Abstract
Antigen 85 (Ag85) complex proteins are major secretory products of Mycobacterium tuberculosis and induce strong cellular and humoral immune responses in infected experimental animals and human beings. We have previously shown that nanogram doses of these 30- to 32-kDa fibronectin-binding proteins inhibit local expression of delayed hypersensitivity by a T-cell fibronectin-dependent mechanism. Circulating levels of Ag85 might be expected to be elevated in patients with active tuberculosis and possibly to play a role in systemic anergy in these patients. To test this hypothesis, Ag85 was measured in serum and urine by a monoclonal antibody-based dot immunobinding assay in 56 patients and controls with known skin test reactivity. Median serum Ag85 levels were 50- to 150-fold higher in patients with active tuberculosis than in patients with active M. avium-intracellulare disease or other nontuberculous pulmonary disease or in healthy controls (P < 0.001). The median and range of serum Ag85 in patients with active tuberculosis was not significantly different between skin test-positive and -negative subjects. Patients with active M. avium disease could be distinguished from those with disease due to M. tuberculosis by monoclonal anti-Ag85 antibodies of appropriate specificities. No increases in urinary Ag85 were detected in any patient, regardless of the Ag85 level in serum. Chromatographic analysis and immunoprecipitation studies of serum revealed that Ag85 existed in the serum of these patients complexed to either fibronectin or immunoglobulin G (IgG). Uncomplexed circulating Ag85 was demonstrable in serum from fewer than 20% of patients with active tuberculosis. In patients with active tuberculosis, Ag85 is therefore likely to circulate primarily as complexes with plasma fibronectin and IgG rather than in unbound form. The existence of Ag85 complexes with plasma proteins would account for its lack of urinary clearance.
Tuberculosis is a global public health problem. A third of the world’s population is estimated to be infected with Mycobacterium tuberculosis, and tuberculosis is the most common cause of death of adults from infectious disease throughout the world (25). The recent increase in tuberculosis incidence in the United States resulting in part from the human immunodeficiency virus (HIV) epidemic has further focused interest on immunity to this disease (5).
Only actively dividing mycobacteria efficiently generate protective cell-mediated immunity to M. tuberculosis (30). Much recent research has therefore been focused on this organism’s secreted proteins. Proteins of the antigen 85 complex (Ag85A, Ag85B, and Ag85C) are major secretory proteins of actively replicating M. tuberculosis (40). They share high sequence homology at the nucleotide and protein level both with each other and with Ag85 from other mycobacterial species (41). This high degree of homology results in a particular Ag85 protein containing common epitopes found in many Ag85, in addition to unique species- and subtype-specific epitopes (11, 34). Ag85 complex proteins are mycolyltransferases (3). As such, they play an essential role in the final stages of mycobacterial cell wall synthesis, since inhibitors of this activity inhibit both the transfer and the deposition of mycolates into the mycobacterial cell wall and cell growth (3). The function of Ag85 complex proteins in mycobacterial physiology and pathogenesis of tuberculosis is otherwise incompletely understood (13, 17, 33).
Ag85 complex proteins induce delayed hypersensitivity, protective immune responses, and specific antibodies in infected mice and guinea pigs (2, 9, 15, 18–20, 27). They also induce readily elicitable cellular immune responses in cultured peripheral blood mononuclear cells of most healthy purified protein derivative of tuberculin (PPD)-positive people and a few patients with clinically active tuberculosis (16, 22). While levels of anti-Ag85 antibodies are often low in healthy PPD-positive subjects, they increase in patients with active tuberculosis (16, 38). Similar patterns of response are exhibited by healthy lepromin-positive subjects and patients with lepromatous leprosy (26).
Ag85 proteins bind to plasma and cellular fibronectins (1, 13), high-molecular-weight glycoproteins found in plasma and tissues that play important roles in cell motility and adhesion, development, phagocytic function, wound healing, and inflammation (23). Although microgram doses of Ag85 elicit delayed hypersensitivity reactions in sensitized guinea pigs, nanogram doses of these proteins inhibit local in vivo expression of delayed hypersensitivity by binding to and inactivating a specialized T-cell fibronectin produced after antigenic stimulation (13). This latter activity led us to hypothesize that patients with active tuberculosis might have high levels of circulating Ag85 proteins that could possibly play a role in the systemic anergy these patients often exhibit.
To examine this hypothesis, we measured Ag85 concentrations in serum and urine from patients and controls with known PPD skin test reactivity. We found serum Ag85 to be significantly increased in patients with active tuberculosis independent of skin test status. Ag85 in these patients circulates primarily as complexes with immunoglobulin G (IgG) and plasma fibronectin.
MATERIALS AND METHODS
Study population.
The study population consisted of 56 patients and healthy controls at Metropolitan Hospital Center (New York, N.Y.). It included white (1 female, 4 males), black (10 females, 15 males), and Hispanic (11 females, 15 males) individuals. Diagnoses included bacteriologically and/or biopsy-confirmed active tuberculosis (13), inactive tuberculosis (history of previously treated tuberculosis) (6), bacteriologically confirmed active M. avium-intracellulare disease (5), nontuberculous lung disease (20), and no disease (healthy controls) (12). Thirteen patients were HIV positive (eight males, five females). Of the patients with tuberculosis, one had pleural tuberculosis, one had tuberculous lymphadenitis, four had disseminated tuberculosis (pulmonary and either bone marrow or lymph node involvement) and seven had pulmonary tuberculosis (cavitary in one patient, associated with pleural effusion in another). Seven patients with tuberculosis were smear positive and six were smear negative. Serum was collected with informed consent from all 56 members of the study population, and urine was collected from 53; samples were stored frozen at −80°C until analyzed. Diagnoses of the three patients not providing a urine sample included active tuberculosis (two subjects) and active M. avium-intracellulare disease (one subject). PPD skin tests were done in the course of standard medical treatment. They were omitted in the case of two patients with active tuberculosis who refused their placement. Serum and urine samples were coded, and the code was not broken until all assay measurements had been completed.
