Abstract
Staphylococcus aureus is the most common etiological agent of bacterial arthritis and acute osteomyelitis and has been shown to bind to type II collagen under static and dynamic conditions. We have previously reported the effect of shear on the adhesion of S. aureus Phillips to collagen and found that this process is shear dependent (Z. Li, M. Höök, J. M. Patti, and J. M. Ross, Ann. Biomed. Eng. 24[Suppl. 1]:S–55). In this study, we used recombinant collagen adhesin fragments as well as polyclonal antibodies generated against adhesin fragments in attempts to inhibit bacterial adhesion. A parallel-plate flow chamber was used in a dynamic adhesion assay, and quantification of adhesion was accomplished by phase contrast video microscopy coupled with digital image processing. We report that both recombinant fragments studied, M19 and M55, and both polyclonal antibodies studied, α-M17 and α-M55, inhibit adhesion to varying degrees and that these processes are shear dependent. The M55 peptide and α-M55 cause much higher levels of inhibition than M19 and α-M17, respectively, at all wall shear rates studied. Our results demonstrate the importance of using a dynamic system in the assessment of inhibitory strategies and suggest the possible use of M55 and α-M55 in clinical applications to prevent infections caused by S. aureus adhesion to collagen.
Staphylococcus aureus is a major human pathogen and is responsible for infections such as bacterial arthritis (7), osteomyelitis (30, 34), and acute infectious endocarditis (25). Staphylococcal infections may be acquired hematogenously or by direct inoculation of wounds.
In the past, antibiotics have provided an effective treatment for staphylococcal infections. However, bacteria in general and staphylococci in particular have developed multidrug resistance. Over the past decades, methicillin resistance in S. aureus has become an increasing problem in the United States (18), and vancomycin remains the only effective antibiotic. With the recent emergence of vancomycin resistance (4), we are facing the possibility of having no effective antibiotic treatment available for combating staphylococcal infections. Alternative approaches must be considered to prevent and treat staphylococcal infections.
Adhesion and colonization of host tissues is a common initial step in the pathogenic process of many infectious diseases and therefore represents an attractive target for novel antibacterial strategies. Bacterial adherence is mediated primarily by proteins on the bacterial surface (adhesins) which bind specifically to complimentary ligands (11).
S. aureus appears to primarily cause infections in the extracellular space and binds several extracellular matrix proteins, including collagen (31), fibronectin (13), fibrinogen (10), laminin (15), bone sialoprotein (29), elastin (19), and vitronectin (5). The bacterial adhesins responsible for these binding activities are currently being identified and characterized in detail. This information could lead to the design of effective inhibitors of bacterial adherence, which may find a clinical use. For example, synthetic short peptides based on the fibronectin binding adhesin have been shown to effectively inhibit staphylococcal adherence to fibronectin-coated surfaces (26). In another study, recombinant fragments of the collagen binding adhesin were found to inhibit S. aureus adhesion to collagen-coated surfaces as well as to cartilage segments (32).
All the studies mentioned above were performed using static binding assays. However, static studies can be misleading, since drag force effects are not incorporated. Drag force, which is the mechanical force generated at a surface as a fluid flows over, may influence the efficacy of inhibitory strategies. Since many S. aureus infections are acquired through hematogenous spread, we decided to examine the staphylococcal attachment process under flow conditions. Initially, we focused on staphylococcal adherence to collagen in the wall shear rate range from 100 to 1,500 s−1 (corresponding to wall shear stress of 1 to 15 dyn/cm2). This shear range is physiologically relevant since typical shear stresses found in the vasculature are between 1 and 76 dyn/cm2 (9) and are predicted to be between 0 and 30 dyn/cm2 in the bone (35, 36). Our results demonstrated that dynamic adhesion is mediated by the collagen binding adhesin, follows first-order kinetics, and is shear dependent. For wall shear rates over 1,500 s−1, adhesion was found to be insignificant (14).
The collagen binding adhesin is a mosaic protein which is composed of an N terminal 55-kDa A domain containing a unique sequence; a B domain, which is composed of 1, 2, 3, or 4 repeats of a 25-kDa unit; and a C-terminal domain containing a cell wall attachment site, a hydrophobic transmembrane segment, and a short, cytoplasmic segment rich in positively charged residues (20). The collagen binding activity has been localized to a 19-kDa subfragment (M19) within the A domain (21). The crystal structure of the 19-kDa subfragment was recently solved (33). The protein appears as a “jelly roll” formed by two β-sheets connected by short α-helices. On one of the β-sheets is a transversing “trench” which represents the collagen binding site as shown by molecular modeling studies and analyses of site-directed mutants.
