Abstract
In accordance with the spectrum theory of metastatic disease, an oligometastatic clinical state has been proposed as an intermediary step along the natural history of cancer with few (typically 1–3) metastatic lesions identifiable on imaging that may be amenable to metastasis-directed therapy. Effective therapy of oligometastatic disease is anticipated to impact cancer evolution by delaying progression and improving patient outcome at a minimal or acceptable cost of toxicity. There has been increasing recognition of oligometastatic disease in prostate cancer with the advent of new-generation imaging agents, most notably the recently approved PET radiotracers based on targeting prostate-specific membrane antigen. Early clinical trials with metastasis-directed therapy of oligometastases have provided evidence for delaying the employment of systematic therapy and improving outcome in selected patients. Despite these encouraging results, much needs to be investigated and learned about the underlying biology of the oligometastatic state along the evolutionary clinical course of prostate cancer, the identification of relevant imaging and nonimaging predictive and prognostic biomarkers, and the development of treatment strategies to optimize short-term and long-term patient outcome. We provide a review of the current status and the lingering challenges of this rapidly evolving clinical space in prostate cancer.
Keywords: oligometastasis, prostate, cancer, PET
Metastatic prostate cancer is incurable despite major strides in the development of novel treatment regimens, including those targeting the androgen axis, the immune system, and certain genomic signatures. Prognosis is associated with metastatic tumor burden and biology. It has been recognized that there is remarkable spatiotemporal clonal diversity in metastases and that not all metastases are clinically alike. In 1995, Hellman and Weichselbaum proposed the existence of an oligometastatic state as an intermediary clinical state of cancer with few identifiable metastatic lesions and limited facility for growth. Oligometastatic disease provides unique opportunities for metastasis-directed therapies (MDTs) that may impact cancer evolution by delaying progression and improving patient outcome at minimal toxicity cost (1,2). The theory behind MDT in this setting is to eliminate the oligometastatic colony before it can evolve biologically into a more aggressive phenotype with untoward consequences, including potential tumor seeding of other sites (3). Additionally, the oligometastatic condition in various clinical scenarios may have differences in underlying biology and need different treatment strategies (4). Oligometastatic lesions may be observed coincident with the untreated primary tumor (synchronous or de novo) or at biochemical recurrence (BCR) after definitive therapy of primary cancer (metachronous), after systemic therapy of polymetastatic disease with few drug-resistant lesions remaining (oligopersistent), or after an initial favorable response to systemic therapy and subsequent development of disease progression at a limited number of new sites (oligoprogression) (5). In this article, we review the investigations on the underlying biology, utility, and contribution of new-generation imaging and clinical studies relevant to oligometastatic prostate cancer (OMPC), with a focus on metachronous oligometastases.
BIOLOGIC BASIS
The exact biologic underpinning of OMPC remains unestablished. The current rudimentary classification of metastatic disease as the oligometastatic phenotype is based on number of metastases identifiable on imaging, which relies fundamentally on the sensitivity of the imaging modality. Such a “moving target” limits comparison of various studies in this clinical space and may be the reason for the large variations in the reported prevalence (6–8). Understanding of the differential genomic, epigenetic, and immunologic features of oligometastatic and polymetastatic phenotypes will enable more robust treatment strategies and improved prognostication (9–13). It is recognized that the spatiotemporal genetic evolution of prostate cancer is complex and that development of metastatic disease is not a passive, random process (14). Primary tumors harbor heterogeneous subpopulations of clonogens with variable metastatic potential, and further, cross metastatic site seeding can occur in combination with dynamic subclonal selection in response to microenvironmental and therapy-induced pressures (15).
Deek et al. retrospectively assessed the mutational landscape of metastatic prostate cancer in patients who underwent clinical-grade sequencing of their tumors (269 primary tumors, 25 metastatic tumors) classified as biochemically recurrent (micrometastatic), metachronous oligometastatic (≤5 lesions), metachronous polymetastatic (>5 lesions), or de novo metastatic lesions at the time of initial diagnosis (16). Mutations in TP53 and double-strand break repair genes were associated with a higher number of metastases. On multivariate analysis, TP53 mutations were independently associated with shorter radiographic progression-free survival (PFS) and development of a castration-resistant phenotype. The authors concluded that the somatic mutational profile of prostate cancer unveils a spectrum of metastatic biology that may aid in defining OMPC beyond simple lesion enumeration on imaging. Several other genomic or genetic signatures are associated with more aggressive biologic behavior in the advanced setting and are being evaluated with a variety of systemic therapies such as the addition of platinum chemotherapy or poly(adenosine diphosphate ribose) polymerase inhibitors (17–19). However, these types of signatures remain to be prospectively tested in cohorts with OMPC in the context of metastatic development, persistence, or progression.
Research is ongoing to identify liquid biopsy biomarkers that may be useful in differential characterization of OMPC from polymetastatic disease. These benchmarks may include circulating tumor cells, cell-free DNA/RNA, and micro-RNA (20–22). Studies have suggested that oligometastases may be less genomically heterogeneous than polymetastatic disease manifested by different micro-RNA profiles (23,24). However, other studies have failed to delineate a serum-derived micro-RNA signature that can discriminate between oligometastatic and polymetastatic disease (25). Research is under way to assess the biologic anchor for the oligometastatic clinical state.
IMAGING DEFINITION AND MANAGEMENT RELEVANCE
There is no consensus on the imaging definition of OMPC with respect to the number or location of metastases (26). Sollini et al. compared an intrapatient lesion radiomics similarity analysis according to the total number of detected lesions (≤3, ≤5, >3, or >5) on 18F-fluoromethylcholine PET/CT in men with BCR after curative treatments for primary prostate cancer (27). Patients with oligometastatic disease defined with a 5-lesion threshold demonstrated lesion heterogeneity comparable to patients with polymetastatic disease. A 3-lesion threshold for defining oligometastatic disease was suggested because of less radiomics heterogeneity than for polymetastatic disease, stratified by various relevant parameters (e.g., Gleason score, prostate-specific antigen [PSA], and androgen deprivation therapy [ADT]). The study was limited by the sensitivity of choline PET/CT, lack of an in vivo biologic framework, and lack of correlation to clinical outcome.
