Abstract
It was reported that protein hydrolysates or derived peptides have more functionalities than their parent protein. Most functional protein hydrolysates or peptides are identified from various food products, including plant, fish, and land-animal protein sources. Within a few decades, the application of food protein-origin functional hydrolysates or peptides could be divided into two main categories according to their applied intentions: 1) preservatives and bioactive packing materials; 2) nutraceutical ingredients. According to the literature, the applications of food protein-origin functional hydrolysates or peptides on food preservative and nutraceutical ingredients have attracted much attention. However, the approach method should be changed. Multi-activities, compound formulation, comprehensive evaluation, and the added value of by-products are possible strategies. Although there have been great results and findings in the functionalities of food protein-origin bioactive hydrolysates or peptides, there is still a big gap between the lab-scale results and practical applications. Via this narrative review on the current research, scientists, the food/health industry, and government authorities should cooperate to dig into the new material sources and the possible practical application.
Keywords: Bioactive packing materials, Nutraceuticals, Peptides, Preservatives, Protein hydrolysates
1. Introduction
Amino acids joined by covalent bonds, also known as amide or peptide bonds, form bioactive peptides, which could positively impact body functions or health conditions [1]. Besides, bioactive peptides play crucial roles in the metabolic functions of living organisms, especially human beings. In many studies, antihypertension, antithrombotic, anti-cancer, antimicrobial, antioxidant, immunomodulatory, and agonist/antagonist properties of bio-peptides and protein hydrolysates have been reported [2]. Many bioactive peptides are identified from protein hydrolysates of various food products, including soybean, cereals, potatoes, nuts, vegetables, dairy products, eggs, and meat proteins [2]. It was mentioned that the protein hydrolysates or peptides produced from various protein sources possess some, or better, beneficial bioactivities compared to those found in the parent proteins [3,4]. Most food protein does not show specific biological activities in the naive sequences, though some biological activities of that food protein can be triggered by enzymatic, chemical, or microbial hydrolysis [5]. Enzymatic hydrolysis is the most effective method of producing functional hydrolysates or peptides. However, different factors, such as processing condition, protein source, amino acid sequence and compositions, molecular weight, charge distribution, pH, and certain chemical treatments, could directly affect the functionalities of generated bioactive hydrolysates or peptides [6].
Regarding bio-functional peptides, Sánchez and Vázquez indicated that many bioactive peptides have a peptide residue length of between 2–20 amino acids in addition to proline, lysine, or arginine groups [1]. Interestingly, bioactive peptides have also been shown to resist the further action of digestion peptidase [7]; therefore, the bioactive peptides could be absorbed under the current bioactive form. Moreover, the correlation between structure and functional properties is still not well understood; therefore, the crude extract, known as hydrolysates, is often acceptable and used widely in practice [8–10].
There always exists a pervasive doubt in the absorption and bioavailability of bioactive peptides or hydrolysates. According to metabolic physiology, the protein is digested and absorbed in the gastrointestinal tract while gastric and pancreatic proteinases conduct luminal digestion. The resultant end products (mostly large peptides) undergo a further hydrolyzation by various peptidases present in the intestinal epithelium brush border membrane [11] Interestingly, several scientific pieces of evidence revealed that luminal amino acids are present as a peptide form (about 80%) rather than the free form (about 20%), and most peptides are 2–6 amino acids [12]. Ganapathy reported that the transport of free amino acids contributes relatively less; mean-while, the protein digestion products enter the enterocytes in dipeptides or tripeptides via specific peptide transport systems [11].
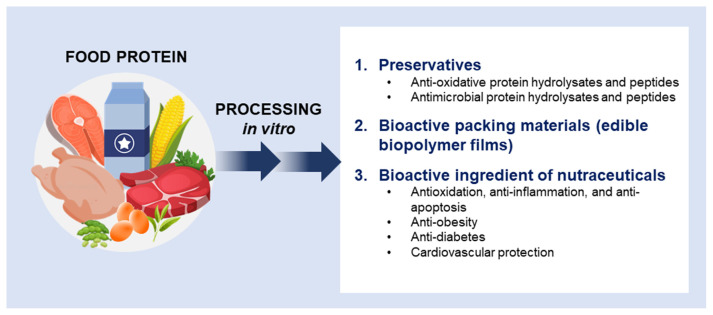
Recently, bioactive peptides or hydrolysates from food by-product proteins have attracted much attention. According to a report from Food and Agriculture Organization of the United Nations (FAO) [13], the global meat output in 2018 is 336.4 million metric tons, in which there are mainly 123.9, 71.1, and 120.5 million tons for poultry, bovine, and pig meats, respectively. As a result, huge amounts of by-products are generated, including feathers, fish scales, blood, bones, skin, and viscera [14,15]. Hence, many researchers should be drawn to the question of how to maximize the utilization of those by-products from livestock, poultry, and aquaculture. It seems that the development of functional protein hydrolysates or bioactive peptides is one of the possible strategies; thus, this article also includes cases generated from food by-product protein. In this article, the application of food protein-origin bioactive peptides or hydrolysates would be discussed as following two major categories according to their applied intentions: 1) preservatives and bioactive packing materials and 2) nutraceutical ingredients (Fig. 1).