In attempts to inhibit bacterial adherence to collagen-coated surfaces under dynamic flow conditions, we used recombinant fragments of the collagen adhesin covering the intact A domain (M55) or ligand binding subfragment (M19) as well as antibodies to M55 and to M17 (a truncated form of M19), some of which have been shown to inhibit soluble collagen binding to staphylococcal cells under static conditions (24). These strategies differ in the mechanisms by which they cause inhibition. While recombinant adhesin fragments cause inhibition by blocking binding sites on the substrate, antibodies block the binding sites on the adhesin present on the surface of bacteria. Either event could prevent the adhesin from binding to the ligand and inhibit adherence. The primary aim of this study was to assess and compare the inhibitory capability of these recombinant adhesin fragments and antibodies in a dynamic environment.
MATERIALS AND METHODS
Bacteria and growth conditions.
The bacterial strain used in this study, S. aureus Phillips, was isolated from a patient diagnosed with osteomyelitis (23). Glycerol stocks were made from overnight cultures in tryptic soy broth (Difco, Detroit, Mich.) and were stored at −70°C. S. aureus cultures were started by inoculation from glycerol stocks into tryptic soy broth. After overnight growth at 37°C under constant rotation, the cells were harvested and resuspended in phosphate-buffered saline (PBS; 138 mM NaCl, 2.7 mM KCl, 0.1% sodium azide [pH 7.4]). Overnight cultures were used since previous studies of S. aureus adhesion to collagen were performed using post-exponential phase cultures (31, 32). The cell density was adjusted to 107 CFU/ml by using a reference standard curve relating absorbance at 600 nm to the number of CFU.
Preparation of collagen-coated coverslips.
Acid-soluble collagen type II from bovine nasal septum was purchased from Sigma Chemical Co., St. Louis, Mo. The purity of the collagen was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Collagen was coated on the glass coverslip by a procedure described previously (27, 28). The collagen was dissolved in deionized water (3.0% glacial acetic acid) to obtain a 2.44-mg/ml solution. Twenty microliters of the collagen solution was placed on a glass coverslip (no. 1, 24 by 50 mm; Corning Inc., Corning, N.Y.) in the region corresponding to the area of flow (approximately 60 mm2). The slide was incubated in a humid atmosphere for approximately 1 h, after which the unbound collagen was rinsed away with 1 ml of PBS before assembly onto the flow chamber. The amount of immobilized collagen on the slide was estimated by assaying the protein content of the rinse using a modified Lowry assay (Sigma Chemical Co.). The collagen concentration on the glass coverslips was 9.8 ± 0.9 μg/cm2. In order to verify that the collagen coated the entire area of flow, preliminary studies to confirm that nonspecific adhesion was insignificant were performed.
Recombinant adhesin fragments.
Segments of the collagen adhesin covering the intact A domain (M55; amino acid [aa] residues 30 to 529) or a collagen binding subfragment (M19; aa residues 151 to 318) were produced as described previously (21). In brief, the collagen adhesin gene fragments from S. aureus FDA 574 were overexpressed in Escherichia coli using the vector pQE-30 (QIAGEN Inc., Chatsworth, Calif.). The recombinant proteins were harvested and purified as described previously (21). These proteins were dissolved in PBS, and a stock solution at a concentration of 1.0 mg/ml was prepared. The collagen II-coated coverslips were preincubated with the recombinant proteins at 37°C for 45 min and subsequently rinsed with 1 ml of PBS before assembly onto the flow chamber. A modified Lowry assay was used to determine the protein content of the rinse, and the surface concentration was subsequently determined. The different amounts of M19 and M55 incubated with collagen and their corresponding surface concentrations are given in Table 1. The surface concentration values represent those prior to perfusion of the cell suspension. Control coverslips were incubated with deionized water, and subsequent rinses were assayed for protein content. No controls had detectable levels of protein, demonstrating that the collagen was not removed. Control surfaces were also generated by using a nonrelated protein similar in size to M55. Bovine serum albumin (BSA; molecular mass, 66 kDa) was used for preincubation of the collagen coverslips by the same procedure mentioned above.
TABLE 1.
