SUMMARY
SETTING:
TBM-KIDS is a phase I/II trial enrolling children with tuberculous meningitis (TBM) in three tertiary referral centers in India and Malawi.
OBJECTIVE:
To describe the challenges encountered in conducting the first randomized clinical trial of antimicrobial agents in pediatric TBM.
DESIGN:
The sources of the data were primarily monthly trial reports, non-enrollment case report forms, study diaries and registers maintained for recruitment, experiences shared by key team members during regular study calls and comments from site review visits. We reviewed, broadly categorized, and describe in detail the challenges encountered by study teams in trial implementation.
RESULTS:
Over 17 months, 3371 children with clinical presentations consistent with meningoencephalitis or undergoing lumbar puncture were assessed for eligibility; 21 (<1%) met enrollment criteria. We encountered challenges related to diagnosis, management of sick children, large catchment areas, adverse event attribution, concomitant medications, infrastructure requirements, expensive pediatric formulations with short expiry, and detection of treatment response in a highly variable disease across the age continuum. Training and adaptation of tools for neurocognitive and neurologic function assessment were necessary. Special care was undertaken to explain study participation to distraught caregivers and manage children longitudinally.
CONCLUSION:
Interventional trials in pediatric TBM are challenging but are critically important for improving the treatment of a disease that disables children physically, cognitively and emotionally. Sharing these challenges may help to address them more effectively as a TB research community and to advance treatments for this at-risk population.
Keywords: tuberculous meningitis, pediatric, diagnosis, treatment, clinical trial
RÉSUMÉ
CONTEXTE:
TBM-KIDS est un essai de phase I/II enrôlant des enfants atteints de méningite tuberculeuse (TBM) dans trois centres de référence tertiaires en Inde et au Malawi.
OBJECTIF:
Décrire les défis rencontrés dans la réalisation du premier essai clinique randomisé de médicaments antimicrobiens de la TBM pédiatrique.
SCHÉMA:
Les sources des données ont été en premier lieu les rapport mensuels de l’essai, des formulaires de rapport de cas non enrôlés, des journaux d’étude et des registres tenus pour le recrutement, des expériences partagées par les membres principaux de l’équipe lors d’appels réguliers pendant l’étude et les commentaires lors de visites des sites. Nous avons revu, rapidement cassé en catégories et décrit en détail les défis rencontrés par les équipes de l’étude dans la mise en œuvre de l’essai.
RÉSULTATS:
En 17 mois, 3371 enfants ayant une présentation clinique compatible avec une méningoencéphalite ou soumis à une ponction lombaire ont été évalués en vue de leur éligibilité; 21 (<1%) ont répondu aux critères d’enrôlement. Nous avons rencontré des défis liés au diagnostic, à la prise en charge des enfants malades, aux vastes zones de desserte, à l’attribution des effets secondaires, aux médicaments concomitants, aux exigences/besoins des infrastructures, au coût des formules pédiatrique de courte durée de conservation et à la détection de la réponse au traitement dans le cadre d’une maladie très variable avec l’âge. Une formation et une adaptation des outils d’évaluation de la fonction neurocognitive et neurologique ont été nécessaires. Nous avons fait particulièrement attention à expliquer la participation à l’étude à des parents éperdus et à prendre en charge les enfants dans la durée.
CONCLUSION:
Les essais d’intervention en matière de TBM sont un défi mais sont cruciaux pour améliorer le traitement de cette maladie qui entrâine chez les enfants un déficit physique, cognitif et émotionnel. Partager ces défis pourrait contribuer à les affronter de manière plus efficace comme une communauté de recherche en matière de TB, pour faire progresser la prise en charge de cette population à risque.
RESUMEN
MARCO DE REFERENCIA:
El ensayo clínico TBM-KIDS es un estudio de fase I/II de niños con meningitis tuberculosa (TBM) en tres centros de referencia de atención terciaria en la India y Malawi.
OBJETIVO:
Describir las dificultades encontradas al llevar a cabo el primer ensayo clínico aleatorizado sobre los fármacos antimicrobianos en casos de TBM en pediatría.
MÉTODO:
Las principales fuentes datos fueron los informes mensuales del ensayo, los formularios de notificación de casos no incluidos, los cuadernos de datos del estudio y los registros mantenidos durante la inclusión de los participantes, las experiencias compartidas por los principales miembros del equipo durante las llamadas corrientes durante el estudio y los comentarios de las visitas de evaluación a los centros. Las dificultades se analizaron, se clasificaron en categorías amplias y se describieron en detalle los problemas encontrados por los equipos del estudio durante su ejecución.