Purified proteins and antibodies.
Ag85 complex proteins were purified from concentrated culture filtrates of M. bovis bacillus Calmette-Guérin (BCG) (8). Purified Ag85 complex contained 90% Ag85A, 6% Ag85B, and 4% Ag85C as judged by Western blotting against monoclonal anti-BCG Ag85 complex antibodies (11) and was stored in sterile aliquots at −80°C. For use as an antigen standard in dot blotting, the initial preparation of Ag85 used was arbitrarily assigned an immunoreactive Ag85 content of 1 mU/mg. Unitage of subsequent preparations was determined by parallel-line analysis of dot blots of initial and subsequent materials (24). Aliquots from a single standard preparation were used for these experiments; unitage was constant between the different aliquots of this preparation. Purified human plasma fibronectin was purchased (New York Blood Center, New York, N.Y.). Purified rabbit anti-fibronectin was a gift from Mary Haak-Frendscho, Promega Corp. (Madison, Wis.). It recognized only a 220- to 250-kDa dimer in human plasma and did not react with purified Ag85. The following mouse monoclonal antibodies were used: IgG1 anti-BCG Ag85, clone 240; IgG1 anti-BCG Ag85, clone 17/4 (11); and IgG1 anti-human fibronectin, clone 248 (28). The antigen specificity of anti-Ag85 clone 240 is identical to that of the previously reported clone 233 (11). It reacted equally strongly with M. tuberculosis Ag85A and Ag85B, weakly with M. tuberculosis Ag85C, and minimally with M. avium Ag85B. It delineated a 30- to 32-kDa doublet on Western blots of the purified Ag85 complex standard. The specific Ag85 epitope recognized by clone 240 is not known (21; unpublished observations). Anti-Ag85 clone 17/4 reacted strongly with M. tuberculosis Ag85A and Ag85C, weakly with M. tuberculosis Ag85B, and strongly with M. avium Ag85B (11). It recognizes a sequence spanning amino acids 261 to 280 in the C-terminal region of Ag85A (21). The antifibronectin clone 248 did not react with Ag85. It recognizes an epitope located on the major cell-binding domain of plasma fibronectin.
Dot immunobinding assay.
For the determination of Ag85 levels in serum and urine, fivefold serial dilutions of 100-μl aliquots of coded samples (diluted in phosphate-buffered saline [PBS], pH 7.2; lowest dilution tested, 1:5) by dot blot with a filtration manifold and monoclonal anti-Ag85 (13). Briefly, serum samples and a half-log serial dilution series of purified Ag85 standard (range, 0.0003 to 1 μU) were adsorbed to nitrocellulose, and the nitrocellulose was dried for 15 min and blocked overnight at 4°C in PBS (pH 7.2) containing 5% nonfat dried milk. Blots were developed with horseradish peroxidase-conjugated second antibodies, enhanced chemiluminescence (ECL or ECL-Plus; Amersham Pharmacia Biotech, Arlington Heights, Ill.), and standard X-ray film (Kodak). Developed X-ray films were evaluated visually by comparison to Ag85 antigen standard included on each blot. Previous studies have indicated that visual and densitometric quantitation yield equivalent results (39; unpublished observations). Results are reported as geometric means in Ag85 immunoreactive units of six to nine determinations in duplicate. For purposes of statistical analysis, samples not reactive at 0.1 μU/ml were assumed to react at 0.01 μU of Ag85 per ml.
Gel filtration chromatography.
Aliquots of sera diluted 1:4 from six patients with active tuberculosis (four PPD positive, two PPD negative), two patients with treated inactive tuberculosis (one PPD positive, one PPD negative), three patients with nontuberculous pulmonary disease (two PPD positive, one PPD negative), and six healthy controls (three PPD positive, three PPD negative) or supernatants from immunoprecipitations were fractionated by high-pressure liquid chromatography on Superose 12 (Amersham Pharmacia Biotech) with a Perkin-Elmer series 400 Bio-pump (Wilton, Conn.). The column was eluted with PBS–0.1 mM phenylmethylsulfonyl fluoride–3 mM NaN3 at a flow rate of 0.5 ml/min, and 0.5-ml fractions were collected. The A280 was continuously monitored (Perkin-Elmer LC-95 UV/vis spectrophotometer) and was analyzed by using Maxima 820 software (Millipore Corporation, Milford, Mass.). Columns were calibrated with proteins of known molecular weights (Pharmacia) or with purified plasma fibronectin. Immunoreactive Ag85 and fibronectin contents in individual fractions were determined by dot blot with monoclonal anti-Ag85 or monoclonal anti-human fibronectin, respectively, and a protocol similar to that described above for the Ag85 dot blot, except that serial dilutions of antigen standard were replaced by a single-dose positive antigen control of Ag85 or plasma fibronectin. Dot blots were quantitated by transmission densitometry in arbitrary units. Mr of fractionated materials were determined from a plot of Kav against the log molecular weight.
Immunoprecipitation.