Incubation amounts of M19 and M55 recombinant fragments and their corresponding surface concentrations
| Fragment | Incubated amount (μg) | Avg surface concn
|
|
|---|---|---|---|
| (μg/cm2) | (nmol/cm2) | ||
| M19 | 15 | 7.64 | 0.40 |
| 30 | 17.9 | 0.94 | |
| 60 | 51.2 | 2.7 | |
| M55 | 10 | 8.7 | 0.16 |
| 20 | 12.7 | 0.23 | |
| 30 | 15.4 | 0.28 | |
Antibodies.
Polyclonal immunoglobulin G (IgG) to the recombinant segments of the collagen adhesin M55 and M17 (a truncated version of M19; aa residues 151 to 297) were raised in rabbits (24). The IgG of the immune sera was purified by affinity chromatography on a protein A-Sepharose column. To prepare Fab fragments, the IgG was cleaved by overnight incubation at 37°C with papain coupled to agarose (10 μg of papain per 1 mg of IgG; Sigma Chemical Co.). The crystalline fragment (Fc) portion and the uncleaved IgG were removed by flowing the mixture through a protein A-Sepharose column. For the inhibition experiments, varying quantities of Fabs were incubated with the cell suspensions for 30 min at 37°C prior to perfusion over the collagen surface. The specificity of the Fabs was tested by flow cytometry, and the optimum titer required was used as the initial concentration for our inhibition experiments. Experiments using control cell suspensions preincubated with a nonrelated protein similar in size to the Fab fragments (∼50 kDa) were also performed. BSA was preincubated with the cell suspensions by the same procedure employed for preincubation of the Fabs.
Calculation of inhibition.
Inhibition for all the experiments was calculated using the following equation:
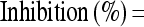 |
 |
The control run refers to experiments that were performed using both untreated cell suspensions and untreated collagen-coated coverslips.
Flow chamber and phase contrast video microscopy.
To mimic dynamic in vivo conditions, a parallel-plate flow chamber was utilized (1). The flow apparatus was cleaned and disinfected before each use. The flow chamber was assembled with the collagen-coated coverslip and filled with PBS. Different wall shear rates were attained by varying the flow rate through the chamber with a syringe pump (Model 44; Harvard Apparatus, South Natick, Mass.), since wall shear rate is a function of the flow chamber geometry (6).
The flow chamber assembly was mounted onto a computer-driven stage (Ludl Mac 2000; Hawthorne, N.Y.) of an Olympus IMT-2 phase contrast microscope (Olympus Corp., Lake Success, N.Y.). The microscope stage was maintained at 37°C in an air curtain incubator. A CCD camera (CCTV Corp., South Hackensack, N.J.) was used to obtain images at four equidistant points on the coverslip. The images from the camera were recorded with a VCR (model HR-VP422U; JVC, Elmwood Park, N.J.). The captured images were digitized by a frame grabber board (LG-3; Scion Corp., Frederick, Md.) on a computer (Quadra 950; Apple Computer) at a rate of 10 frames per second. The public domain NIH-Image program (written by Wayne Rasband at the U.S. National Institutes of Health and available from the Internet by anonymous ftp from zippy.nimh.nih.gov or on floppy disk from NTIS, 5825 Port Royal Rd., Springfield, Va. 22161; part no. PB93-504868) was used to analyze the captured images. The field of view used for counting adherent cells was 0.0206 mm2. The cell counts obtained from all four images were averaged for each experiment. The adherent cell densities at randomly selected points on the coverslip were not significantly different. We assumed that adhering cells did not affect the downstream bulk cell concentration, since the number of adherent cells compared to the bulk concentration of the cell suspension was negligible.
Statistical analysis.
Analysis of the statistical significance of the inhibition data was performed using the single-factor analysis of variance technique. In all cases, a probability value of 0.05 (95% confidence) was used to test for significance. The mean values were calculated for experiments based on data from at least three replicates. The error bars represent standard errors of the means.
RESULTS
Qualitative description of dynamic cell adhesion behavior.