Physicians of the Society of Urologic Oncology were surveyed to gather information on their clinical practices as related to OMPC (28). With a 12.9% response rate, most physicians (35.29%) defined OMPC as fewer than 3 bone or lymph node metastases evident on standard imaging, with only 27% of the responders using PET in their practice. Clinical benefit from MDT of OMPC was considered worthwhile if there would be an increase in the rate of 1-y ADT-free survival. The Korean investigators performed a survey of 326 radiation oncologists caring for patients with OMPC. With a high response rate of 82%, most physicians (75%) agreed on 5 or fewer lesions as the definition for oligometastases (29). The Dutch multidisciplinary consensus meeting stated that OMPC comprises 3–5 metastases in a maximum of 2 organs in the hormone-sensitive setting and for either a synchronous or a metachronous presentation. The panel considered prostate-specific membrane antigen (PSMA) PET/CT as currently the most accurate diagnostic imaging modality for the identification of oligometastases (30).
CLINICAL MANAGEMENT STUDIES
The optimal management of OMPC is debated (31–33). However, patients with OMPC are potential candidates for MDT with or without systemic therapy to target micrometastases (34,35). The type of MDT may depend on various factors, including when OMPC is temporally manifested along the disease trajectory, lesion location, and other pertinent clinical factors, with the goal of locally eradicating the lesion while inducing the least toxicity to the surrounding normal tissue. There is no consensus on the optimal treatment strategy for OMPC, which may involve several approaches, including observation, metastatectomy, stereotactic ablative radiation therapy (SABR), radioligand therapy, or potentially a combination of these therapies with or without ADT (36–40). In particular, SABR has been recognized as an effective and safe treatment strategy with minimal toxicity despite variable nonstandardized radiotherapy regimens (41–45). Generally, however, data are currently lacking on the impact of a selected treatment strategy on cancer-specific or overall survival.
In a systematic review and metaanalysis of patients with oligometastatic disease from a variety of cancers (21 studies, 943 patients, 1,290 oligometastases [≤5 extracranial metastases], with prostate cancer as the most common primary tumor, comprising 22.9% of cancers), MDT of oligometastases was determined to be associated with clinically acceptable rates of 1-y local-control PFS and an acceptable 13% rate of acute and late toxic effects of grades 3–5 (46). A systematic review of literature focused on OMPC that included 7 studies of acceptable quality reported choline PET/CT as the most common imaging modality for identification of oligometastases, with nodal, bone, and visceral metastases treated in 78%, 21%, and 1% of patients, respectively (47). Overall, half of patients across the studies were progression-free (various definitions or not reported) 1–3 y after salvage MDT, with grade 2 toxicity in 8.5% of patients. In another systematic review and metaanalysis of 356 abstracts and 10 studies that included 653 patients with 3–5 metachronous OMPC (based on new-generation imaging with PET/CT in 92% of cases, most commonly performed with radiolabeled choline), the summary 2-y biochemical PFS, radiographic PFS, and ADT-free survival were 33% (95% CI, 11%–55%), 39% (95% CI, 24%–54%), and 52% (95% CI, 41%–62%), respectively (48). Viani et al. also provided a metaanalysis of 23 observational studies on the efficacy and toxicity of SABR for OMPC (49). The proportional rate of local control at the treated sites, PFS, and ADT-free survival were 0.976 (95% CI, 0.96–0.98), 0.413 (95% CI, 0.378–0.477), and 20.1 mo (95% CI, 14.5–25.6), respectively. The rate of any early or late toxicity of grade 2 or higher was 1.3% and 1.2%, respectively.
It is pertinent to decipher the long-term management impact of MDT in OMPC. Deek et al. performed a retrospective multiinstitutional study on 258 men who had MDT of 474 lesions. The authors reported on the patterns of recurrence and modes of progression after MDT in castration-sensitive OMPC with a median follow-up of 25.2 mo. About half the patients were receiving concurrent ADT. Most patients had long-term control (no recurrence at ≥18 mo; 40.9%) or showed oligoprogression (≤3 lesions at recurrence; 36%). Bone was the favored site of progression (50). In another retrospective multicenter study of 359 patients who had metachronous oligometastatic lesions after prostatectomy and were treated with MDT, about one third of the patients progressed within a median follow-up of 16 mo (51).
There have been several studies on OMPC, including ongoing clinical trials (52,53). We summarize below some of the major clinical studies, organized on the basis of the imaging method that has been used for OMPC identification. Table 1 summarizes the features of the selected clinical trials on OMPC that are discussed in detail below.
TABLE 1.
Summary of Features in Selected Clinical Trials on OMPC
| Trial | Imaging modality | Oligometastatic definition | Oligometastatic therapy | Outcome measure | Reference |
|---|---|---|---|---|---|
| ORIOLE | CT, BS | ≤3 bone or LN | SABR vs. observation | Progression at 6 m | 54 |
| POPSTAR | 18F-NaF PET/CT | ≤3 bone | SABR | Radiographic PFS | 55 |
| RAVENS | CT, BS | ≤3 bone or ST (at least 1 bone) | SABR + 223RaCl2 | PFS | 56 |
| STOMP | Choline PET/CT | ≤3 bone or LN | Surveillance vs. SABR | ADT-free survival | 61 |
| LOCATE | 18F-fluciclovine PET/CT | ≤5 extraprostatic (≤3 in any single organ) | NA | Change in management | 64 |
| TROD 09-004 | PSMA PET/CT | ≤5 bone | SABR | 2-y PFS | 73 |
| OLI-P | PSMA PET/CT | ≤5 bone or LN | SABR | Time to ADT initiation; time to PSA progression | 75 |
| BULLSEYE | PSMA PET/CT | ≤5 bone or LN | 177Lu-PSMA-617 vs. SOC (deferred ADT) | Progression at 24 wk | 78 |
BS = bone scan; LN = lymph node; NA = not applicable; SOC = standard of care; ST = soft tissue.
Contrast-Enhanced Abdominopelvic CT and Bone Scanning
The phase 2 nonmasked randomized ORIOLE trial (Observation vs. Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer; NCT02680587) randomized, in a 2:1 ratio, 54 men with recurrent castration-sensitive oligometastatic disease (1–3 metastases and not receiving ADT within 6 mo of enrollment or ≥3 y total) to receive SABR or observation, with a primary clinical endpoint of progression at 6 mo by PSA level increase, radiographically on standard imaging, symptomatology, ADT initiation for any reason, or death. There was a statistically significant lower progression at 6 mo in the patients receiving SABR than in those who were observed (19% vs. 61%, P = 0.005). Treatment with SABR improved median PFS (not reached vs. 5.8 mo; hazard ratio, 0.30; 95% CI, 0.11–0.81; P = 0.002) (54).