Sera from the six patients with active tuberculosis, two patients with treated inactive tuberculosis, three patients with nontuberculous pulmonary disease, and six healthy controls analyzed by gel filtration chromatography were analyzed by immunoprecipitation. Sera from an additional two patients with treated tuberculosis (both PPD positive) and from four patients with nontuberculous pulmonary disease (two PPD positive, two PPD negative) were also analyzed by immunoprecipitation. IgG was precipitated from aliquots of diluted samples by overnight incubation at 4°C with an excess of protein A-conjugated agarose beads (Sigma Chemical Co., St. Louis, Mo.), followed by centrifugation and washing of the pellet, which was then dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The completeness of IgG removal was confirmed by Western blotting of the immunoprecipitate supernatants. Aliquots of dissolved pellets, purified Ag85 and human IgG (Miles Laboratories, Tarrytown, N.Y.), were analyzed for immunoreactive Ag85 and IgG by Western blotting (SDS–15% PAGE) (12) with monoclonal anti-Ag85, clone 240, and horseradish peroxidase-conjugated second antibodies (Jackson ImmunoResearch, West Grove, Pa.) or with horseradish peroxidase-conjugated goat anti-human IgG with minimal cross-reactivity to bovine, horse, and mouse IgG. Bound antibodies were visualized by using ECL technology (Amersham Pharmacia Biotech).
Fibronectin was precipitated from the supernatant remaining after IgG removal by incubation for 3 h at 23°C with an aliquot of rabbit anti-fibronectin or normal rabbit serum, followed by incubation overnight at 4°C with protein A/G Plus-agarose beads (Santa Cruz Labs, Santa Cruz, Calif.), centrifugation, and washing of the pellet. Fibronectin-Ag85 complexes were generated in vitro by incubating 2 μg of purified human plasma fibronectin and 4.5 μU of purified Ag85 in PBS (pH 7.2) for 2 h at 23°C; they were then immunoprecipitated in parallel with other samples. Dissolved pellets were analyzed by SDS-PAGE and Western blotting with mouse monoclonal anti-Ag85 or with rabbit anti-fibronectin with appropriate horseradish peroxidase-conjugated second antibodies and ECL as described above.
Statistical analysis.
Significance of differences in medians was determined by Kruskal-Wallis one-way analysis of variance by ranks and a Dunn multiple comparison post test (7).
RESULTS
Circulating Ag85 in patients with active tuberculosis.
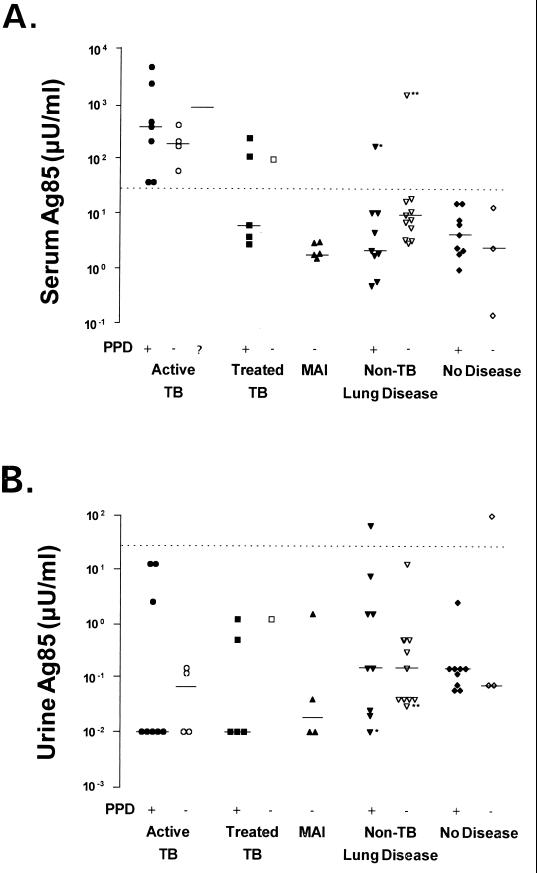
Median levels of circulating Ag85 proteins measured with clone 240 anti-Ag85 were 50- to 150-fold higher in patients with active tuberculosis than in patients with active M. avium-intracellulare disease or other nontuberculous pulmonary disease or in healthy controls (P < 0.001, Dunn multiple comparison test) (Fig. 1). Median increases in Ag85 in patients with active tuberculosis were independent of PPD skin reactivity, acid-fast bacilli smear positivity, or stage of disease. There was also no significant relationship between circulating Ag85 levels and skin test reactivity in patients without tuberculosis. All 13 patients with active tuberculosis had serum Ag85 levels of >30 μU/ml. Only 5 of 43 patients without active tuberculosis (three with treated tuberculosis, one with PPD-positive and HIV-positive nontuberculous pulmonary disease, and one with sarcoidosis) had serum Ag85 levels above this level (Fig. 1A).
FIG. 1.
Levels of serum (A) and urine (B) Ag85 complex proteins in patients with active tuberculosis (•, ○,  ), inactive treated tuberculosis (■, □), active infection with M. avium-intracellulare (MAI) (▴), other pulmonary disease (▾, ▿), or healthy controls (⧫, ◊), as well as positive (+), negative (−), or unknown (?) skin test reactivities to PPD. (Two patients with active tuberculosis with unknown PPD reactivity refused placement of a skin test). Ag85 measured by dot blot immunobinding with clone 240 anti-M. bovis BCG Ag85 and evaluated by comparison to a purified native BCG Ag85 complex standard arbitrarily defined as containing 1 mU of immunoreactive Ag85 complex/mg of protein. Geometric means of six to nine determinations in duplicate (median is indicated by solid line). For purposes of statistical analysis, samples not reactive at 0.1 μU/ml were assumed to react at 0.01 μU of Ag85 per ml. See Materials and Methods for details of this assay. The dotted line indicates the Ag85 concentration (30 μU/ml). Mean serum Ag85 levels were significantly higher in patients with active tuberculosis than in patients with M. avium disease or other nontuberculosis pulmonary disease or in healthy controls (P < 0.001, Dunn multiple comparison test). There was no statistical difference in urinary Ag85 means between any group. ∗, HIV-positive patient with nontuberculous pulmonary disease; ∗∗, patient with pulmonary sarcoidosis.