Fig. 1.
Trends and applications of food protein-origin bioactive peptides and hydrolysates.
2. Preservatives and bioactive packing materials in the market
2.1. Antioxidative protein hydrolysates and peptides
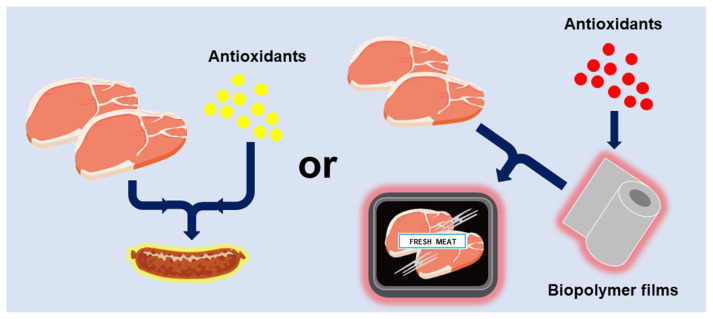
Excessive free radicals could produce oxidants, which may reduce the quality of oleaginous foods, cause lipid oxidation, and shorten the shelf life of food [16]. Lipid oxidation always causes a great problem for the food industry and consumers because it leads to undesirable off-flavors, odors, and potentially toxic reaction products [17]. Because it is very practical to retard lipid peroxidation occurring in foodstuffs to maintain the quality and extend the shelf life of foods [18], many synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tertiary butylhydroquinone (TBHQ), are used as food additives to prevent rancidity. Antioxidants for use in food processing must be inexpensive, nontoxic, effective at low concentrations (0.001–0.02%), capable of surviving processing (carry-through), stable in the finished products, and devoid of undesirable color flavor and odor effects [18]. Generally, antioxidants in food products could normally be added as either direct additives or indirectly through diffusion from packaging materials [19] (Fig. 2). Although synthetic antioxidants show stronger antioxidant activities than natural ones, such as α-tocopherol and ascorbic acid, there is still a doubt that these chemical compounds may cause health concerns due to the induction of DNA damage and toxicity [20–23]. Hence, there is a credit to looking for a natural source of antioxidants in applying food products.
Fig. 2.
Direct and indirect strategies of antioxidants in food-product preservation.
Notably, natural antioxidant peptides originating from food proteins have captured scientists’ attention due to their advantages of eco-friendliness, sustainability, and a lack of toxic side effects [24]. Plant proteins have been considered a new source of antioxidant peptides or hydrolysates, which delay the lipid peroxidation of food, save energy, and strengthen the treatment of oxidation-related diseases, thus decreasing food waste and improving the quality of life, respectively [25]. Decades ago, the antioxidative properties of whey and soy hydrolysates were revealed [26] (Table 1). Recently, some agricultural by-products, such as tea dregs and Phoenix dactylifera L. seed, were also found to be good sources of antioxidative hydrolysates [27,28] (Table 1). It was reported that peptide- and polyphenol-rich dark red kidney bean (Phaseolus vulgaris L.) hydrolysates could reduce the oxidation process of plain yogurt products during storage at room temperature for 3 days, and their antioxidative stability is higher than that of ascorbic acid [29]. Moreover, Gomes and Kurozawa reported that the rice protein hydrolysate as an encapsulated matrix in linseed oil microparticles enhances the stability of the unsaturated fatty acid-rich lipid [30]. Nowadays, antioxidative plant protein hydrolysates successfully apply to various food systems, i.e., beverages, yogurt, oil, and meat (Table 1).
Table 1.
