualized by a UV transilluminator and photographed.
IFN-γ assay for ELISA.
The IFN-γ titer was determined by a two-site sandwich enzyme-linked immunosorbent assay (ELISA). Wells of enzyme immunoassay plates (Costar) were precoated with 1.5 μg of rat anti-mouse IFN-γ monoclonal antibody R4-6A2 per ml and 0.5% bovine serum albumin in carbonate-bicarbonate buffer (pH 9.6). Next, the test supernatant or standard murine IFN-γ was added to each well. After incubation for 60 min, the plates were washed with PBS-Tween and incubated with rabbit anti-mouse IFN-γ polyclonal antibody for 60 min. The plates were then washed, and peroxidase-conjugated anti-rabbit IgG (Zymed) was added. After incubation for 60 min, the plates were washed and a substrate solution (100 ml of orthophenylenediamine [0.4 mg/ml] in phosphate-citrate buffer [pH 5.0] containing 0.03% H2O2) was added. The A490 was measured after termination of the reaction with 50 μl of 2.5 N H2SO4.
ELISPOT assay.
To determine the antigen-specific IFN-γ-producing cells, an enzyme-linked immunospot (ELISPOT) assay was carried out as described previously (17). Briefly, nylon wool-nonadherent T cells were stimulated with HKLM in the presence of antigen-presenting cells for 18 h. After being washed, the cells were resuspended in RPMI 1640 containing 10% FCS and seeded at graded concentrations of 5 × 103 to 1 × 106 cells/100 μl/well in nylon-based 96-well plates (Millipore) which had been coated with rat anti-mouse IFN-γ monoclonal antibody and blocked with RPMI 1640 containing 10% FCS. After further incubation for 20 h, the plates were thoroughly washed with PBS-Tween and incubated with rabbit anti-mouse IFN-γ polyclonal antibody for 90 min. After being washed, the plates were incubated with peroxidase-conjugated goat anti-rabbit IgG for 90 min. After repeated washings, spots representing IFN-γ-producing cells were developed by the addition of 100 μl of 3-amino-9-ethylcarbazole (0.27 mg/ml) in 0.1 M phosphate-citrate buffer (pH 5.0). The spots were counted under a dissecting microscope.
Adoptive transfer of antilisterial protection by spleen T cells.
Mice were immunized with L. monocytogenes ATCC 15313 mixed with LLO-liposome on days 0 and 2. Other groups of mice were immunized with ATCC 15313 or LLO-liposome only. Spleen cells from immunized or control mice were obtained on day 6, and T cells were prepared by using nylon wool columns. Mice were injected intravenously with 107 nylon wool-enriched T cells. Immediately after transfer of T cells, mice were challenged intravenously with 2 × 104 CFU of viable L. monocytogenes EGD. After 2 days, CFU counts in spleens and livers were determined to assess the level of protection conferred by T-cell transfer.
Cytotoxicity assay.
As the source of target cells, 7-day-old BMMΦ cultured without antibiotics were used. BMMΦ were infected with viable L. monocytogenes EGD at a multiplicity of infection of 1:10 for 90 min. BMMΦ were then washed twice and resuspended in RPMI 1640 containing 10% FCS and 5 μg of gentamicin per ml overnight to selectively kill the extracellular bacteria. The BMMΦ were collected with a cell strainer, and the viability of the cells was determined by the trypan blue dye exclusion method. Viable target cells (106; >90% viable) were labeled with 100 μCi of Na51CrO4 (NEN Life Science Products, Boston, Mass.) for 2 h. After washing, target cells (104/well) were cultured with the effector cells at various ratios for 4 h. Then 100 μl of supernatant was removed, and 51Cr activity was determined in an automated gamma counter. Percent specific lysis was calculated as (experimental value − spontaneous release)/(maximum release − spontaneous release) × 100.
Statistics.
The statistical significance of the data was determined by Student’s t test, and a P value of <0.05 was taken as significant.
RESULTS
Preparation of LLO-liposome and phagocytosis by macrophages in vitro.
To confirm that LLO molecules were encapsulated successfully in the multilamellar liposome, LLO-liposome was disrupted by 0.2% Triton X-100 and centrifuged. The supernatant was subjected to SDS-PAGE and then transferred electrophoretically to a nitrocellulose sheet for Western blot analysis.
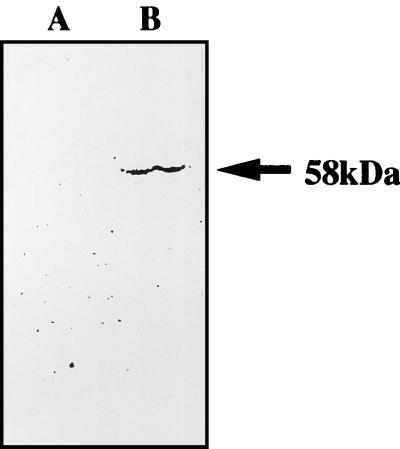
As shown in Fig. 1, the LLO-specific monoclonal antibody detected the presence of LLO in the supernatant of disrupted LLO-liposome. Since titration of LLO released from Triton-treated LLO-liposome was difficult, we applied known concentrations of the original LLO preparation to SDS-PAGE and Western blotting in the same way for comparison. It was estimated that about 6% of the LLO molecules of original LLO solution were encapsulated in the LLO-liposome (LLO concentration, 30 μg/ml).
