Abstract
Background
Neoadjuvant chemoradiotherapy (nCRT) before total mesorectal excision (TME) and followed systemic chemotherapy is widely accepted as the standard therapy for locally advanced rectal cancer (LARC). This meta-analysis was to evaluate the current evidence regarding nCRT in combination with induction or consolidation chemotherapy for rectal cancer in terms of oncological outcomes.
Methods
A systematic search of medical databases (PubMed, EMBASE and Cochrane Library) was conducted up to the end of July 1, 2021. This meta-analysis was performed to evaluate the efficacy of TNT in terms of pathological complete remission (pCR), nCRT or surgical complications, R0 resection, local recurrence, distant metastasis, disease-free survival (DFS) and overall survival (OS) in LARC.
Results
Eight nRCTs and 7 RCTs, including 3579 patients were included in the meta-analysis. The rate of pCR was significantly higher in the TNT group than in the nCRT group, (OR 1.85, 95% CI 1.39–2.46, p < 0.0001), DFS (HR 0.80, 95% CI 0.69–0.92, p = 0.001), OS (HR 0.75, 95% CI 0.62–0.89, p = 0.002), nCRT complications (OR 1.05, 95% CI 0.77–1.44, p = 0.75), surgical complications (OR 1.02, 95% CI 0.83–1.26, p = 0.83), local recurrence (OR 1.82, 95% CI 0.95–3.49, p = 0.07), distant metastasis (OR 0.77, 95% CI 0.58–1.03, p = 0.08) did not differ significantly between the TNT and nCRT groups.
Conclusion
TNT appears to have advantages over standard therapy for LARC in terms of pCR, R0 resection, DFS, and OS, with comparable nCRT and postoperative complications, and no increase in local recurrence and distant metastasis.
Introduction
Neoadjuvant chemoradiotherapy (nCRT) before total mesorectal excision (TME) and followed systemic chemotherapy is widely accepted as the standard therapy for locally advanced rectal cancer (LARC). Although nCRT and preoperative systemic chemotherapy are the preferred approach for LARC, it does not provide better results in terms of disease-free survival (DFS) and overall survival (OS) compared with surgery and adjuvant chemotherapy [1]. The concept of total neoadjuvant therapy (TNT), in which chemotherapy and chemoradiation are administered before TME, can be administered exclusively for patients with widespread LARC [2, 3]. TNT has also become a platform for studying systemic chemotherapy, new radiation sensitizers and immunotherapy agents for LARC [4, 5]. The NCCN guidelines have approved the use of TNT [6], however, the current evidence is preliminary, we tried to further consolidate the evidence through meta-analysis of related studies.
Methods
Literature search strategy
A systematic search of medical databases (PubMed, EMBASE and Cochrane Library) was conducted up to the end of July 1, 2021. We used the following search MeSH terms: neoadjuvant therapy, neoadjuvant chemoradiotherapy, preoperative chemoradiotherapy, neoadjuvant chemoradiation, chemoradiotherapy, total neoadjuvant therapy, total mesorectal excision, rectal cancer. We also searched bibliographies of identified reports for additional references.
Study inclusion and exclusion criteria
Studies inclusion criteria in the meta-analysis were list as follow: (a) randomized controlled trials (RCTs) or non-randomized controlled trials (nRCTs), (b) TNT versus nCRT in LARC, (c) interest outcomes describing the details of pCR, chemoradiotherapy or surgical complications, R0 resection, local recurrence, distant metastasis, DFS and OS. Review/case reports, no comparable data, repeat publications were excluded.
Data extraction and quality assessment
Two authors performed study selection, evaluation, and data extraction independently, and discrepancies were resolved by consensus including a third author. The primary endpoint was pCR, The secondary endpoints were chemoradiotherapy or surgical complications, R0 resection, local recurrence, distant metastasis, DFS and OS. Study characteristics were extracted independently by two researchers. Corresponding authors were contacted via e-mail for missing data when necessary. The methodological quality of the included studies was assessed using the Jadad Score [total score from 0 (poor) to 5 (excellent)] for RCT or the Newcastle-Ottawa scale (NOS) [total score from 0 (poor) to 9 (excellent)] for nRCTs [7, 8]. A Jadad scale score≥3 points for RCTs and NOS score≥6 points for nRCTs were considered high quality.
Statistical analysis
Continuous variables were analysed using weighted mean differences (WMD) and 95% confidence intervals (CIs). Pooled odds ratios (ORs) and 95% CIs were calculated for dichotomous variables. Statistical heterogeneity between trials was assessed by using the I2 test, a value of 50% or greater suggests moderate to substantial inconsistency among studies. Random effects models were used if high heterogeneity between study existed, Otherwise, fixed-effects models were used. Subgroup analysis and sensitivity analysis was performed to identify potential heterogeneity [9, 10]. Publication bias was investigated using Begg’s and Egger’s tests [11]. All statistical tests were performed using Review Manager Version 5.3 and STATA/SE version 13.1. The significance level was set at p < 0.05.
