Abstract
Background
COVID-19 disease is accompanied by a dysregulated immune response and hypercoagulability. The Anti-Coronavirus Therapies (ACT) inpatient trial aimed to evaluate anti-inflammatory therapy with colchicine and antithrombotic therapy with the combination of rivaroxaban and aspirin for prevention of disease progression in patients hospitalised with COVID-19.
Methods
The ACT inpatient, open-label, 2 × 2 factorial, randomised, controlled trial was done at 62 clinical centres in 11 countries. Patients aged at least 18 years with symptomatic, laboratory confirmed COVID-19 who were within 72 h of hospitalisation or worsening clinically if already hospitalised were randomly assigned (1:1) to receive colchicine 1·2 mg followed by 0·6 mg 2 h later and then 0·6 mg twice daily for 28 days versus usual care; and in a second (1:1) randomisation, to the combination of rivaroxaban 2·5 mg twice daily plus aspirin 100 mg once daily for 28 days versus usual care. Investigators and patients were not masked to treatment allocation. The primary outcome, assessed at 45 days in the intention-to-treat population, for the colchicine randomisation was the composite of the need for high-flow oxygen, mechanical ventilation, or death; and for the rivaroxaban plus aspirin randomisation was the composite of major thrombosis (myocardial infarction, stroke, acute limb ischaemia, or pulmonary embolism), the need for high-flow oxygen, mechanical ventilation, or death. The trial is registered at www.clinicaltrials.gov, NCT04324463 and is ongoing.
Findings
Between Oct 2, 2020, and Feb 10, 2022, at 62 sites in 11 countries, 2749 patients were randomly assigned to colchicine or control and the combination of rivaroxaban and aspirin or to the control. 2611 patients were included in the analysis of colchicine (n=1304) versus control (n=1307); 2119 patients were included in the analysis of rivaroxaban and aspirin (n=1063) versus control (n=1056). Follow-up was more than 98% complete. Overall, 368 (28·2%) of 1304 patients allocated to colchicine and 356 (27·2%) of 1307 allocated to control had a primary outcome (hazard ratio [HR] 1·04, 95% CI 0·90–1·21, p=0·58); and 281 (26·4%) of 1063 patients allocated to the combination of rivaroxaban and aspirin and 300 (28·4%) of 1056 allocated to control had a primary outcome (HR 0·92, 95% CI 0·78–1·09, p=0·32). Results were consistent in subgroups defined by vaccination status, disease severity at baseline, and timing of randomisation in relation to onset of symptoms. There was no increase in the number of patients who had at least one serious adverse event for colchicine versus control groups (87 [6·7%] of 1304 vs 90 [6·9%] of 1307) or with rivaroxaban and aspirin versus control groups (85 [8·0%] vs 91 [8·6%]). Among patients assigned to colchicine, 8 (0·61%) had adverse events that led to discontinuation of study drug, mostly gastrointestinal in nature. 17 (1·6%) patients assigned to the combination of rivaroxaban and aspirin had bleeding compared with seven (0·66%) of those allocated to control (p=0·042); the number of serious bleeding events was two (0·19%) versus six (0·57%), respectively (p=0·18). No patients assigned to rivaroxaban and aspirin had serious adverse events that led to discontinuation of study drug.
Interpretation
Among patients hospitalised with COVID-19, neither colchicine nor the combination of rivaroxaban and aspirin prevent disease progression or death.
Funding
Canadian Institutes for Health Research, Bayer, Population Health Research Institute, Hamilton Health Sciences Research Institute, Thistledown Foundation.
Translations
For the Portuguese, Russian and Spanish translations of the abstract see Supplementary Materials section.
Introduction
Most patients who are infected with the SARS-CoV-2 virus remain asymptomatic or have only mild symptoms, but those in whom the disease progresses can have respiratory failure and death.1, 2 Interventions that directly target the virus and those that suppress inflammation can prevent disease progression and save lives,3 but are often not affordable, incompletely effective, and might be associated with life-threatening toxicity.4 Vaccines have reduced the burden of COVID-19 disease but cannot be accessed in many low-income countries,5 reluctance to accept them by some segments of the population has limited their uptake,6 and protection provided by vaccines wanes over time.7 Additional efficacious, safe, and inexpensive therapies that are widely available and affordable are needed.
Research in context.
Evidence before this study
We searched PubMed from Jan 1, 2020, to the present using the search terms, “anticoagulation OR anticoagulant OR thromboprophylaxis OR antithrombotic”, “heparin” OR UFH OR unfractionated-heparin OR LMWH OR “low molecular weight heparin” OR dalteparin OR enoxaparin OR NOAC OR DOAC OR “direct oral anticoagulant” OR “novel oral anticoagulant” OR “non-vitamin K antagonist oral anticoagulant” OR “apixaban” OR “rivaroxaban” OR “edoxaban” OR “dabigatran”, “coronavirus” OR “COVID” OR “coronavirus disease-2019” OR “coronavirus 2019” OR “COVID19” OR “covid-19”. Meta-analyses of randomised trials indicated no overall benefit of colchicine in patients hospitalised with COVID-19. However, in the three largest colchicine trials, one found no evidence of benefit with up to 10 days of colchicine treatment, and the other two suggested a benefit with 15 to 30 days of treatment. These data raised the possibility that a longer course of treatment might be of benefit. Meta-analysis of trials of intensified anticoagulant therapy showed a reduction in venous thromboembolism but no mortality benefit. None of the trials of intensified anticoagulant therapy tested anticoagulation in combination with an antiplatelet agent.
Added value of this study
The ACT inpatient trial found no benefit of 28 days of colchicine or with the combination of rivaroxaban and aspirin for prevention of disease progression in patients hospitalised with COVID-19.
Implications of all the available evidence
The results of the ACT inpatient trials taken in the context of the totality of the data as summarised in updated meta-analyses provide clear evidence that neither colchicine nor the combination of rivaroxaban and aspirin benefits patients hospitalised with COVID-19.