FIG. 1.
Western blot analysis of LLO in the supernatant of the disrupted liposome used in this study. Liposome (LLO concentration, 30 μg/ml) was disrupted by 0.2% Triton X-100; 100 μl of supernatant fluid was analyzed by SDS-PAGE, and then electrophoresed proteins were transferred to a nitrocellulose sheet. Transferred protein was analyzed by immunoblotting using the anti-LLO monoclonal antibody 7D10E12. Lane A, PBS-liposome; lane B, LLO-liposome.
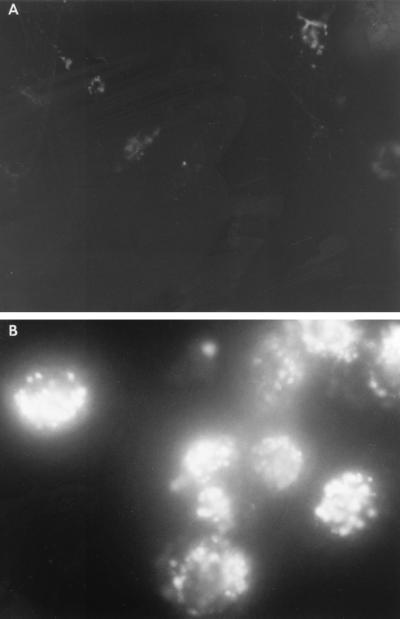
To determine whether the liposome can be effectively entrapped by macrophages, we examined phagocytosis in vitro. LY-labeled peritoneal exudate macrophages were incubated for 60 min at 37°C with or without 1 ml of liposome suspension. The cells were washed to remove nonphagocytosed liposome and examined under fluorescence microscopy. Strong fluorescence indicative of massive phagocytosis and phagosome-lysosome (P-L) fusion was observed in macrophages added with liposome, while staining with LY alone did not reveal this level of fluorescence (Fig. 2).
FIG. 2.
Phagocytosis and P-L fusion assay in macrophages. LY-labeled peritoneal exudate macrophages (106/ml) were incubated with liposome for 1 h (B) or without liposome (A). After washing to remove nonphagocytosed liposomes, cells were examined under fluorescence microscopy.
Cytokine gene expression by PEC stimulated with LLO-liposome.
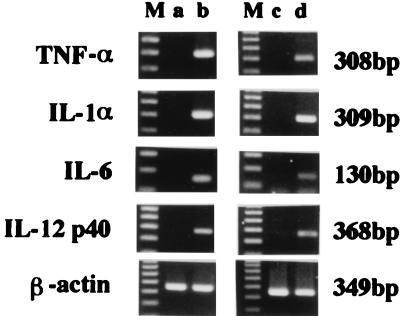
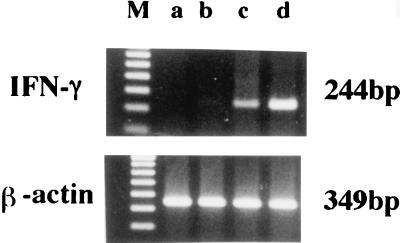
PEC were stimulated in vitro with PBS-liposome or LLO-liposome for 60 min and then washed to remove nonphagocytosed liposome. After a further 6 h of incubation, total cellular RNA was extracted and cytokine-specific mRNA expression was examined by RT-PCR. For comparison, we stimulated PEC with soluble LLO (10 μg/ml) for 6 h and similarly extracted total cellular RNA. PCR products specific for IL-1α, IL-6, IL-12, and TNF-α could be detected after stimulation with LLO-liposome, but cytokine mRNA was not induced by PBS-liposome (Fig. 3). The profile of cytokines induced by stimulation with LLO-liposome was similar to that induced by stimulation with the soluble form of purified LLO.
FIG. 3.
RT-PCR detection of cytokine-specific mRNA after in vitro stimulation with soluble LLO or LLO-liposome. PEC were unstimulated (lane a) or stimulated with 10 μg of soluble LLO (lane b), PBS-liposome (lane c), or LLO-liposome (lane d) per ml. After stimulation for 60 min, cells were washed to remove nonphagocytosed liposome. Total cellular RNA was extracted for RT-PCR after 6 h of stimulation. Lanes M, 100 bp ladder.
In vivo cytokine gene expression after intravenous injection of LLO-liposome.