), inactive treated tuberculosis (■, □), active infection with M. avium-intracellulare (MAI) (▴), other pulmonary disease (▾, ▿), or healthy controls (⧫, ◊), as well as positive (+), negative (−), or unknown (?) skin test reactivities to PPD. (Two patients with active tuberculosis with unknown PPD reactivity refused placement of a skin test). Ag85 measured by dot blot immunobinding with clone 240 anti-M. bovis BCG Ag85 and evaluated by comparison to a purified native BCG Ag85 complex standard arbitrarily defined as containing 1 mU of immunoreactive Ag85 complex/mg of protein. Geometric means of six to nine determinations in duplicate (median is indicated by solid line). For purposes of statistical analysis, samples not reactive at 0.1 μU/ml were assumed to react at 0.01 μU of Ag85 per ml. See Materials and Methods for details of this assay. The dotted line indicates the Ag85 concentration (30 μU/ml). Mean serum Ag85 levels were significantly higher in patients with active tuberculosis than in patients with M. avium disease or other nontuberculosis pulmonary disease or in healthy controls (P < 0.001, Dunn multiple comparison test). There was no statistical difference in urinary Ag85 means between any group. ∗, HIV-positive patient with nontuberculous pulmonary disease; ∗∗, patient with pulmonary sarcoidosis.
Immunoassay specificity was critically dependent on the antibody used. Even though clone 240 anti-BCG Ag85 showed weak reactivity with M. avium Ag85 in a previous study (11), it detected serum Ag85 in none of the five patients with active M. avium-intracellulare disease (Fig. 1A). When the remaining serum aliquots from eight patients with active tuberculosis and three patients with active M. avium-intracellulare disease were reassayed with an anti-BCG Ag85 monoclonal antibody (clone 17/4) that was strongly cross-reactive with M. avium Ag85 in vitro (11), elevated levels of serum Ag85 were detected in both groups of patients (data not shown). The difference in median serum Ag85 levels detected by clones 240 and 17/4 in sera from patients with M. avium disease was significant (P < 0.01, Dunn’s multiple comparison test), while the difference in median Ag85 levels detected by these two antibodies in patients with M. tuberculosis disease was not (P > 0.05).
Urinary levels of Ag85 were uniformly lower than serum levels in all patients (Fig. 1B). They did not correlate with serum Ag85 concentration, patient diagnosis, or skin test reactivity.
Physical nature of circulating Ag85 in patients with active tuberculosis.
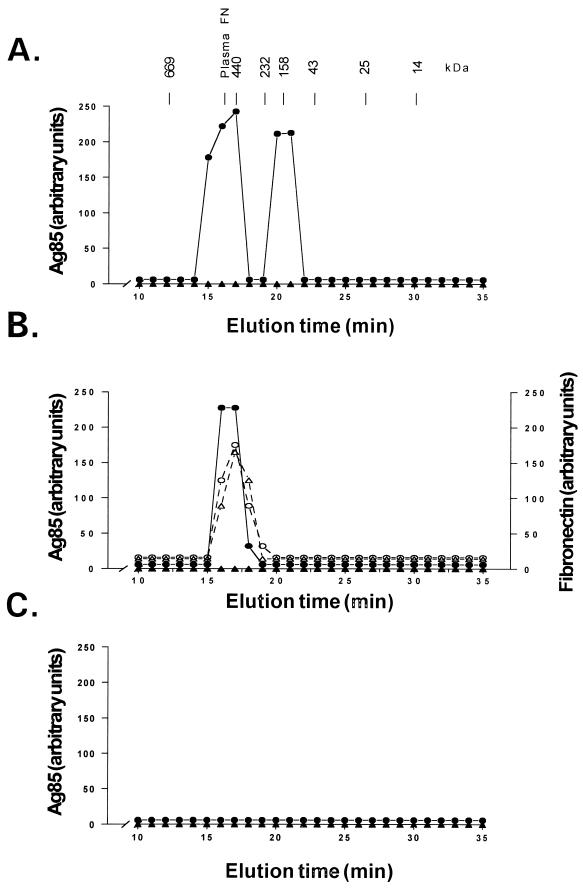
Proteins of the Ag85 complex are small enough (30 to 32 kDa) to be cleared from the circulation by the kidney. The lack of urinary Ag85 in patients with active tuberculosis in the face of high levels of serum Ag85 suggested that Ag85 circulated as complexes with plasma proteins. To confirm this hypothesis, sera from six patients with active tuberculosis, two patients with treated inactive tuberculosis, three patients with nontuberculous pulmonary disease, and six healthy controls were fractionated by gel filtration chromatography, and the Ag85 content of each fraction was determined by immunoassay with clone 240 anti-Ag85. Sera from all patients with tuberculosis contained immunoreactive Ag85 associated with 440- to 580-kDa and 200-kDa materials, regardless of skin test reactivity. Figure 2A shows the results obtained with one such serum. These Mr values correspond to complexes of Ag85 with plasma fibronectin and IgG, respectively. In one of six patients, Ag85 was also reproducibly demonstrable in the 30-kDa region corresponding to unbound (“free”) Ag85 (data not shown). The presence of free Ag85 could not be related to an unusually high concentration of Ag85, as this particular serum contained only 210 μU of Ag85 per ml, which is slightly less than the median level of 300 μU/ml for this group. No Ag85 immunoreactivity was present in any serum fraction from healthy controls (Fig. 2) or from patients with treated inactive tuberculosis (not shown) or nontuberculous pulmonary disease (not shown).