RESULTADOS:
Durante 17 meses se evaluó la aptitude para participar en el ensayo de 3371 niños con cuadros clínicos indicativos de meningoencefalitis o en quienes se practicaba una punción lumbar; 21 niños cumplieron los criterios de inclusión (<1%). Se encontraron dificultades relacionadas con el diagnóstico, el tratamiento de los niños enfermos, las amplias zonas de cobertura, la atribución de los acontecimientos adversos, los medicamentos concomitantes, las necesidades de infraestructura, el alto costo de las formulaciones pediátricas con cortos periodos de caducidad y la detección de la respuesta al tratamiento de una enfermedad sumamente variable en las diferentes edades. Fue necesario utilizar herramientas de capacitación y adaptación para la evaluación de la función neurocognoscitiva y neurológica. Se tuvo especial cuidado en explicar a los cuidadores angustiados la participación al estudio y el manejo longitudinal de los niños.
CONCLUSIÓN:
Los estudios clínicos de intervención en casos de TBM en pediatría son difíciles de realizar pero de gran importancia para mejorar el tratamiento de esta enfermedad que causa discapacidad física, cognitiva y emocional. Compartir estas dificultades puede ayudar a la comunidad de investigación en tuberculosis a abordarlas con mayor eficacia y a proponer tratamientos dirigidos a estos grupos vulnerables de la población.
IN 2017, THERE WERE AN ESTIMATED 1 million children with TB, with 250 000 deaths in children <15 years old.1 Due to their immature immune systems, young children (<5 years) are at high risk of developing disseminated TB following exposure, including tuberculous meningitis (TBM), the most devastating form of the disease. Prompt and accurate diagnosis of TBM is key,2 however, early diagnosis is challenging given the low number of bacilli in the cerebrospinal fluid (CSF). It is particularly difficult in children given they present with non-specific symptoms and signs which mimic other conditions. Even with treatment, mortality from pediatric TBM is about 20%, with > 50% of survivors experiencing permanent neurologic sequelae.3–5 In addition, pediatric TBM affects the developing mind, with Mycobacterium tuberculosis infecting microglia, cells that contribute to neurocognitive development.6,7 Sequelae of TBM that are unique to children include developmental delay and emotional disability.8
Clinicians are often faced with providing urgent, empiric treatment for TBM without diagnostic confirmation.9 But which drugs to give? Regimens for TBM are not experimentally derived but are extrapolated from studies of treatment for adult pulmonary TB—same drugs, same doses—despite differences in pathophysiology and drug penetration into the sites of infection. However, some advances in therapeutics for adult TBM have recently emerged, raising the possibility of improved outcomes with more rational treatment.10–14
In children, drug disposition differs from adults, and, additionally, TBM treatment outcomes are different in children and adults, with children suffering developmental disabilities and perhaps lower mortality. Considering the high risk of functional and neurodevelopmental impairment following pediatric TBM, better treatment may impact both mortality and quality of life. US Food and Drug Administration (FDA) guidelines specify that findings from clinical trials of novel treatments conducted in adults can be extrapolated to children via pharmacokinetic (PK) and safety studies, provided treatment responses are expected to be similar. Efficacy studies are required when response to treatment is anticipated to be different in the two populations.15,16 Accordingly, for TBM, while PK and safety studies are helpful, trials that characterize outcomes (namely functional and neurocognitive outcomes) and their relationship to drugs or drug exposures are needed to quantify the effects of new regimens comprehensively.
To date, there has not been a randomized clinical trial of antimicrobial regimens for pediatric TBM.17 The TBM-KIDS trial is the first to evaluate the PK, safety, and efficacy of high-dose rifampin (with or without levofloxacin) as part of multidrug treatment compared to standard treatment for pediatric TBM, building on efficacy data from adults.10,12,18–21 There have been a few studies detailing the challenges and emphasizing the importance of conducting clinical trials in children in general.22–26 These studies focus on ethical, regulatory, operational, pathophysiological, social, and economical factors. However, there is no published literature addressing the challenges of conducting pediatric TBM and/or meningitis trials in resource limited settings. The TBM-KIDS trial is open and enrolling in India and Malawi, and has provided a vital opportunity to learn lessons from the field. In this study, we describe the unique challenges encountered in the development and conduct of this interventional trial.
METHODS
Trial design
TBM-KIDS (NCT02958709) is a phase I/II, multisite, randomized, open-label clinical trial in children aged 6 months to 12 years with probable or definite TBM.27 The trial schema is shown in Figure 1.
Figure 1.