Application of antioxidative protein hydrolysates and peptides as a preservative in the food system.
| Protein source | Hydrolysates or peptides | Incorporated food system | Amount of active ingredients | References |
|---|---|---|---|---|
| Plant protein | ||||
| Date seed protein | Hydrolysates | Ground salmon | 200 ppm | [28] |
| Dark red kidney bean (Phaseolus vulgaris L.) | Hydrolysates | Plain yogurt | 3 g/L | [29] |
| Tea residue protein | Hydrolysates | Chicken surimi | 0.1, 0.5, and 1.0% | [27] |
| Zein | Hydrolysates | Oil-in-water emulsions prepared by myofibrillar protein | 1.25, 2.5, 5, and 10 mg/mL | [95] |
| Fish protein | ||||
| Amur sturgeon (Acipenser schrenckii) skin gelatin | Peptides | Japanese sea bass (Lateolabrax japonicus) mince | 25 ppm | [33] |
| Capelin (Mallotus villosus) protein | Hydrolysates | Cooked meat | 3% | [32] |
| Gelatin from blacktip shark (Carcharhinus limbatus) skin | Hydrolysates | Cooked comminuted pork | 100, 500, and 1000 ppm | [34] |
| Goby (Zosterissessor ophiocephalus) muscle | Hydrolysates | Turkey meat sausage | 0.01–0.04% | [35] |
| Land-animal protein | ||||
| Camel milk | Hydrolysates | Minced fish | 5% and 10% | [39] |
| Casein | Peptides | Ground beef/deboned poultry meat | 20 mg/g | [37] |
| Deboned chicken residue | Hydrolysates | Cantonese sausage | 2% | [40] |
| Milk protein | Peptides | Cooked beef | 200 and 800 μg/g | [38] |
| Porcine blood | Hydrolysates | Pork meat emulsion | 900 μg/g | [41] |
| Camel milk | Hydrolysates | Minced fish | 5% and 10% | [39] |
| Whey protein isolate and soy protein | Hydrolysates | Meat patties | 2% | [26] |
Fish can serve as a source of functional materials, such as polyunsaturated fatty acids, polysaccharides, minerals and vitamins, antioxidants, enzymes, and bioactive peptides. Recently, a topic focused on identifying and characterizing bioactive murine peptides’ structure, composition, and sequence. Antioxidant peptides and hydrolysates from marine sources and their by-products are also revealed [18,31]. 3% caplin-protein-hydrolysate addition in porcine meat increased cooking yield by 4% and inhibited oxidation [32]. The concentration of hydrolysates was up 3% in cases where the opposite effects occurred. Nikoo et al. also revealed similar results as the anti-oxidative-peptide study. The antioxidative peptide from Amur sturgeon skin gelatin was effective in the minced Japanese sea bass muscle model system [33]. Processed meat foods were also chosen for further application, as indicated in Table 1. Among those meat models, the optimal addition levels of antioxidant hydrolysates are different [34,35]. These cases indicated that addition levels were crucial factors, and meanwhile, the addition levels also depended on the various properties of different food systems. Although antioxidants from fish and their by-products were effective, their usage was limited due to their flavor and odor. Therefore, the solid results of antioxidative hydrolysates or peptides should be developed in a specific food system, and the influence on the sensory evaluation of the final product should also be included.
In addition, land-animal protein is a good source for deriving antioxidant hydrolysates or peptides because its proteins contain plenty of essential amino acids with a high bioavailability that defeats plant proteins. The well-known antioxidant dipeptides of carnosine (β-alanyl-L-histidine) and anserine (β-alanyl-3-methylhistidine) endogenously exist in muscle tissue, acting as free radical scavengers and metal ion chelators [36]. As seen in Table 1, the land-animal protein sources were milk protein and slaughter remnant protein. Rossini et al. found that casein peptides exhibited a good antioxidative capacity in the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical method [37]; a similar result was displayed in studies of bovine milk protein-derived peptides and camel milk hydrolysates [38,39]. Sun et al. reported that deboned chicken-residue hydrolysates decrease oxidation and Mallard reaction products in Cantonese sausage without significant influences of sensory properties [40].
Furthermore, Verma et al. proved that porcine-blood hydrolysate has possible antioxidant and antimicrobial abilities in pork meat emulsion [41]. The hydrolysate incorporation may have more opportunities to maintain multi-functional characteristics, i.e., antioxidation, antimicrobial, and physic-chemical property enhancements, than a single bioactive peptide. Thus, it has high potential in practice, especially in the food industry. Overall, those studies indicated that the antioxidative protein hydrolysate could be used to retard lipid peroxidation in oxidizable products, such as the unsaturated fatty acid-rich product. However, the optimal application should be further measured depending on the properties of the individual food system.