FIG. 2.
Chromatographic characterization of Ag85 in serum from a PPD-positive patient with active tuberculosis (•) and a PPD-positive healthy control (▴). (A) Ag85 determined by dot immunobinding and densitometry in serum from a PPD-positive patient with active tuberculosis. Ag85 was detected in association with 440- and 200-kDa materials. (B) The supernatant from protein A immunoprecipitation of serum from the PPD-positive patient shown in panel A no longer contains 200-kDa Ag85 but still contains 440-kDa Ag85. Ag85 coelutes with plasma fibronectin, as determined by dot immunobinding and densitometry, in patient serum (○) but not in the control serum (▵). (C) Ag85 is no longer present in the IgG-depleted supernatant shown in panel B following further immunoprecipitation with anti-human plasma fibronectin. Ag85 was not demonstrable in fractionated sera or supernatant from any healthy control either before or after the immunoprecipitations. The molecular sizes and the elution time of protein standards (in kilodaltons) and the units of plasma fibronectin (plasma FN) used to calibrate the column are indicated. See Materials and Methods for more details.
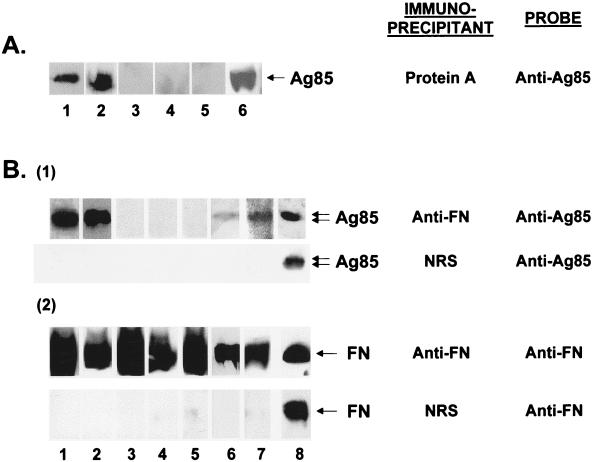
Removal of IgG from the serum of tuberculosis patients with insolubilized protein A caused the disappearance of the 200-kDa but not the 440- to 580-kDa peak of Ag85 immunoreactivity on gel filtration chromatography of the residual supernatant (Fig. 2B). This remaining high Mr peak of Ag85 coeluted with immunoreactive fibronectin (Fig. 2B). IgG immune complexes precipitated by protein A contained large amounts of immunoreactive material with Mr values identical to those for purified Ag85 complex (Fig. 3A, lane 6) only in patients with active tuberculosis regardless of skin test status (Fig. 3A, lanes 1, 2). No immunoreactive Ag85 was present in the IgG immune complexes from other patient groups or from healthy controls (Fig. 3A, lanes 3 to 5).
FIG. 3.
Immunoprecipitation characterization of Ag85 in sera from patients with active tuberculosis and from controls. (A) Western blot analysis (SDS–10% PAGE gel) of protein A immunoprecipitates of serum from a PPD-positive patient with active tuberculosis (lane 1), a PPD-negative patient with active tuberculosis (lane 2), a PPD-positive patient with treated tuberculosis (lane 3), a PPD-positive patient with nontuberculous lung disease (lane 4), and a PPD-positive healthy control (lane 5). Lane 6 contains 10 μU of immunoreactive purified M. bovis BCG Ag85 complex proteins. Samples separated on SDS-PAGE, electroblotted to nitrocellulose, and developed with mouse clone 240 anti-Ag85 and ECL. Ag85 is present in circulating immune complexes only in patients with active tuberculosis. (B) Western blot analysis (SDS–10% PAGE gel) of rabbit anti-plasma fibronectin (anti-FN) or normal rabbit serum (NRS) immunoprecipitates of serum previously immunoprecipitated with protein A from a PPD-positive patient with active tuberculosis (lane 1), a PPD-negative patient with active tuberculosis (lane 2), a PPD-positive patient with treated tuberculosis (lane 3), a PPD-positive patient with nontuberculous lung disease (lane 4), a PPD-positive healthy control (lane 5), and a PPD-negative healthy control (lane 6) or of a mixture of purified human plasma fibronectin and purified M. bovis BCG Ag85 complex proteins (lane 7). Double arrows indicate the positions of the 32- and 30-kDa components of the Ag85 complex. Lane 8 contains 4.5 μU of immunoreactive purified M. bovis BCG Ag85 complex proteins. After removal of IgG, sera were incubated with monospecific rabbit anti-FN or NRS and then immunoprecipitated with protein A/G. The immunoprecipitates were separated by SDS-PAGE, electroblotted to nitrocellulose, and developed with either mouse clone 240 anti-Ag85 or rabbit anti-FN and ECL. See Materials and Methods for more details.
The complete removal of IgG from each protein A-immunoprecipitated supernatant was confirmed by Western blotting; no residual IgG heavy and light chains were observed in any sample after protein A immunoprecipitation (data not shown). Subsequent immunoprecipitation of these IgG-depleted supernatants with anti-plasma fibronectin produced (i) supernatants in which the previously seen 440- to 580-kDa peak of immunoreactive Ag85 on gel filtration chromatography was absent (Fig. 2C) and (ii) immunoprecipitates containing large amounts of immunoreactive material with Mr values identical to those for purified Ag85 complex (Fig. 3B1, lane 8) in all patients with active tuberculosis regardless of their PPD skin reactivity (Fig. 3B1, lanes 1 and 2). Fibronectin immunoprecipitates from all patients who did not have active tuberculosis contained either no immunoreactive material with Mr values identical to those for Ag85 complex (Fig. 3B1, lanes 3 to 5) or, in the case of 2 of 6 healthy control subjects (one PPD positive, one PPD negative), small amounts of immunoreactive material with Mr values identical to those for Ag85. Figure 3B1, lane 6, shows a fibronectin immunoprecipitate from a healthy, PPD-negative subject.