By visually analyzing real-time video from the experiment, we observed the interactive behavior between the bacterial cells and the collagen surface under varying levels of shear. At a wall shear rate of 100 s−1, intermittent transient interactions between some S. aureus Phillips cells and the surface were observed. In some cases, the transiently interacting cell became firmly adherent, whereas in other cases the cell would continue to have transient interactions and move out of the field of view. Not all cells in the population exhibited this behavior; some cells simply became firmly adherent on contact with the surface. However, at higher wall shear rates (≥500 s−1), intermittent transient interactions were not observed, and some cells appeared to become firmly adherent immediately on contact with the surface. Once cells were firmly adherent, no detachment from the surface at any level of shear was observed. In fact, raising the shear to 5,000 s−1 (a wall shear rate too high to allow initial adhesion), did not detach the bacteria. S. aureus PH100, a collagen adhesin negative strain derived from S. aureus Phillips (23) also exhibited the same transient interaction described above at a wall shear rate of 100 s−1. Also, at higher shear (≥500 s−1), this transient interaction was not observed. However, the PH100 cells were unable to become firmly adherent to the collagen-coated surface at any wall shear rate.
In the case of the inhibition experiments, we observed qualitatively similar cell behavior for experiments with recombinant peptides as well as antibodies. At a wall shear rate of 100 s−1, we observed intermittent transient interactions that were similar to the controls. However, the ability of the cells to become firmly adherent in the presence of inhibitors was diminished. While at higher wall shear rates (≥500 s−1), we did not observe any qualitative differences compared to control experiments, quantitative differences in the level of firm adhesion were clear and are described in detail below.
Inhibition of S. aureus adhesion to collagen by M19 and M55.
We examined the ability of the recombinant adhesin segments M55 and M19 to inhibit adhesion of a heterologous strain, S. aureus Phillips, to collagen-coated surfaces. Inhibition at wall shear rates of 100, 500, and 1,100 s−1 was studied. These wall shear rates were chosen based on our previous results, which demonstrated shear-dependent adhesion within that range as well as no significant adhesion at a wall shear rate of >1,500 s−1 (14).
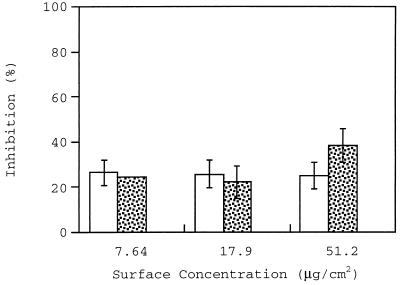
The inhibition caused by preincubating collagen with M19 (surface concentration range, 7.64 to 51.2 μg/cm2, corresponding to 0.40 to 2.7 nmol/cm2) is shown in Fig. 1. The highest levels of inhibition caused by M19 at wall shear rates of 100 and 500 s−1 were 25 and 40%, respectively. A statistical analysis of inhibition percentages at different surface concentrations of recombinant protein showed that there was no significant difference between values for these wall shear rates. M19 was completely ineffective at a wall shear rate of 1,100 s−1.
FIG. 1.
Inhibition of adhesion caused by preincubation of the collagen surface with M19 fragment at wall shear rates of 100 s−1 (open bars), 500 s−1 (stippled bars), and 1,100 s−1 (closed bars [not shown because zero value]).
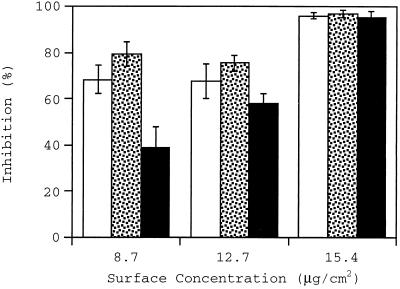
As discussed above, the inhibition caused by the M19 peptide is not high, especially at the high wall shear rate of 1,100 s−1. The inhibitory activity of a larger form of the adhesin was therefore investigated. Figure 2 shows the percentage of inhibition caused by M55 in a surface concentration range of 8.7 to 15.4 μg/cm2 (corresponding to 0.16 to 0.28 nmol/cm2). A maximum inhibition of over 95% was achieved at all wall shear rates at the highest M55 concentration. A statistical analysis of inhibition percentage at the lowest surface concentration of M55 shows that the inhibition caused at 1,100 s−1 is significantly lower than inhibition at 100 and 500 s−1. In comparison with M19, the M55 peptide caused higher levels of inhibition at all the wall shear rates, even at surface concentrations severalfold lower. Experiments were also performed using control surfaces coated with BSA instead of M55. No significant level of inhibition was observed in these experiments.
FIG. 2.
Inhibition of adhesion caused by preincubation of the collagen surface with M55 fragment at wall shear rates of 100 (open bars), 500 (stippled bars), and 1,100 (filled bars) s−1.
Inhibition of S. aureus adhesion by adhesin antibodies.
