Abstract
Background
Recently, attention has been focused on mesenchymal stem cells (MSC) because of their unique ability to suppress inflammation induced by cytokine storms caused by COVID-19. Several patients have been successfully treated in this manner. After one year of treatment with Wharton's jelly-derived MSC injections, this study evaluated the safety and efficacy of injecting MSCs intravenously in patients with COVID-19.
Methods
This study treated four patients with severe COVID-19 with Wharton's jelly-derived mesenchymal stem cells. In this study, patients were followed up for routine tests, tumor markers, and whole-body imaging (spiral neck CT scan (with contrast), spiral chest CT scan (with & without contrast), and spiral abdominopelvic CT scan (with IV & Oral contrast)) one year after cell therapy.
Results
The results indicated that lymphocyte; lymph count significantly increased, and neutrophil, ESR, ferritin, and CRP significantly decreased. LDH showed a non-significant decrease (P-value<0.05). One year after the WJ-MSC injection, the tumor markers were normal, and no tumors were observed in patients after one year. Also, the CT scan result was normal.
Conclusions
In patients, no serious complications were observed after a one-year follow-up. After monitoring the patient via laboratory tests, tumor markers, and whole-body imaging, we concluded that the Wharton jelly-derived mesenchymal stem cells did not cause severe complications, including tumor formation, in severe COVID19 patients within a year. More clinical trials with higher sample sizes need to be performed on cell therapy with Wharton jelly-derived mesenchymal stem cells in the future.
Keywords: One-year follow-up, COVID-19, WJ-MSC, Cell therapy
1. Introduction
Coronavirus disease 2019 (COVID-19) has emerged as a global epidemic and has caused diverse clinical conditions, from asymptomatic carriers to severe acute respiratory distress syndrome (Huang et al., 2020; Tang et al., 2020; Xu et al., 2020). COVID-19 symptoms subside within 2 to 3 weeks, but approximately 10 % of patients experience symptoms several months after the infection. Long-term follow-up studies have been reported for severe COVID-19 patients discharged from hospitals (Huang et al., 2021; Wu et al., 2021). COVID19 is categorized into three categories based on the severity of the symptoms (Huang et al., 2020; Wu and McGoogan, 2020; Shi et al., 2020). Mild cases present symptoms such as fatigue, cough, fever, diarrhea, headache, and whether or not mild pneumonia is present. Dyspnea, decreased blood oxygen saturation, pulmonary infiltrates, acute respiratory stress, multiple peripheral ground-glass patches on both lungs, and so forth are symptoms of severe cases. Septic shock and respiratory failure are symptoms of critical cases. COVID-19 is estimated to have a mortality rate of 2.3 %, ranging from 6 to 41 days after the onset of symptoms (Shi et al., 2020; Wang et al., 2020).
Most cases are treated with supportive and symptomatic measures, but several antiviral agents, an antimalarial drug (chloroquine), and antibiotics have been used to treat milder and more severe cases. In addition to convalescent plasma therapy and mesenchymal stem cell therapy, other options have also been proposed to modulate the immune response in severely and critically ill patients (Shen et al., 2020; Leng et al., 2020; Orleans et al., 2020).
Mesenchymal stem cells can self-regeneration and differentiate into numerous types of cells (Meirelles et al., 2006; Chamberlain et al., 2007). Because MHC-I is not expressed to a high degree, and MHC-II is expressed in low numbers, mesenchymal stem cells are not immunogenic (Lee et al., 2014; Hass et al., 2011). That is why MSCs can be used for allogeneic cell transplantation. Several disease models have shown that MSCs can modulate the immune system and regenerate tissue (Corcione et al., 2006; Le Blanc and Davies, 2015; Wang et al., 2013; Forbes et al., 2014). There have been significant developments in stem cell technology with promising therapeutic prospects for treating various diseases, such as respiratory diseases (Fatima and Nawaz, 2015). In a previous study in 2020 conducted by our research team, Wharton Jelly-derived mesenchymal stem cells were performed in 5 patients with severe forms of COVID19. After a 1-month follow-up, Wharton's jelly-derived stem cells were safe and well-tolerated by the patient (Saleh et al., 2021).