The pilot study for the POPSTAR trial (Patients with Oligometastases from Prostate Cancer Treated with Stereotactic Ablative Radiotherapy) enrolled 33 patients with OMPC who received SABR to a total of 50 oligometastases and were followed for 2 y. Patients were screened with bone scanning using 18F-NaF PET/CT, with oligometastases defined as 3 or fewer bone metastases. The local and distant radiographic PFS was 97% (95% CI, 91%–100%) and 58% (95% CI, 43%–77%), respectively, at 1 y and 93% (95% CI, 84%–100%) and 39% (95% CI, 25%–60%), respectively, at 2 y. The treatment strategy approach was safe and avoided hormone therapy in almost half the patients at 2 y. Moreover, there were no significant differences from baseline quality-of-life measures based on the European Organization for Research and Treatment of Cancer QLQ-C30 and QLQ-BM22 at 1, 3,12, and 24 mo (55).
The phase 2 nonmasked randomized RAVENS trial (223RaCl2 and SABR vs. SABR for Oligometastatic Prostate Cancers; NCT04037358) will test the hypothesis that SABR plus 223RaCl2 will double the median PFS (defined by the PCWG2 criteria) from 10 mo in the SABR arm to 20 mo in the combined-therapy arm. A metachronous oligometastatic disease is defined as up to 3 asymptomatic metastatic tumors of the bone or soft tissue (with at least 1 bone metastasis) (56).
Choline
In a phase 2 trial, 128 oligometastatic lesions identified on 11C-choline PET/CT in 89 patients were treated with SABR. The biochemical PFS was 40% at 1 y and 21% at 2 y. Baseline high levels of effector memory T cells were associated with improved biochemical PFS. The investigators suggested that the design of future randomized trials on castrate-resistant OMPC may benefit from incorporation of immune-based markers (57). In another multicenter, retrospective study on patients with castration-sensitive BCR after definitive primary treatment and fewer than 6 oligometastases on choline PET/CT, MDT plus ADT improved the biochemical PFS compared with MDT alone (48.4 mo vs. 34.2 mo, respectively), reaching statistical significance for M1b disease (58). The Italian investigators reported their experience with SABR of up to 3 oligometastases identified on 18F-fluoromethylcholine PET/CT in 46 patients (59). The median systemic therapy–free survival was 39.1 mo (95% CI, 6.5–68.6 mo), with 12- and 24-mo ratios of 74% and 63%, respectively. A similar study of 43 patients who underwent MDT of metachronous oligometastases (≥5 metastases) on 18F-fluoromethylcholine reported a longer time to a castration-resistant state in the intervention group than in a historical control (66.6 mo vs. 36.4 mo, respectively; P = 0.02) (60).
In the phase 2 multicenter, randomized STOMP trial (Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence; NCT01558427), 62 patients with BCR after curative primary therapy were randomly assigned (1:1) to either surveillance (PSA follow-up every 3 mo, with repeated imaging at PSA or clinical progression) or MDT (surgery or SABR) of 3 or fewer choline PET/CT–identified oligometastases (61). The primary endpoint was ADT-free survival with a median follow-up of 3 y. The median ADT-free survival was longer with MDT than with surveillance (21 mo vs. 13 mo, respectively; P = 0.11), with similar quality of life at the 3-mo and 1-y follow-ups.
One investigation compared choline PET/CT (10 patients) and PSMA PET/CT (40 patients) in identifying OMPC (≤4 metastases and no local recurrence) in patients eligible to undergo SABR. The primary endpoint was ADT-free survival. Secondary endpoints were PSA response after SABR and time to PSA rise after SABR. The PSMA PET/CT group had a significantly longer PSA response duration and ADT-free survival than did the choline PET group, probably because of lead time bias from the higher sensitivity in detection of disease sites at a lower PSA level with PSMA PET/CT (62). Moreover, having more than a single oligometastasis appears to be a significant prognostic factor for progression after choline PET/CT–guided SABR (hazard ratio, 2.74; P = 0.03) (63).
18F-Fluciclovine
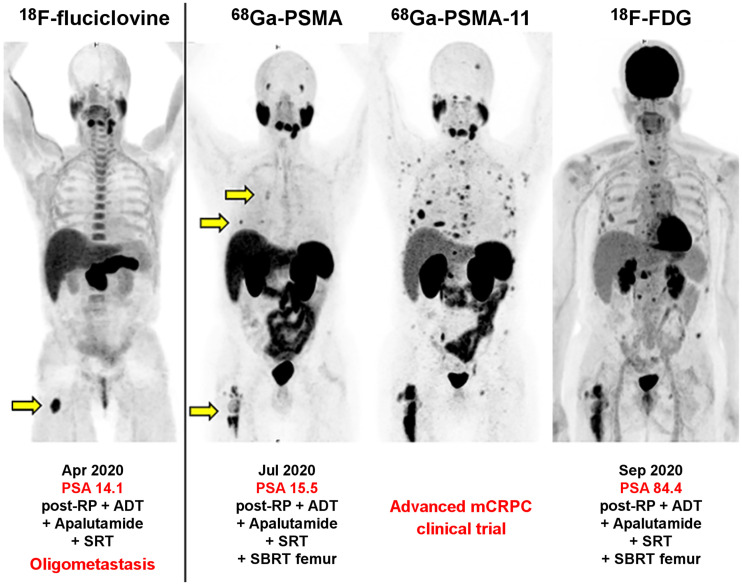
The LOCATE trial (18F-Fluciclovine PET/CT in Patients with Rising PSA After Initial Prostate Cancer Treatment; NCT02680041) evaluated the impact of 18F-fluciclovine (Axumin; Blue Earth Diagnostics) PET/CT on the management of men with BCR after definitive primary treatment and uninformative standard imaging. A cohort of men with OMPC (1–5 extraprostatic lesions with 3 or fewer lesions in any single organ system) and no imaging evidence of recurrence in the treated prostate bed were further analyzed. Of the total 213 enrolled patients, 53 patients (25%) had OMPC, with 38% of these patients having a serum PSA level of 1 ng/mL or less and 79% of them experiencing a change in their clinical management plan (64). The phase 3 cooperative group INDICATE trial (NCT04423211) will use PET/CT with 18F-fluciclovine to select patients who will undergo stratification and then randomization into 1 of 4 therapy arms. This trial will examine the comparative impact of MDT in a subpopulation of patients with oligometastases outside the standard salvage radiotherapy fields (65). In a retrospective investigation, we observed an incidence rate of 23.6% metachronous oligometastatic prostate cancer (≤5 metastases) in 21 patients with a first BCR of prostate cancer after definitive primary therapy who had negative or equivocal findings on conventional imaging. Treatment management was affected in 57.1% of patients with oligometastatic prostate cancer. Figure 1 is an example of a 18F-fluciclovine PET/CT demonstration of oligometastatic prostate cancer in a CRPC patient with BCR on ADT (66).