Results
Fig 1 shows the selection of relevant studies for inclusion in the meta-analysis. A total of 8 nRCTs with 1502 and 7 RCTs with 2077 enrolled patients enrolled patients were included for this meta-analysis [12–26] (Fig 1), there were 1812 patients in the TNT group and 1767 patients in the nCRT group. The main characteristics of the included studies are summarized in Table 1.
Fig 1. Flow chart indicating the selection process for this meta-analysis.
Table 1. Characteristics of the studies included in the meta-analysis.
| First author/Year | Study design | CRTvsTNT | TNT regimen | CRT regimen | Follow-up (months) | Study quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Male gender (%) | Age (year) (mean ± SD) | T4 | N2 | CRM+ | RT Dose (Gys) | ||||||
| Marechal R/2012 | RCT | 29vs28 | 75vs55 | 62.5±8.8vs62 ±14.5 | NR | NR | NR | 45vs45 | FOLFOX *2→CRT→Sur | CRT(5FU)→Sur | NR | −/3 |
| Fernandez C/2015 | RCT | 62vs60 | 70vs65 | 62±8.3vs60±9.5 | 3vs2 | NR | 5vs- | NR | XELOX * 4→CRT→Sur | CRT→Sur→CT | 69 | −/3 |
| Bujko K/2016 | RCT | 254vs261 | 67vs70 | NR | NR | NR | NR | 50.4vs25 | SCRT→FOLFOX *3→Sur | CRT→ Sur | 35 | −/4 |
| Moore J/2017 | RCT | 24Vs25 | 75vs73 | 60.5 ±12.6 vs59.7 ±9.9 | 163vs165 | NR | NR | NR | CRT→5FU * 3→Sur | CRT→Sur | NR | −/4 |
| Kim SY/2018 | RCT | 55Vs53 | 84vs68 | 55± 8 vs56± 10 | 1vs5 | 19vs15 | 14vs16 | 50.4vs50.4 | CRT→XELOX *2→Sur | CRT→Sur | NR | −/4 |
| Conroy T/2020 | RCT | 230Vs231 | NR | NR | 10vs9 | NR | NR | 50.4vs50.4 | FOLFIRINOX*6→CRT→Sur | CRT→Sur→ FOLFOX | 46.5 | −/3 |
| Bahadoer RR/2021 | RCT | 450vs462 | 69vs65 | 62±2.2vs62±2.3 | 35vs41 | NR | NR | 25vs50.4 | CRT→XELOX *6/→Sur | CRT→Sur→ CT | 54 | −/4 |
| Cui J/2020 | Re | 61vs83 | 56vs69 | 56 ±13.5vs54 ±12 | 20vs15 | NR | NR | NR | CRT→XELOX * 2→Sur | NR | NR | 7★/− |
| Liang HQ/2018 | Re | 80vs76 | NR | 64vs75 | 44vs50 | 11vs21 | NR | NR | CRT→mFOLFOX *2–4→Sur | CRT→Sur | 32vs30 | 6★/− |
| Cercek A/2018 | Re | 320vs308 | 60vs59 | NR | 57vs62 | 45vs36 | NR | NR | NR | NR | 46vs23 | 7★/− |
| Bhatti AB /2015 | Re | 61vs93 | 69vs68 | NR | 20vs36 | NR | 8vs17* | 50.4vs50.4 | mFOLFOX6 *4→ CRT→Sur | CRT→Sur | NR | 8★/− |
| Thakur N./2020 | Pro | 13vs15 | -NR | NR | NR | NR | NR | 25vs25 | CRT→mFOLFOX6 *2→Sur | CRT→Sur | NR | 8★/− |
| van Zoggel D/2018 | Pro | 71vs58 | 57Vs79 | 65±9vs64 ±10 | NR | NR | NR | NR | FOLFOX *4→ CRT→Sur | CRT→Sur | NR | 7★/− |
| Garcia-Aguilar J/2015 | Pro | 60vs65 | 62vs63 | 57 ±13.3vs58 ±9.8 | NR | NR | NR | NR | CRT→mFOLFOX6 *6→Sur | CRT→Sur | NR | 7★/− |
| Markovina S/2017 | Pro | 69vs69 | 67vs71 | 56.6 ±12.9vs57.2 ±12.6 | 1vs3 | NR | NR | NR | CRT→mFOLFOX6 →Sur | NR | 54.3 vs49.4 | 8★/− |
★ number of stars for Nottingham Ottawa scale for each included trial,* Postradiotherapy, NOS = Nottingham-Ottawa scale, CRT = Chemoradiotherapy, CT = chemotherapy, TNT = total neoadjuvant therapy, Pro = prospective, Sur = surgery; Re = retrospective, RCTs = randomized controlled trial, NR = no report.