This study examined patients for one year for routine laboratory tests, tumor markers, and whole-body imaging examinations.
2. Materials and methods
Five patients with severe COVID-19 were treated with Wharton Jelly-derived mesenchymal stem cells in a previous pilot study at Shariati Hospital. By signing the consent form, patients entered the study from July 21, 2020, to August 21, 2020. HWj-MSC cells prepared by cell Tech Pharmed were injected into patients in 150 × 106 cells via IV in 3 doses on days 0, 3, and 6 (Saleh et al., 2021). In the previous study, these patients were monitored after cell injection for 0, 3, 6, and 14 days (myocardial enzymes, hematology parameters, biochemistry, and inflammatory tests) and one year after cell therapy. In this study, patients (4 patients and one patient's information is not available) were followed up for routine tests, tumor markers, and whole-body imaging (spiral neck CT scan (with contrast), spiral chest CT scan (with & without contrast) and spiral abdominopelvic CT scan (with IV & Oral contrast)) one year after cell therapy. The Ethics Committee approved this study at the Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1400.035).
One year after cell therapy, all patients were evaluated for adverse events through clinical examinations, measurements of vital signs, and routine tests. One year after cell therapy, the following parameters were monitored: heart rate, respiration, blood pressure, body temperature, and oxygen saturation. Routine blood tests, biochemical indicators, myocardial enzymes, and Inflammatory Markers were performed before and after one year of cell therapy.
Beta-hCG, AFP, CEA, CA125, CA19-9, CA15-3, TPSA, and FPSA levels were measured in serum samples one year after cell therapy (ECL, HITACHI).
3. Statistical analysis
GraphPad Prism version 8.00 (GraphPad Software, Inc.) analyzed the data. We compared the means of two related groups using paired t-tests and one-way ANOVA for multi-group comparisons. The data were analyzed as mean ± S.D. P < 0.05 was considered to be statistically significant.
4. Results
Cell therapy was administered to patients using WJ-MSC in this research. Heparin and dexamethasone were administered to all patients as common treatments. Demographic data of patients such as gender, age, weight, Initial vital signs at day 0 and 1 year after cell injection, Physical examination, and diagnosis are listed in Table 1 . It was found that no serious complications were associated with WJ-MSC stem cells in this study. Wj-MSC is safe and tolerable for patients, as indicated above.
Table 1.
Demographic data's and physical examination.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Gender | Female | Female | Male | Male | ||||
| Age | 50–59 | 50–59 | 50–59 | 40–49 | ||||
| Weight | 94 | 70 | 88 | 95 | ||||
| Initial vital sign | Day 0 before cell therapy | 1 year after cell therapy | Day 0 before cell therapy | 1 year after cell therapy | Day 0 before cell therapy | 1 year after cell therapy | Day 0 before cell therapy | 1 year after cell therapy |
| RR breaths/min | 22 | 15 | 28 | 18 | 42 | 15 | 36 | 12 |