FIGURE 1.
BCR (PSA, 14.1 ng/mL) in patient with CRPC who was previously treated with radical prostatectomy, salvage radiation therapy to prostate bed, and ADT. 18F-fluciclovine (Axumin) PET/CT (first panel) showed single oligometastasis in proximal right femur (arrow), which was treated with SABR, with initial transient decline in PSA level but rapid subsequent rise within 3 mo to 15.5 ng/mL. 68Ga-PSMA PET/CT (second panel) showed heterogeneous activity in proximal right femur and additional metastatic lesions in right ribs (arrows). Serum PSA level rose rapidly again within 2 mo to 84.4 ng/mL, at which time repeat 68Ga-PSMA-11 PET/CT (third panel) showed numerous metastases. Companion 18F-FDG PET/CT showed that some metastases also exhibited high glycolytic phenotype (fourth panel). Patient was enrolled in clinical trial. All images are maximum-intensity projections. RP = radical prostatectomy; SBRT = stereotactic body radiation therapy. (Courtesy of Jeremie Calais, UCLA.)
PSMA
PSMA PET/CT–guided SABR has been investigated in OMPC (67,68). In a small retrospective study, 20 patients with BCR after radical prostatectomy and with 3 or fewer oligometastases on PSMA PET/CT were treated with MDT, which postponed the initiation of ADT for 2 y in 74% of patients (69). In another study of 86 patients with recurrent OMPC who were treated with MDT and followed for a median of 26 mo, the 3-y overall survival and biochemical PFS were 84% and 55%, respectively; the median time of ADT-free survival was 13.5 mo (70). In a multicenter prospective clinical trial of PSMA PET/CT in restaging biochemically relapsed prostate cancer in 238 patients, the oligometastatic rate was 16.4% (39 of 238 patients) on standard imaging, but 41% (16 of 39 patients) of these patients were upstaged to polymetastatic disease with PSMA PET/CT (71). Interestingly, in 199 patients (83.6% of the total) with negative findings on standard imaging, 148 (74%) showed PSMA-avid metastases, including 113 patients (57%) with oligometastases. Of those patients with oligometastatic prostate cancer on PSMA PET/CT, nearly two thirds had localized disease in the prostate bed, seminal vesicles, or pelvic lymph nodes only. Notably, in 35% of patients with oligometastatic prostate cancer outside the pelvis and invisible on standard imaging, the disease would have not been covered in the standard salvage pelvic radiation therapy field. In a phase 2, single-center, single-arm investigation, 37 patients with BCR and oligometastases on 18F-DCFPyL (Pylarify; Progenics Pharmaceuticals) PET/CT/MRI were treated with surgical resection or SABR (72). The overall complete or partial biochemical response rate was 60%, with only 1 case of grade 3 toxicity.
In the multiinstitutional, retrospective TROD 09-004 trial of the Turkish Society for Radiation Oncology group, 74 men with bone-only oligometastatic castration-sensitive prostate cancer based on 68Ga-PSMA-11 PET/CT (36.5% synchronous, 63.5% metachronous) were treated with SABR with a median dose of 20 Gy (73). The 2-y PFS was 72%, with multivariate analysis demonstrating that a single metastasis and PSA response (defined as a ≥25% decline in PSA from the baseline level) were significantly associated with improved PFS. The TROD 09-002 multicenter study of 176 patients with 356 oligometastases (33.5% synchronous, 66.5% metachronous) receiving MDT showed a 2-y local control rate of 93.2% at the treated sites, with no grade 3 or more acute toxicity. In multivariate analysis, an untreated primary tumor and an increased number of oligometastatic sites were negative predictors of overall survival (74).
In the nonrandomized, prospective investigator-initiated phase 2 OLI-P trial (Oligoprogression in Androgen-Sensitive Patients; NCT02264379), the toxicity and therapeutic efficacy of local ablative radiotherapy was assessed in 63 patients with 5 or fewer nonvisceral oligometastases on PSMA PET/CT (75). The median time to ADT initiation was 20.6 mo. The median time to PSA progression was 13.2 mo, and 21.4% of patients were free of PSA progression after 3 y. There was no treatment-related grade 2 or greater toxicity for up to 2 y after local ablative radiotherapy.
Farolfi et al. performed a systematic review of 3 clinical trials (choline, n = 1; 18F-NaF, n = 1; PSMA, n = 1) and 21 observational PET/CT studies (choline, n = 7; PSMA, n = 11; both choline and PSMA, n = 3) that included castration-sensitive patients with metachronous OMPC recurrence after radical treatment of primary prostate cancer (76). The number of oligometastases ranged from 2 to 5 lesions. PSMA PET/CT was associated with a higher percentage range of PFS than was choline PET/CT (19%–100% for PSMA vs. 16%–93% for choline).