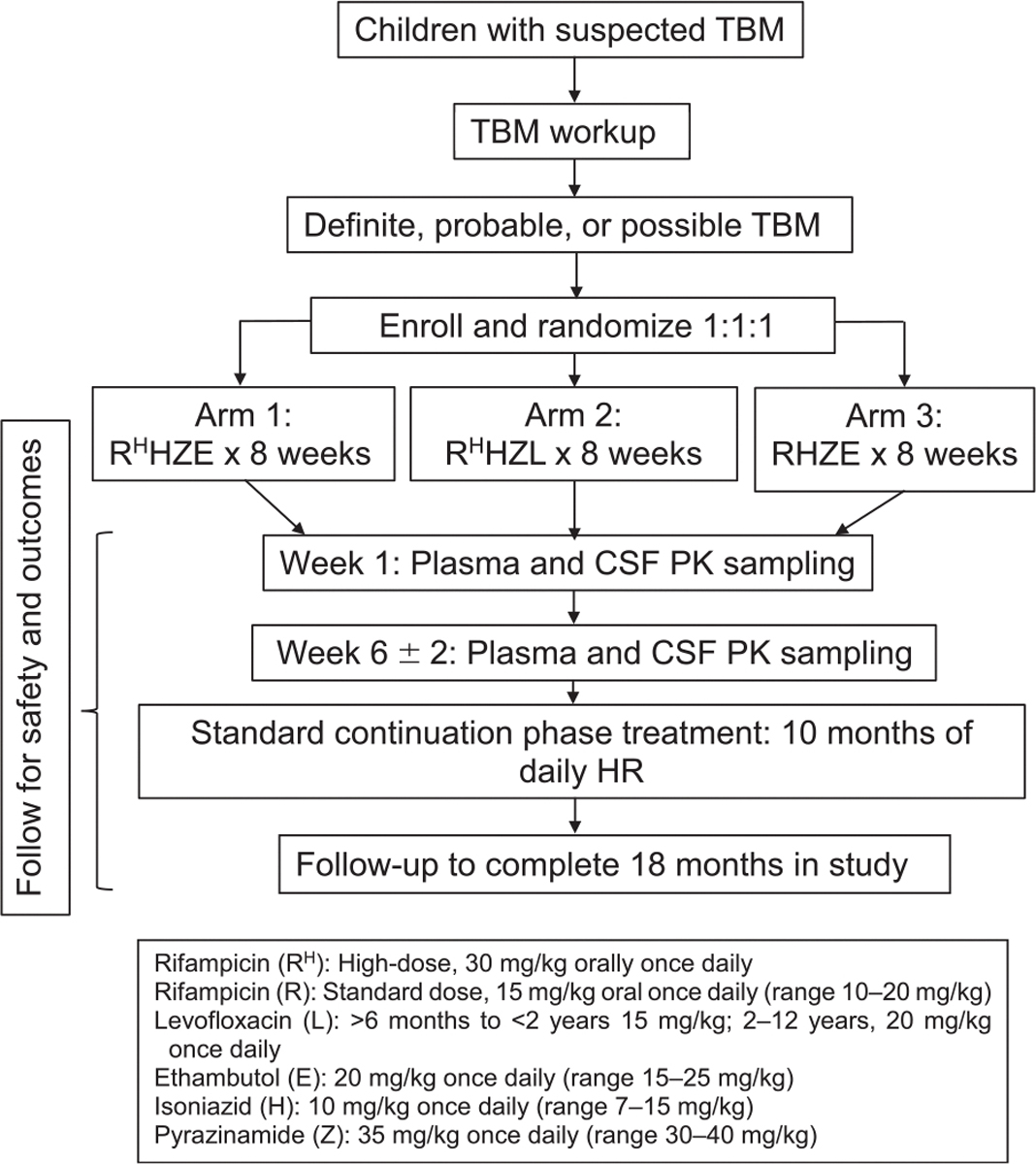
TBM-KIDS Trial study schema. TBM = tuberculous meningitis; CSF = cerebrospinal fluid; PK = pharmacokinetics.
Selection of study sites and participants
Clinical trial sites had to have a close affiliation with 24-h hospitals with pediatric inpatient and emergency care; specialty care for sick children; a fully functional and equipped intensive care unit; standardized laboratory facilities; neuroimaging; and experience in pediatric research and clinical trials. Pediatric TBM had to be common locally. Sites also had to be able to randomize patients according to the WHO-recommended control arm; this was not possible in settings where national guidelines required a very different treatment.28
Participants are enrolled from three sites, all at tertiary referral hospitals, in Chennai and Pune, India, and Lilongwe, Malawi, which met all site selection criteria. The trial was approved by local ethics committees at each site and the Johns Hopkins Institutional Review Board, Baltimore, MD, USA. Exclusion criteria include known exposure to multidrug-resistant (MDR) TB, grade ≥2 elevations of creatinine, alanine aminotransferase (ALT) or direct bilirubin, TB treatment for > 10 days, and antiretroviral treatment (ART) with nevirapine or protease inhibitors.
Study procedures
The study staff at each site underwent extensive training in protocol and manual procedures by the study Principal Investigator (PI) and the Global study coordinator before site initiation. Furthermore, each site PI and coordinator additionally retrained the staff on these aspects and the standard operating procedures. All the site staff are trained in human subject protection, and good clinical practices, and the laboratory staff underwent training in clinical laboratory practices.