| PR beats/min | 71 | 75 | 92 | 84 | 89 | 72 | 66 | 90 |
| Sys BP, mm Hg | 141 | 100 | 103 | 148 | 124 | 100 | 139 | 130 |
| Dias BP, mm Hg | 76 | 60 | 71 | 100 | 89 | 60 | 90 | 80 |
| So2 (without oxygen mask) | 87 | 98 | 70 | 98 | 79 | 95 | 80 | 98 |
| Other | ||||||||
| Physical examination | 51 years old woman infected by COVID19 with severe lung involvement, she was a candidate for MSC therapy | One year after the MSC transplant, She is well with No problem. Ph/E (Lung: clear, Heart: Normal) | 53 years old woman infected by COVID19 with severe lung involvement, she was a candidate for MSC therapy | One year after the MSC transplant, She is well with No problem. Ph/E (Lung: clear, Heart: Normal) | 55 years old man infected by COVID19 with severe lung involvement, he was a candidate for MSC therapy | One year after the MSC transplant, He is well with No problem. Ph/E (Lung: clear, Heart: Normal) | 45 years old man infected by COVID19 with severe lung involvement, he was a candidate for MSC therapy | One year after the MSC transplant, He is well with No problem. Ph/E (Lung: clear, Heart: Normal) |
| Diagnosis | COVID 19 | Fully recovered | COVID 19 | Fully recovered | COVID 19 | Fully recovered | COVID 19 | Fully recovered |
Routine laboratory tests such as hematology (WBC, PLT, Hb, neutrophil and lymphocyte percentage, absolute lymphocyte count, and ESR), myocardial enzymes (LDH), biochemical tests (Cr, BUN, ALT, AST, total and direct bilirubin), inflammation tests (ferritin, and CRP) were conducted for all patients on days base, 3, 6, 14, and 1 year after cell therapy which are shown in Table 2 . Testing results have improved over time, according to the results. We statistically examined seven of these tests that are more important for COVID-19 patients of our study, including LDH, neutrophil, lymphocyte, lymph count, CRP, ESR, and ferritin, among which lymphocyte; lymph count showed a significant increase and neutrophil, ESR, ferritin, and CRP showed a significant decrease. LDH showed a non-significant decrease (P-value<0.05) (Fig. 1 ).
Table 2.
Laboratory tests (base, Day3, Day6, Day14 & 1 year).
| Variables | Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day0 | Day3 | Day6 | Day14 | Year 1 | Day0 | Day3 | Day6 | Day14 | Year 1 | Day0 | Day3 | Day6 | Day14 | Year 1 | Day0 | Day3 | Day6 | Day14 | Year 1 | ||
| Routine blood tests | Normal range | ||||||||||||||||||||
| WBC count (×109/L) | 3400 - 12,500 | 10,940 | 14,910 | 16,710 | 14,220 | 7500 | 9600 | 7560 | 10,620 | 8050 | 5400 | 10,400 | 11,040 | 10,950 | 11,630 | 6200 | 6100 | 12,740 | 10,050 | 9040 | 7190 |
| Hb (g/L) | M:14–18 | 12.1 | 12.7 | 15.1 | 13.7 | 12.5 | 11.5 | 11 | 11 | 13.5 | 13 | 13.1 | 14.2 | 13.5 | 15.1 | 15 | 14.7 | 15.1 | 14.4 | 13.8 | 16.9 |
| PLT count (×109/L) | F:12–16 | 146 | 195 | 227 | 218 | 204 | 233 | 321 | 428 | 295 | 220 | 287 | 243 | 192 | 205 | 228 | 307 | 328 | 183 | 189 | 226 |
| Neutrophil (%) | 150,000-450,000 | NA | 88 | 88 | 70 | 54 | 90 | 80 | 83 | 62 | 50 | 90 | 82 | 70 | 71 | 54 | 86 | 91 | 71 | 81 | 57 |