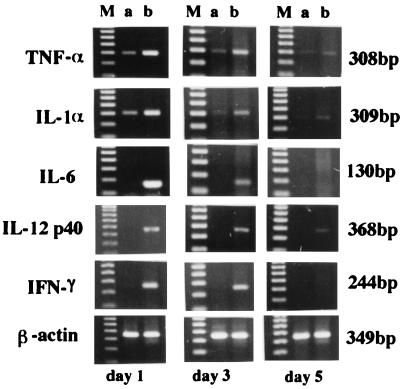
Mice were injected intravenously with PBS-liposome or LLO-liposome, each at 200 μl/head. No harmful effects were observed for mice after systemic administration of LLO-liposome during the course of immunization. The cardiotoxicity of soluble LLO could be overcome by encapsulating it into liposome. On days 1, 3, and 5, spleens were removed to determine the expression of various cytokines. Total cellular RNA was extracted, and then cytokine-specific mRNA expression was examined by RT-PCR. PCR products for IL-1α, IL-6, IL-12, TNF-α, and IFN-γ were detected on days 1 and 3. The pattern of cytokine expression in vivo was similar to the profile of cytokine expression in the spleen cells stimulated in vitro with soluble LLO (Fig. 4). The profile was also similar to that observed in the spleens of mice infected with viable, virulent L. monocytogenes as reported previously (49). Several reports have shown that liposome concentrates in the liver and spleen and is phagocytosed by macrophages when injected intravenously (9, 20, 46). Therefore, it seemed that LLO-liposome was delivered to the liver and spleen, where endogenous cytokines were induced just as in organs of mice immunized with the LLO-producing strain of L. monocytogenes.
FIG. 4.
PCR detection of cytokine-specific mRNA expression after in vivo injection of LLO-liposome. Mice were immunized intravenously with PBS-liposome (lanes a) or LLO-liposome (lanes b). At the time points indicated, total cellular RNA in the spleen was extracted and subjected to RT-PCR. Lanes M, 100 bp ladder.
Induction of acquired resistance in mice immunized with the combination of avirulent viable L. monocytogenes ATCC 15313 and LLO-liposome.
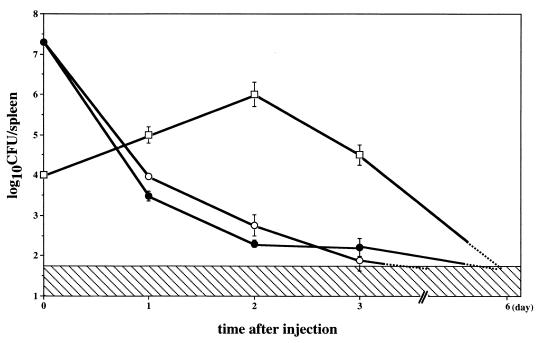
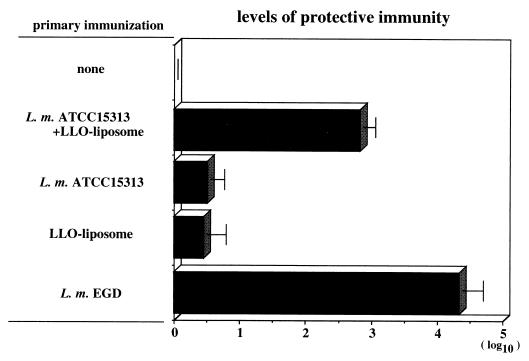
As previously reported, L. monocytogenes ATCC 15313 is an LLO(−) avirulent strain incapable of inducing protective immunity even after immunization at a high dosage (31, 32). This strain could not induce endogenous IFN-γ in mice immunized even with a high dosage of bacteria (Fig. 5). To determine whether protective immunity can be induced by an LLO(−) strain with the assistance of LLO-liposome-induced cytokines, mice were intravenously immunized with the mixture of L. monocytogenes ATCC 15313 and LLO-liposome. Simultaneous administration of LLO-liposome did not affect the kinetics of elimination of ATCC 15313 in the spleen (Fig. 6). Six days after the immunization, bacteria in the mice were reduced to a nondetectable level. The immune mice were challenged with 2 × 104 CFU of viable, virulent L. monocytogenes EGD, and the CFU counts in spleens were determined 2 days later to assess protective immunity. As shown in Fig. 7, mice given the viable strain EGD and those immunized with a combination of strain ATCC 15313 and LLO-liposome, but not mice that received LLO-liposome only or strain ATCC 15313 only, exhibited a high level of protective immunity.
FIG. 5.
Expression of IFN-γ mRNA after immunization with L. monocytogenes ATCC 15313 or EGD as determined by RT-PCR. Mice were immunized with 2 × 107 CFU of ATCC 15313 (lane b), ATCC 15313 plus 200 μl of LLO-liposome (lane c), or 2 × 103 CFU of EGD (lane d). Lane a, control; lane M, 100 bp ladder. Total cellular RNA was extracted from the spleen 24 h after stimulation for RT-PCR.
FIG. 6.