Children with symptoms of meningoencephalitis and/or undergoing lumbar puncture are approached, with review of charts for clinical and laboratory data to assess the probability of meningoencephalitis. Collection and testing of additional CSF is performed as part of pre-screening consent at Indian sites. Children with findings possibly consistent with TBM are fully screened. Screening evaluations include informed consent, followed by collection of information required to calculate the TBM score (e.g., symptom screening, magnetic resonance imaging [MRI], Xpert testing) to see if the child meets criteria for probable or definite TBM, based on the consensus research definition, as assessed by the site PI.27 Eligible participants are randomized to one of three treatment arms (and are provided study drugs for 1 year). They undergo plasma and CSF sampling (for PK analysis, glucose level, cell count, protein) within the first week and then at 4–8 weeks, serial safety assessments (complete blood counts, chemistries), and assessment of functional (neurologic) and neurocognitive outcomes over 12–18 months (Supplementary Table S1 gives the schedule of evaluations).29
Enumeration of challenges
The source of the challenges data was primarily the monthly trial reports prepared by the respective site teams, which are based on the reasons for non-screening and non-enrollment captured on the ‘non-enrollment’ case report form (CRF). These CRFs were completed by the respective site clinician who was primarily responsible for ascertaining the eligibility of the potential participants for the trial. The other sources include the study diaries/registers maintained for study recruitment, experiences shared by the key team members during the regular study calls as well as during site review visits by the central team members. The challenges encountered by study teams during the recruitment and management of participants are reviewed, broadly categorized, and described in detail.
RESULTS
Study progress to date
The study opened at Byramjee Jeejeebhoy Government Medical Centre (BJGMC) in Pune in February 2017, University of North Carolina Project Malawi/Kamuzu Central Hospital in Lilongwe in May 2017, and the National Institute for Research in Tuberculosis (NIRT)/Institute for Child Health (ICH) in Chennai in June 2017. By August 2018, 3371 children with clinical presentations consistent with possible infectious meningoencephalitis or undergoing lumbar puncture were assessed for eligibility. Of these, 1288 were pre-screened, 50 were screened, and 21 were enrolled. Figure 2 shows the reasons children did not qualify.
Figure 2.

CONSORT (Consolidated Standards of Reporting Trials) diagram showing number of children approached, pre-screened, screened, and enrolled to date in the TBM-KIDS Trial. TB = tuberculosis; LP = lumbar puncture; TBM = tuberculous meningitis; HIV = human immunodeficiency virus; ART =antiretroviral therapy; CT =computed tomography.
Finding and supporting study sites for pediatric TBM interventional trials
As noted above, potential clinical trials sites had to meet all criteria for participation, and these sites were rare. As TBM is uncommon, even in TB-endemic settings, multiple sites should be involved to achieve enrollment goals. Each site requires full-time staff, ongoing training (neurologic and neurocognitive assessments), and resources both for the science and for patient care.
Diagnostic challenges
Headache, fever, vomiting, or irritability can be signs of TBM. Among children presenting with these symptoms, few will have TBM (Figure 2). Even those children with early stage disease who do have TBM may not meet criteria for probable or definite TBM at the time of screening, as their CSF and clinical findings are not specific, and their CSF AFB stain and Xpert are often negative. Children with more advanced disease may have signs of meningeal irritation, cranial nerve palsies, motor or sensory deficits, and altered level of consciousness. Diagnosis of TBM in these children may be hindered by clinician concerns about the safety of lumbar puncture (LP) or by the quantity of CSF required to make the diagnosis. All the tools required for diagnosis, including Xpert and neuroimaging were available at all the sites. However, accessibility was a challenge occasionally and Xpert Ultra, while more sensitive, is not marketed in many countries, and official procedures and permission for importation and use can delay its use. CT and MRI technology and neuroradiology expertise are not universally available, and machines may not be regularly serviced. In addition, the high local incidence of infectious diseases that present with fever, seizures, and encephalopathy (such as malaria or pneumococcus) can delay diagnosis of TBM, and bacterial meningitis may represent a higher proportion of meningitis cases in settings where pneumococcal and meningococcal vaccines are not offered routinely.30,31
High screening-to-enrollment ratio
Because TBM in children is rare, almost all the children presenting to the hospital wards or emergency departments with CNS signs and symptoms or who have laboratory samples sent for CSF studies are approached, pre-screened, and followed by study team members until a TBM diagnosis or an alternative is reached. Hence a large number of children need to be followed to identify eligible participants (60–200 children pre-screened and 4–8 screened for one enrollment, Figure 2). More recently, we are including children with longer pre-treatment (up to 10 days) and with possible TBM, where the clinician has committed the patient to TBM treatment. A considerable amount of human, laboratory, and financial resources need to be considered in the trial budget. As the catchment area for large tertiary care centers is large, some of the children diagnosed with TBM and initiated on anti-TB treatment are from neighboring states and speak a different language, so they cannot be recruited because their understanding of study procedures and risks cannot be assured (consent forms are available in many, but not all local languages). Care and retention of migrant children or children from distant towns, similarly, cannot be assured. Lastly, some families declined participation due to personal beliefs or social reasons, or left the hospital with their children to pursue traditional or comfort care only.