| Lymphocyte (%) | 45–75 | NA | 2 | 10 | 19 | 39 | 4 | 9 | 11 | 26 | 38 | 5 | 10 | 20 | 20 | 33 | 8 | 2 | 21 | 14 | 35 |
| LYM count (×109/L) | 20–40 | NA | 298.2 | 1671 | 2702 | 2925 | 384 | 680.4 | 1168.2 | 2093 | 2052 | 520 | 1104 | 2190 | 2326 | 2046 | 640 | 254.8 | 2110.5 | 1265.6 | 2517 |
| ESR | M: 0 to 20 F: 0 to 25 |
96 | 73 | 23 | 26 | 20 | 104 | NA | NA | 51 | 15 | 74 | 9 | 25 | 10 | 6 | 37 | 5 | 64 | 17 | 1 |
| Myocardial enzymes | |||||||||||||||||||||
| LDH (U/L) | 240–480 | 723 | 939 | 462 | 374 | 269 | 860 | 483 | 427 | 545 | 350 | 542 | 465 | 392 | 398 | 313 | 1458 | 1117 | 615 | 417 | 354 |
| Biochemical indicators | |||||||||||||||||||||
| Total Bili (mg/dl) | 0.1–1.2 | 0.26 | 0.7 | 1 | 0.6 | 0.7 | 1.1 | 0.54 | 0.43 | 0.9 | 0.8 | 0.7 | 0.6 | 0.7 | 1.3 | 0.8 | 1.6 | 1.6 | 1.6 | 0.9 | 1 |
| Direct Bili (mg/dl) | Up to 0.3 | 0.1 | 0.3 | 0.3 | 0.1 | 0.2 | 0.3 | 0.2 | 0.02 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 1.2 | 0.6 | 0.3 | 0.2 | 0.3 |
| ALT (U/L) | M:up to 41 | 55 | 29 | 40 | 43 | 11 | 98 | 41 | 42 | 39 | 21 | 16 | 43 | 30 | 11 | 15 | 136 | 106 | 55 | 75 | 29 |
| AST (U/L) | F: up to 31 | 23.5 | 20 | 25 | 20 | 17 | 68 | 47.5 | 25 | 18 | 26 | 19 | 40 | 15 | 35 | 18 | 145 | 50 | 29 | 27 | 23 |
| BUN (mmol/L) | M:up to 38 | 6 | 23 | 24 | 16 | 13 | 23 | 54 | 43 | 21 | 14 | 25.2 | 16 | 21 | 21 | 16 | 25 | 14 | 11 | 11 | 11 |
| Cr (μmol/L) | F: up to 31 | 0.8 | 0.8 | 0.7 | 0.8 | 0.6 | 0.5 | 0.9 | 1 | 1.1 | 0.74 | 0.9 | 0.8 | 1.1 | 0.9 | 0.8 | 0.8 | 0.7 | 0.8 | 0.7 | 1 |
| Inflammatory markers | |||||||||||||||||||||
| CRP | UP TO 10 | 60.5 | 6 | 3 | 0.48 | 2 | 107 | 101 | 20 | 3.84 | 2 | 87 | 53.5 | 24.5 | 4.44 | 3.9 | 45 | 82 | 35 | 1.47 | 2 |
| Ferritin | M: 24 to 336 F: 11 to 307 |
1979 | 959 | 231 | 169 | 55 | 798 | 986 | 686 | 659 | 120 | 896 | 707 | 600 | 500 | 142 | 1087 | 2666 | 742 | 878 | 229 |
Fig. 1.
Laboratory tests at day 0, 3, 6, 14, and 1 year after WJ-MSC injection.
This research examined tumor markers (beta-hCG, AFP, CEA, CA125, CA19-9, CA15-3, TPSA, and FPSA) and whole-body imaging one year after cell therapy, shown in Table 3 and Fig. 2a & b, respectively. The results show 1 year after WJ-MSC injection, tumor markers were normal, and no tumors were observed in patients after one year (Table 3).
Table 3.
Tumor marker.
| Variables | Patient 1 | Patient2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|---|
| Tumor marker | Gender | Female | Female | Male | Male |
| Normal range | |||||
| beta-hCG | F:6–24 M: up to 2 micIU/ml |
<0.1 | 1.59 | <0.1 | <0.1 |
| AFP | 0.89–8.78 ng/dl | 1.23 | 6.14 | 2.3 | 1.71 |
| CEA | Smoker up to 10 Nonsmoker up to 5 ng/ml |
1.04 | 2.34 | 2.49 | 3.21 |
| CA125 | <46 U/ml | 7.2 | 8.7 | 17.2 | 13.8 |
| CA19-9 | 0 and 37 U/ml | 2.91 | 22.32 | 12.04 | 6.06 |
| CA15-3 | <30 U/ml | 14.4 | 17.7 | 23.4 | 29.8 |
| TPSA | 0–4.0 ng/ml | 0 | 0 | 0.6 | 0.74 |
| FPSA | up to 2 ng/ml | 0 | 0 | 1 | 0.6 |
Beta-HCG: Beta-human chorionic gonadotropin, AFP: Alpha-fetoprotein, CEA: Carcinoembryonic antigen, CA125: Cancer antigen 125, CA19-9: Cancer antigen 19-9, CA15-3: Cancer antigen 15-3, TPSA: Total prostate-specific antigen, FPSA: Free prostate-specific antigen.