Another strategy to inhibit bacterial adhesion is to block the ligand binding site on the adhesin with an inhibiting antibody. We studied the inhibition caused by preincubation of S. aureus Phillips with α-M17 and α-M55 antibodies at wall shear rates of 100, 500, and 1,100 s−1.
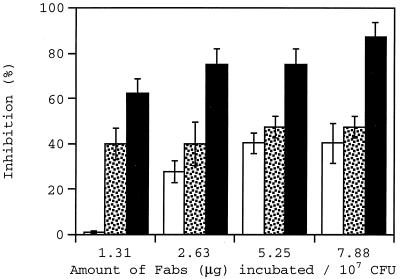
Figure 3 shows the adhesion inhibition caused by preincubation of the bacterial cell suspension with α-M17 antibodies in a Fab concentration range of 1.31 to 7.9 μg/ml. While the highest levels of inhibition achieved at wall shear rates of 100 and 500 s−1 were 40 and 47%, respectively, up to 87% inhibition was caused at a higher wall shear rate of 1,100 s−1. A statistical analysis of inhibition percentages shows that at a wall shear rate of 100 s−1, inhibition at 1.31 μg/ml was significantly lower than values found at higher concentrations of Fabs. At 500 s−1, no statistically significant difference in the values of inhibition at different concentrations was found. At 1,100 s−1, the inhibition at 7.9 μg/ml was significantly higher than the inhibition at 1.31 μg/ml.
FIG. 3.
Inhibition of adhesion at wall shear rates of 100 (open bars), 500 (stippled bars), and 1,100 (filled bars) s−1 caused by preincubation of S. aureus Phillips with α-M17 antibodies (Fabs).
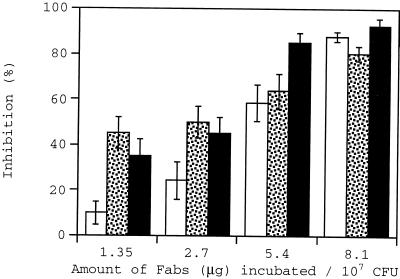
Figure 4 summarizes the inhibition caused by preincubation of cell suspensions with α-M55 antibodies in a Fab concentration range of 1.35 to 8.1 μg/ml. These antibodies were generated against a larger fragment of the collagen adhesin and hence presumably bind more epitopes on the adhesin protein. Over 80% inhibition was caused by α-M55 at all wall shear rates studied at a Fab concentration of 8.1 μg/ml of cell suspension. A statistical analysis of inhibition percentages showed that inhibition at higher Fab concentrations was significantly higher than values at lower Fab concentrations. This behavior is different from that found with α-M17, possibly due to greater diversity in the epitope binding region of α-M55 antibodies. Experiments using control cell suspensions incubated with BSA were performed, and no significant level of inhibition was observed. Experiments to determine if α-M55 antibodies were capable of detaching adherent cells were also performed. Fab concentrations up to 16.2 μg/ml and wall shear rates up to 5,000 s−1 were used for these experiments. No detachment was observed.
FIG. 4.
Inhibition of adhesion at wall shear rates of 100 (open bars), 500 (stippled bars), and 1,100 (filled bars) s−1 caused by preincubation of S. aureus Phillips with α-M55 antibodies (Fabs).
DISCUSSION
The use of adhesin fragments and antiadhesin antibodies to prevent adhesion has been previously suggested (2, 22). In fact, earlier studies using static assays suggest that these strategies can be successful (24, 26, 32). However, the results may be misleading since shear forces were not accounted for in these experiments. A dynamic study not only mimics in vivo conditions more closely, but also leads to determination of shear ranges in which particular compounds are effective.