Kinetic change in the CFU of L. monocytogenes EGD and ATCC 15313 after intravenous immunization. Mice were immunized with 104 CFU of EGD (□) or with 2 × 107 CFU of ATCC 15313 alone (•) or with LLO-liposome (200 μl/head) (○). At the time points indicated, CFU counts in the spleen were determined. The hatched area indicates the nondetectable level. Data represent mean counts for three mice ± standard deviation.
FIG. 7.
Protective immunity induced by immunization with a combination of 2 × 107 CFU of L. monocytogenes (L. m.) ATCC 15313 and LLO-liposome. The level in mice immunized with virulent strain EGD is shown as positive control. Six days after immunization, mice were challenged intravenously with 2 × 104 CFU of strain EGD, and the CFU number in the spleen was determined 2 days later. The level of protective immunity was calculated as log10 CFU in nonimmune control − log10 CFU in the immune group. Data represent mean counts for three mice ± standard deviation.
Adoptive transfer of antilisterial protection by spleen T cells.
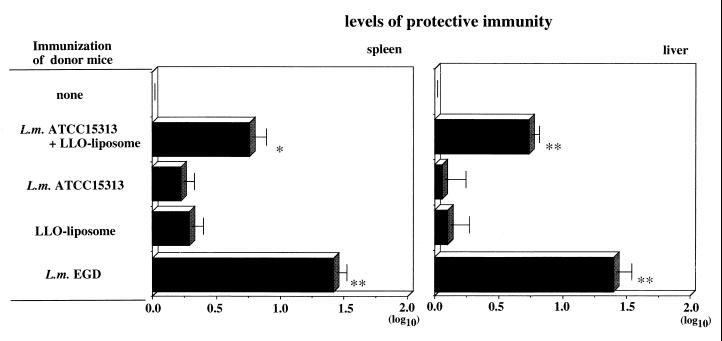
To determine whether protective T cells were induced by immunization with a combination of ATCC 15313 and LLO-liposome, spleen T cells obtained from the immunized mice were passively transferred. As shown in Fig. 8, a significant level of protection could be transferred by T cells from mice immunized with a combination of ATCC 15313 and LLO-liposome but not by T cells from other groups of mice. This finding indicated that T-cell-mediated protective immunity was induced by using LLO and avirulent LLO(−) strain, ATCC 15313.
FIG. 8.
Level of protective immunity conferred by adoptive transfer of T cells. Spleen T cells from each group of immunized mice were transferred into naive recipient mice, and the recipients were challenged intravenously with 2 × 104 CFU of strain EGD. The number of bacteria in the spleen and liver was determined 2 days later. The level of protective immunity was calculated as for Fig. 7. Data represent mean counts for three mice ± standard deviation. **, P < 0.001; *, P < 0.05 compared with the nonimmune control.
Generation of Listeria-specific IFN-γ-producing T cells.
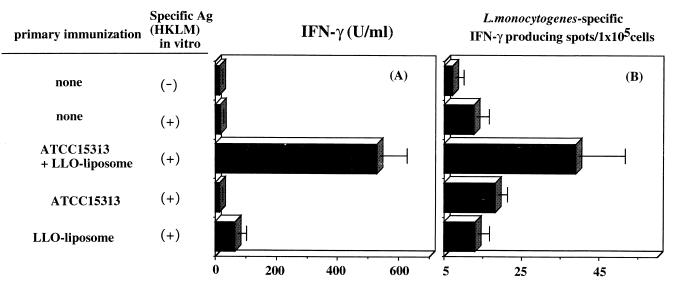
In mice immunized with viable L. monocytogenes EGD, CD4+ T cells contributed to the acquired protective immunity by producing macrophage-activating cytokines, especially IFN-γ (44, 16). Generation of Listeria-specific IFN-γ-producing cells was determined both by IFN-γ titration by ELISA in the culture supernatant and by ELISPOT assay. As shown in Fig. 9, significant levels of antigen-specific, IFN-γ-producing cells were observed by ELISA, and IFN-γ-producing cells were more numerous in spleens of mice immunized with a combination of strain ATCC 15313 and LLO-liposome. The negative selection of immune spleen cells for CD4+ or CD8+ cells by using monoclonal antibody plus complement revealed that Listeria-specific, IFN-γ-producing cells were of the CD4+ phenotype (data not shown).
FIG. 9.
Generation of antigen-specific, IFN-γ-producing cells in mice immunized with a combination of ATCC 15313 and LLO-liposome. Spleen cells (106) obtained from each group of immunized mice were stimulated with 107 HKLM in vitro. IFN-γ titer in the supernatant (A) was determined by ELISA 48 h after stimulation, and ELISPOT assay for the number of antigen (Ag)-specific, IFN-γ-producing T cells (B) was done 18 h after stimulation. Data represent mean counts for three wells ± standard deviation.
Assessment of CTL activity in mice immunized with a combination of ATCC 15313 and LLO-liposome.