Vogel et al. analyzed the outcome biochemical PFS in 292 patients with local recurrence or pelvic lymph node metastases and up to 5 distant metastases on 68Ga-PSMA PET/CT who underwent MDT (77). Patients with a PSA level of less than 0.8 ng/mL and local relapse with or without metastatic pelvic lymph nodes had the highest mean biochemical PFS. A prospective phase 2 open-label 2-arm randomized clinical BULLSEYE trial (177Lu-PSMA-617 in Oligo-Metastatic Hormone Sensitive Prostate Cancer; NCT04443062) is ongoing to assess the efficacy and toxicity of radioligand therapy with 177Lu-PSMA-617 in treating metachronous OMPC (≤5 bone or lymph nodes) evident on PSMA PET/CT (SUVmax > 15) in 58 patients with castration-sensitive prostate cancer after prior definitive primary treatment and a PSA doubling time of less than 6 mo (78). The patients will be randomized in a 1:1 ratio between the standard-of-care (deferred ADT) arm and the interventional arm (2 cycles of 7.4 GBq of 177Lu-PSMA-617 at a 6-wk interval) and monitored every 3 wk for 24 wk. The primary outcome measure is the fraction of patients who show disease progression during the study follow-up. Disease progression is defined as a 100% increase in PSA from baseline or clinical progression. Figures 2–4 are 3 illustrative case examples of oligometastatic identification on PSMA PET/CT and the impact on subsequent treatment management and outcome.
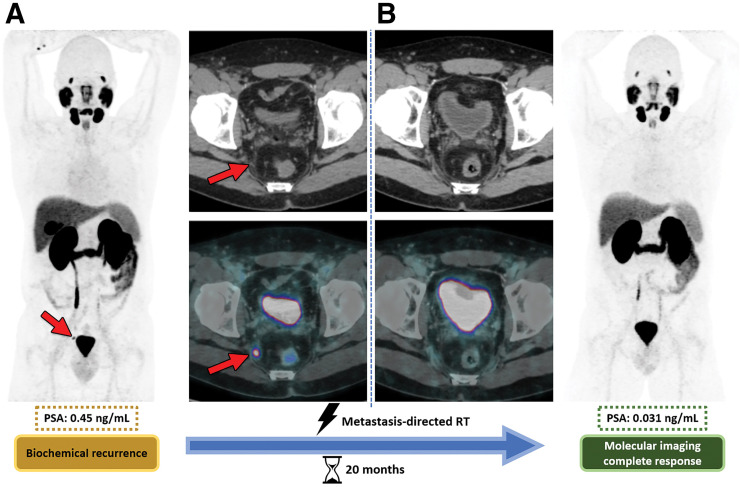
FIGURE 2.
Metachronous oligometastasis in 48-y-old man with BCR (PSA, 0.45 ng/mL) of prostate cancer (Gleason score, 8; International Society of Urologic Pathologists grade, 4) after prostatectomy. (A) Baseline 68Ga-PSMA PET/CT shows subcentimeter 68Ga-PSMA–avid metastasis in right internal iliac lymph node (arrows). (B) Follow-up 68Ga-PSMA PET/CT obtained 20 mo after metastasis-directed SABR shows complete local response, with PSA decline to 0.031 ng/mL. Shown are maximum-intensity projection images (rightmost and leftmost panels), axial CT images (upper middle panels), and axial PET/CT images (lower middle panels). RT = radiation therapy. (Reprinted with permission of (35)).
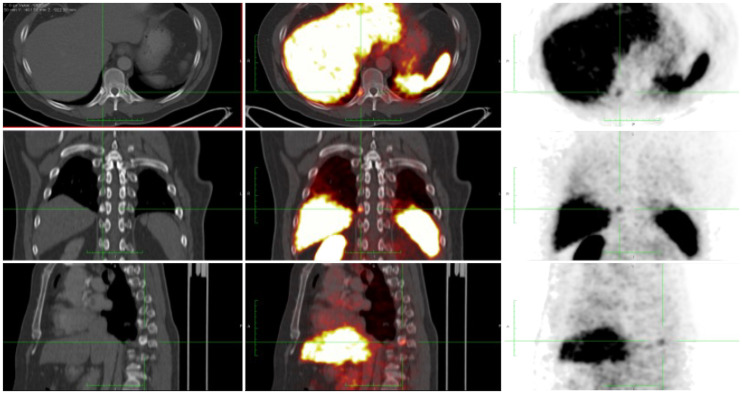
FIGURE 3.
BCR (PSA, 0.63 ng/mL) in patient with prostate cancer (pT3bN0M0; Gleason score, 4 + 3) treated with radical prostatectomy. 68Ga-PSMA-11 PET/CT showed single rib lesion (green crossbar), which was subsequently treated with SABR (45 Gy × 5). Follow-up serum PSA levels at 3 and 26 mo were 0.03 and 0.01 ng/mL, respectively. Shown are CT images (left panel), PET/CT images (middle panel), and PET images (right panel); all panels depict axial (top), sagittal (middle), coronal (bottom) views. (Courtesy of Jeremie Calais, UCLA.)
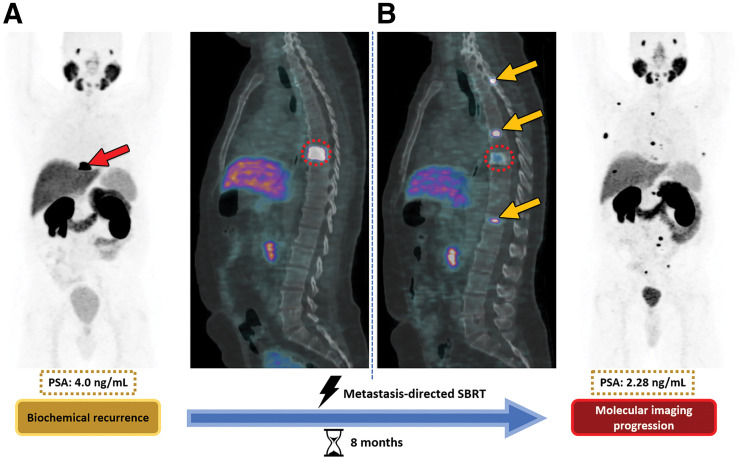
FIGURE 4.
Metachronous oligometastasis in 73-y-old man with BCR (PSA, 4.0 ng/mL) after prostatectomy and adjuvant radiation therapy for prostate cancer (Gleason score, 9; International Society of Urologic Pathologists grade, 5). (A) Baseline 68Ga-PSMA PET/CT shows single osseous lesion at T8 vertebral body (arrow in A, dotted circles in A and B). (B) Follow-up 68Ga-PSMA PET/CT obtained 8 mo after metastasis-directed SABR of T8 lesion shows partial radiographic local response of lesion but development of new lesions elsewhere in spine despite biochemical response, with overall PSA decline to 2.28 ng/mL. Arrows in B show new metastatic lesions in spine. Shown are maximum-intensity projections (first and fourth panels) and sagittal PET/CT images (second and third panels). SBRT = stereotactic body radiation therapy. (Reprinted with permission of (35)).