Colchicine is a simple, inexpensive, anti-inflammatory drug that accumulates in neutrophils and monocytes and inhibits the nucleotide binding oligomerisation domain (NOD)-like pyrin domain 3 (NLRP3) inflammasome which is activated by the SARS-CoV-2 virus.8 The results of randomised trials of colchicine and antithrombotic therapies in patients with COVID-19 have been inconclusive and additional data are required to clarify their role.9, 10 Aspirin and rivaroxaban are effective antithrombotic drugs when used alone or in combination in patients with cardiovascular disease,11, 12 but the combination has not been tested in patients with COVID-19.
The Anti-Coronavirus Therapies (ACT) trials are factorial studies that evaluated anti-inflammatory therapy with colchicine and antithrombotic therapy with the combination of rivaroxaban and aspirin (inpatient trial) or aspirin alone (outpatient trial) in patients with COVID-19.13 Here we report the results of the ACT inpatient trial which aimed to test colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19. The results of the ACT outpatient trial are reported separately.14
Methods
Study design
Briefly, this is a 2 × 2 factorial trial in which patients hospitalised with COVID-19 were randomised to colchicine or control, as well as to the combination of rivaroxaban and aspirin or control. All participating trial centres obtained ethics approval before commencing recruitment and all patients (or their surrogates) provided informed consent. The protocol was modified during the course of the trial in response to emerging (masked) data,13 including an increase in sample size (from 1500 to 2500) and a change in the primary outcome (for the colchicine comparison—original primary outcome was mechanical ventilation or death, revised primary outcome was requirement for high-flow oxygen, mechanical ventilation, or death; for the antithrombotic comparison—original primary outcome was mechanical ventilation or death, revised primary outcome was major thrombotic events, requirement for high-low oxygen, mechanical ventilation or death).
The Population Health Research Institute (McMaster University, ON, Canada) coordinated the ACT inpatient trial and was responsible for all aspects of the trial conduct. The steering committee designed the study and approved the protocol. An independent data and safety monitoring committee met periodically to review study data. The trial steering committee met regularly to assess study progress and to discuss necessary interventions or protocol amendments as needed.
The design of the ACT inpatient trial has been published previously,13 and the protocol is available online. The statistical analysis plan was finalised before any investigator was made aware of the trial results.
Participants
Patients were eligible for inclusion if they were symptomatic with laboratory-confirmed COVID-19 disease, aged at least 18 years, and within 72 h of admission to hospital or worsening clinically, if already hospitalised. Patients were excluded if they had advanced kidney or liver disease, were pregnant or lactating, already ventilated for more than 72 h, and had a medical indication or a contraindication to the trial interventions. Detailed eligibility criteria are summarised in appendix 4 (p 1).
Randomisation and masking
Patients were randomly assigned (1:1) to receive colchicine or control, and in a second random assignment (1:1) to rivaroxaban and aspirin or control. Following written informed consent, randomisation was done by means of a centralised computerised system using block randomisation and with stratification according to centre. The randomisation sequence was concealed. Investigators, patients, and those doing analysis were not masked to treatment allocation.
Procedures
Patients received either colchicine 1·2 mg followed by 0·6 mg 2 h later and then 0·6 mg twice daily in tablet form for 28 days or control, and in a second random assignment received rivaroxaban 2·5 mg twice daily in tablet form and aspirin 100 mg once daily in tablet form for 28 days or control. Treatments were continued for 28 days. Additional details of the dosing regimens are provided in appendix 4 (p 2). Controls received usual care, as established by the local investigator. Patient data were collected throughout hospitalisation and all patients were followed at day 45 to collect information on adherence, adverse events, and outcomes.
Outcomes
Outcomes were assessed at day 45. The primary outcome for the comparison between colchicine and control was a composite of need for high-flow oxygen, mechanical ventilation, or death, and the secondary outcome was a composite of need for high-flow oxygen, mechanical ventilation, or respiratory death. The primary outcome for the comparison between the combination of rivaroxaban and aspirin with control was the composite of major thrombosis (includes pulmonary embolism, acute limb ischaemia, stroke, and myocardial infarction), need for high-flow oxygen, mechanical ventilation, or death, and the secondary outcomes were a composite of need for high flow oxygen, mechanical ventilation, or respiratory death, and any thrombosis. Appendix 4 provides additional details of primary and secondary outcomes (appendix 4 p 3) and their definition (appendix 4 p 4).
Statistical analysis
All analyses were done according to the intention-to-treat principle and included all patients from the time of randomisation. Before examining the independent effects of the two randomised treatment comparisons a formal test for interaction was done. For efficacy outcomes, Kaplan-Meier curves were used for a survival analysis and stratified Cox proportional hazard model with treatment group as a predictive variable and stratified by the other group of the factorial design was used to estimate the hazard ratio and 95% CIs. The proportional hazards assumption was tested by including a time by treatment interaction term in the stratified Cox model. Prespecified subgroup analyses included age, sex, comorbidities at baseline, disease severity at baseline (including oxygen therapy, admission to the intensive care unit), vaccination status, and in a post-hoc analysis the timing of enrolment according to the phase of the pandemic. The significance of any difference in safety outcomes was examined using a χ2 test or Fisher exact test.
The ACT inpatient trial aimed to enroll 2500 patients, which would provide at least 80% power with a two-sided α of 0·05 to detect a 20% relative risk reduction for each intervention versus control assuming an overall incidence rate of the primary outcome of 22% at 45 days and allowing for up to 1% loss to follow-up.
A two-sided p value of less than 0·05 was considered to indicate significance. There was no adjustment for multiplicity of testing because there was only one primary outcome for each randomisation. The statistical analysis plan prespecified that secondary and other outcomes would be considered as supportive if the results were consistent with the primary outcome.