We have used two recombinant forms of the collagen binding adhesin; a 19-kDa fragment that contains the smallest segment with collagen binding activity and a 55-kDa fragment that encompasses the entire ligand binding domain. These two recombinant proteins inhibit adhesion of a heterologous strain to varying extents, and the degree of inhibition is dependent on the level of shear. The larger protein, M55, caused much higher inhibition than the smaller M19 fragment at all wall shear rates studied. At a wall shear rate of 1,100 s−1, M19 did not cause any inhibition. One hypothesis to explain this behavior is that the M19 fragment does not bind collagen tightly enough to withstand drag forces at the surface generated by higher shear, since this peptide mimics the minimum collagen binding domain. For recombinant adhesin fragments to remain stably bound to the substrate ligand and cause inhibition, they should not only have the ability to attach to the ligand but should also be capable of resisting detachment due to fluid flow. It is possible that a threshold wall shear rate exists above which M19 is incapable of remaining bound to collagen. This could explain why M19 causes inhibition at 100 and 500 s−1 and not at 1,100 s−1. The M55 peptide was able to cause nearly complete inhibition (at a surface concentration of 15.4 μg/cm2) at all wall shear rates studied, possibly due to its ability to remain bound to the collagen under shear conditions. Presumably, M55 should bind more tightly, since it mimics the entire A domain of the collagen adhesin. However, at the lowest M55 surface concentrations studied, inhibition at 1,100 s−1 was significantly less than inhibition at 100 and 500 s−1. One explanation of this result is that at 1,100 s−1, lower amounts of M55 are retained compared to the M55 retained at 100 and 500 s−1. Although more peptide may be detached from collagen at a higher wall shear rate, a high initial surface concentration may still ensure saturation of binding sites on the collagen, causing almost complete inhibition at the highest surface concentration studied.
The second part of the study involved the use of antibodies generated against recombinant forms of collagen adhesin segments. The antibodies for our study were raised against M17 (a truncated version of M19) and against the M55 proteins. We found that these antibodies cause adhesion inhibition to different degrees. In general, the inhibition was found to be shear dependent with higher levels of inhibition observed at higher wall shear rates. This behavior was expected, since stronger cell-matrix interaction is required for adhesion at a higher wall shear rate because the drag force that opposes attachment is higher. Since the antibodies partially block binding sites on the adhesin, the cell-matrix interaction that leads to adhesion is weakened. This leads to lower levels of adhesion at a higher wall shear rate. At lower wall shear rates, however, the weakened cell-matrix interaction may still be sufficient to cause significant levels of adhesion. This, in essence, amounts to lower inhibition. The shear effect on inhibition is most evident in the case of α-M17 antibodies which cause almost twice the level of inhibition at 1,100 s−1 as at the lower wall shear rates. The α-M55 antibodies cause significantly higher levels of inhibition than the α-M17 antibodies. These antibodies cause up to 85% inhibition, even at the lower wall shear rates. Since α-M55 antibodies are generated against a larger fragment of the collagen adhesin, they presumably bind to a higher number of epitopes than α-M17 antibodies and should more effectively block the collagen binding site. Also, there is a low dose dependence of inhibition in case of α-M17 antibodies. This may be due to the fact that these antibodies are generated against a smaller peptide and hence bind to fewer number of epitopes than α-M55 antibodies.
The results presented above emphasize the importance of assessing the inhibitory capabilities of different compounds in a dynamic environment. Based on the visual qualitative observations, we hypothesize that both the recombinant adhesin peptides and antibodies inhibit firm adhesion mediated by the collagen adhesin. However, since intermittent transient interactions were observed in the presence of both inhibitors at a wall shear rate of 100 s−1, it appears that the cells are able to interact with the collagen matrix via interactions that are weaker than those required for adhesion. These transient intermittent interactions could be due to the presence of a broad specificity adhesin which has been shown to have a low affinity for collagen (12, 16). Also, adhesion is irreversible since there is no detachment observed.
This study demonstrates the effect of shear on two different strategies for adhesion inhibition. The adhesin peptides cause lower inhibition at higher wall shear rates while the converse is true for inhibition caused by antibodies. This phenomenon can be explained based on the fluid dynamics within the flow chamber. The shear rate (and hence the drag force) due to fluid flow is maximum at the collagen surface and decreases linearly toward the center of the flow field (3). Therefore, the recombinant peptides bound to the collagen surface are exposed to the highest level of shear in the chamber. In contrast, cells incubated with antibody are exposed to lower levels of shear due to their position in the flow chamber. In addition, the physical forces on the antibody-adhesin bond are lower since the cells are not bound to the surface and can translate force into cell motion (8).
A comparison between different recombinant adhesin truncates and different antibodies for use in strategies to prevent staphylococcal infections is also obtained from this study. Studies previously performed with antibodies to the collagen adhesin (24, 32) had shown that these completely inhibited collagen adhesion under static conditions. However, M19 and α-M17 did not cause inhibition as effectively in a dynamic environment, while M55 and α-M55 cause high levels of inhibition.