Ag85 could also be demonstrated in Ag85-fibronectin complexes generated in vitro (Fig. 3B1, lane 7). All fibronectin immunoprecipitates contained large amounts of immunoreactive fibronectin (Fig. 3B2, lanes 1 to 7). No Ag85 or fibronectin was precipitated by normal rabbit serum (Fig. 3B1, lanes 1 to 7) or if anti-fibronectin antibodies were omitted from the reaction mixture (data not shown).
Complexes of Ag85 with IgG and fibronectin were stable to repeated freeze-thaw cycles. IgG-Ag85 complexes could be demonstrated by immunoprecipitation even after three freeze-thaw cycles, and fibronectin-Ag85 complexes were demonstrable by immunoprecipitation even after six freeze-thaw cycles (data not shown). Thus, Ag85 proteins circulate in patients with tuberculosis in relatively stable complexes with IgG and fibronectin.
DISCUSSION
The pathophysiology of Ag85 proteins in patients with tuberculosis is complex. Median serum Ag85 levels were significantly higher in patients with active tuberculosis than in patients with active M. avium disease or other pulmonary diseases or with no disease, regardless of skin test reactivity or stage of disease. Although nanogram doses of Ag85 were previously shown to inhibit the local expression of delayed hypersensitivity in vivo (13), much higher serum levels in patients with active tuberculosis bore no relationship to PPD responsiveness. Moreover, high levels of Ag85 antigenemia in tuberculosis patients were not associated with appreciable Ag85 antigenuria. Ag85 is a small enough protein to expect that it would be readily cleared by the kidney from the circulation, much as other small secreted mycobacterial proteins are (36). The discovery that circulating Ag85 in patients with active tuberculosis existed primarily as complexes with fibronectin and IgG provided a reasonable explanation for its low renal clearance. Ag85 antigenuria in the presence of Ag85 complexes could of course occur if rapid dissociation of the circulating complexes led to the release of free antigen at some point in time (37). The relative stability of the complexes to freeze-thaw cycles, as well as their low dissociation constants (likely to be in the range of 10−5 to >10−10 M for Ag85-IgG by analogy with other polyclonal antibodies generated in response to repeated antigenic stimulation [27] and 10−7 M for Ag85-fibronectin [28]) makes rapid complex dissociation in the circulation appear unlikely.
It has been recognized for some time that mycobacterial growth products are detectable in tissues and tissue fluids of patients with active infections (6, 10, 29, 32, 35, 36, 42). Analysis of the pathophysiological interaction of growth products such as Ag85 with the host has previously focused on analyzing immune responses (16, 22, 26, 38) or the composition of circulating immune complexes (31). The availability of an immunoassay able to detect both unbound and complexed Ag85 has permitted the examination of other aspects of Ag85 pathophysiology. This ability to detect complexed Ag85 by immunoassay confirms an earlier report of detection of mycobacterial antigenemia by immunoassay in the presence of circulating immune complexes (35).
Large amounts of Ag85 proteins were demonstrable in plasma protein complexes only in sera from patients with active tuberculosis. No Ag85 was present in plasma fibronectin-Ag85 and IgG immune complexes in sera from patients with inactive tuberculosis or other pulmonary diseases. While no Ag85 was present in IgG immune complexes in sera from healthy controls, small amounts of Ag85 proteins were present in fibronectin-Ag85 complexes in sera from two of six healthy controls. The reason(s) for the presence of immunoreactive 32-kDa material associated with fibronectin detected by immunoprecipitation in the sera from normal individuals may reflect differences in sensitivity between dot blotting and immunoprecipitation. They are, however, also consistent with the low levels of circulating Ag85 detected by dot blot in individuals not suffering from active tuberculosis. Incomplete removal of human IgG immune complexes from these samples is highly unlikely, since complete IgG removal was confirmed on each sample by Western blotting. Clone 240 anti-Ag85 is variably cross-reactive with Ag85 from many nonpathogenic mycobacteria (11), and the low levels of immunoreactive Ag85 bound to plasma fibronectin and measured in sera from patients not diagnosed as having active tuberculosis could reflect this cross-reactivity, which perhaps results from transient or chronic colonization by nonpathogenic mycobacteria. The occurrence of immunoreactive Ag85 in healthy controls could even be a result of a normally silent initial infection with M. tuberculosis itself. Ag85 bound to fibronectin might be detectable for an extended period of time after it was generated, since such complexes, unlike IgG immune complexes, are not known to be preferentially removed from the circulation.
The Ag85 complex of M. tuberculosis consists of two bands seen in SDS-PAGE, one at 32 kDa containing Ag85A and Ag85C and the other at 30 kDa containing Ag85B (11, 14). Because clone 240 anti-Ag85 reacts equally well with Ag85A and Ag85B (11) and can recognize a 30- to 32-kDa doublet on Western blots of the purified Ag85 complex standard, the single band in Western blots of immunoprecipitates or purified Ag85 complex proteins is not simply due to lack of antibody specificity. While it could be indicative of large amounts of Ag85B not being present in the circulation of patients with active tuberculosis, it could also merely reflect assay variability that leads to a loss of resolution as a result of the particular doses of Ag85 complex proteins applied to the SDS-PAGE gel.