An independent data and safety monitoring committee (DSMC) oversaw the ACT trials and did a formal interim analysis when approximately two-thirds of the target sample size had been enrolled. The interim analyses were guided by the Haybittle-Peto boundary of three SDs to indicate benefit. If this boundary were crossed, it had to be confirmed at a subsequent analysis done at least 1 month later for the trial to be stopped early for efficacy. The DSMC also examined the consistency of results across the inpatient and outpatient trials. No modification to the level of significance of the primary outcome was needed because of the extreme boundaries applied.
In order to contextualise our results, we did a literature search of electronic databases (PubMed) to identify trials of intensified anticoagulant therapy in patients hospitalised with COVID-19. The search strategy is provided in appendix 4 (p 5). Trials were eligible for inclusion if they involved at least 100 adult patients hospitalised with COVID-19 who were randomly allocated to receive intensified anticoagulation versus control, and reported mortality, which was the main outcome of interest. The data were pooled using a random effects Mantel-Haenszel model and are reported as risk ratios and 95% CIs with a χ2 p value for heterogeneity. These pooled analyses were not prespecified. The ACT inpatient trial is registered at ClinicalTrials.gov, NCT04324463.
Role of the funding source
The funder of the study had no role in study design, patient recruitment, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The trial was done at 62 sites in 11 countries, with the first patient enrolled on Oct 2, 2020, and the last on Feb 10, 2022.
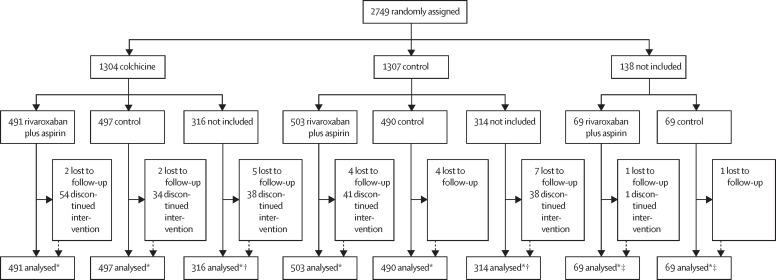
Patient flow is presented in figure 1 . 2611 patients were included in the analysis of colchicine (n=1304) versus control (n=1307) and 2119 patients were included in the analysis of rivaroxaban and aspirin (n=1063) vs control (n=1056). In Argentina, investigators were given the option of participating in either the colchicine or rivaroxaban plus aspirin randomisation, or both, whereas investigators in all other countries participated in both randomisations, thereby accounting for the difference in number of patients in the two randomisations.
Figure 1.
Trial profile
2611 patients were included in the analysis of colchicine (n=1304) vs control (n=1307). 2119 patients were included in the analysis of rivaroxaban and aspirin (n=1063) vs control (n=1056). *Information on screening eligibility or reasons for exclusion was not collected. †Patients not included in the rivaroxaban and aspirin vs control analysis. ‡Patients not included in the colchicine vs control analysis.
There were 23 protocol deviations (eligibility 7, use of prohibited medications 6, not administered per protocol 5, study procedures 2, and other 3; appendix 4 pp 6–8). Among patients who completed day 45 follow-up, adherence, defined by taking at least 80% of study drug was 930 (89·9%) of 1034 for the comparison of colchicine versus control and 804 (93·2%) of 863 for rivaroxaban plus aspirin versus control.
Table 1 presents baseline characteristics and clinical features of patients randomly assigned to colchicine versus control, and for those randomly assigned to the combination of rivaroxaban and aspirin versus control. Baseline characteristics were well balanced between the groups. At baseline or during the trial, 85 to 90% of patients received corticosteroids: colchicine 2297 (88·0%) of 2611 or rivaroxaban plus aspirin 1815 (85·6%) of 2119.
Table 1.
Baseline characteristics
|
Colchicine versus control group (n=2611) |
Rivaroxaban plus aspirin versus control group (n=2119) |
||||
|---|---|---|---|---|---|
| Colchicine | Control | Rivaroxaban plus aspirin | Control | ||
| Randomised | 1304 | 1307 | 1063 | 1056 | |
| Age, years | 56·1 (16·7) | 56·0 (16·0) | 55·0 (16·0) | 54·8 (15·7) | |
| <50 | 501 (38·4%) | 487 (37·3%) | 420 (39·5%) | 427 (40·4%) | |
| 50–69 | 489 (37·5%) | 538 (41·2%) | 417 (39·2%) | 435 (41·2%) | |
| ≥70 | 314 (24·1%) | 282 (21·6%) | 226 (21·3%) | 194 (18·4%) | |
| Female | 542 (41·6%) | 511 (39·1%) | 414 (38·9%) | 464 (43·9%) | |
| Male | 762 (58·4%) | 796 (60·9%) | 649 (61·1%) | 592 (56·1%) | |
| Ethnicity* | |||||
| Arab | 113 (17·0%) | 110 (16·5%) | 112 (16·7%) | 111 (16·8%) | |
| White European | 53 (8·0%) | 53 (7·9%) | 55 (8·2%) | 51 (7·7%) | |
| Latinx | 63 (9·5%) | 69 (10·3%) | 67 (10·0%) | 65 (9·8%) | |
| South Asian | 302 (45·5%) | 293 (43·9%) | 288 (43·0%) | 307 (46·4%) | |
| Other Asian | 80 (12·0%) | 90 (13·5%) | 86 (12·8%) | 84 (12·7%) | |