2.2. Antimicrobial protein hydrolysates and peptides
Microbial intrinsic antimicrobial peptides, known as bacteriocin, have been an interesting research subject for a long time and developing the foundation of modern antibiotics [42]. Nowadays, microbial peptides from probiotics are also applied to the entire food system [43], though food protein hydrolysates and peptides were not emphasized in this study. Pane et al. proclaimed that antimicrobial peptides could also be produced by enzymatic hydrolysis of food proteins in vitro [44]. The isolation and characterization of antimicrobial peptides from food proteins have also been well studied. Moreover, various antimicrobial agents, such as food protein-origin bioactive peptides and protein hydrolysates, are applied as bio-preservatives for different food products (Table 2). The antimicrobial activity of porcine blood protein hydrolysates was also observed against spoilage microbes, such as Listeria monocytogenes, Staphylococcus aureus, E. coli, and Bacillus cereus in the pork emulsion during storage [41]. It was reported that the whey acidic protein-derived peptide has good antimicrobial activity against Staphylococcus aureus in milk [45].
Table 2.
Application of antimicrobial protein hydrolysates and peptides as a preservative in the food system.
| Protein source | Hydrolysates or peptides | Incorporated food system | Amount of active ingredients | References |
|---|---|---|---|---|
| Snakin-1-derived from potato tubers | Peptides | Fanta orange, cranberry juice, and apple juice | 50, 100, 200, and 400 μg/mL | [96] |
| Porcine blood | Hydrolysates | Pork meat emulsion | 900 μg/g | [41] |
| Whey acidic protein | Peptides | Milk | 31.2 and 15.6 μg/mL | [45] |
In most research cases, the antimicrobial activity of peptides was demonstrated only in vitro and not shown on any trials in commercial practices because food ingredients, such as proteins, proteases, fats, and metal ions, may limit the interaction of antimicrobial peptides with their target pathogens [42,46]. Therefore, before peptidic preservatives are introduced to the market, their stability during food processing and storage requires further assessment; potential safety and sensory problems also warrant an evaluation [47]. Moreover, the clean-label requirement in the market could push the industry or government to develop natural-origin (non-synthetic) food additives. From this point of view, food hydrolysates or peptides with an antimicrobial ability still require comprehensive study, and the real commercial application still has a long way to go.
2.3. Protein hydrolysates and peptides as food preservatives of edible biopolymer films
Recently, protein hydrolysates and bioactive peptides as food preservatives on green packaging materials, biodegradable and edible films and coatings have attracted much attention from food scientists. Rangaraj et al. reported that edible bioactive packaging materials had been applied to food storage and preservation [48]. The main function of food packaging systems is to separate food from the surrounding environment, thereby reducing interaction with spoilage factors, such as microorganisms, water vapor, oxygen, and off-flavor, and avoiding losses of desirable compounds, for example, flavor volatiles, thus extending the shelf life of food products [49]. Although coatings and edible films are not expected to replace conventional wrapping materials fully, they can retain food stability by reducing the exchange of moisture, lipids, volatiles, and gasses between the food and the surrounding environment. As we know, avoiding surface contamination increases the efficiency of food packaging, thus reducing the need for petroleum-derived polymers. The main components of biodegradable and edible films are usually an animal or vegetable proteins, polysaccharides, fats, and waxes. Meanwhile, they are primary packaging made from edible ingredients. Moreover, it is possibly used directly in the food system by coating, immersion, and spraying [50,51].
It has been reported that hydrolysates from fish by-products can be a source of biologically active peptides with high antioxidative activity (Table 3). The raw materials are mainly from cuttlefish, rainbow trout, silver carp, squid, and tilapia. An active two-layer coating consisting of furcellaran and gelatin hydrolysates from carp skins had been fully discussed [52–54]. Interestingly, many cases use hydrolysates and gelatin film from the same origin, which may be due to the concept of full application [55–59] (Table 3). It was demonstrated that milk protein-based edible films could be applied to preserve food products [56,57]. These selective films are effective oxygen, fat, and aroma barriers but are still permeable to moisture. Hence, they could be applied to various forms of high-protein dairy preparations, such as total milk protein powder, skim milk powder, caseinates, and whey protein concentrates [60,61]. Mukherjee and Haque (2016) proclaimed that antioxidative coatings incorporating cheddar whey casein hydrolysates could reduce the protein carbonylation in steak and fish fillet systems.
Table 3.