In a separate recent study, it was demonstrated that the α-M55 antibodies effectively protected mice from S. aureus-induced sepsis when provided in passive immunization strategies whereas α-M17 were much less effective (17). The current study, in which α-M55 was found to be more effective in inhibiting dynamic adhesion of staphylococcal cells than α-M17 antibodies, raises the possibility that the protective effect in the mouse model of these antibodies was based on its ability to inhibit bacterial adherence rather than promote opsonization and phagocytosis. If this mode of action for the protective antibodies can be substantiated, it may suggest that other inhibitors of bacterial adhesion can find clinical applications.
ACKNOWLEDGMENTS
This work was supported by grants from the Whitaker Foundation to J.M.R., from the National Institutes of Health (AR44415) to M.H., and from the Arthritis Foundation to J.M.P.
REFERENCES
- 1.Abbassi O, Kishimoto T K, McIntire L V, Anderson D C, Smith C W. E-Selectin supports rolling in vitro under conditions of flow. J Clin Investig. 1993;92:2719–2730. doi: 10.1172/JCI116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beachey E H. Bacterial adherence: adhesion receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981;143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 3.Bird R B, Stewart W E, Lightfoot E N. Transport phenomena. New York, N.Y: John Wiley & Sons Inc.; 1960. [Google Scholar]
- 4.Centers for Disease Control. Reduced susceptibility of S. aureus to vancomycin—Japan 1996. Morbid Mortal Weekly Rep. 1997;46:624–626. [PubMed] [Google Scholar]
- 5.Chhatwal G S, Preissner K T, Müller-Berghaus G, Blobel H. Specific binding of human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987;55:1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frangos J A, McIntire L V, Eskin S G. Shear stress induced stimulation of mammalian cell metabolism. Biotechnol Bioeng. 1988;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg D L, Reed J I. Bacterial arthritis. N Engl J Med. 1985;312:764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- 8.Goldman A J, Cox R G, Brenner H. Slow viscous motion of a sphere parallel to a plane wall. II. Couette Flow. Chem Eng Sci. 1967;22:653–660. [Google Scholar]
- 9.Goldsmith H L, Turrito V T. Rheological aspects of thrombosis and haemostasis: basic principles and applications. Thromb Haemostasis. 1986;55:415–435. [PubMed] [Google Scholar]
- 10.Harwiger J S, Timmons S, Strong D D, Cottrell B A, Riley M, Dolittle R F. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982;21:1407–1413. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- 11.Höök M, McGavin M J, Switalski L M, Raja R, Raucci G, Lindgren P, Lindberg M, Signas C. Interaction of bacteria with extracellular matrix proteins. Cell Differ Dev. 1990;32:433–438. doi: 10.1016/0922-3371(90)90060-a. [DOI] [PubMed] [Google Scholar]
- 12.Jönsson K, McDevitt D, McGavin M H, Höök M. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J Biol Chem. 1995;270:21457–21460. doi: 10.1074/jbc.270.37.21457. [DOI] [PubMed] [Google Scholar]
- 13.Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978;276:718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- 14.Li, Z., M. Höök, J. M. Patti, and J. M. Ross. 1996. The effect of shear stress on the adhesion of Staphylococcus aureus to collagen I, II, IV and VI. Ann. Biomed. Eng. 24(Suppl. 1):S–55.
- 15.Lopes J D, dos Reis M, Brentani R R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985;229:275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- 16.McGavin M H, Krajewska-Pietrasik D, Rydén C, Höök M. Identification of a Staphylococcus aureus extracellular matrix-binding protein with broad specificity. Infect Immun. 1993;61:4438–4443. doi: 10.1128/iai.61.6.2479-2485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson T M, Patti J M, Bremell T, Höök M, Tarkowski A. Vaccination with a recombinant fragment of collagen adhesin provides protection against Staphylococcus aureus-mediated septic death. J Clin Investig. 1998;101:2640–2649. doi: 10.1172/JCI1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panlilio A L, Culver D H, Gaynes R P. Methicillin-resistant Staphylococcus aureus in U. S. hospitals. Infect Control Hosp Epidemiol. 1992;13:582–586. doi: 10.1086/646432. [DOI] [PubMed] [Google Scholar]
