Summary
Merkel cell carcinoma (MCC) of the conjunctiva is rare. We report the case of a 73-year-old man who presented with unilateral foreign body sensation and blurred vision. A rapidly enlarging conjunctival lesion was identified and excised. The histopathological diagnosis was poorly differentiated squamous cell carcinoma, later reclassified as neuroendocrine / Merkel cell carcinoma following excision on subsequent recurrence. The patient developed lymph node and widespread metastatic disease. The challenges of diagnosing MCC at this site are discussed and the literature on treatment options for this aggressive disease is reviewed.
Introduction
Merkel cell carcinoma (MCC) is an aggressive tumor, with high rates of metastasis—up to 22% to regional lymph nodes and up to 38% with distant metastases.1–3 MCC of the periocular region, including the eyelids, is uncommon. The published literature on MCC of the conjunctiva is limited.
Periocular MCC often presents near the eyelid margin and can be associated with partial or complete eyelash loss.4 Clinically, it appears as a rapidly growing, painless, violaceous mass.4 The upper eyelid is the most commonly involved site in this region. The most important predictor for survival is lack of involvement of regional lymph nodes.5 Other factors associated with poor prognosis include lymphovascular invasion (twofold increase of mortality), p63 expression, and immunosuppression.5
Case Report
A 73-year-old male fork lift driver presented at the Mater Hospital, Brisbane, with foreign body sensation and blurred vision in his right eye. His past medical history included a T1N0 well differentiated rectal adenocarcinoma, treated with lower anterior resection 1 month prior to presentation. In addition, he had hypertension, dyslipidemia, ischemic heart disease, and impaired left ventricular systolic function. Notable risk factors included significant occupation-related sun exposure and a smoking history of 35 packs per year.
On examination, there was a large, ulcerated lesion arising from the temporal conjunctiva extending from the 7 o’clock to the 1 o’clock position. The lesion was red and had superficial ulceration and blood vessel abnormalities resembling squamous cell carcinoma (SCC). Best-corrected visual acuity was 6/9 in the right eye and 6/6 in the left eye. His intraocular pressures were within normal limits.
The patient proceeded to excision of the right limbal lesion, which measured 20 mm × 20 mm × 9 mm. At this point, the histopathology indicated an ulcerated, poorly differentiated SCC, positive for the immunostains MNF116, p63, and CK5/6 and negative for S100 and LCA. There was extensive margin involvement.
The lesion recurred 2 months later, prior to planned re-excision, as a large red mass in the same area, measuring 28 mm in the largest diameter (Figure 1). Ultrasound biomicroscopy showed no evidence of intraocular invasion. Positron emission tomography staging demonstrated the primary tumor as well as an avid ipsilateral parotid lymphadenopathy, which was confirmed as metastases on fine-needle aspirate. At this stage, there were no distant metastases, in particular, no demonstrable gastrointestinal lesion.
Figure 1.
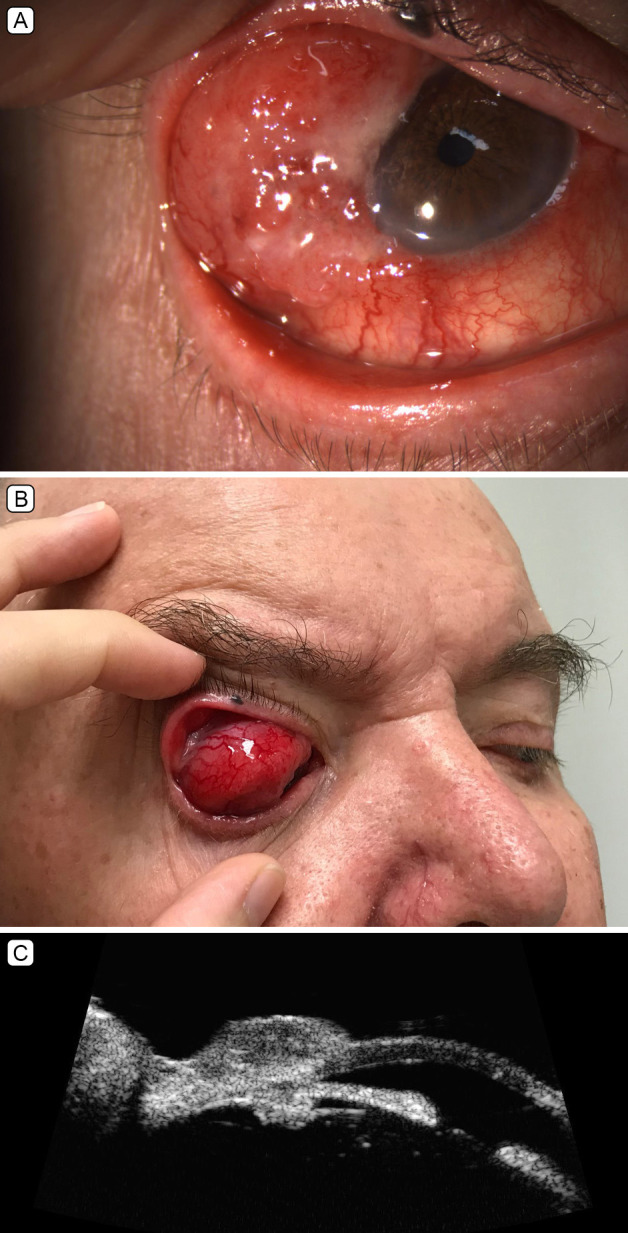
Clinical images of recurrent right superotemporal conjunctival mass. A, Visible mass under right upper eyelid at rest. B, Posterior extent of mass visible on downgaze. C, Slit-lamp biomicroscopy image of right anterior segment showing the lesion crossing the limbus.