Challenges in enrolling and caring for very sick children
The trial centers are tertiary referral hospitals capable of caring for very ill children, so many children present with advanced disease, having been managed at smaller centers before transfer. They often arrive in a moribund condition. Enrollment procedures cannot interfere with critical or surgical care (e.g. a fitting a ventriculoperitoneal [VP] shunt for hydrocephalus for two participants, for which temporary transfer to a neurosurgery center was required; for another child, critical illness including the need for a VP shunt precluded participation). Special care should be taken in communicating with the parents of very ill children about study participation: even with thoughtful patient counselling, many parents are hesitant to enroll their ill child in a trial due to concerns about experimental regimens and invasive procedures such as LP. Lastly, care expenses are high for such ill children. Clinical trials insurance, which is compulsory, costs tens of thousands of dollars per year (even when trial participants are few).
Once enrolled in the study, the assessment of adverse events and establishing a relatedness to study medications is a challenge. Seizures, for example, can be due to the disease or its treatment (isoniazid, levofloxacin). Serious adverse events will happen in very ill children. Strict study stopping rules based on toxicity grading or use of prohibited medications can result in children being removed from study unnecessarily, depriving a child in need of the benefit of the comprehensive care and monitoring that comes with the trial, harming the trial itself and the possible benefits for future children with TBM. Careful assessment of causality and relationship to the study drugs is essential.
Treatment challenges
Current WHO-recommended doses of first-line drugs for children are isoniazid (H, 5–10 mg/kg), rifampin (R, 10–15 mg/kg), pyrazinamide (Z, 25–35 mg/kg), and ethambutol (E, 20 mg/kg).32 Only one fixed-dose combination (FDC) formulation is available with drug ratios that make it possible to achieve those doses of RHZ (75:50:150) (intensive phase) or RH (75:50) (continuation phase).32 For that reason, we use these WHO-prequalified FDCs, which also are palatable and dispersible. For two arms, supplemental rifampin is required to reach the 30 mg/kg dose. Most stand-alone rifampin is either in tablet form (unsuitable dose size for young children), or in suspension formulations that are sticky or display poor bioavailability. Ethambutol is available only in 100 mg tablets. A new dispersible formulation of levofloxacin is used. Our dosing tables are complex, with dosing information for children weighing 6–40 kg. Notably, study drugs should be stored at cool temperatures, and many study families do not have refrigeration in their homes. The biggest challenge for the pediatric formulations is their availability and short shelf life. Currently only one company manufactures WHO-compliant pediatric FDC of RHZ and RH for children. The minimum number of doses in one manufactured batch is 1 650 000 tablets, and our trial drug requirements are low. Further, as the shelf life of study drugs is short (2 years), mid-study resupplying is necessary. Drug costs are very high. Recently, the Global Drug Facility (GDF) has made drugs available for trials and so we have shifted our purchasing to GDF.
As many children with TBM have pre-existing or disease-related comorbid conditions (such as seizures) requiring treatment, drug interactions with rifampin (including high-dose) should be considered. In Malawi, six children with HIV infection were excluded due to current or planned protease inhibitor (PI) use (there are no data on high-dose rifampin with PIs); 3 had definite TBM. While initiation of ART is contraindicated in adults with TBM, there are no international guidelines for timing of ART initiation in children with HIV-associated TBM.33 Hospital-acquired infections are common in critically ill children and are often treated with drugs that have some activity against TBM. Furthermore, pre-existing conditions (developmental delay, cerebral palsy) complicate evaluations of important study endpoints including neurocognitive outcomes.
Measuring treatment effect
More effective TBM treatment may result in better functional outcomes and improved neurocognitive function. Given the lack of previous pediatric TBM trials, there were no standard outcomes measures of neurologic function to use. The six-point modified Rankin Scale (mRS) considers disability and independence with tasks and is useful for assessing functional status. Issues with the mRS are that the tool has been used predominantly in the adult stroke population, and has been shown to have inter-rater variability as the assessment tool is subjective. We have used the gross motor function classification system to measure neurologic status more objectively and as a guidance tool for assigning mRS score, the primary outcome, with ongoing video training by a seasoned neurologist. For neurocognitive testing, there is no single test that can be used across the pediatric age span. The use of neurocognitive test results as a study outcome across multiple study sites spanning different continents with varied languages and cultures poses challenges. The Mullen Scales of Early Learning, an individually-administered comprehensive measure of cognitive functioning that may be used from birth through 68 months of age (the most common age for TBM), was selected for our study given its high reliability, validity demonstrated in groups of children at risk for developmental delay, and use in multicultural settings.34,35 The measure had to be adapted for cultural fairness (while still assessing relevant brain function) which included translation of instructions into multiple local languages, and testing in normally-developing children to give study results context is still in progress. Training of testers requires significant dedication by a professional neuropsychologist (with videotaped practice sessions and detailed feedback), and administration of the adapted test in medically complex children with brain disease is challenging, as they are often uncooperative and longer duration is needed for testing with breaks as these children are often irritable and lethargic. Nevertheless, longitudinal testing of functional status and neurocognitive function allows us to assess treatment response relationships in a comprehensive fashion.