- 19.Park P W, Roberts D D, Grosso L E, Parks W C, Rosenbloom J, Abrams W R, Mecham R P. Binding of elastin to Staphylococcus aureus. J Biol Chem. 1991;266:23399–23406. [PubMed] [Google Scholar]
- 20.Patti J M, Jonsson H, Guss B, Switalski L M, Wiberg K, Lindberg M, Höök M. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J Biol Chem. 1992;267:4766–4772. [PubMed] [Google Scholar]
- 21.Patti J M, Boles J O, Höök M. Identification and biochemical characterization of the ligand binding domain of the collagen adhesin from Staphylococcus aureus. Biochemistry. 1993;32:11428–11435. doi: 10.1021/bi00093a021. [DOI] [PubMed] [Google Scholar]
- 22.Patti J M, Höök M. Microbial adhesins recognizing extracellular matrix macromolecules. Curr Opin Cell Biol. 1994;6:752–758. doi: 10.1016/0955-0674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 23.Patti J M, Bremell T, Pietrasik D K, Abdelnour A, Tarkowski A, Rydén C, Höök M. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun. 1994;62:152–161. doi: 10.1128/iai.62.1.152-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patti, J. M., K. House-Pompeo, J. O. Boles, N. Garza, S. Gurusiddappa, and M. Höök. Critical residues in the ligand-binding site of the Staphylococcus aureus collagen-binding adhesin (MSCRAMM). J. Biol. Chem. 270:12005–12011. [DOI] [PubMed]
- 25.Pelletier L, Petersdorf R G. Infectious endocarditis: a review of 125 cases from the University of Washington Hospitals. 1963–1972. Medicine. 1977;56:287–313. [PubMed] [Google Scholar]
- 26.Raja R H, Raucci G, Höök M. Peptide analogs to a fibronectin receptor inhibit attachment of Staphylococcus aureus to fibronectin-containing substrates. Infect Immun. 1990;58:2593–2598. doi: 10.1128/iai.58.8.2593-2598.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross J M, McIntire L V, Moake J L, Rand J H. Platelet adhesion and aggregation on human type VI collagen surfaces under physiological flow conditions. Blood. 1995;85:1826–1835. [PubMed] [Google Scholar]
- 28.Ross J M, McIntire L V, Moake J L, Kuo H, Qian R, Glanville R W, Schwartz E, Rand J H. Fibrillin containing elastic microfibrils support platelet adhesion under dynamic conditions. Thromb Haemostasis. 1998;79:155–161. [PubMed] [Google Scholar]
- 29.Rydén C, Yacoub A I, Maxe I, Heinegård D, Oldberg Å, Frazén A, Ljungh Å, Rubin K. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur J Biochem. 1989;184:331–336. doi: 10.1111/j.1432-1033.1989.tb15023.x. [DOI] [PubMed] [Google Scholar]
- 30.Rydén C. Osteomyelitis and staphylococcal infections. In: Wadstrom T, Eliasson I, Holder I, Ljungh A, editors. Pathogenesis of wound and bio-material associated infections. New York, N.Y: Springer-Verlag; 1990. pp. 69–75. [Google Scholar]
- 31.Speziale P, Raucci G, Visai L, Switalski L M, Timpl R, Höök M. Binding of collagen to Staphylococcus aureus Cowan 1. J Bacteriol. 1986;167:77–81. doi: 10.1128/jb.167.1.77-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Switalski L M, Patti J M, Butcher W, Gristina A G, Speziale P, Höök M. A collagen receptor on Staphylococcus aureus strains isolated from patients with septic arthritis mediates adhesion to cartilage. Mol Microbiol. 1993;7:99–107. doi: 10.1111/j.1365-2958.1993.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 33.Symersky J, Patti J M, Carson M, House-Pompeo K, Teale M, Moore D, Jin L, Schneider A, DeLucas L J, Höök M, Narayana S V L. Structure of the collagen-binding domain from a Staphylococcus aureus adhesin. Nat Struct Biol. 1997;4:833–838. doi: 10.1038/nsb1097-833. [DOI] [PubMed] [Google Scholar]
- 34.Waldvogel F A, Vasey H. Osteomyelitis: the past decade. N Engl J Med. 1980;303:360–369. doi: 10.1056/NEJM198008143030703. [DOI] [PubMed] [Google Scholar]
- 35.Weinbaum S, Cowin S C, Zeng Y. A model for the fluid shear stress excitation of membrane ion channels in osteocytic processes due to bone strain. In: Vanderby R Jr, editor. Procedural advances in bioengineering 1991. New York, N.Y: American Society of Mechanical Engineers; 1991. pp. 317–320. [Google Scholar]
- 36.Zeng Y, Cowin S C, Weinbaum S. A fiber matrix model for fluid flow and streaming potentials in the caniliculi of an osteon. Ann Biomed Eng. 1994;22:280–292. doi: 10.1007/BF02368235. [DOI] [PubMed] [Google Scholar]