| Other | 53 (8·0%) | 52 (7·8%) | 62 (9·3%) | 43 (6·5%) | |
| Smoking or vaping | |||||
| Current | 75 (5·8%) | 83 (6·4%) | 65 (6·1%) | 54 (5·1%) | |
| Former | 213 (16·3%) | 232 (17·8%) | 166 (15·6%) | 152 (14·4%) | |
| Never | 1013 (77·7%) | 990 (75·7%) | 832 (78·3%) | 850 (80·5%) | |
| Diabetes | 288 (22·1%) | 295 (22·6%) | 243 (22·9%) | 238 (22·5%) | |
| Hypertension | 483 (37·0%) | 476 (36·4%) | 373 (35·1%) | 386 (36·6%) | |
| Dyslipidaemia | 58 (8·7%) | 57 (8·5%) | 52 (7·8%) | 63 (9·5%) | |
| Cardiovascular disease | 96 (7·4%) | 91 (7·0%) | 66 (6·2%) | 72 (6·8%) | |
| Coronary disease or myocardial infarction | 54 (4·1%) | 44 (3·4%) | 32 (3·0%) | 40 (3·8%) | |
| Stroke | 27 (2·1%) | 29 (2·2%) | 21 (2·0%) | 23 (2·2%) | |
| Peripheral artery disease* | 2 (0·2%) | 0 (0·0%) | 1 (0·1%) | 1 (0·2%) | |
| Lung disease | 73 (5·6%) | 63 (4·8%) | 51 (4·8%) | 39 (3·7%) | |
| Kidney disease | 14 (1·1%) | 14 (1·1%) | 10 (0·9%) | 16 (1·5%) | |
| Immunosuppressed | 23 (1·8%) | 16 (1·2%) | 12 (1·1%) | 13 (1·2%) | |
| Active cancer | 11 (0·8%) | 7 (0·5%) | 7 (0·7%) | 3 (0·3%) | |
| Vaccination status | |||||
| Nil | 737 (56·5%) | 748 (57·2%) | 672 (63·2%) | 687 (65·1%) | |
| Partial | 146 (11·2%) | 174 (13·3%) | 101 (9·5%) | 118 (11·2%) | |
| Full | 150 (11·5%) | 138 (10·6%) | 128 (12·0%) | 111 (10·5%) | |
| Unknown | 271 (20·8%) | 247 (18·9%) | 162 (15·2%) | 140 (13·3%) | |
| Symptoms | |||||
| Fever | 781 (59·9%) | 794 (60·7%) | 560 (52·7%) | 575 (54·5%) | |
| Cough* | 575 (86·6%) | 589 (88·2%) | 592 (88·4%) | 572 (86·4%) | |
| Muscle pain* | 387 (58·3%) | 386 (57·8%) | 380 (56·7%) | 393 (59·4%) | |
| Breathlessness | 1113 (85·4%) | 1118 (85·5%) | 857 (80·6%) | 820 (77·7%) | |
| Loss of smell or taste* | 162 (24·4%) | 172 (25·7%) | 167 (24·9%) | 167 (25·2%) | |
| Diarrhoea* | 125 (18·8%) | 138 (20·7%) | 128 (19·1%) | 135 (20·4%) | |
| Fatigue* | 432 (65·1%) | 441 (66%) | 420 (62·7%) | 453 (68·4%) | |
| Headaches* | 239 (36·0%) | 225 (33·7%) | 230 (34·3%) | 234 (35·3%) | |
| Examination | |||||
| Temperature, °C | 36·9 (0·8) | 37·0 (1·5) | 37·0 (0·8) | 37·0 (0·8) | |
| Heart rate | 88·6 (15·1) | 88·5 (14·9) | 89·3 (13·7) | 88·5 (13·8) | |
| Systolic blood pressure in mmHg | 125·1 (16·7) | 125·7 (16·9) | 125·3 (16·0) | 125·0 (15·9) | |
| Diastolic blood pressure in mmHg | 77·0 (10·4) | 77·4 (10·2) | 77·2 (9·8) | 77·2 (10·8) | |
| Respiratory rate | 21·9 (5·0) | 22·1 (5·6) | 22·0 (5·5) | 22·0 (5·3) | |
| Body-mass index in kg/m2 | 29·0 (5·8) | 29·0 (6·1) | 28·9 (6·0) | 29·0 (5·7) | |
| Admission status at randomisation | |||||
| General or COVID ward | 1140 (87·4%) | 1135 (86·8%) | 935 (88·0%) | 938 (88·8%) | |
| Intensive care unit | 164 (12·6%) | 172 (13·2%) | 128 (12·0%) | 118 (11·2%) | |
| Respiratory support | |||||
| Oxygen at baseline | 1048 (80·4%) | 1056 (80·8%) | 819 (77·0%) | 801 (75·9%) | |
| High-flow oxygen | 300 (23·0%) | 290 (22·2%) | 223 (21·0%) | 207 (19·6%) | |
| Non-invasive mechanical ventilation | 106 (8·1%) | 98 (7·5%) | 75 (7·1%) | 72 (6·8%) | |
| Invasive mechanical ventilation or extracorporeal membrane oxygenator | 35 (2·7%) | 27 (2·1%) | 20 (1·9%) | 19 (1·8%) | |
| Symptom onset to randomisation, days* | 7·0 (4·0) | 7·1 (3·8) | 7·0 (4·0) | 7·1 (3·8) | |
| Tertile 1 (0–4 days) | 196 (29·5%) | 177 (26·5%) | 180 (26·9%) | 193 (29·2%) | |
| Tertile 2 (5–6 days) | 148 (22·3%) | 152 (22·8%) | 169 (25·2%) | 131 (19·8%) | |
| Tertile 3 (7–28 days) | 319 (48·0%) | 339 (50·7%) | 320 (47·8%) | 338 (51·1%) | |
| Diagnosis to randomisation, days | 3·3 (3·2) | 3·3 (3·2) | 3·1 (3·1) | 3·4 (3·4) | |
Data are n (%) or mean (SD).
Denominator excludes 1279 colchicine and 787 rivaroxaban plus aspirin patients enrolled in Argentina because data were not available. Percentages do not always add up to 100 because some extreme outliers were excluded.
Among 2611 patients randomly assigned to colchicine versus control, mean age was 56·1 years (SD 16·3) and 1558 (59·7%) were male. Ethnicity was not reported for Argentina but in the rest of the trial population, 595 (44·7%) were South Asian, 223 (16·7%) Arab, 132 (9·9%) Latinx, and 106 (8·0%) White European. At the time of randomisation, 2104 (80·6%) of 2611 were on supplemental oxygen and 247 (10·2%) were receiving either non-invasive (204 [7·8%]) or invasive (62 [2·4%]) mechanical ventilation. Among patients for whom the data were available, the most common symptoms at baseline were cough (1164 [87·4%] of 1332]), breathlessness (2231 [85·4%] of 2611), fatigue (873 [65·5%] of 1332), fever (1575 [60·3%] of 2611), and muscle pain (773 [58·0%] of 1332).