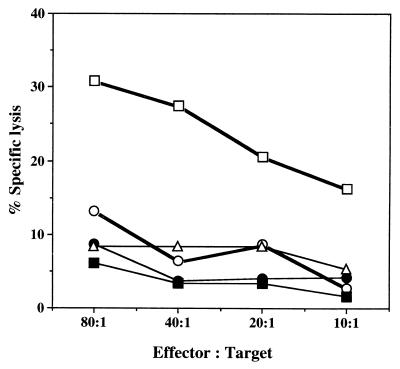
The importance of LLO-specific cytotoxic T lymphocytes (CTLs) in the expression of protective immunity to L. monocytogenes has been documented elsewhere (14, 16, 34). In the present experimental system, there was a possibility that CTLs were generated in addition to Listeria-specific Th1 cells. To rule out this possibility, a 51Cr release assay was carried out with Listeria-infected macrophages. BMMΦ were infected overnight with the LLO-producing strain L. monocytogenes EGD and were subsequently used as targets for possible CTLs in the spleen cells from immunized mice. A 4-h Cr release assay was used at various effector/target ratios. As shown in Fig. 10, immune T cells obtained from mice immunized with L. monocytogenes EGD lysed Listeria-infected BMMΦ in vitro. However, no CTL activity was observed in cells from the other groups of mice, including those receiving combination immunization. This result suggested that CTLs were not actively induced by immunization of mice with a combination of L. monocytogenes ATCC 15313 and LLO-liposome.
FIG. 10.
CTL activity in the spleen as determined ex vivo by 51Cr release assay. Mice were immunized with L. monocytogenes EGD (□), a combination of L. monocytogenes ATCC 15313 and LLO-liposome (○), ATCC 15313 alone (▵), or LLO-liposome alone (•) for 6 days. Cells obtained from nonimmune mice (■) were used as the control. 51Cr-labeled target cells (BMMΦ) infected with EGD) were cultured with effector cells form each group of mice at ratios ranging form 80:1 to 10:1 for 4 h, and the percent specific lysis was calculated. Data represent mean counts for three wells.
DISCUSSION
In this present study, we have shown that immunization of mice with a combination of purified LLO and an LLO(−) strain was highly effective in the generation of T cells protective against L. monocytogenes. Neither the LLO preparation nor strain ATCC 15313 afforded protective immunity when injected alone into mice.
The novel approach utilized in this study was the use of liposome in the delivery of purified LLO. Because of the cardiotoxicity of LLO upon intravenous injection into mice (18), it has been difficult to test the role of LLO in inducing protective immunity in vivo by intravenous injection of the soluble form of purified LLO. Encapsulation of LLO into liposome enabled the intravenous injection of LLO into mice without any apparent harmful effects. It has been shown that liposome is phagocytosed by macrophages, and then the phospholipid bilayers of the liposome are disrupted under the influence of lysosomal phospholipase (46). We also confirmed that phagocytosis of LLO-liposome and P-L fusion occurred in macrophages by using LY staining of macrophages in vitro (Fig. 2). Although we could not determine whether the same process took place in vivo, it was likely that most of the LLO-liposome along with strain ATCC 15313 reached the spleen and liver, where LLO molecules induced cytokine expression while somatic antigens of ATCC 15313 were immunologically processed.
Among the many virulence factors identified so far, LLO is regarded widely as the major one, being involved especially in the escape of L. monocytogenes from the phagosomal compartment into the cytosolic space of macrophages (2, 15, 35, 36). ATCC 15313, the avirulent LLO(−) strain used in our study, could not grow in the spleen or liver in infected mice and could not induce protective immunity (Fig. 6 and 7). It has been reported that viable ATCC 15313 cannot induce protective immunity even if used at a very high dose, suggesting that the dose of antigenic may not be the factor critical for this inability (26, 49).
The primary role of LLO administered in vivo was the induction of endogenous cytokines. We have shown in previous studies that purified LLO is highly capable of inducing various inflammatory cytokines in macrophages and spleen cells in vitro (31, 45, 52). Among the cytokines inducible by LLO, IFN-γ appears to be essential because a direct relationship between IFN-γ-inducing ability and the ability to induce protective immunity was found upon screening of various strains (32, 49). Besides, IFN-γ is regarded as the major cytokine in the priming of T cells to differentiate into IFN-γ-producing mature Th1 cells as reported by Macatonia et al. (21). We recently confirmed an essential role of endogenous IFN-γ in the generation of protective Th1 cells; induction of protective T cells in mice immunized with a virulent strain was almost completely abrogated when the endogenous IFN-γ was neutralized by in vivo administration of anti-IFN-γ antibody to mice at the initial stage of immunization (51). As expected, expression of IFN-γ and other cytokines was successfully induced by injection of LLO-liposome in vivo (Fig. 4). LLO-liposome-induced expression of IFN-γ lasted more than 3 days after immunization. It has been reported that a significant level of IFN-γ production in the murine spleen occurred within 24 to 48 h of immunization with a virulent strain (28, 30). Therefore, LLO-liposome was effective in the induction of endogenous IFN-γ just as found for natural immunization with viable LLO-producing bacteria. In addition to the established role of LLO as virulence factor, the present results suggest that LLO may also act as an essential adjuvant in the immune response of the infected host.