Aside from targeted therapy with SABR or radioligand, there is little consensus about the ideal type or duration of systemic therapy that should be given in addition to such targeted therapies. Generally, on the basis of data from biochemical or local relapse after primary therapy, most groups favor the addition of a least 4–6 mo of ADT to SABR or radioligand therapy even though prospective data are currently lacking (79). Extrapolation of data from trials such as STAMPEDE (Systemic Therapy for Advancing or Metastatic Prostate Cancer; NCT00268476), a multiarm, multistage randomized trial using abiraterone, is often used to support the addition of androgen receptor–targeted agents to ADT for treating OMPC (80). Proposed and ongoing trials will test the benefit of the addition of these and other novel agents.
CONCLUSION
OMPC is viewed as an opportunity for a potential cure or a delay in systemic therapy using MDT, essentially altering the disease trajectory. However, despite ongoing clinical interest and few randomized trials, the evidence remains immature because of the small number of patients, short follow-up durations, lack of harmonized endpoints across studies, and little information on the impact on cancer-specific survival. Furthermore, much yet needs to be learned about the underlying biology of the various forms of the oligometastatic clinical state, predictive and prognostic imaging and nonimaging biomarkers, and evidence-based treatment strategies, including integrated local (prostate bed and metastases) and systemic approaches, to establish OMPC firmly as a clinically distinct and actionable disease state in the management of patients with prostate cancer.
REFERENCES
- 1. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. [DOI] [PubMed] [Google Scholar]
- 2. Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6:8491–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jadvar H. Oligometastatic prostate cancer: molecular imaging and clinical management implications in the era of precision oncology. J Nucl Med. 2018;59:1338–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tosoian JJ, Gorin MA, Ross AE, et al. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel PH, Palma D, McDonald F, Tree AC. The dandelion dilemma revisited for oligoprogression: treat the whole lawn or weed selectively. Clin Oncol (R Coll Radiol). 2019;31:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lecouvet FE, Oprea-Lager DE, Liu Y, et al. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC imaging group. Lancet Oncol. 2018;19:e534–e545. [DOI] [PubMed] [Google Scholar]
- 7. deSouza NM, Liu Y, Chiti A, et al. Strategies and technical challenges for imaging oligometastatic disease: recommendations from the European Organization for Research and Treatment of Cancer imaging group. Eur J Cancer. 2018;91:153–163. [DOI] [PubMed] [Google Scholar]
- 8. Joice GA, Rowe SP, Pienta KJ, Gorin MA. Oligometastatic prostate cancer: shaping the definition with molecular imaging and an improved understanding of tumor biology. Curr Opin Urol. 2017;27:533–541. [DOI] [PubMed] [Google Scholar]
- 9. Gutiontov SI, Pitroda SP, Weichselbaum RR. Oligometastasis: past, present, future. Int J Radiat Oncol Biol Phys. 2020;108:530–538. [DOI] [PubMed] [Google Scholar]
- 10. Fraser M, Kontz B, Emmenegger U, et al. What is oligometastatic prostate cancer? Eur Urol Focus. 2019;5:159–161. [DOI] [PubMed] [Google Scholar]
- 11. Foster CC, Weichselbaum RR, Pitroda SP. Oligometastatic prostate cancer: reality or figment of imagination? Cancer. 2019;125:340–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morgans AK, Hussain M. Risk stratification in oligometastatic prostate cancer: where are we and what do we need? Curr Opin Urol. 2017;27:547–552. [DOI] [PubMed] [Google Scholar]
- 13. Sonpavde G. The biology of prostate cancer metastases: does oligo differ from polymetastatic? Curr Opin Urol. 2017;27:542–546. [DOI] [PubMed] [Google Scholar]
- 14. Van Etten JL, Dehm SM. Clonal origin and spread of metastatic disease. Endocr Relat Cancer. 2016;23:R207–R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hong MKH, Macintyre G, Wedge DC, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun. 2015;6:6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deek MP, Van der Eecken K, Phillips R, et al. The mutational landscape of metastatic castration-sensitive prostate cancer: the spectrum theory revisited. Eur Urol. 2021;80:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beltran H, Tomlins S, Aparacio A, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10:eaam7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corrao G, Zaffaroni M, Bergamaschi L, et al. Exploring miRNA signature and other potential biomarkers for oligometastatic prostate cancer characterization: the biological challenge behind clinical practice—a narrative review. Cancers (Basel). 2021;13:3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stelcer E. Konkol M, Glebka A, Suchorska WM. Liquid biopsy in oligometastatic prostate cancer: a biologist’s point of view. Front Oncol. 2019;9:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uppal A, Ferguson MK, Posner MC, et al. Towards a molecular basis of oligometastatic disease: potential role of micro-RNAs. Clin Exp Metastasis. 2014;31:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khodarev NN, Pitroda SP, Weichselbaum RR. microRNAs and oligometastases. Aging (Albany NY). 2015;7:146–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastases. PLoS One. 2011;6:e28650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dhondt B, De Bleser E, Claeys T, et al. Discovery and validation of a serum microRNA signature to characterize oligo- and polymetastatic prostate cancer: not ready for prime time. World J Urol. 2019;37:2557–2564. [DOI] [PubMed] [Google Scholar]
- 26. Savir-Baruch B, Choyke PL, Rowe SP, et al. Role of 18F-fluciclovine and prostate-specific membrane antigen PET/CT in guiding management of oligometastatic prostate cancer: AJR expert panel narrative review. AJR. 2021;216:851–859. [DOI] [PubMed] [Google Scholar]