Primary endpoints
pCR
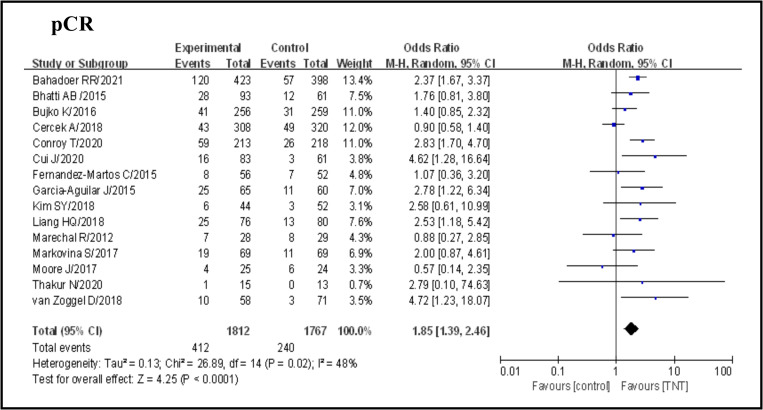
Overall, including the 15 studies selected, 652 of 3579 patients (18.2%) achieved a pCR after neoadjuvant therapy, 412 of 1812 (22.7%) in the TNT group and 240 of 1767 (13.6%) in the standard group (OR 1.85, 95% CI 1.39–2.46, p < 0.0001), with certain statistical heterogeneity (I2 = 48%, p = 0.02)(Fig 2). A subgroup analysis showed that single-center or multi-center studies, sample size, RCT or nRCT, neoadjuvant therapy were not causes of heterogeneity in view of the dissimilarity between subgroups (Table 2). The sensibility analysis performed showed stability of the pCR when excluding each study at a time (Table 3).
Fig 2. Forest plot for pCR, OR are shown with 95% confidence interval.
Table 2. Subgroup analyses of PCR based on the study type, setting, sample size and chemoradiotherapy sequence.
| Outcome | Subgroup | No. of studies | No. of patients | Study Heterogeneity | Model | Meta-analysis | |||
|---|---|---|---|---|---|---|---|---|---|
| TNTvsCRT | I 2 (%) | P Value | OR (95%CI) | P Value | |||||
| pCR | Study type | RCT | 7 | 1045vs 1032 | 45 | 0.09 | Fixed | 1.75 [1.21, 2.54] | 0.003 |
| Prospective | 4 | 207 vs 213 | 0 | 0.76 | Fixed | 2.65 [1.56, 4.50] | 0.0003 | ||
| Retrospective | 4 | 560 vs 522 | 70 | 0.02 | Random | 1.42 [1.03, 1.96] | 0.03 | ||
| Setting | Single-center | 7 | 702vs 675 | 55 | 0.04 | Random | 2.01 [1.20, 3.39] | 0.009 | |
| Multi-center | 8 | 1117vs1095 | 32 | 0.20 | Fixed | 2.19 [1.62, 2.50] | < 0.00001 | ||
| Sample size | ≥200 | 4 | 1200 vs 1195 | 81 | 0.001 | Random | 1.71 [1.02, 2.84] | 0.04 | |
| < 200 | 11 | 619 vs 565 | 0 | 0.50 | Fixed | 2.24 [1.62, 2.91] | < 0.00001 | ||
| Chemoradiotherapy | CT first | 4 | 498 vs 381 | 31 | 0.19 | Fixed | 2.23 [1.61, 3.27] | < 0.0001 | |
| Radiation first | 11 | 1421 vs 1389 | 49 | 0.03 | Random | 1.81 [1.30, 2.51] | 0.0004 | ||
CRT = Chemoradiotherapy, TNT = total neoadjuvant therapy, OR = odds ratio, CI = confidence interval.