Among 2119 patients randomly assigned to the combination of rivaroxaban and aspirin versus control, 1241 (58·6%) were male and mean age was 54·9 years (SD 15·9). The distribution of other baseline characteristics and clinical features were similar to those for the colchicine randomisation.
There was no statistical evidence of an interaction between the two randomised treatments for the primary or secondary outcomes.
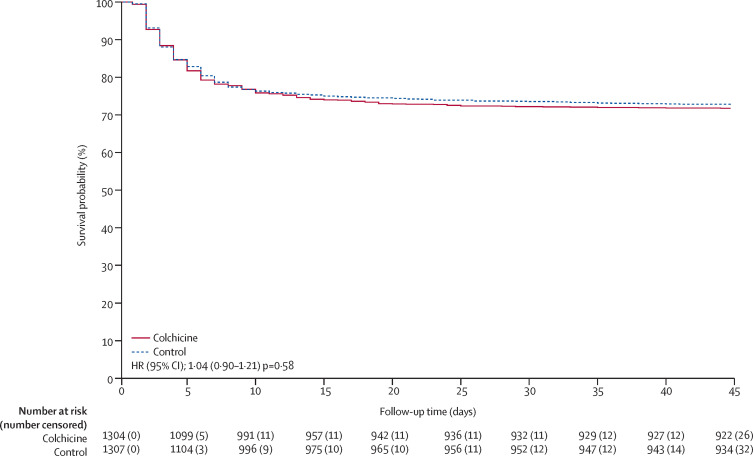
Outcomes for the colchicine vs control comparison are summarised in table 2 and a Kaplan-Meier curve for the primary outcome is shown in figure 2 . Follow-up for the primary outcome at day 45 was 98·9% complete. There was no statistical evidence for violation of the proportional hazards assumption for the primary or secondary outcomes. Colchicine compared with control did not significantly reduce the primary outcome of high-flow oxygen, ventilation, or death (368 [28·2%] events in 1304 participants vs 356 [27·2%] events in 1307 participants, HR 1·04, 95% CI 0·90–1·21, p=0·58) or the secondary outcome of high-flow oxygen, ventilation, or respiratory death (343 [26·3%] vs 323 [24·7%], HR 1·07, 95% CI 0·92–1·25, p=0·38). There was no evidence of benefit of colchicine in prespecified subgroups (all p values for interaction were non-significant; appendix 4 p 12).
Table 2.
Colchicine vs control: outcomes
| Colchicine group (n=1304) | Control Group (n=1307) | Hazard ratio (95% CI) | p value | ||
|---|---|---|---|---|---|
| High-flow oxygen, ventilation, or death* | 368 (28·2%) | 356 (27·2%) | 1·04 (0·90–1·21) | 0·58 | |
| High-flow oxygen, ventilation, or respiratory death† | 343 (26·3%) | 323 (24·7%) | 1·07 (0·92–1·25) | 0·38 | |
| High-flow oxygen or ventilation | 246 (18·9%) | 252 (19·3%) | 0·98 (0·82–1·17) | 0·84 | |
| Death | 264 (20·2%) | 249 (19·1%) | 1·08 (0·91–1·29) | 0·38 | |
| Respiratory death | 203 (15·6%) | 181 (13·8%) | 1·14 (0·93–1·40) | 0·19 | |
Data are n (%) unless stated otherwise.
Primary outcome.
Secondary outcome.
Figure 2.
Kaplan Meier curve showing the effect of colchicine compared with control on the primary outcome of high flow oxygen, ventilation, or death
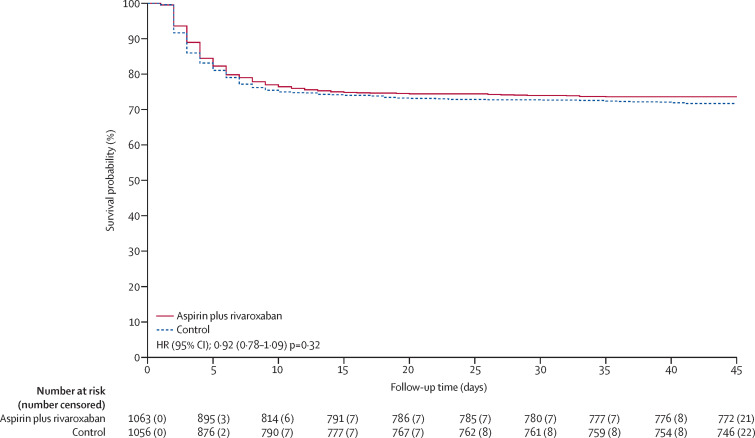
Outcomes for the rivaroxaban plus aspirin versus control groups are summarised in table 3 and a Kaplan-Meier curve for the primary outcome is shown in figure 3 . Follow-up for the primary outcome at day 45 was 99·1% complete. There was no statistical evidence for violation of the proportional hazards assumption for the primary or secondary outcomes. The combination of rivaroxaban and aspirin compared with control did not significantly reduce the primary outcome of major thrombosis, high-flow oxygen, ventilation, or death (281 [26·4%] events in 1063 participants vs 300 [28·4%] events in 1056 participants, HR 0·92; 95% CI 0·78–1·09, p=0·32) or the secondary outcome of any thrombosis, high-flow oxygen, ventilation, or respiratory death (269 [25·3%] vs 280 [26·5%], HR 0·95; 95% CI 0·80–1·12, p=0·53). There was no evidence of benefit of rivaroxaban and aspirin in prespecified subgroups (p values for interaction were non-significant) except for diabetes versus no diabetes (p=0·027; appendix 4 p 13). In analysis of safety, there was no increase in serious adverse events with colchicine versus control (87 events [6·7%] of 1304 vs 90 [6·9%] of 1307) or with rivaroxaban and aspirin versus control (85 [8·0%] vs 91 [8·6%]). Among patients randomly assigned to colchicine, 8 (0·61%) had adverse events that led to discontinuation of study drug, mostly gastrointestinal. For the antithrombotic randomisation, 17 (1·6%) patients randomly assigned to the combination of rivaroxaban and aspirin had bleeding events compared with seven (0·66%) of those allocated to control (p=0·042). The number of serious bleeding events was two (0·19%) versus 6 (0·57%) respectively (p=0·18). No patients randomly assigned to the combination of rivaroxaban and aspirin had serious adverse events that led to discontinuation of study drug. A listing of serious adverse events is provided in appendix 4 (colchicine versus control pp 6–8; combination of rivaroxaban and aspirin versus control pp 9–11).