The recurrent lesion was excised from the temporal conjunctiva of the right eye (Figure 2). Expanded immunohistochemical panel revealed positivity with CK20 (perinuclear dot pattern), synaptophysin, chromogranin, TTF1 and CD56. CK7, CK5/6, CDX2, and S100 were negative. The lesion was reclassified as a poorly differentiated carcinoma with neuroendocrine differentiation / neuroendocrine carcinoma; CK20 reactivity raised the possibilities of skin (MCC) or salivary gland origin. Multiple involved margins were again noted.
Figure 2.
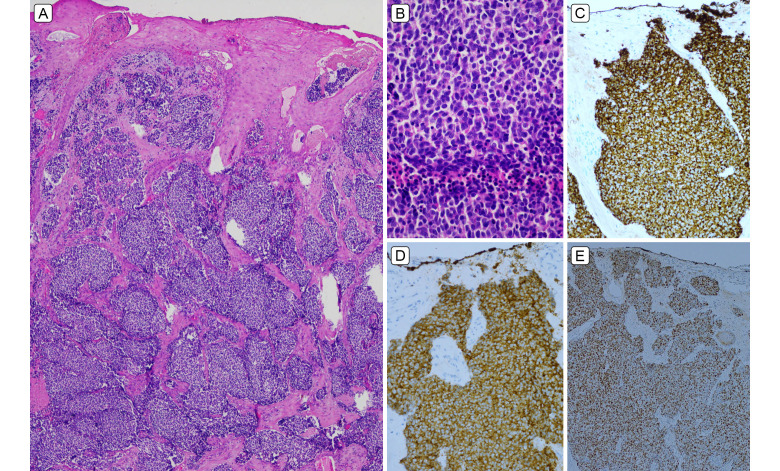
A, Beneath ulcerated, focally acanthotic squamous epithelium, there was infiltration by confluent sheets of basaloid carcinoma cells within desmoplastic stroma (hematoxylin and eosin [H&E], original magnification ×40). B, Mitotically and apoptotically active, medium-large tumor cells have high N:C ratio and small amount of amphophilic cytoplasm, without overt nucleoli or significant nuclear molding (H&E original magnification ×400). C, CK20 paranuclear dot positivity. D, Synaptophysin positivity. E, TTF1 positivity.
Following a multidisciplinary team discussion, the patient was planned for definitive radiotherapy to the eye, parotid, and neck. However, restaging before commencing radiotherapy revealed further progression with innumerable liver metastases.
The patient was instead commenced on four cycles of carboplatin/etoposide chemotherapy. Further staging after four cycles of chemotherapy revealed progression of the liver metastases and diffuse skeletal metastatic deposits scattered throughout the axial and appendicular skeleton, as well as recurrence at the primary disease site. The patient was commenced on the checkpoint inhibitor avelumab. He went on to complete palliative radiation therapy to his right orbit in the same month (16Gy in 4 fractions).
In the face of extensive disease burden, the patient died 10 months after initial presentation.