Table 3. Sensitivity analysis for pathological complete response.
| Study Excluded | Random Effect | Heterogeneity | |||
|---|---|---|---|---|---|
| OR | 95% CI | p Value | I2 (%) | I2- P Value | |
| van Zoggel D/2018 | 1.79 | 1.34, 2.38 | < 0.00001 | 48 | 0.02 |
| Garcia-Aguilar J/2015 | 1.80 | 1.33, 2.42 | 0.0001 | 50 | 0.02 |
| Markovina S/2017 | 1.84 | 1.36, 2.50 | < 0.0001 | 52 | 0.01 |
| Thakur N/2020 | 1.85 | 1.38, 2.47 | < 0.0001 | 52 | 0.01 |
| Bhatti AB /2015 | 1.86 | 1.37, 2.53 | < 0.0001 | 52 | 0.01 |
| Cercek A/2018 | 2.08 | 1.66, 2.61 | < 0.00001 | 13 | 0.31 |
| Cui J/2020 | 1.79 | 1.34, 2.38 | < 0.0001 | 48 | 0.02 |
| Liang HQ/2018 | 1.81 | 1.33, 2.45 | 0.0001 | 50 | 0.02 |
| Bahadoer RR/2021 | 1.79 | 1.30, 2.45 | 0.0003 | 46 | 0.03 |
| Bujko K/2016 | 1.92 | 1.40, 2.62 | < 0.0001 | 49 | 0.02 |
| Conroy T/2020 | 1.76 | 1.30, 2.37 | 0.0002 | 45 | 0.03 |
| Fernandez-Martos C/2015 | 1.91 | 1.42, 2.56 | < 0.0001 | 50 | 0.02 |
| Kim SY/2018 | 1.83 | 1.36, 2.46 | < 0.0001 | 51 | 0.01 |
| Marechal R/2012 | 1.92 | 1.43, 2.56 | < 0.0001 | 49 | 0.02 |
| Moore J/2017 | 1.92 | 1.45, 2.55 | < 0.00001 | 46 | 0.03 |
OR = odds ratio; CI = confidence interval.
Secondary endpoints
Chemoradiotherapy complications
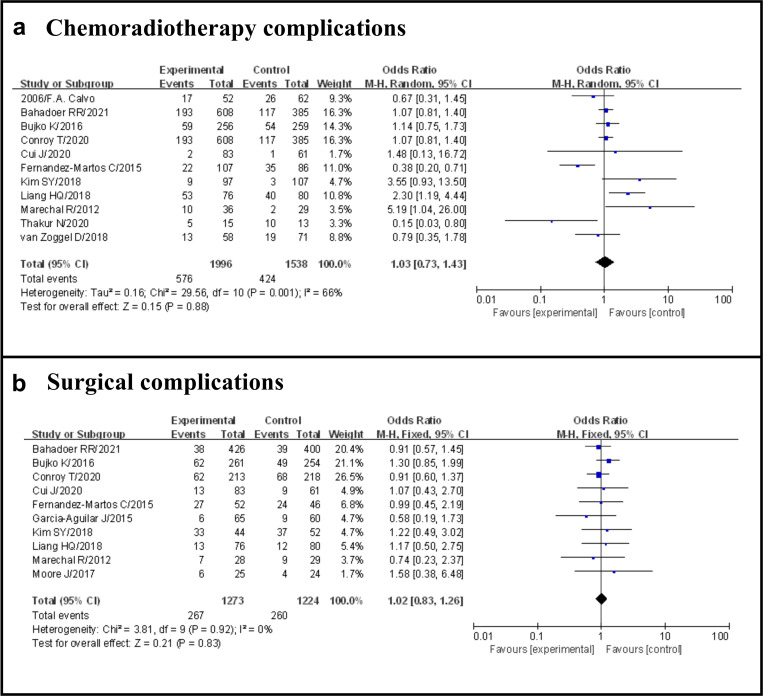
Overall, including the 11 studies selected, 1000 of 3534 patients (28.3%) occurred G3–4 adverse events after neoadjuvant therapy, 576 of 1996 (28.9%) in the TNT group and 424 of 1538 (27.6%) in the standard group (OR 1.03, 95% CI 0.73–1.43, p = 0.88), with certain statistical heterogeneity (I2 = 66%, p = 0.001) (Fig 3A).
Fig 3. Forest plot for chemoradiotherapy complications and surgical complications, OR are shown with 95% CIs.
Surgical complications
Overall, including the 10 studies selected, 527 of 2497 patients (21.1%) occurred surgical complications, 267 of 1273 (21.0%) in the TNT group and 260 of 1224 (21.2%) in the standard group (OR 1.02, 95% CI 0.83–1.26, p = 0.83), with no evidence of significant heterogeneity (I2 = 0%, p = 0.92) (Fig 3B).