The increased circulating levels of Ag85 displayed by patients with active tuberculosis were similar in PPD-positive and PPD-negative (anergic) patients, and there was no significant difference in median serum levels of Ag85 between these two patient groups. Ag85 inhibits local expression of delayed hypersensitivity by binding to T-cell fibronectin (13), and increased levels of this secretory product might be expected to modify local host effector response at tissue sites of mycobacterial growth (32). However, elevated circulating levels of Ag85 had no apparent effect on systemic reactivity to PPD. This lack of effect of Ag85 on systemic expression of delayed-type hypersensitivity in these patients might reflect inhibition of its activity after formation of complexes with plasma proteins. Studies to confirm this hypothesis are currently in progress in our laboratory.
Although the numbers of tuberculosis patients examined were small, increases in serum Ag85 appeared to be independent of the stage of tuberculosis disease or the presence of acid-fast bacilli on sputum smears. Monoclonal anti-Ag85 antibodies of appropriate specificity distinguished patients with active tuberculosis from those with active M. avium disease. Elevated levels of serum Ag85 were also detected in a single patient with sarcoidosis, a disease of unknown etiology which has been suggested to be due to mycobacterial infection (4). These results suggest that measurement of circulating Ag85 might be developed into a diagnostic test for active mycobacterial infection that was independent of host immune response. Studies on larger numbers of patients with different stages of tuberculosis to examine this possibility are currently in progress.
In sum, median levels of circulating Ag85 are significantly higher in patients with active tuberculosis than in patients with other diseases or healthy controls. No increases in urinary Ag85 were detected in any patient, regardless of the serum Ag85 level. In patients with active tuberculosis, Ag85 circulates primarily as complexes with immunoglobulins and plasma fibronectin rather than in unbound form. The existence of Ag85 complexes with plasma proteins accounts for its lack of urinary clearance.
ACKNOWLEDGMENTS
This work was supported by grant AI37014 from the U.S. National Institutes of Health, The Public Health Service, and the Department of Health and Human Services and by grant NFWO 3.0020.89 from the Belgium National Research Foundation.
REFERENCES
- 1.Abou-Zeid C, Ratliff T L, Wiker H G, Harboe M, Bennedsen J, Rook G A W. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988;56:3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin S L, D’Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I A. Evaluation of new vaccine in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Basra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biosynthesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 4.Bocart D, Lecossier D, de Lassence A, Valeyre D, Battesti J-P, Hance A J. A search for mycobacterial DNA in granulomatous tissues from patients with sarcoidosis using the polymerase chain reaction. Am Rev Respir Dis. 1992;145:1142–1148. doi: 10.1164/ajrccm/145.5.1142. [DOI] [PubMed] [Google Scholar]
- 5.Cantwell M F, Snider D E, Jr, Cauthen G M, Onorato I M. Epidemiology of tuberculosis in the United States, 1985 through 1992. JAMA. 1994;272:535–539. [PubMed] [Google Scholar]
- 6.Daniel T M. Antibody and antigen detection for the immunodiagnosis of tuberculosis: Why not? What more is needed? Where do we stand today? J Infect Dis. 1988;158:678–680. doi: 10.1093/infdis/158.4.678. [DOI] [PubMed] [Google Scholar]
- 7.Daniel W W. Applied non-parametric statistics. Boston, Mass: PWS-Kent Publishers; 1990. [Google Scholar]
- 8.De Bruyn J, Huygen K, Bosmans R, Fauville M, Lippens R, Van Vooren J P, Falmagne P, Wiker H G, Harboe M, Turneer M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987;2:351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- 9.Denis O, Lozes E, Huygen K. Induction of cytotoxic T-cell responses against culture filtrate antigens in Mycobacterium bovis bacillus Calmette-Guérin-infected mice. Infect Immun. 1997;65:676–684. doi: 10.1128/iai.65.2.676-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doan C A. Diagnostic significance of precipitin test with Anderson phosphatide fractions from human, bovine and avian tubercle bacilli. Proc Soc Exp Biol Med. 1929;26:672–677. [Google Scholar]
- 11.Drowart A, De Bruyn J, Huygen K, Damiani G, Godfrey H P, Stelandre M, Yernault J-C, Van Vooren J-P. Isoelectric characterization of protein antigens present in mycobacterial culture filtrates and recognized by monoclonal antibodies directed against the Mycobacterium bovis BCG antigen 85 complex. Scand J Immunol. 1992;36:697–702. doi: 10.1111/j.1365-3083.1992.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey H P, Canfield L S, Kindler H L, Angadi C V, Tomasek J J, Goodman J W. Production of a fibronectin-associated lymphokine by cloned mouse T cells. J Immunol. 1988;141:1508–1515. [PubMed] [Google Scholar]