- 27. Sollini M, Bartoli F, Cavinato L, et al. [18F]FMCH PET/CT biomarkers and similarity analysis to refine the definition of oligometastatic disease. EJNMMI Res. 2021;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herrera-Caceres JO, Gleave A. Lajkosz K, et a. Defining oligometastatic hormone sensitive prostate cancer and clinically significant outcomes: implications on clinical trials? Urol Oncol. 2021;39:431.e1–431.e8. [DOI] [PubMed] [Google Scholar]
- 29. Bae SH, Jang WI, Kang H-C, et al. Current usage of stereotactic body radiotherapy for oligometastatic prostate cancer in Korea: patterns of care survey (KROG 19-08). Ann Transl Med. 2021;9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aluwini SS, Mehra M, Lolkerma MP, et al. Oligometastatic prostate cancer: results of a Dutch multidisciplinary consensus meeting. Eur Urol Oncol. 2020;3:231–238. [DOI] [PubMed] [Google Scholar]
- 31. Deek MP, Phillips RM, Tran PT. Local therapies in oligometastatic and oligoprogressive prostate cancer. Semin Radiat Oncol. 2021;31:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Battaglia A, De Meerleer G, Tosco L, et al. Novel insights into the management of oligometastatic prostate cancer: a comprehensive review. Eur Urol Oncol. 2019;2:174–188. [DOI] [PubMed] [Google Scholar]
- 33. Murphy DG, Sweeney CJ, Tombal B. Gotta catch ‘em all’, or do we? Pokemet approach to metastatic prostate cancer. Eur Urol. 2017;72:1–3. [DOI] [PubMed] [Google Scholar]
- 34. Habl G, Straube C, Schiller K, et al. Oligometastases from prostate cancer: local treatment with stereotactic body radiotherapy (SBRT). BMC Cancer. 2017;17:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barbosa FG, Queiroz MA, Ferraro DA, et al. Prostate specific membrane antigen PET: therapy response assessment in metastatic prostate cancer. Radiographics. 2020;40:1412–1430. [DOI] [PubMed] [Google Scholar]
- 36. Connor MJ, Smith A, Miah S, et al. Targeting oligometastasis with stereotactic ablative radiation therapy or surgery in metastatic hormone-sensitive prostate cancer: a systematic review of prospective clinical trials. Eur Urol Oncol. 2020;3:582–593. [DOI] [PubMed] [Google Scholar]
- 37. Kucharczyk MJ, So J, Gravis G, et al. A combined biological and clinical rationale for evaluating metastasis directed therapy in the management of oligometastatic prostate cancer. Radiother Oncol. 2020;152:80–88. [DOI] [PubMed] [Google Scholar]
- 38. Privé BM, Janssen MJR, van Oort IM, et al. Lutetium-177-PSMA I&T as metastases directed therapy in oligometastatic hormone sensitive prostate cancer, a randomized controlled trial. BMC Cancer. 2020;20:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hijazi S, Meller B, Leitsmann C, et al. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA positron emission tomography/computed tomography. Prostate. 2015;75:1934–1940. [DOI] [PubMed] [Google Scholar]
- 40. Schick U, Jorcano S, Nouet P, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 2013;52:1622–1628. [DOI] [PubMed] [Google Scholar]
- 41. Marvaso G, Volpe S, Pepa M, et al. Oligorecurrent prostate cancer and stereotactic body radiotherapy: where are we now? A systematic review and meta-analysis of prospective studies. Eur Urol Open Sci. 2021;27:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vilela RA, Navarro NF, Faria ET, et al. Use of stereotactic body radiation therapy for oligometastatic recurrent prostate cancer: a systematic review. J Med Imaging Radiat Oncol. 2018;62:692–706. [DOI] [PubMed] [Google Scholar]
- 43. De Bleser E, Tran PT, Ost P. Radiotherapy as metastasis-directed therapy for oligometastatic prostate cancer. Curr Opin Urol. 2017;27:587–595. [DOI] [PubMed] [Google Scholar]
- 44. Saluja R, Cheung P, Zukotynski K, Emmenegger U. Disease volume and distribution as drivers of treatment decisions in metastatic prostate cancer: from chemohormonal therapy to stereotactic ablative radiotherapy of oligometastases. Urol Oncol. 2016;34:225–232. [DOI] [PubMed] [Google Scholar]
- 45. Ahmed KA, Barney BM, Davis BJ, et al. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front Oncol. 2013;2:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lehrer EJ, Singh R, Wang M, et al. Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer: systematic review and meta-analysis. JAMA Oncol. 2021;7:92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67:852–863. [DOI] [PubMed] [Google Scholar]
- 48. Yan M, Moideen N, Bratti VF, et al. Stereotactic body radiotherapy (SBRT) in metachronous oligometastatic prostate cancer: a systemic review and meta-analysis on the current prospective evidence. Br J Radiol. 2020;93:20200496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Viani GA, Arruda CV, Hamamura AC, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer recurrence: a meta-analysis. Am J Clin Oncol. 2020;43:73–81. [DOI] [PubMed] [Google Scholar]
- 50. Deek MP, Taparra K, Dao D, et al. Patterns of recurrence and modes of progression after metastasis-directed therapy in oligometastatic castration-sensitive prostate cancer. Int J Radiat Oncol Biol Phys. 2021;109:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmidt-Hegemann NS, Kroeze SGC, Henkenberens C, et al. Influence of localization of PSMA-positive oligometastases on efficacy of metastasis-directed external beam radiotherapy: a multicenter retrospective study. Eur J Nucl Med Mol Imaging. 2020;47:1852–1863. [DOI] [PubMed] [Google Scholar]
- 52. De Bruycker A, Tran PT, Achtman AH, et al. Clinical perspectives from ongoing trials in oligometastatic or oligorecurrent prostate cancer: an analysis of clinical trials registries. World J Urol. 2021;39:317–326. [DOI] [PubMed] [Google Scholar]
- 53. Sutera P, Phillips RM, Deek M, et al. The promise of metastasis-directed therapy for oligometastatic prostate cancer: going beneath the surface with molecular imaging. J Nucl Med. 2022;63:339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Phillips R, Shi WY, Deek M, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomised clinical trial. JAMA Oncol. 2020;6:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Siva S, Bressel M, Murphy DG, et al. Stereotactic ablative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74:455–462. [DOI] [PubMed] [Google Scholar]