R0 resection
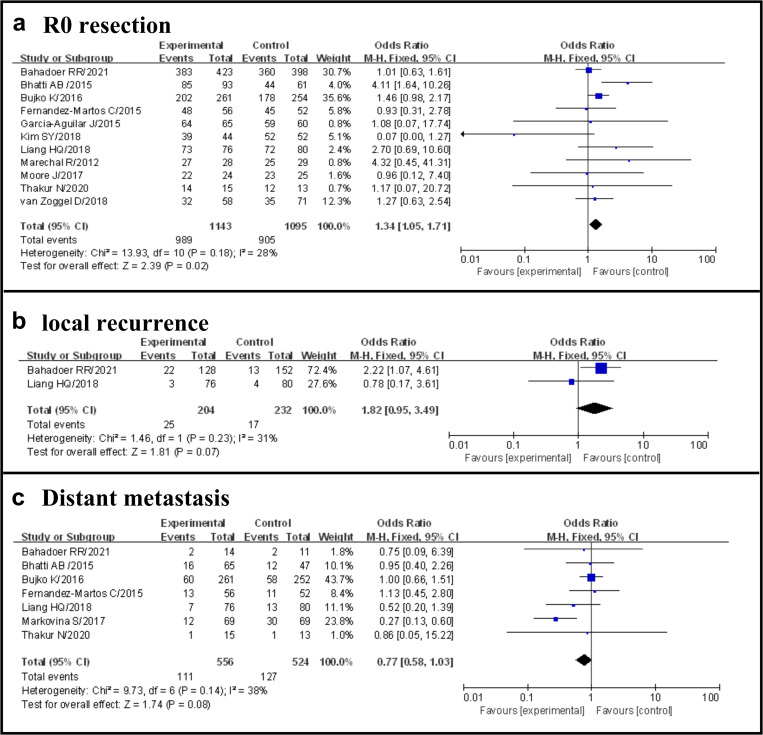
Overall, including the 11 studies selected, 1894 of 2238 patients (84.6%) achieved R0 resection, 989 of 1143 (86.5%) in the TNT group and 905 of 1095 (82.6%) in the standard group (OR 1.34, 95% CI 1.05–1.71, p = 0.02), with no evidence of significant heterogeneity (I2 = 28%, p = 0.18) (Fig 4A).
Fig 4. Forest plot for R0 resection and distant metastasis, OR are shown with 95% Cis.
Local recurrence
Overall, including the two studies selected, 42 of 436 patients (9.6%) occurred local recurrence, 25 of 204 (12.2%) in the TNT group and 17 of 232 (7.3%) in the standard group (OR 1.82, 95% CI 0.95–3.49, p = 0.07), with no evidence of significant heterogeneity (I2 = 31%, p = 0.23) (Fig 4B).
Distant metastasis
Overall, including the 7 studies selected, 238 of 1080 patients (22.0%) occurred distant metastasis, 111 of 556 (20.0.5%) in the TNT group and 127 of 524 (24.2%) in the standard group (OR 0.77, 95% CI 0.58–1.03, p = 0.08), with no evidence of significant heterogeneity (I2 = 38%, p = 0.14) (Fig 4C).
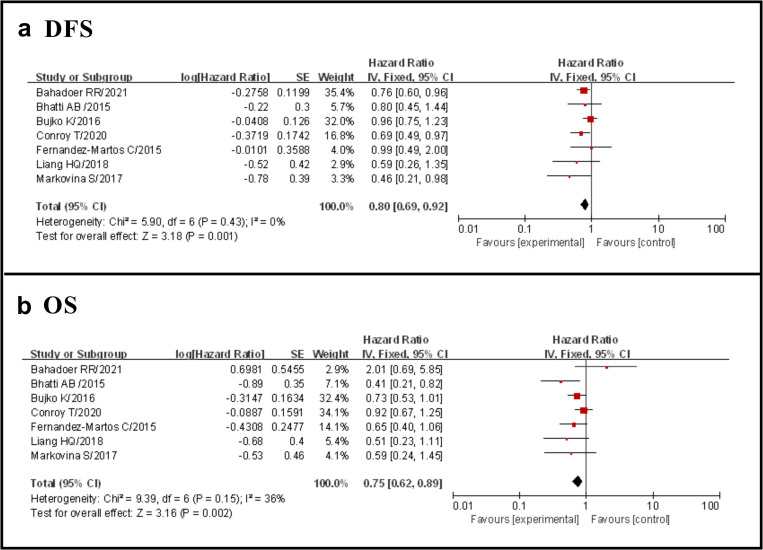
DFS
Seven studies reported DFS and the results showed that the TNT group had a significantly longer DFS than the standard group (HR 0.80, 95% CI 0.69–0.92, p = 0.001), with no evidence of significant heterogeneity (I2 = 0%, p = 0.43) (Fig 5A).
Fig 5. Forest plot for DFS and OS, HR are shown with 95% Cis.