Discussion
Neuroendocrine neoplasms (NEN) comprise a heterogeneous group of tumors of epithelial and neuroendocrine origin that arise at almost any anatomical site. MCC of the eyelid is well documented, and May et al demonstrated low numbers of native Merkel cells at the eyelid immunohistochemically using CK20, although the same authors were unable to demonstrate Merkel cells in the conjunctival or corneal epithelium.6 In the 2018 WHO Classification of Tumors of the Eye, neuroendocrine tumor is listed in the ―secondary tumors‖ sections of conjunctival and lacrimal gland chapters. Thus, in our case and potentially in the previous single case report of conjunctival MCC, a metastasis cannot be fully excluded. Even so, in a recent literature review, ocular sites of NEN metastasis tended to be to uvea (30 cases [46%]), orbit (27 cases [42%]), retina (3 cases), eyelid (2 cases), and none reported to conjunctiva.7
The histologic diagnosis of NEN entails a number of criteria (Table 1). In Chan’s review of CK20 expression in NEC, CK20 reactivity in NEC strongly predicted cutaneous MCC (100%) or those from salivary gland (60%), especially if the majority of cells are positive.8 Only rare pulmonary and cervical small cell carcinoma cases were focally positive for CK20 (3% and 9%, respectively).8 Although the normal epithelium of urothelium and gastrointestinal tract is CK20 positive, NEC/small cell carcinomas of these sites are uniformly CK20 negative.8 A confounding factor in our case was reactivity to TTF1, which is traditionally a pulmonary and thyroid marker, but which loses its specificity in poorly differentiated NEC, where it can be expressed regardless of site of origin. In our case, no skin primary site of MCC could be identified, while the right parotid lesion has been interpreted as nodal metastasis rather than a primary salivary lesion.
Table 1.
Criteria for histologic diagnosis of neuroendocrine neoplasms
Treatment of the primary tumor includes surgical excision, with a margin of 5 mm in the eyelid region.9 Postoperative radiotherapy is the standard of care for cutaneous MCC for primary tumors >1 cm, with close margins and lymphovascular invasion or lymph node involvement to improve locoregional control and survival.10 However, the delivery of radiotherapy to doses of 50–66 Gy is challenging for periorbital cases because of the sensitivity of ocular structures to radiotherapy. The role of adjuvant chemotherapy post operatively for locoregional disease is controversial. In fact, there have been concerns about the immunosuppressive nature of chemotherapy in MCC, a disease in which the immune system plays an important role.
In the past, platinum-based chemotherapy was the standard of care in the setting of metastatic disease. More recently, immunotherapy has become the first-line approach. Approximately 50% of cases of MCC overexpress receptors related to programmed-death-ligand-1 (PDL-1).11 MCC expresses PDL-1, which binds to PD-1 receptors on cytotoxic T cells, blocking the immune response to tumor. Checkpoint inhibition immunotherapy agents against the PD-1 pathway include avelumab, nivolumab, and pembrolizumab. Avelumab was used in our case. It is a monoclonal antibody that inhibits the PD-1/PD-L1 pathway and therefore generates an endogenous antitumor T cell effect.12
The phase II JAVELIN Merkel 200 trial included 88 patients with metastatic MCC and progression of disease with previous chemotherapy who were treated with avelumab.13 Over a follow-up period of 2 years, it was found that 33% of patients had an objective response, with 11% having a complete response. The 2-year progression-free survival rate was 26%; the overall survival rate was 36%. The incidence of significant adverse effects was low. Abdalla et al14 recently reported a case in which avelumab was used in the neoadjuvant setting, with a complete response. I-MAT is a future clinical trial launching later in 2020 evaluating avelumab in the adjuvant setting.15 The GoTHAM clinical trial will investigate the role of radiation in inducing immunogenic cell death and improving the anti-tumor efficacy when combined with avelumab.16
Chemotherapy has a role in the treatment of metastatic MCC where immunotherapy is contraindicated or is ineffective. The disadvantages of chemotherapy include nondurable responses, limited effect on survival, and potential for considerable toxicity.13 Novel targeted therapy under investigation currently include pazopanib (tyrosine kinase inhibitor) and lanreotide (somatostatin analog).17,18
In summary, there is paucity of literature on conjunctival MCC, even as a site of metastasis. Our case also highlights the rapid aggressive and fatal course from detection of conjunctival mass at initial presentation. The rarity of this entity at this site initially led to an initial histologic assessment of an SCC, potentially delaying appropriate urgent treatment, including early involvement of oncology multidisciplinary team.