Adherence and retention challenges
TBM patients are from a large catchment area. Caregivers travel far, and return for follow-up visits can result in lost wages and child care issues for siblings. TBM management in a trial is challenging because the care involves an inpatient stay, long duration of treatment, and frequent visits for complications. To ensure caregivers administer study drugs properly, training in administering the medications, adherence counselling, and dispensing medications in day-wise pill covers for tablets and small boxes for syrup is critical. The study team has experienced behavioral, familial, parental, marital and social factors which directly or indirectly affect treatment adherence, compliance with study procedures, and retention. At one site, two children left the study early due to financial and family issues that led to relocation.
DISCUSSION
TBM is the most severe and disabling form of TB, and young children are at particular risk. The disease and its treatment occur in children who are learning to walk, talk, and interact, so the consequences of inadequate treatment may be lifelong. In spite of this, we have no clinical trials data to guide treatment in this population, with drug selection and dosing extrapolated from adult trials or pulmonary TB.
Without trials specifically conducted in children, we will not know if antimicrobial optimization, host directed treatment, or both will help children with functional and neurocognitive sequelae that are unique to them. However, pediatric TBM trials are difficult to conduct for a broad variety of reasons. Recognizing and addressing these challenges may help us advance better, more efficient treatments for this therapeutically orphaned subpopulation (Table).
Table.
Challenges encountered in the implementation of a therapeutic trial for pediatric TBM, and resources and advancements needed to facilitate future research in this area
| Challenges in conducting TBM trials | Requirements for optimal implementation |
|---|---|
|
| |
| Tertiary hospital setting required, high screening-to-enrollment ratios, and large catchment areas | Substantial financial and human resources |
| TBM is rare in any one location | More resources for multisite recruitment |
| Diagnosis of TBM is hard to make and accrual is slow. | Advanced and rapid diagnostics with high sensitivity for confirming TBM • Xpert Ultra, a test with higher sensitivity for detecting M. tuberculosis can be used—import approval into India has just been received • Further work on CSF diagnostics and biomarkers may be useful • Screening of all children with severe forms of TB to look for TBM may help identify TBM Low cost and child-friendly drug formulations with longer shelf life, |
| Anti-TB drug formulations are expensive, not readily available for pediatric populations, have shorter expiry periods and it is challenging to optimize the dose across weight bands | Low cost and child-friendly drug formulations with longer shelf life, allowing appropriate dosing across the different weight bands, is readily available and accessible • Pediatric formulations, particularly stand-alone rifampin, would be useful for (commonly under-dosed) intrathoracic TB, as well as TBM in children • The Global Drug Facility is now allowing investigators to purchase study drugs for clinical trials from their facility, reducing the amount of drug that should be purchased, by batching orders to the company |
| Training in assessing functional status and neurocognitive function is intensive and should be ongoing | Capacity building to develop or build upon existing expertise—neurology, neurocognitive and neuroradiological assessments—at international sites in areas where TB is common is key |
| It may be challenging to detect treatment response in a highly variable disease in children across the age continuum | Validated surrogate markers of treatment response for TBM in children so that we can discriminate efficiently among experimental therapies and identify those regimens that work best for neurologic, functional, and neurocognitive recovery. Longitudinal assessments of functional status or neurocognitive function may be useful in that regard, coupled with exposureresponse assessments |
| Children suffer comorbid conditions (hospital-acquired infections, seizures, nephrotic syndrome, malignancies, etc.) which should be managed, with attention to drug interactions, adverse event determination, and unintended anti-TB effects of concomitant medications | Laboratory capacity to perform drug-level monitoring, and for diagnosing alternative etiologies to aid the accurate assessment of the relationship of study and/or concomitant medications to the adverse events |
| Importantly, families and children suffer terribly from this disease, so extreme care is needed in explaining risks and benefits of study participation and in longitudinal care of these children and their caregivers over months-long treatment | Qualitative research to help us understand the sociodemographic, behavioral, and other barriers to trial participation and treatment adherence would aid in improving enrollment strategies and support structures |
Pediatric TBM trials should take place in tertiary hospitals, have high screening-to-enrollment ratios, involve large catchment areas, and have substantial financial and human resources. Since TBM in any one location is rare, multiple sites are needed, and even then accrual is slow. The diagnosis is hard to make. Drugs are not formulated for pediatric use or have short expiry dates, they have storage and temperature requirements, are expensive, and dosing varies substantially by weight. Children suffer comorbid conditions (hospital-acquired infections, seizures) which need to be managed, with attention to drug interactions, adverse event determination, and unintended anti-TB effects of concomitant medications. Training in assessing functional status and neurocognitive function is intensive and should be ongoing. It may be challenging to detect treatment response in a highly variable disease in children across the age continuum. Importantly, families and children suffer terribly from this disease, so extreme care is needed in explaining the risks and benefits of study participation and in longitudinal care of these children and their caregivers over many months of treatment.