OS
Seven studies reported OS and the results showed that the TNT group had a significantly longer OS than the standard group (HR 0.75, 95% CI 0.62–0.89, p = 0.002), with no evidence of significant heterogeneity (I2 = 36%, p = 0.15) (Fig 5B).
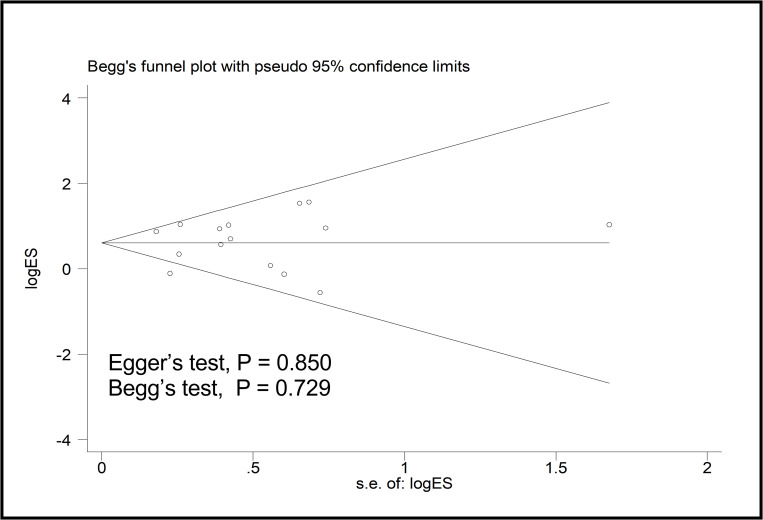
Sensitivity analysis and publication bias
The sensibility analysis performed showed stability of the pooled OR with pCR when excluding each study at a time. There was no significant publication bias in the analyses of pCR (Egger’s test, p = 0.850; Begg’s test, p = 0.729) (Fig 6).
Fig 6. Funnel plot using Begg method for pCR.
Discussion
In the meta-analysis, we compared the efficacy of TNT with that of standard therapy for LARC. TNT appears to have advantages over standard therapy for LARC in terms of pCR, R0 resection, DFS, and OS, with comparable nCRT and postoperative complications, and no increase in local recurrence and distant metastasis.
In this meta-analysis, we observed that the overall pCR rate of TNT may increase compared with chemoradiotherapy. Our results were consistent with previous reports [27]. However, our meta-analysis included more and newer studies, which further confirmed the advantage of TNT in rectal cancer. In the review of 16 studies involving a total of 3363 patients, patients who experienced pCR had a 75%, lower risk of distant (8.7%) and local (0.7%) recurrence than patients who did not [28]. Compared with patients with incomplete response, patients with pCR after neoadjuvant therapy are less likely to have local recurrence and more likely to have better survival outcomes [29]. pCR is considered to be the key prognostic criterion for the long-term prognosis of LARC [30]. The use of TNT can significantly reduce tumor volume, which may lead to more patients adopting non-surgical observation and waiting strategies in the future. Habr-Gama et al introduced the watch-and-wait strategy [31], they reported that after 10 years of follow-up, patients who gave up surgery after obtaining complete clinical response had an OS rate of 97.7% and a DFS rate of 84%. pCR for rectal cancer is associated with excellent long-term prognosis, independent of treatment strategy. Surgical resection may not improve outcomes while increasing the incidence of temporary or permanent stoma and unnecessary morbidity and mortality. Another potential advantage of simultaneous chemotherapy and radiochemotherapy is that it can avoid or delay surgery when a complete clinical response is observed. The watch-and-wait approach may be considered a better treatment strategy, because surgery may lead to intestinal or bladder incontinence and sexual dysfunction, as well as short-term or permanent stoma [32–35].
Adjuvant chemotherapy is recommended by current guidelines, however, patient compliance is poor and survival benefit is unclear. Although the nCRT approach has made significant improvements in local control, the incidence of distal metastasis has not decreased. In our meta-analysis, the rate of DFS and OS was significantly higher in the TNT group than in the nCRT group, although studies have shown that watch-and-wait approach is associated with higher rectal preservation, which may be at the expense of lower OS rates and increased risk of distant metastasis [36]. In addition, treatment-related toxicity appeared to be acceptable in all patients who completed TNT therapy. Although TNT was associated with an increased incidence of pelvic fibrosis and a longer interval from nCRT to surgery, TNT did not lead to an increase in surgical difficulty or postoperative complications [17]. However, these results should be interpreted with caution, as the lack of standardization of postoperative morbidity reports limits the interpretation of the results. Another disadvantage is the short follow-up period, so the assessment of long-term distant metastases needs to be cautious.