- 56. Hasan H, Deek MP, Phillips R, et al. A phase II randomized trial of RAdium-223 dichloride and SABR Versus SABR for oligomEtastatic prostate caNcerS (RAVENS). BMC Cancer. 2020;20:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang H, Orme JJ, Abraha F, et al. Phase II evaluation of stereotactic ablative radiotherapy (SABR) and immunity in 11C-choline-PET/CT identified oligometastatic castration-resistant prostate cancer. Clin Cancer Res. 2021;27:6376–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gravis G, Autret A, Guibert-Broudic M, et al. Prognostic risk classification for biochemical relapse-free survival in oligometastatic recurrent prostate cancer determined by choline PET. Clin Genitourin Cancer. 2021;19:346–353. [DOI] [PubMed] [Google Scholar]
- 59. Pasqualetti F, Panichi M, Sollini M, et al. [18F]fluorocholine PET/CT-guided stereotactic body radiotherapy in patients with recurrent oligometastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bouman-Wammes EW, van Dodewaard-De Jong JM, Dahele M, et al. Benefits of using stereotactic body radiotherapy in patients with metachronous oligometastases of hormone-sensitive prostate cancer detected by [18F]fluoromethylcholine PET/CT. Clin Genitourin Cancer. 2017;15:e773–e782. [DOI] [PubMed] [Google Scholar]
- 61. Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. [DOI] [PubMed] [Google Scholar]
- 62. Deijen CL, Vrijenhoek GL, Schaake EE, et al. PSMA-11-PET/CT versus choline PET/CT to guide stereotactic ablative radiotherapy for androgen deprivation therapy deferral in patients with oligometastatic prostate cancer. Clin Transl Radiat Oncol. 2021;30:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cysouw M, Bouman-Wammes E, Hoekstra O, et al. Prognostic value of [18F]-fluoromethylcholine positron emission tomography/computed tomography before stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2018;101:406–410. [DOI] [PubMed] [Google Scholar]
- 64. Kim EH, Siegel BA, Teoh EJ, et al. Prostate cancer recurrence in patients with negative or equivocal conventional imaging: a role for 18F-fluciclovine-PET/CT in delineating sites of recurrence and identifying patients with oligometastatic disease. Urol Oncol 2021;39:365-e9-365.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vapiwala N, Chen Y-H, Cho SY, et al. PET-directed local or systemic therapy intensification in prostate cancer patients with post-prostatectomy biochemical recurrence: a trial of the ECOG-ACRIN cancer research group (EA8191) [abstract]. J Clin Oncol. 2021;39(suppl): TPS267. [Google Scholar]
- 66. Anderson R-C, Velez EM, Jadvar H. Management impact of metachronous oligometastatic disease identified on 18F-fluciclovine (AXUMINTM) PET/CT in biochemically recurrent prostate cancer. Mol Imaging Biol. May 23, 2022. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 67. Ong WL, Koh TL, Lim Joon D, et al. Prostate-specific membrane antigen-positron emission tomography/computed tomography (PSMA PET/CT) guided stereotactic ablative body radiotherapy for oligometastatic prostate cancer: a single-institution experience and review of the published literature. BJU Int. 2019;124(suppl 1):19–30. [DOI] [PubMed] [Google Scholar]
- 68. Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol. 2018;1:531–537. [DOI] [PubMed] [Google Scholar]
- 69. Artigas C, Flamen P, Charlier F, et al. 68Ga-PSMA PET/CT-based metastasis-directed radiotherapy for oligometastatic prostate cancer recurrence after radical prostatectomy. World J Urol. 2019;37:1535–1542. [DOI] [PubMed] [Google Scholar]
- 70. Koerber SA, Sprute K, Kratochwil C, et al. Clinical outcome of PSMA-guided radiotherapy for patients with oligorecurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McCarthy M, Francis R, Tang C, et al. A multicenter prospective clinical trial of 68gallium PSMA HBED-CC PET-CT restaging in biochemically relapsed prostate carcinoma: oligometastatic rate and distribution compared with standard imaging. Int J Radiat Oncol Biol Phys. 2019;104:801–808. [DOI] [PubMed] [Google Scholar]
- 72. Glicksman RM, Metser U, Vines D, et al. Curative-intent metastasis-directed therapies for molecularly-directed oligorecurrent prostate cancer: oligometastasis hypothesis. Eur Urol. 2021;80:374–382. [DOI] [PubMed] [Google Scholar]
- 73. Onal C, Ozyigit G, Akgun Z, et al. Oligometastatic bone disease in castration-sensitive prostate cancer patients treated with stereotactic body radiotherapy using 68Ga-PSMA PET/CT: TROD 09-004 study. Clin Nucl Med. 2021;46:465–470. [DOI] [PubMed] [Google Scholar]
- 74. Hurmuz P, Onal C, Ozyigit G, et al. Treatment outcomes of metastasis-directed treatment using 68Ga-PSMA PET/CT for oligometastatic or oligorecurrent prostate cancer: Turkish Society for Radiation Oncology group study (TROD 09-002). Strahlenther Onkol. 2020;196:1034–1043. [DOI] [PubMed] [Google Scholar]
- 75. Holscher T, Baumann M, Kotzerke J, et al. Toxicity and efficacy of local ablative, image-guided radiotherapy in gallium-68 prostate-specific membrane antigen targeted positron emission tomography-staged, castration-sensitive oligometastatic prostate cancer: the OLI-P phase 2 clinical trial. Eur Urol Oncol 2021;5:44–51. [DOI] [PubMed] [Google Scholar]
- 76. Farolfi A, Hadaschik B, Hamdy FC, et al. Positron emission tomography and whole-body magnetic resonance imaging for metastasis-directed therapy in hormone-sensitive oligometastatic prostate cancer after primary radical treatment: a systematic review. Eur Urol Oncol. 2021;4:714–730. [DOI] [PubMed] [Google Scholar]
- 77. Vogel MME, Kroeze SG, Henkenberens C, et al. Prognostic risk classification for biochemical relapse-free survival in patients with oligorecurrent prostate cancer after [68Ga]PSMA-PET-guided metastasis-directed therapy. Eur J Nucl Med Mol Imaging. 2020;47:2328–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Privé BM, Janssen MJR, van Oort IM, et al. Update to a randomized controlled trial of lutetium-177-PSMA in oligometastatic hormone-sensitive prostate cancer: the BULLSEYE trial. Trials. 2021;22:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rosenthal SA, Hu C, Sartor O, et al. Effect of chemotherapy with docetaxel with androgen suppression and radiotherapy for localized high-risk prostate cancer: the randomized phase III NRG oncology RTOG 0521 trial. J Clin Oncol. 2019;37:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Attard G, Sydes MR, Mason MD, et al. Combining enzalutamide with abiraterone, prednisone, and androgen deprivation therapy in the STAMPEDE trial. Eur Urol. 2014;66:799–802. [DOI] [PubMed] [Google Scholar]