Table 3.
Rivaroxaban plus aspirin versus control outcomes
| Rivaroxaban plus aspirin group (n=1063) | Control group (n=1056) | Hazard ratio (95% CI) | p value | ||
|---|---|---|---|---|---|
| Major thrombosis, high-flow oxygen, ventilation, or death* | 281 (26·4%) | 300 (28·4%) | 0·92 (0·78–1·09) | 0·32 | |
| Any thrombosis, high-flow oxygen, ventilation, or respiratory death† | 269 (25·3%) | 280 (26·5%) | 0·95 (0·80–1·12) | 0·53 | |
| Major thrombosis‡ | 10 (0·9%) | 18 (1·7%) | 0·56 (0·26–1·21) | 0·14 | |
| Any thrombosis§ | 17 (1·6%) | 20 (1·9%) | 0·85 (0·45–1·63) | 0·63 | |
| Venous thromboembolism | 13 (1·2%) | 10 (0·9%) | 1·31 (0·58–3·00) | 0·52 | |
| High-flow oxygen or ventilation | 191 (18·0%) | 210 (19·9%) | 0·89 (0·73–1·09) | 0·27 | |
| Death | 193 (18·2%) | 186 (17·6%) | 1·05 (0·86–1·28) | 0·66 | |
| Respiratory death | 145 (13·6%) | 138 (13·1%) | 1·06 (0·84–1·34) | 0·61 | |
Data are n (%) unless stated otherwise.
Primary outcome.
Secondary outcome.
Includes stroke, myocardial infarction, acute limb ischaemia, and pulmonary embolism.
Includes major thrombosis plus deep vein thrombosis.
Figure 3.
Kaplan-Meier curve showing the effect of rivaroxaban plus aspirin compared with control on the primary outcome of major thrombosis, high flow oxygen, ventilation, or death
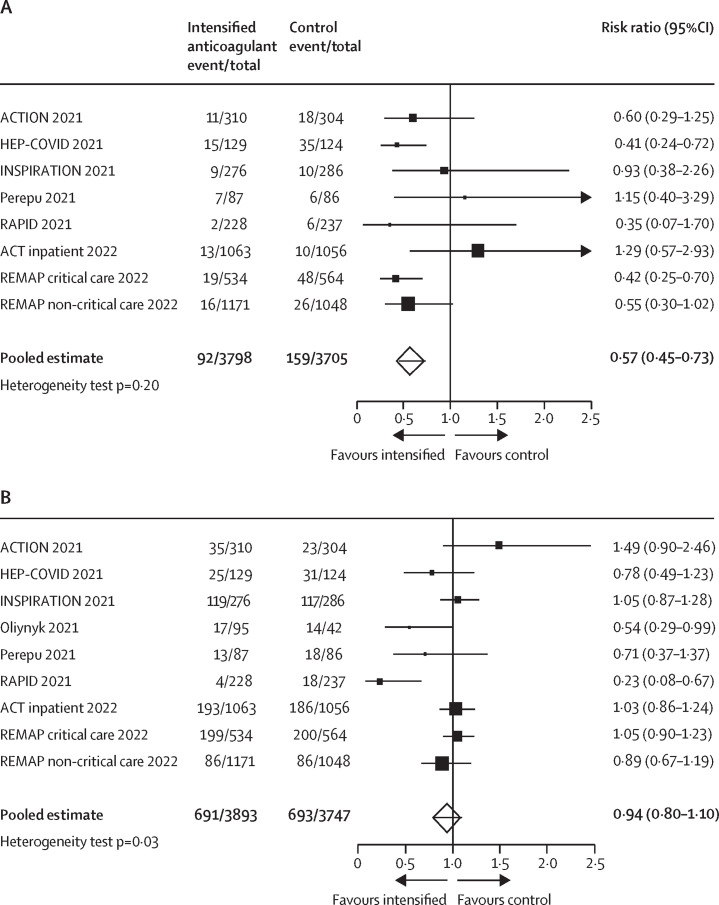
Our search identified nine trials (including our ACT inpatient trial) that compared intensified anticoagulation using therapeutic or intermediate dose unfractionated or low molecular weight heparin versus control in patients hospitalised with COVID-19.15, 16, 17, 18, 19, 20, 21, 22 Among 7503 patients, 92 (2·4%) of 3798 allocated to intensified anticoagulation compared with 159 (4·3%) of 3705 of those allocated to control had venous thromboembolism (risk ratio 0·57; 95% CI 0·45–0·73, pheterogeneity=0·20; (figure 4A ). Among 7640 patients, 691 (17·7%) of 3893 allocated to intensified anticoagulation compared with 693 (18·5%) of 3747 of those allocated to control died (risk ratio 0·94; 95% CI 0·80–1·10, pheterogeneity=0·027; figure 4B). Statistical evidence for heterogeneity appeared to be driven primarily by the results of two smaller trials,18, 20 which suggested implausibly large reductions in mortality (46% to 77% relative risk reductions) with intensified anticoagulation.
Figure 4.