Assessment of quality of life (QOL) was not included in this meta-analysis, in the EXPERT-C trial, intensified neoadjuvant strategy for QoL and bowel function did not appear to be significantly affected [37]. This study was conducted at an appropriate time, because enough data was available to use meta-analytical methods which enabled us to provide the most up-to-date information on this topic.
Our study has the following limitations. First, although the meta-analysis including RCT is ideal, the limited number of RCTs prevents us from drawing clear conclusions. Second, the follow-up included in this meta-analysis is short, and the long-term survival benefit remains to be confirmed. However, this meta-analysis was completed at the appropriate time, and we provide the latest information in this area.
In conclusion, TNT appears to have advantages over standard therapy for LARC in terms of pCR, R0 resection, DFS, and OS, with comparable nCRT and postoperative complications, and no increase in local recurrence and distant metastasis. If these findings can be applied clinically, more patients with LARC will be eligible for organ preservation, which will avoid surgical sequelae and improve quality of life.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper.
Funding Statement
the Natural Science Foundation of Gansu Province (No.20JR10RA371, L.LY), Fundamental Research Funds for the Central Universities (No.lzujbky-2019-kb21 L.L.Y & No.lzujbky-2020-kb22 L.Y), and Institute Scientific Research Fund Project/Youth Project(No. 20GSSY4-8 L.L.Y). The funders (Laiyuan Li and Yang Luo) had a role in study design, data collection, analysis, the decision to publish, and preparation of the manuscript.
References
- 1.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355(11): 1114–23. doi: 10.1056/NEJMoa060829 [DOI] [PubMed] [Google Scholar]
- 2.Chau I, Brown G, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol 2006; 24(4): 668–74. doi: 10.1200/JCO.2005.04.4875 [DOI] [PubMed] [Google Scholar]
- 3.Cercek A, Roxburgh CSD, Strombom P, et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol 2018; 4(6): e180071. doi: 10.1001/jamaoncol.2018.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman KA. Total neoadjuvant therapy for rectal cancer. Cancer Radiother 2018; 22(5): 459–65. doi: 10.1016/j.canrad.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 5.Franke AJ, Parekh H, Starr JS, Tan SA, Iqbal A, George TJ Jr. Total Neoadjuvant Therapy: A Shifting Paradigm in Locally Advanced Rectal Cancer Management. Clin Colorectal Cancer 2018; 17(1): 1–12. doi: 10.1016/j.clcc.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. NCCN guidelines: rectal cancer. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1461. Accessed Version 1.2021.
- 7.Clark HD, Wells GA, Huet C, et al. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials 1999; 20(5): 448–52. doi: 10.1016/s0197-2456(99)00026-4 [DOI] [PubMed] [Google Scholar]
- 8.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25(9): 603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 9.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22(4): 719–48. [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7(3): 177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 11.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ 1997; 315(7121): 1533–7. doi: 10.1136/bmj.315.7121.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy T., Lamfichekh N., Etienne P., E, et al. Total neoadjuvant chemotherapy with mFOLFIRINOX versus preoperative chemo-radiation in patients with locally advanced rectal cancer: Final results of the PRODIGE 23 phase III trial, a UNICANCER GI trial. J. Clin. Oncol. 2020, 38, 4007. 2020. [Google Scholar]
- 13.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22(1): 29–42. doi: 10.1016/S1470-2045(20)30555-6 [DOI] [PubMed] [Google Scholar]
- 14.Bhatti AB, Waheed A, Hafeez A, et al. Can induction chemotherapy before concurrent chemoradiation impact circumferential resection margin positivity and survival in low rectal cancers? Asian Pac J Cancer Prev 2015; 16(7): 2993–8. doi: 10.7314/apjcp.2015.16.7.2993 [DOI] [PubMed] [Google Scholar]