The results in Fig. 7 to 9 clearly indicate the generation of IFN-γ-producing Th1 cells effective for conferring protective immunity to a virulent strain of L. monocytogenes in mice after immunization with a combination of an avirulent strain and LLO-liposome. Immunization of mice with either LLO-liposome or ATCC 15313 alone was not successful, where as the combination of antigens and IFN-γ inducer was highly effective. This finding may be relevant to those of Miller et al., who reported that coinjection of IL-12 with heat-killed bacteria (24) or synthetic LLO peptides (25) elicited intense antigen-specific T-cell responses conferring protective immunity against challenge infection with L. monocytogenes. Though phenotypic characterization of effector T cells was not carried out, the authors concluded that the CD4+ T-cell response can be sufficient for the immunity achieved by coinjection based on the class II major histocompatibility complex (MHC)-binding ability of the active peptide used for immunization (25). Our present results have shown also that IL-12 was induced in vivo by LLO-liposome (Fig. 4). IL-12 is the macrophage-derived cytokine known to be capable of inducing IFN-γ production by NK cells (42, 43); therefore, it is possible that macrophage-derived IL-12 was induced first, and then IL-12-induced IFN-γ probably deriving from NK cells (31) alone or in combination with IL-12 served as the central cytokine(s) to promote the functional differentiation of antigen-primed T cells into Th1-type effector cells. Future studies will be designed to determine whether neutralization of LLO-liposome-induced cytokines, especially that of IFN-γ, actually abolishes LLO-liposome–ATCC 15313-induced protective immunity as reported by Yang et al. (51).
Antigen-specific, IFN-γ-producing T-cells are among the critical effector T cells indispensable for the activation of macrophages resulting in the enhanced microbicidal activity in immune animals (17, 44). In addition to the important role of Th1 cells for macrophage activation, the involvement of CTLs in the expression of protective immunity has been documented (16). Pamer et al. reported that LLO peptide (positions 91 to 99) serves as an antigenic epitope recognized by CTLs established from mice immunized with a virulent strain of L. monocytogenes (34). Bouwer et al. reported that the presentation of L. monocytogenes-derived peptides by class I MHC molecules occurs only when the LLO-producing bacteria escape into the cytosol of macrophages (3). Darji et al. showed that immunization of mice with mixtures of soluble LLO together with various passenger proteins resulted in strong CTL response to each exogenous protein (5). Since the immunization of mice with LLO-liposome alone did not generate protective immunity, it was unlikely that these kinds of LLO-specific CTLs were induced in our immunization protocol. Lee et al. reported that the delivery of LLO into the cytosolic space of macrophages from LLO-containing liposome in vitro and liposome containing LLO and ovalbumin efficiently delivered ovalbumin to the cytosolic pathway for MHC class I antigen processing (20). It is not clear whether LLO molecules used in our study after encapsulation into liposome are processed in the context of class I MHC; however, the present result may indicate that LLO alone is not sufficient to induce effective CTLs. Although the induction of L. monocytogenes-specific CTLs by immunization with LLO alone has not been documented, we did observe generation of CTLs in mice immunized with strain EGD; however, CTL activity was not observed at the same level in the spleen even after immunization with LLO-liposome plus ATCC 15313, which afforded protective immunity. In the present assessment of CTL activity, we used an ex vivo experiment without additional stimulation and therefore cannot exclude the possibility that CTL activity is increased in vitro by further stimulation with antigen plus IL-2. Although it is possible that CTLs contribute to the expression of acquired protection in mice immunized with a virulent strain, the present results suggest that any such role is minor at best.
The results of this study strongly suggest that the inability of an LLO(−) avirulent strain of L. monocytogenes, and possibly of killed bacteria, is not due to the lack of a central T-cell epitope(s) in avirulent or killed bacteria but rather is due to the lack of ability to induce production of endogenous cytokines, especially IFN-γ, at the early stage of immunization. In other words, LLO may be a highly effective adjuvant in the induction of Th1-dependent immunity. An appropriate use of LLO at least in animal models may be effective in the induction of antigen-specific IFN-γ-producing T cells against various kind of intracellular parasitic pathogens. It is now of interest to determine the domain of LLO effective for cytokine induction.
ACKNOWLEDGMENTS
This study was supported by the Research for the Future program of the Japan Society for the Promotion of Science, by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Culture, and Sports, and by a grant from the Ministry of Health and Welfare, Japan.
We thank Jack L. Schnell for the critical reading of the manuscript.