Meta-analysis of randomised trials of the effects of intensified anticoagulation versus control on venous thromboembolism (A) and mortality (B) in patients hospitalised with COVID-19
Discussion
The ACT inpatient trial results provide no evidence for a benefit of either colchicine or the combination of rivaroxaban and aspirin for the prevention of disease progression or death in patients hospitalised with COVID-19. Results were consistent for primary and secondary outcomes and there was no suggestion of benefit in any of the prespecified subgroups except for a nominally significant interaction for diabetes which is almost certainly the play of chance. Thrombosis rates were low, and the combination of rivaroxaban and aspirin did not reduce thrombotic events. As expected, colchicine was associated with an increase in gastrointestinal side-effects and the combination of rivaroxaban and aspirin was associated with an increase in bleeding.
Inflammation is a consistent feature of COVID-19 disease progression, with elevated blood markers of inflammation independently predictive of poor outcome.22 Inflammation is thought to play a direct causal role in the development and progression of respiratory failure in patients with COVID-19, with multiple randomised trials showing that glucocorticoids and interleukin 6 inhibitors improve survival in patients with severe disease.24, 25 Colchicine has a wide range of anti-inflammatory actions, including specific inhibition of NLRP3 inflammasome, which is activated in patients with COVID-19, suggesting that it might have a particular role in these patients.8 However, the lack of benefit of colchicine in the ACT inpatient trial suggests that colchicine might not be sufficiently potent to suppress inflammation or that activation of the NLRP3 inflammasome does not play a major causal role in COVID-19 disease progression.
Our results with colchicine are consistent with those of the RECOVERY trial which showed no benefit of colchicine in 11 340 patients in the UK hospitalised with COVID-19.26 The COLCOVID trial, involving 1279 patients in Argentina hospitalised with COVID-19, showed a non-significant 17% reduction compared with control in the primary outcome of new requirement for mechanical ventilation or death,27 and the COLCORONA trial involving 4488 outpatients with COVID-19 showed a non-significant 21% reduction compared with placebo in the primary outcome of hospitalisation or death.28 However combining the results of previous trials with those of the ACT trials, including the ACT outpatient trial, provides no evidence for a benefit of colchicine in patients with COVID-19.14
Coagulation activation is a consistent feature of moderate and severe COVID-19 disease, with elevated blood levels of D-dimer, a marker of coagulation activation, independently associated with disease progression and survival.29 A causal role of hypercoagulability is further suggested by post-mortem findings of extensive microvascular thrombosis in patients who die of COVID-19.30 Despite these observations, intensified antithrombotic therapies have not been shown in previous randomised trials to reduce COVID-19 mortality.10 The NIH multiplatform trial suggested that in the subset of patients without severe disease (ie, hospitalised not requiring admission to the intensive care unit at the time of randomisation) therapeutic anticoagulation with heparin or low-molecular-weight heparin reduces the need for organ support,21 but this did not translate into a mortality benefit. Other trials using intermediate or therapeutic doses of unfractionated or low-molecular weight heparin, direct oral anticoagulants,10 or antiplatelet therapy31, 32 have not shown a benefit in patients hospitalised with COVID-19. The results of our updated meta-analysis are consistent with an earlier analysis,10 and provides no evidence that intensified anticoagulation reduces mortality in patients hospitalised with COVID-19, although there is a substantial reduction in venous thromboembolism.
The strengths of our study are that despite numerous challenges,13 we recruited several thousand high-risk patients and achieved high levels of adherence and follow-up, thereby ensuring that our hypotheses were rigorously tested. We tested therapies in the context of usual care including the use of corticosteroids, in both vaccinated and unvaccinated patients, and over the course of several waves of the pandemic during which patients would have been exposed to different variants. Our study also has some limitations. First, the trial was open label which raises the possibility for ascertainment and reporting biases and the differential use of other therapies. However, disease progression assessed by the need for high- flow oxygen and mechanical ventilation can be objectively determined, and death reporting is unbiased. Furthermore, we found no evidence of differential use of supportive therapies including corticosteroids according to treatment allocation (used in 85 to 90% of trial participants in both groups). Second, the ACT inpatient trial was done over a period of approximately 18 months during which different SARS-CoV-2 viral variants emerged, potentially with different susceptibility to interventions. Although therapies for COVID-19 evolved during the trial, we found no evidence of time dependent effects of treatments evaluated in the ACT inpatient trial. Third, a growing proportion of patients over time had been vaccinated before study entry, which might have affected the response to treatment. However, 56 to 65% of patients enrolled in the ACT inpatient trial were unvaccinated, event rates in the inpatients remained consistently elevated throughout the study period, and we found no evidence of differential treatment effect according to baseline vaccination status.
In conclusion, among patients hospitalised with COVID-19, colchicine and the combination of rivaroxaban and aspirin do not prevent disease progression or death. The lack of evidence of benefit of colchicine and of the combination of rivaroxaban and aspirin suggests that these treatments should not be used for the treatment of patients hospitalised with COVID-19.
Data sharing
Study materials are available from PHRI. Individual participant data will not be made available. After completion and publication of the results of long-term follow-up the ACT Trials steering committee will consider reasonable requests for specific additional analyses on a cost recovery basis (waived for low-income and middle-income countries).