What can be done? First, Xpert Ultra, with higher sensitivity for detecting M. tuberculosis can be used—and approval for import into India has recently been received. Further work on CSF diagnostics and biomarkers may be useful. Screening of all children with severe forms of TB to look for TBM may help identify CNS TB. Second, the Global Drug Facility is now allowing investigators to purchase study drugs for clinical trials from their facility, so lowering cost. Pediatric formulations, particularly stand-alone rifampicin, would be useful for (commonly under-dosed) intrathoracic as well as CNS TB in children. Third, capacity building to develop or build upon existing expertise—neurology, neurocognitive and neuroradiological assessments—at international sites in areas where TB is common is key. Fourth, qualitative research to help us understand the sociodemographic, behavioural, and other barriers to trial participation and treatment adherence would aid in improving enrolment strategies and support structures, Finally, we need validated surrogate markers of treatment response for TBM in children to discriminate efficiently among experimental therapies and identify those regimens that work best for neurologic, functional, and neurocognitive recovery. Longitudinal assessments of functional status or neurocognitive function may be useful in that regard, coupled with exposure-response assessments.
CONCLUSIONS
Interventional trials in pediatric TBM are challenging for a broad variety of reasons, but by overcoming these challenges we can better understand how to treat this formidable disease that disables children physically, cognitively and emotionally. Without trials specifically conducted in children, we will not know if antimicrobial optimization can help children with functional and neurocognitive sequelae that are unique to them. Delineating and sharing these challenges may help to address them more effectively as a TB research community and, in turn, advance treatments for this therapeutically-orphaned population. In sharing the practical difficulties with TB practitioners, families, and researchers, we hope to provide insights that will inform future work in this long-neglected arena.
Supplementary Material
Acknowledgements
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD074944). The authors report no conflicts of interest. We acknowledge the following study team members for their contributions: A Nijampurkar, A Nagargoje, D Shere, G Wani, J Chandane, K Muttha, M Ithape, M Bendre, N Khan, N Pradhan, P Kapre, P Pawar, P Onawale, R Dhage, R Madewar, R Ahire, S Khwaja, S Dalimbkar, S Meshram, S Agiwal, S Nimkar, S Kante, U Balasubramanian, V Nadgeri, V Kulkarni, V Jadhav, V Shaikh, Z Shaikh (Byramjee Jeejeebhoy Government Medical Centre, Pune); A Maniselvi, G Arasan, K Yadav, L Jennifer, M Ganesan, M Venkatesan, O Puspha, P Arul, S Ganesh, S Kandan, S Balaji, S K Pramila, S Mary, S Karuppaiah, S Hissar (National Institute for Research in Tuberculosis, Chennai, India); A Mbewe, D Gadama, D Sichali, E Msiska, I Z Phiri, M Chiunda, M Chunga, N Mumba, P Kamthunzi, V Palichina (University of North Carolina Project Malawi, Lilongwe, Malawi); L Wolf (Johns Hopkins University, Baltimore, MD, USA).
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2017. WHO/HTM/TB/2017.23. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 2.Mezochow A, Thakur K, Vinnard C. Tuberculous meningitis in children and adults: new insights for an ancient foe. Curr Neurol Neurosci Rep 2017; 17(11): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang SS, Khan FA, Milstein MB, et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14(10): 947–957. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson RJ, Rohlwink U, Misra UK, et al. Tuberculous meningitis. Nat Rev Neurol 2017; 13: 581. [DOI] [PubMed] [Google Scholar]
- 5.Merkler AE, Reynolds AS, Gialdini G, et al. Neurological complications after tuberculous meningitis in a multi-state cohort in the United States. J Neurol Sci 2017; 375: 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker EW, Pokkali S, Zhang Z, et al. Microglia activation in a pediatric rabbit model of tuberculous meningitis. Dis Models Mech 2016; 9(12): 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Be NA, Kim KS, Bishai WR, Jain S K Pathogenesis of central nervous system tuberculosis. Current molecular medicine. 2009; 9(2): 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie D, Rashid H, El-Bashir H, et al. Impact of meningitis on intelligence and development: a systematic review and meta-analysis. PLoS ONE 2017; 12(8): e0175024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin J H Tuberculous meningitis: diagnostic and therapeutic challenges. Neurol Clin Pract 2014; 4(3): 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Török ME. Tuberculous meningitis: advances in diagnosis and treatment. Br Med Bull 2015; 113(1): 117–131. [DOI] [PubMed] [Google Scholar]