Fig. 2.
a. Chest CT scan.
a-1: A-D: A: day 0, B: day 14, C: day 30, and D: 1 year after WJ-MSC infection.
P1: Patient 1.
a-2: A-D: A: day 0, B: day 14, C: day 30, and D: 1 year after WJ-MSC infection.
P4: Patient 4.
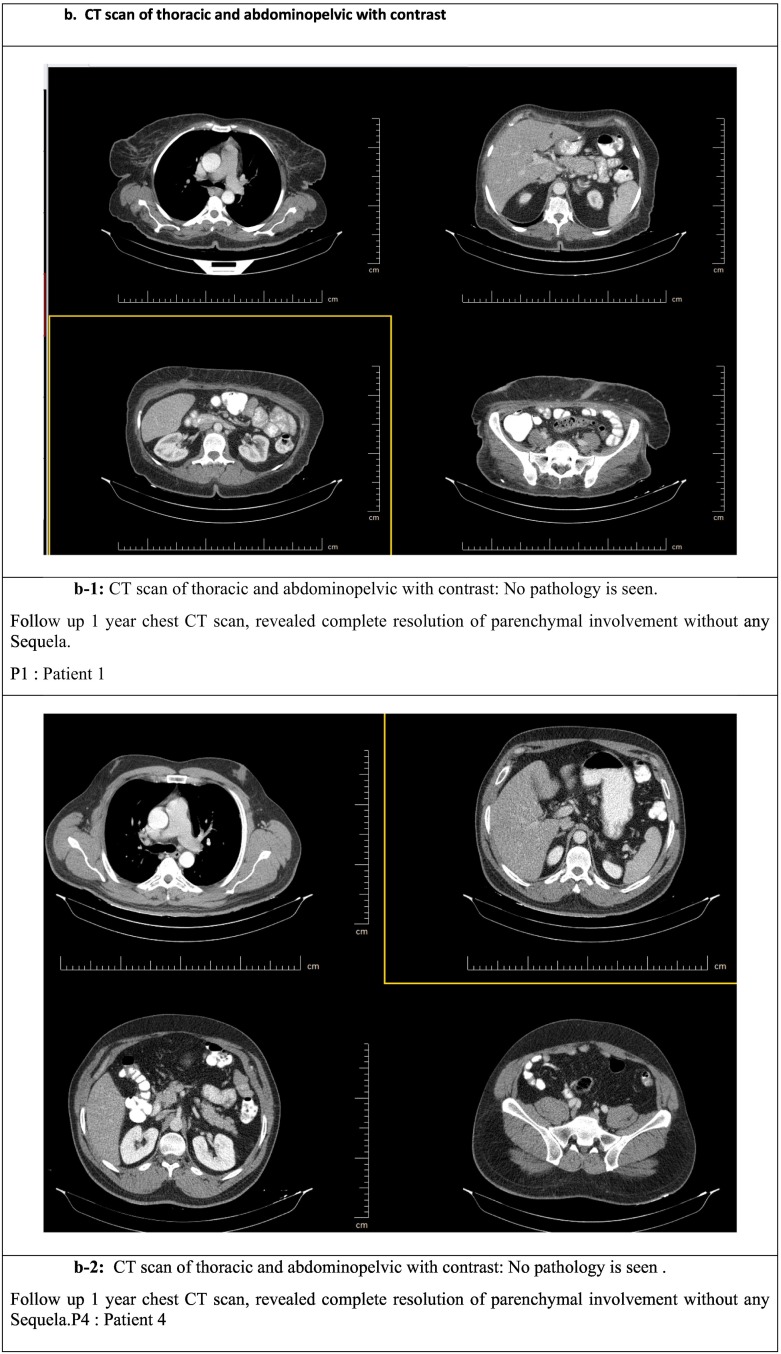
b. CT scan of thoracic and abdominopelvic with contrast.
b-1: CT scan of thoracic and abdominopelvic with contrast: No pathology is seen.