Declaration of interests
JWE reports grant or in-kind support from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Pfizer, Janssen, Sanofi-Aventis and honoraria from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Daiichi-Sankyo, Eli-Lilly, Glaxo-Smith-Kline, Merck, Pfizer, Janssen, Sanofi-Aventis, Servier. SSJ reports grant support from Boston Scientific, honoraria from Medtronic, Penumbra. EPB-C reports grant support from Bayer, Roche, BMS-Pfizer. RPW reports grant support from Bayer, Roche, BMS-Pfizer, grant and honorarium from Boehringer-Ingelheim, and consultancy fees from Atricure and Phasebio. MLD reports grant support from the Population Health Research Institute (PHRI) to manage the ACT study in Argentina. RD reports grant support from PHRI to manage the ACT study in Argentina. AA reports institutional grant support from Bayer and EMS, and lecture fees from Bayer and Sanofi-Aventis. SW reports grant support from NIH, honoraria from Pfizer, and safety monitoring committee of an AIDS Clinical Trial Group. RDL reports institutional grant support from Bristol Myers Squibb, Glaxo Smith Kline, Medtronic, Pfizer, and Sanofi, consulting fees from Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, Sanofi, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck, and Portola, honoraria from Pfizer and meeting travel support from IQVIA. OB reports grant support from Astra Zeneca, Bayer, Amgen, Novartis, Servier, Pfizer. SSA reports grants from CIHR and PHAC, honoraria and consulting fees from Bayer and Janssen Pharma, meeting support from Heart and Stroke Canada, is committee member at the American Heart Association, the Canadian Cardiovascular Society, and Heart and Stroke Canada. JB reports consulting fees from Bayer. SC reports ACT study funding from Bayer, owns Bayer stock, is a board member of Canadian Arrhythmia Network, is an institutional advisory board member at the Institute of Circulatory and Respiratory Health, Canadian Institutes for Health Research. MEF reports institutional grants from Amgen, Astra Zeneca, Novartis, and Novo Nordisk and consulting fees from Otitopic. ML reports participation in vaccine advisory boards for Medicago, Pfizer, and Sanofi and is on the data safety and monitoring board for CanSino Biologics. SY reports institutional grant support from Bayer and honoraria, and travel costs for lectures from Bayer. SR, LX, LH, SIB, AY, SKS, WMT, MH, WH, AA, OD, CF, and PL-J have no disclosures to report.
Acknowledgments
Acknowledgments
This study was funded by the Canadian Institutes for Health Research (VR3-172627), Bayer, the Population Health Research Institute, Hamilton Health Sciences Research Institute, and the Thistledown Foundation.
Contributors
JWE, SSJ, EPB-C, RPW, SR, WH, SSA, JB, SC, MEF, ML, and SY conceived thestudy. LX and LH accessed and validated the raw data. LX, LH, and SIB did the formal analysis. JWE, SSJ, EPB-C, RPW, PL-J, AA, CF, SSA, SC, MEF, ML, and SY acquired the funding. All authors were involved in the investigation. JWE, SSJ, EPB-C, RPW, LX, LH, SIB, WH, SSA, JB, SC, MEF, ML, and SY were responsible for the methodology. JWE, SSJ, SR, and SY were responsible for project administration and supervision. JWE wrote the original draft. The steering committee vouches for the accuracy and completeness of the data and for the adherence to the trial protocol. All authors were responsible for the writing review and editing and the decision to submit the manuscript.
Supplementary Materials
References
- 1.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 2.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 3.Bartoletti M, Azap O, Barac A, et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect. 2022;28:222–238. doi: 10.1016/j.cmi.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3:e543–e552. doi: 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollyky TJ, Nuzzo J, Huhn N, Kiernan S, Pond E. Global vaccination must be swifter. Nature. 2022;603:788–792. doi: 10.1038/d41586-022-00809-w. [DOI] [PubMed] [Google Scholar]
- 6.Pavia CS. Pasteur, vaccines, and the refusal to become fully vaccinated in the midst of the COVID-19 pandemic. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.815816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaventura A, Vecchié A, Dagna L, Tangianu F, Abbate A, Dentali F. Colchicine for COVID-19: targeting NLRP3 inflammasome to blunt hyperinflammation. Inflamm Res. 2022;71:293–307. doi: 10.1007/s00011-022-01540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanghavi D, Bansal P, Kaur IP, et al. Impact of colchicine on mortality and morbidity in COVID-19: a systematic review. Ann Med. 2022;54:775–789. doi: 10.1080/07853890.2021.1993327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wills NK, Nair N, Patel K, et al. Efficacy and safety of intensified versus standard prophylactic anticoagulation therapy in patients with Covid-19: a systematic review and meta-analysis. Open Forum Infect Dis; 9: ofac285. [DOI] [PMC free article] [PubMed]
- 11.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 12.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eikelboom J, Rangarajan S, Jolly SS, et al. The anti-coronavirus therapies (ACT) trials: design, baseline characteristics, and challenges. CJC Open. 2022;4:568–576. doi: 10.1016/j.cjco.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eikelboom JW, Jolly SS, Belley-Cote EP, et al. Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir Med. 2022 doi: 10.1016/S2213-2600(22)00299-5. published online Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliynyk O, Barg W, Slifirczyk A, et al. Comparison of the effect of unfractionated heparin and enoxaparin sodium at different doses on the course of COVID-19-associated coagulopathy. Life (Basel) 2021;11 doi: 10.3390/life11101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perepu US, Chambers I, Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: A multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021;19:2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sholzberg M, Tang GH, Rahhal H, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375 doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734. doi: 10.1186/s13063-020-04641-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Group RC. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet Respir Med. 2021;9:1419–1426. doi: 10.1016/S2213-2600(21)00435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz R, Orlandini A, Castellana N, et al. Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.41328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tardif JC, Bouabdallaoui N, L'Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021;9:924–932. doi: 10.1016/S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leentjens J, van Haaps TF, Wessels PF, Schutgens REG, Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year. Lancet Haematol. 2021;8:e524–e533. doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiese A, Manetti AC, La Russa R, et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2021;17:279–296. doi: 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradbury CA, Lawler PR, Stanworth SJ, et al. Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2022;327:1247–1259. doi: 10.1001/jama.2022.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group RC. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:143–151. doi: 10.1016/S0140-6736(21)01825-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited References
- 23.Wang JH, Chen RD, Yang HK, et al. Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19. J Med Virol. 2021;93:2908–2917. doi: 10.1002/jmv.26771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study materials are available from PHRI. Individual participant data will not be made available. After completion and publication of the results of long-term follow-up the ACT Trials steering committee will consider reasonable requests for specific additional analyses on a cost recovery basis (waived for low-income and middle-income countries).