- 11.Caraffa E, Russo G, Vita S, et al. Intracranial tuberculous mass lesions treated with thalidomide in an immunocompetent child from a low tuberculosis endemic country: a case report. Medicine 2018; 97(29): e11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savic RM, Ruslami R, Hibma JE, et al. Pediatric tuberculous meningitis: model-based approach to determining optimal doses of the anti-tuberculosis drugs rifampin and levofloxacin for children. Clin Pharmacol Ther 2015; 98(6): 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Lu J, Liu J, Zhao Y, Ni X, Zhao S. Linezolid is associated with improved early outcomes of childhood tuberculous meningitis. Pediatr Infect Dis J 2016; 35(6): 607–610. [DOI] [PubMed] [Google Scholar]
- 14.De Vita M V, Silvestro E, Canavese C, Pennacchietti V, Scolfaro C. Use of linezolid in a child with tuberculous meningitis. Pediatr Infect Dis J 2018; 37(5): 499. [DOI] [PubMed] [Google Scholar]
- 15.Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics 2011; 128(5): e1242–e1249. [DOI] [PubMed] [Google Scholar]
- 16.Zisowsky J, Krause A, Dingemanse J. Drug development for pediatric populations: regulatory aspects. Pharmaceutics 2010; 2(4): 364–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran P, Duraipandian M, Nagarajan M, Prabhakar R, Ramakrishnan CV, Tripathy SP. Three chemotherapy studies of tuberculous meningitis in children. Tubercle 1986; 67(1): 17–29. [DOI] [PubMed] [Google Scholar]
- 18.Yunivita V, Dian S, Ganiem AR, et al. Pharmacokinetics and safety/tolerability of higher oral and intravenous doses of rifampicin in adult tuberculous meningitis patients. Int J Antimicrob Agents 2016; 48(4): 415–421. [DOI] [PubMed] [Google Scholar]
- 19.Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis 2013; 13(1): 27–35. [DOI] [PubMed] [Google Scholar]
- 20.Te Brake L, Dian S, Ganiem AR, et al. Pharmacokinetic/pharmacodynamic analysis of an intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis. Int J Antimicrob Agents 2015; 45(5): 496–503. [DOI] [PubMed] [Google Scholar]
- 21.Thwaites GE, Bhavnani SM, Chau TTH, et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob Agents Chemother 2011; 55(7): 3244–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health 2018; 17(1): 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern S E Challenges in conducting clinical trials in children: approaches for improving performance. Expert Rev Clin Pharmacol 2009; 2(6): 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph PD, Caldwell PHY, Tong A, Hanson CS, Craig JC. Stakeholder views of clinical trials in low- and middle-income countries: a systematic review. Pediatrics 2016; 137(2): e20152800. [DOI] [PubMed] [Google Scholar]
- 25.Lang T, Siribaddana S. Clinical trials have gone global: is this a good thing? PLoS Med 2012; 9(6): e1001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph PD, Craig JC, Caldwell P H Y Clinical trials in children. Br J Clin Pharmacol 2015; 79(3): 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marais S, Thwaites G, Schoeman JF, et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 2010; 10(11): 803–812. [DOI] [PubMed] [Google Scholar]
- 28.Department of Health, South Africa. National guidelines for the management of tuberculosis in children 2013, South Africa. Pretoria, South Africa: DoH, 2013. [Google Scholar]
- 29.US National Library of Medicine. Phase I/II Randomized, Open-label Trial to Evaluate the PK, safety, and outcomes of treatment including high dose rifampicin +/− levofloxacin vs standard treatment for pediatric tuberculous meningitis (TBM). Bethesda, MD, USA: US National Library of Medicine, 2016. https://clinicaltrials.gov/ct2/show/NCT02958709. Accessed August 2019. [Google Scholar]
- 30.Hirose TE, Maluf EM, Rodrigues C O Pneumococcal meningitis: epidemiological profile pre- and post-introduction of the pneumococcal 10-valent conjugate vaccine. J Pediatr (Rio J) 2015; 91(2): 130–135. [DOI] [PubMed] [Google Scholar]
- 31.Madhi S A Pneumococcal conjugate vaccine and changing epidemiology of childhood bacterial meningitis. J Pediatr (Rio J) 2015; 91(2): 108–110. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2nd ed. WHO/HTM/TB/2014.03. Geneva, Switzerland: WHO, 2014. http://www.who.int/tb/publications/childtb_guidelines/en/2014. Accessed August 2019. [PubMed] [Google Scholar]
- 33.Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)—associated tuberculous meningitis. Clin Infect Dis 2011; 52(11): 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akshoomoff N Use of the Mullen Scales of early learning for the assessment of young children with autism spectrum disorders. Child Neuropsychol 2006; 12(4–5): 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullen E M Mullen Scales of early learning. Circle Pines, MN, USA: American Guidance Service, 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