REFERENCES
- 1.Berche P, Gaillard J, Sansonetti P J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. [PubMed] [Google Scholar]
- 2.Bielecki J, Youngman P, Connelly P, Portnoy D A. Bacillus subtilis expressing a hemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature (London) 1990;345:175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- 3.Bouwer H G, Nelson C S, Gibbins B L, Portnoy D A, Hinrichs D. Listeriolysin O is a target of the immune response to Listeria monocytogenes. J Exp Med. 1992;175:1467–1471. doi: 10.1084/jem.175.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colette L B, James P B, Henry A F, Peter A V. Kinetics of phagocytosis and phagosome-lysosome fusion in hamster lung and peritoneal macrophages. J Leukoc Biol. 1991;50:229–239. doi: 10.1002/jlb.50.3.229. [DOI] [PubMed] [Google Scholar]
- 5.Darji A, Chakraborty T, Wehland J, Weiss S. TAP-dependent major histocompatibility complex class I presentation of soluble protein using listeriolysin. Eur J Immunol. 1997;27:1353–1359. doi: 10.1002/eji.1830270609. [DOI] [PubMed] [Google Scholar]
- 6.de Chastellier C, Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994;62:543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deneer H G, Boychuk I. Species-specific detection of Listeria monocytogenes by DNA amplification. Appl Environ Microbiol. 1991;57:606–609. doi: 10.1128/aem.57.2.606-609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geoffroy C, Gaillard J L, Alouf J E, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunol Today. 1990;11:89–97. doi: 10.1016/0167-5699(90)90034-7. [DOI] [PubMed] [Google Scholar]
- 10.Haak F M, Kurtz R S, Czuprynski C J. rIL-1 alpha enhances adoptive transfer of resistance to Listeria monocytogenes infection. Microb Pathog. 1991;10:385–392. doi: 10.1016/0882-4010(91)90083-m. [DOI] [PubMed] [Google Scholar]
- 11.Havell E A, Moldawer L L, Helfgott D, Kilian P L, Sehgal P B. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1490. [PubMed] [Google Scholar]
- 12.Iizawa Y, Brown J F, Czuprynski C J. Early expression of cytokine mRNA in mice infected with Listeria monocytogenes. Infect Immun. 1992;60:4068–4073. doi: 10.1128/iai.60.10.4068-4073.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins E M, Njoku-Obi A N, Adams E A. Purification of the soluble hemolysins of Listeria monocytogenes. J Bacteriol. 1964;88:418–424. doi: 10.1128/jb.88.2.418-424.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Gregory S H, Wing E J. Immune CD8+ T lymphocytes lyse Listeria monocytogenes-infected hepatocytes by a classical MHC class I-restricted mechanism. J Immunol. 1997;158:287–293. [PubMed] [Google Scholar]
- 15.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufmann S H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura I, Tsukada H, Yoshikawa H, Fujita M, Nomoto K, Mitsuyama M. IFN-γ-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J Immunol. 1992;148:2887–2893. [PubMed] [Google Scholar]
- 18.Kingdon G C, Sword C P. Cardiotoxic and lethal effects of Listeria monocytogenes hemolysin. Infect Immun. 1970;1:373–379. doi: 10.1128/iai.1.4.373-379.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kingdon G C, Sword C P. Biochemical and immunological effects of Listeria monocytogenes hemolysin. Infect Immun. 1970;1:363–372. doi: 10.1128/iai.1.4.363-372.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K D, Oh Y K, Portnoy D A, Swanson J A. Delivery of macromolecules into cytosol using liposomes containing hemolysin from Listeria monocytogenes. J Biol Chem. 1996;271:7249–7252. [PubMed] [Google Scholar]
- 21.Macatonia S, Hsieh C-S, Murphy K M, O’Garra A. Dendritic cells and macrophages are required for Th1 development of CD4+ T cells from αβ, TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-γ production is IFN-γ-dependent. Int Immunol. 1993;5:1119–1128. doi: 10.1093/intimm/5.9.1119. [DOI] [PubMed] [Google Scholar]
- 22.Marquis H, Bouwer H G, Hinrichs D J, Portnoy D A. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect Immun. 1993;61:3756–3760. doi: 10.1128/iai.61.9.3756-3760.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mengaud J, Braun-Breton C, Cossart P. Identification of phosphatidylinositol-specific phospholipase C activity in Listeria monocytogenes: a novel type of virulence factor? Mol Microbiol. 1991;5:367–372. doi: 10.1111/j.1365-2958.1991.tb02118.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller M A, Skeen M J, Ziegler H K. Nonviable bacterial antigens administered with IL-12 generate antigen-specific T cell responses and protective immunity against Listeria monocytogenes. J Immunol. 1995;155:4817–4828. [PubMed] [Google Scholar]
- 25.Miller M A, Skeen M J, Ziegler H K. A synthetic peptide administered with IL-12 elicits immunity to Listeria monocytogenes. J Immunol. 1997;159:3675–3679. [PubMed] [Google Scholar]