References
- 1.Kase S, Ishijima K, Ishida S, Rao NA. Merkel cell carcinoma of the conjunctiva. Ophthalmology. 2010;117:637.e1–2. doi: 10.1016/j.ophtha.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Sniegowski MC, Warneke CL, Morrison WH, et al. Correlation of American Joint Committee on Cancer T category for eyelid carcinoma with outcomes in patients with periocular Merkel cell carcinoma. Ophthalmic Plast Reconstr Surg. 2014;30:480–5. doi: 10.1097/IOP.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 3.Peters GB, 3rd, Meyer DR, Shields JA, et al. Management and prognosis of Merkel cell carcinoma of the eyelid. Ophthalmology. 2001;108:1575–9. doi: 10.1016/s0161-6420(01)00701-1. [DOI] [PubMed] [Google Scholar]
- 4.Merritt H, Sniegowski MC, Esmaeli B. Merkel cell carcinoma of the eyelid and periocular region. Cancers (Basel) 2014;6:1128–37. doi: 10.3390/cancers6021128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moshiri AS, Nghiem P. Milestones in the staging, classification, and biology of Merkel cell carcinoma. J Natl Compr Canc Netw. 2014;12:1255–62. doi: 10.6004/jnccn.2014.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May CA, Osterland I. Merkel cell distribution in the human eyelid. Eur J Histochem. 2013;57:e33. doi: 10.4081/ejh.2013.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Salvia A, Persano I, Trevisi E, et al. Ocular metastases from neuroendocrine tumors: a literature review. Semin Oncol. 2020;47:144–7. doi: 10.1053/j.seminoncol.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Chan JKC, Suster S, Wenig BM, Tsang WYW, Chan JBK, Lau ALW. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol. 1997;21:226–34. doi: 10.1097/00000478-199702000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Lebbe C, Becker JC, Grob JJ, et al. European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO), the European Organization for Research and Treatment of Cancer (EORTC) Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2396–403. doi: 10.1016/j.ejca.2015.06.131. [DOI] [PubMed] [Google Scholar]
- 10.Esmaeli B, Naderi A, Hidaji L, Blumenschein G, Prieto VG. Merkel cell carcinoma of the eyelid with a positive sentinel node. Arch Ophthalmol. 2002;120:646–8. [PubMed] [Google Scholar]
- 11.Cugley DR, Roberts-Thomson SJ, McNab AA, Pick Z. Biopsy-proven metastatic Merkel cell carcinoma to the orbit: case report and review of literature. Ophthalmic Plast Reconstr Surg. 2018;34:e86–8. doi: 10.1097/IOP.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute Localized radiation therapy or recombinant interferon beta and avelumab with or without cellular adoptive immunotherapy in treating patients with metastatic Merkel cell carcinoma. ClinicalTrials.gov identifier NCT02584829. Accessed September 1, 2020 https://clinicaltrials.gov/ct2/show/NCT02584829?term=02584829&rank=1. [Google Scholar]
- 13.D’Angelo SP, Bhatia S, Brohl AS, et al. Avelumab in patients with previously treated metastatic Merkel cell carcinoma: long-term data and biomarker analyses from the single-arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer. 2020;8:e000674. doi: 10.1136/jitc-2020-000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdallah N, Nagasaka M, Chowdhury T, Raval K, Hotaling J, Sukari A. Complete response with neoadjuvant avelumab in Merkel cell carcinoma—a case report. Oral Oncol. 2019;99:104350. doi: 10.1016/j.oraloncology.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Melanoma and Skin Cancer Trials Limited Immunotherapy adjuvant trial in patients with stage I-III Merkel cell carcinoma (I-MAT) ClinicalTrials.gov identifier NCT04291885. Accessed September 1, 2020 https://clinicaltrials.gov/ct2/show/NCT04291885. [Google Scholar]
- 16.Sandhu S. Targeted Therapy and avelumab in Merkel cell carcinoma (GoTHAM) ClinicalTrials.gov Identifier: NCT04261855. Accessed November 1, 2020 https://clinicaltrials.gov/ct2/show/NCT04261855. [Google Scholar]
- 17.A trial of pazopanib for Merkel cell skin cancer (UKMCC-01) Cancer Research UK trial number CRUK/11/015. Accessed September 1, 2020 www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-of-pazopanib-for-merkel-cell-carcinoma-ukmcc-01/.
- 18.Cirillo F, Filippini L, Lima GF, Caresana G, Alquati P. Merkel cell tumor: report of case and treatment with octreotide [in Italian] Minerva Chir. 1997;52:1359–65. [PubMed] [Google Scholar]