- 13.Godfrey H P, Feng Z-H, Mandy S, Mandy K, Huygen K, De Bruyn J, Abou-Zeid C, Wiker H G, Nagai S, Tasaka H. Modulation of expression of delayed hypersensitivity by mycobacterial antigen 85 fibronectin-binding proteins. Infect Immun. 1992;60:2522–2528. doi: 10.1128/iai.60.6.2522-2528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harth G, Lee B-Y, Wang J, Clemens D L, Horwitz M A. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3036–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasløv K, Andersen Å B, Nagai S, Gottschau A, Sørensen T, Andersen P. Guinea pig immune responses to proteins secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:804–810. doi: 10.1128/iai.63.3.804-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havlir D V, Wallis R S, Boom W H, Daniel T M, Chervenak K, Ellner J J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991;59:665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetland G, Wiker H G. Antigen 85C on Mycobacterium bovis BCG and M. tuberculosis promotes monocyte-CR3-mediated uptake of microbeads coated with mycobacterial products. Immunology. 1994;82:445–449. [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz M A, Lee B W, Dillon B J, Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, De Witt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficiency of a tuberculosis DNA vaccine. Nat Med. 1996;2:857–859. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 20.Huygen K, Ljungqvist L, ten Berg R, Van Vooren J-P. Repertoires of antibodies to culture filtrate antigens in mouse strains infected with Mycobacterium bovis BCG. Infect Immun. 1990;58:2192–2197. doi: 10.1128/iai.58.7.2192-2197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, De Bruyn J, Van Vooren J-P, DeLeys R. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huygen K, Van Vooren J P, Turneer M, Bosmans R, Dierckx P, De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32-kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988;27:187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 23.Hynes R O. Fibronectins. New York, N.Y: Springer Verlag; 1990. [Google Scholar]
- 24.Jesty J, Godfrey H P. Parlin, a general microcomputer program for parallel-line analysis of bioassays. Am J Clin Pathol. 1986;85:485–489. doi: 10.1093/ajcp/85.4.485. [DOI] [PubMed] [Google Scholar]
- 25.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 26.Launois P, Huygen K, De Bruyn J, N’Diaye M, Diouf B, Sarthouj L, Grimaud J, Millan J. T cell response to purified filtrate antigen 85 from Mycobacterium bovis Bacilli Calmette-Guérin (BCG) in leprosy patients. Clin Exp Immunol. 1991;86:286–290. doi: 10.1111/j.1365-2249.1991.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lozes E, Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Vandenbussche P, Van Vooren J P, Drowart A, Ulmer J, Liu M A. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–833. doi: 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 28.Mandy S, Feng Z, Canfield L S, Mandy K, Quan X, Rowehl R A, Khan M Y, Akiyama S K, Godfrey H P. Inhibition of expression of delayed hypersensitivity by neutralizing monoclonal anti-T cell fibronectin antibody. Immunology. 1994;83:582–588. [PMC free article] [PubMed] [Google Scholar]
- 29.Odham G, Larsson L, Mardh P A. Demonstration of tuberculostearic acid in sputum from patients with tuberculosis by selected ion monitoring. J Clin Invest. 1979;63:813–819. doi: 10.1172/JCI109380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orme I. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance, in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988;56:3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raja A, Narayananm P R, Mathew R, Prabhakar R. Characterization of mycobacterial antigens and antibodies in circulating immune complexes from pulmonary tuberculosis. J Lab Clin Med. 1995;125:581–587. [PubMed] [Google Scholar]
- 32.Rambukkana A, Das P K, Krieg S, Faber W S. Association of the mycobacterial 30-kDa region proteins with the cutaneous infiltrates of leprosy lesions: evidence for the involvement of the major mycobacterial secreted proteins in the local immune response of leprosy. Scand J Immunol. 1992;36:35–48. doi: 10.1111/j.1365-3083.1992.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 33.Ratliff T L, McGarr J A, Abou-Zeid C, Rook G A W, Stanford J L, Aslanzadeh J, Brown E J. Attachment of mycobacteria to fibronectin-coated surfaces. J Gen Microbiol. 1988;134:1307–1313. doi: 10.1099/00221287-134-5-1307. [DOI] [PubMed] [Google Scholar]
- 34.Rinke de Wit T F, Bekelie S, Osland A, Wieles B, Janson A A M, Thole J E R. The Mycobacterium leprae antigen 85 complex gene family: identification of the genes for the 85A, 85C, and related MPT51 proteins. Infect Immun. 1993;61:3642–3647. doi: 10.1128/iai.61.9.3642-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sada E, Aguilar D, Torres M, Herrera T. Detection of lipoarabinomannan as a diagnostic test for tuberculosis. J Clin Microbiol. 1992;30:2415–2418. doi: 10.1128/jcm.30.9.2415-2418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sippola A A, Gillespie S L, Lewis J A, Daniel T M. Mycobacterium avium antigenuria in patients with AIDS and disseminated M. avium disease. J Infect Dis. 1993;168:466–468. doi: 10.1093/infdis/168.2.466. [DOI] [PubMed] [Google Scholar]
- 37.Taylor A E, Granger D N. Exchange of macromolecules across the microcirculation. In: Renkin E M, Michel C C, editors. Handbook of physiology. Section 2: the cardiovascular system. IV, part 1. Bethesda, Md: American Physiological Society; 1984. pp. 467–520. [Google Scholar]
- 38.Turneer M, Van Vooren J P, De Bruyn J, Serruys E, Dierckx P, Yernault J C. Humoral immune response in human tuberculosis: immunoglobulins G, A, and M directed against the purified P32 protein antigen of Mycobacterium bovis bacillus Calmette-Guérin. J Clin Microbiol. 1988;26:1714–1719. doi: 10.1128/jcm.26.9.1714-1719.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Vooren J P, Turneer M, Yernault J C, De Bruyn J, Burton E, Legros F, Farber C M. A multidot immunobinding assay for the serodiagnosis of tuberculosis. Comparison with an enzyme-linked immunosorbent assay. J Immunol Methods. 1988;113:45–49. doi: 10.1016/0022-1759(88)90380-8. [DOI] [PubMed] [Google Scholar]
- 40.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiker H G, Harboe M, Nagai S, Patarroyo M E, Ramirez C, Cruz N. MPB59, a widely cross-reacting protein of Mycobacterium bovis BCG. Int Arch Allergy Appl Immunol. 1986;81:307–314. doi: 10.1159/000234154. [DOI] [PubMed] [Google Scholar]
- 42.Yáñez M A, Coppola M P, Russo D A, Delaha E, Chaparas S D, Yeager H. Determination of mycobacterial antigens in sputum by enzyme immunoassay. J Clin Microbiol. 1986;23:822–825. doi: 10.1128/jcm.23.5.822-825.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]