- 26.Mitsuyama M, Igarashi K, Kawamura I, Ohmori T, Nomoto K. Difference in the induction of macrophage interleukin-1 production between viable and killed cells of Listeria monocytogenes. Infect Immun. 1990;58:1254–1260. doi: 10.1128/iai.58.5.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsuyama M, Takeya K, Nomoto K, Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978;106:165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- 28.Nakane A, Numata A, Asano M, Kohanawa M, Chen Y, Minagawa T. Evidence that endogenous gamma interferon is produced early in Listeria monocytogenes infection. Infect Immun. 1990;58:2386–2388. doi: 10.1128/iai.58.7.2386-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakane A, Minagawa T, Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988;56:2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakane A, Minagawa T, Kohanawa M, Chen Y, Sato H, Moriyama M, Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989;57:3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishibori T, Xiong H, Kawamura I, Arakawa M, Mitsuyama M. Induction of cytokine gene expression by listeriolysin O and roles of macrophages and NK cells. Infect Immun. 1996;64:3188–3195. doi: 10.1128/iai.64.8.3188-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishibori T, Cooray K, Xiong H, Kawamura I, Fujita M, Mitsuyama M. Correlation between the presence of virulence-associated genes as determined by PCR and actual virulence to mice in various strains of Listeria spp. Microbiol Immunol. 1995;39:343–349. doi: 10.1111/j.1348-0421.1995.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 33.Njoku-Obi A N, Jenkins E M, Njoku-Obi J C, Adams J, Convington V. Production and nature of Listeria monocytogenes hemolysins. J Bacteriol. 1963;86:1–8. doi: 10.1128/jb.86.1.1-8.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pamer E G, Harty J T, Bevan M J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature (London) 1991;353:852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portnoy D A, Tweten R K, Kehoe M, Bielecki J. Capacity of listeriolysin O, streptolysin O, and perfringolysin O to mediate growth of Bacillus subtilis within mammalian cells. Infect Immun. 1992;60:2710–2717. doi: 10.1128/iai.60.7.2710-2717.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poston R M, Kurlander R J. Cytokine expression in vivo during murine listeriosis. Infection with live, virulent bacteria is required for monokine and lymphokine messenger RNA accumulation in the spleen. J Immunol. 1992;149:3040–3044. [PubMed] [Google Scholar]
- 38.Raveneau J, Geoffroy C, Beretti J L, Gaillard J L, Alouf J E, Berche P. Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect Immun. 1992;60:916–921. doi: 10.1128/iai.60.3.916-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tilney L G, Connelly P S, Portnoy D A. Actin filament nucleation by the bacterial pathogen, Listeria monocytogenes. J Cell Biol. 1990;111:2979–2998. doi: 10.1083/jcb.111.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 44.Tsukada H, Kawamura I, Arakawa M, Nomoto K, Mitsuyama M. Dissociated development of T cells mediating delayed-type hypersensitivity and protective T cells against Listeria monocytogenes and their functional difference in lymphokine production. Infect Immun. 1991;59:3589–3595. doi: 10.1128/iai.59.10.3589-3595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukada H, Kawamura I, Fujimura T, Igarashi K, Arakawa M, Mitsuyama M. Induction of macrophage interleukin-1 production by Listeria monocytogenes hemolysin. Cell Immunol. 1992;140:21–30. doi: 10.1016/0008-8749(92)90173-m. [DOI] [PubMed] [Google Scholar]
- 46.Van Rooijen N, Sanders A. Liposome-mediated depletion of macrophage: mechanism of action, preparation of liposome and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 47.Vazquez B, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:1334–1339. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y L, Goren M B. Differential and sequential delivery of fluorescent lysosomal probes into phagosomes in mouse peritoneal macrophages. J Cell Biol. 1987;104:1749–1754. doi: 10.1083/jcb.104.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiong H, Kawamura I, Nishibori T, Mitsuyama M. Cytokine gene expression in mice at an early stage of infection with various strains of Listeria spp. differing in virulence. Infect Immun. 1994;62:3649–3654. doi: 10.1128/iai.62.9.3649-3654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto T, Naito M, Moriyama H, Umezu H, Matsuo H, Kiwada H, Arakawa M. Repopulation of murine Kupffer cells after intravenous administration of liposome-encapsulated dichloro-methylene diphosphonate. Am J Pathol. 1996;149:1271–1286. [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J, Kawamura I, Mitsuyama M. Requirement of the initial production of gamma interferon in the generation of protective immunity of mice against Listeria monocytogenes. Infect Immun. 1997;65:72–77. doi: 10.1128/iai.65.1.72-77.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshikawa H, Kawamura I, Fujita M, Tsukada H, Arakawa M, Mitsuyama M. Membrane damage and interleukin-1 production in murine macrophages exposed to listeriolysin O. Infect Immun. 1993;61:1334–1339. doi: 10.1128/iai.61.4.1334-1339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]