Follow up 1 year chest CT scan, revealed complete resolution of parenchymal involvement without any Sequela.
P1: Patient 1.
b-2: CT scan of thoracic and abdominopelvic with contrast: No pathology is see.
Follow up 1 year chest CT scan, revealed complete resolution of parenchymal involvement without any Sequela. P4: Patient 4.
Spiral neck CT scan (with contrast), spiral chest CT scan (with & without contrast), and spiral abdominopelvic CT scan (with IV & Oral contrast) results were analyzed by an expert radiologist. One year after cell therapy, in all patients: (Fig. 2a & b).
In the spiral neck CT scan, thyroid lobes were normal. Muscular structures have normal shapes and configurations. Vascular structures were intact with smooth walls. No significant cervical LAP was depicted.
The spiral chest CT scan showed no parenchymal abnormalities in both lungs. All patients showed no signs of pulmonary fibrosis. No pleural effusions were seen. No significant mediastinal LAP was noted. Heart and great vessels size were within normal limits. Moreover, there was no abnormal post-contrast enhancement.
In the spiral abdominopelvic CT scan, the Liver was within normal limit, and no intrahepatic focal mass lesion was noted. Gall bladder, bile ducts, spleen, kidneys, and pancreas appeared normal. No obvious abdominopelvic abnormality was found.
5. Discussion
The growing evidence of MSCs' therapeutic effectiveness has been shown in preclinical and clinical studies. Many studies indicate that the short-lived viability of MSCs after the injection may also account for low engraftment (Wang et al., 2014; Von Bahr et al., 2012). MSCs have started to appear as a new treatment option for COVID-19 patients (Hashemian et al., 2021; Feng et al., 2020). After MSCs are injected, many of them are trapped in the lungs, resulting in reduced cells that reach the target site (Mäkelä et al., 2015). Since the most common infection of the COVID-19 virus is the lung, intravenous injection of these mesenchymal stem cells is beneficial in these patients.
However, new therapies, such as cell therapy, face several challenges. The tumorigenic properties of stem cells are essential to consider when using them. Several studies have evaluated the risk associated with tumorigenesis following stem cell transplantation. Both stem cells, and tumor cells can survive, proliferate, and prevent death (Bellagamba et al., 2016). After one year of cell therapy, we evaluated beta-hCG, AFP, CEA, CA125, CA19-9, CA15-3, TPSA, and FPSA. We did not include any patients with cancer diagnoses in this study. Therefore, we cannot correlate the elevation of these biomarkers with preexisting tumorigenesis conditions.
There is evidence that cancer biomarkers, such as CEA, and CA, are elevated during various inflammatory conditions of the lungs. For instance, smoking increases CEA levels, and chronic obstructive pulmonary disease increases CA125 levels (Stockley et al., 1986; Barouchos et al., 2015).
In this study, there were no complications, including tumor formation. Tumor marker results besides whole-body imaging demonstrate the safety of Wharton jelly-derived mesenchymal cells after one year.
In another study, in line with our safety results on a large scale, UC-MSC cells were used in patients with severe COVID-19. These patients were monitored for one year. They concluded that administering UC-MSCs to severe COVID-19 patients for some time could reduce lung lesions and offer good symptom improvement, indicating that UC-MSC administration as adjunctive therapy for COVID-19 patients is feasible (Shi et al., 2022).
6. Conclusion
This study showed that cell therapy using Wharton jelly-derived mesenchymal stem cells after one year did not have serious complications, including tumorigenesis, and the patient tolerated the patient well. It is best to do this study on a larger scale and do more research on tumor formation.
Abbreviation
Funding
The Tehran University of Medical Sciences supported this work.
Tehran University of Medical Sciences and Health Services provided funding for this study (ethics code IR.TUMS.MEDICINE.REC.1400.035).