- 15.Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 x 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 2016; 27(5): 834–42. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trialdagger. Ann Oncol 2015; 26(8): 1722–8. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015; 16(8): 957–66. doi: 10.1016/S1470-2045(15)00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SY, Joo J, Kim TW, et al. A Randomized Phase 2 Trial of Consolidation Chemotherapy After Preoperative Chemoradiation Therapy Versus Chemoradiation Therapy Alone for Locally Advanced Rectal Cancer: KCSG CO 14–03. Int J Radiat Oncol Biol Phys 2018; 101(4): 889–99. doi: 10.1016/j.ijrobp.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 19.Liang HQ, Dong ZY, Liu ZJ, et al. Efficacy and safety of consolidation chemotherapy during the resting period in patients with local advanced rectal cancer. Oncol Lett 2019; 17(2): 1655–63. doi: 10.3892/ol.2018.9804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marechal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol 2012; 23(6): 1525–30. doi: 10.1093/annonc/mdr473 [DOI] [PubMed] [Google Scholar]
- 21.Markovina S, Youssef F, Roy A, et al. Improved Metastasis- and Disease-Free Survival With Preoperative Sequential Short-Course Radiation Therapy and FOLFOX Chemotherapy for Rectal Cancer Compared With Neoadjuvant Long-Course Chemoradiotherapy: Results of a Matched Pair Analysis. Int J Radiat Oncol Biol Phys 2017; 99(2): 417–26. doi: 10.1016/j.ijrobp.2017.05.048 [DOI] [PubMed] [Google Scholar]
- 22.Moore J, Price T, Carruthers S, et al. Prospective randomized trial of neoadjuvant chemotherapy during the ’wait period’ following preoperative chemoradiotherapy for rectal cancer: results of the WAIT trial. Colorectal Dis 2017; 19(11): 973–9. doi: 10.1111/codi.13724 [DOI] [PubMed] [Google Scholar]
- 23.van Zoggel D, Bosman SJ, Kusters M, et al. Preliminary results of a cohort study of induction chemotherapy-based treatment for locally recurrent rectal cancer. Br J Surg 2018; 105(4): 447–52. doi: 10.1002/bjs.10694 [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 2010; 28(5): 859–65. doi: 10.1200/JCO.2009.25.8541 [DOI] [PubMed] [Google Scholar]
- 25.Cui J, Dou X, Sun Y, Yue J. Consolidation chemotherapy may improve pathological complete response for locally advanced rectal cancer after neoadjuvant chemoradiotherapy: a retrospective study. PeerJ 2020; 8: e9513. doi: 10.7717/peerj.9513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thakur N, Seam RK, Gupta MK, et al. A Prospective Observational Study Comparing Long-Course Conventional Neoadjuvant Chemoradiotherapy with Short-Course Radiotherapy Followed by Consolidation Chemotherapy with Delayed Surgery in Locally Advanced Rectal Cancer. South Asian J Cancer 2020; 9(2): 80–5. doi: 10.1055/s-0040-1721220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Jiang T, Xiao L, et al. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021; 26(9): e1555–e66. doi: 10.1002/onco.13824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 2012; 99(7): 918–28. doi: 10.1002/bjs.8702 [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum 2003; 46(3): 298–304. doi: 10.1007/s10350-004-6545-x [DOI] [PubMed] [Google Scholar]
- 30.Omejc M, Potisek M. Prognostic Significance of Tumor Regression in Locally Advanced Rectal Cancer after Preoperative Radiochemotherapy. Radiol Oncol 2018; 52(1): 30–5. doi: 10.1515/raon-2017-0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004; 240(4): 711–7; discussion 7–8. doi: 10.1097/01.sla.0000141194.27992.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun V, Grant M, Wendel CS, et al. Sexual Function and Health-Related Quality of Life in Long-Term Rectal Cancer Survivors. J Sex Med 2016; 13(7): 1071–9. doi: 10.1016/j.jsxm.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thyo A, Emmertsen KJ, Laurberg S. The Rectal Cancer Female Sexuality Score: Development and Validation of a Scoring System for Female Sexual Function After Rectal Cancer Surgery. Dis Colon Rectum 2018; 61(6): 656–66. doi: 10.1097/DCR.0000000000001064 [DOI] [PubMed] [Google Scholar]
- 34.Lewis OA, McCallum IJ, Dixon S, Katory M. Longterm -ostomy as a quality marker: Comparison of outcomes from a six year series of laparoscopic surgery in MRI defined low rectal cancer. Int J Surg 2015; 23(Pt A): 108–14. doi: 10.1016/j.ijsu.2015.09.054 [DOI] [PubMed] [Google Scholar]
- 35.Wu X, Lin G, Qiu H, Xiao Y, Wu B, Zhong M. Loop ostomy following laparoscopic low anterior resection for rectal cancer after neoadjuvant chemoradiotherapy. Eur J Med Res 2018; 23(1): 24. doi: 10.1186/s40001-018-0325-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JJ, Strombom P, Chow OS, et al. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol 2019; 5(4): e185896. doi: 10.1001/jamaoncol.2018.5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sclafani F, Peckitt C, Cunningham D, et al. Short- and Long-Term Quality of Life and Bowel Function in Patients With MRI-Defined, High-Risk, Locally Advanced Rectal Cancer Treated With an Intensified Neoadjuvant Strategy in the Randomized Phase 2 EXPERT-C Trial. Int J Radiat Oncol Biol Phys 2015; 93(2): 303–12. doi: 10.1016/j.ijrobp.2015.03.038 [DOI] [PubMed] [Google Scholar]