Ethics approval and consent to participate
Written informed consent was obtained from each patient or the patient's legally authorized surrogate before the conduct of study-specific procedures.
Consent for publication
Not applicable.
Code availability
Not applicable.
CRediT authorship contribution statement
MS proposed initial idea, study design and writing of the manuscript. MS, AAV, LA, NA, MB, AAS and JV were responsible for the reference selection and writing of the manuscript. MS, NA, MB, and LA took care of the patients and performed the follow-up checks. MS collected and analyzed the data. MS and LA analyzed the CT. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Edited by Jormay Lim
Footnotes
Trial registration: IRCT, IRCT20190717044241N2. Registered April 22, 2020, https://www.irct.ir/trial/47110.
Data availability
All of the data generated and analyzed during this study are included in our manuscript.
References
- Barouchos N., Papazafiropoulou A., Iacovidou N., Vrachnis N., Barouchos N., Armeniakou E., et al. Comparison of tumor markers and inflammatory biomarkers in chronic obstructive pulmonary disease (COPD) exacerbations. Scand. J. Clin. Lab. Invest. 2015;75(2):126–132. doi: 10.3109/00365513.2014.992944. [DOI] [PubMed] [Google Scholar]
- Bellagamba B.C., Abreu B.R.Rd., Grivicich I., Markarian C.F., Camassola M., Nardi N.B., et al. Human mesenchymal stem cells are resistant to cytotoxic and genotoxic effects of cisplatin in vitro. Genet. Mol. Biol. 2016;39:129–134. doi: 10.1590/1678-4685-GMB-2015-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain G., Fox J., Ashton B., Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Corcione A., Benvenuto F., Ferretti E., Giunti D., Cappiello V., Cazzanti F., et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Fatima F., Nawaz M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin. J. Cancer. 2015;34(3):1–13. doi: 10.1186/s40880-015-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Huang J., Wu J., Xu Y., Chen B., Jiang L., et al. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: a pilot study. Cell Prolif. 2020;53(12) doi: 10.1111/cpr.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes G.M., Sturm M.J., Leong R.W., Sparrow M.P., Segarajasingam D., Cummins A.G., et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin. Gastroenterol. Hepatol. 2014;12(1):64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Hashemian S.-M.R., Aliannejad R., Zarrabi M., Soleimani M., Vosough M., Hosseini S.-E., et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):1–12. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass R., Kasper C., Böhm S., Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011;9(1):1–14. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K., Davies L.C. Mesenchymal stromal cells and the innate immune response. Immunol. Lett. 2015;168(2):140–146. doi: 10.1016/j.imlet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Lee M., Jeong S.Y., Ha J., Kim M., Jin H.J., Kwon S.-J., et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2014;446(4):983–989. doi: 10.1016/j.bbrc.2014.03.051. [DOI] [PubMed] [Google Scholar]
- Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., et al. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä T., Takalo R., Arvola O., Haapanen H., Yannopoulos F., Blanco R., et al. Safety and biodistribution study of bone marrow–derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17(4):392–402. doi: 10.1016/j.jcyt.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Meirelles Ld.S., Chagastelles P.C., Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Orleans L., Is Vice H., Manchikanti L. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]
- Saleh M., Vaezi A.A., Aliannejad R., Sohrabpour A.A., Kiaei S.Z.F., Shadnoush M., et al. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther. 2021;12(1):1–13. doi: 10.1186/s13287-021-02483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Yuan X., Yao W., Wang S., Zhang C., Zhang B., et al. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley R., Shaw J., Whitfield A., Whitehead T., Clarke C., Burnett D. Effect of cigarette smoking, pulmonary inflammation, and lung disease on concentrations of carcinoembryonic antigen in serum and secretions. Thorax. 1986;41(1):17–24. doi: 10.1136/thx.41.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C., et al. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front. Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bahr L., Batsis I., Moll G., Hägg M., Szakos A., Sundberg B., et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- Wang D., Zhang H., Liang J., Li X., Feng X., Wang H., et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267–2277. doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15(11):1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir. Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data generated and analyzed during this study are included in our manuscript.