Effect of edible biopolymer films with the addition of protein hydrolysates and biopeptides on the quality of food products during their storage.
| Source of bioactive hydrolysate or peptide | Type of biopolymer matrix | References |
|---|---|---|
| Carp skin gelatin hydrolysates | Polysaccharide-furcellaran film | [54] |
| Casein hydrolysates | Cheddar whey-based coating film | [56,57] |
| Cuttlefish (Sepia Officinalis) protein hydrolysates | Cuttlefish skin gelatin film | [58] |
| Gelatin hydrolysate extracted from Scomberomorus commerson skin | Fish skin gelatin, commercial gelatin, commercial bovine gelatin film | [59] |
| Rapeseed protein hydrolysates | Chitosan film | [97] |
| Squid gelatin hydrolysates | Squid skin gelatin films | [55] |
Various antimicrobial compounds incorporated into edible films have also been interesting, while integrating natural derivatives in edible films was successfully executed (Table 3). Although scientific results are abundant and remarkable, the gap between laboratory to commercial practices still warrants investigation. There is no individual natural polymer to provide all the desired edible film properties, so the challenge is to select integrated and synergistic composite ingredients to fulfill the desired film properties. Furthermore, there are difficulties in up-scaling the laboratory research to industrial applications. Consumer acceptance, industrial interests, and governmental regulations would be other challenges.
3. Bioactive ingredient of nutraceuticals
The global nutraceutical market was valued at USD 382.51 billion in 2019 and is expected to expand at a Compound Annual Growth Rate (CAGR) of 8.3% from 2020 to 2027 [62]. A favorable outlook toward increasing cardiovascular disorders and malnutrition application is observed [62]. The consumers’ positive attitude toward functional foods fuels market growth because these added health and wellness benefits. Overall, rising concern about healthcare costs and the growing elderly population worldwide assist the global functional food industry. Besides, the rising disposable income, changing lifestyle, and shifting preferences for healthier dietary intake are expected to drive nutraceuticals in the Asia Pacific area, where the major market share was 31.01% in 2019 [62]. Jakubczyk et al. mentioned that bioactive protein hydrolysates or peptides are a trend as new sources of therapeutic strategy [63]. The main research targets on the biofunctions of bioactive protein hydrolysates or peptides could be divided into two categories: (1) antioxidation, anti-inflammation, and anti-apoptosis; (2) metabolic factor-related targets including anti-obesity, anti-diabetes, cardiovascular protection, and hypolipidemic effect. As we know, cardiovascular disease (CVD) and diabetes mellitus (DM) are two popular research topics, while metabolic syndrome is a crucial issue and challenge in human health worldwide. A summary of recent research is shown in Table 4.
Table 4.
Recent research targets the functionalities of protein hydrolysates and biopeptides.
| Protein resources | Hydrolysates or peptides | Functionality | Details | Ref. |
|---|---|---|---|---|
| Anti-oxidation, anti-inflammation, & anti-apoptosis | ||||
| Chicken liver | Hydrolysates | In vivo antioxidative effects in serum and organs in D-galactose injected mice | 1.2 g D-galactose kg−1 BW + 50 and 250 mg CLH kg−1 BW on male C57BL/6 mice for 6 weeks | [67] |
| Chicken liver | Hydrolysates | In vivo antioxidation and anti-inflammation in thioacetamide-induced mice | 100 mg TAA kg−1 BW + 200 and 600 mg CLH kg−1 BW on male Wistar rat for 10 weeks | [68] |
| Chicken liver | Hydrolysates | Cardiac muscle anti-inflammation in high-fat-diet-induced mice | HFD (46.5% energy as fat) + 170 and 510 mg CLH kg−1 BW on male C57BL/6 mice for 20 weeks | [70] |
| Potato protein | Hydrolysates | Cardiac muscle apoptosis attenuation in high-fat-diet-fed hamsters | HFD (60% of energy as fat) + 15, 45, and 75 mg CLH kg−1 BW on male hamster for 50 days | [69] |
| Ovalbumin | Hydrolysates | Anti-inflammatory activity in LPS-induced RAW264.7 macrophages | 0.1, 0.5, and 2 mg OVA mL−1 in LPS-induced (100 g mL−1) RAW 264.7 cells | [75] |
| Egg white protein | Peptides | Antioxidative effect in H2O2-induced cells | 20 μm peptide (VYLPR) in HEK-293 cells | [76] |
| Scallop protein | Hydrolysates | Antioxidative activity and protective effect in H2O2-induced cytotoxicity in vitro | 10 mg mL−1 SPH in DPPH and ABTS assays | [77] |
| Anti-obesity | ||||
| Chicken liver | Hydrolysates | Body weight gain decreases in HFD-induced mice | HFD (46.5% energy as fat) + 170 and 510 mg CLH kg−1 BW on male C57BL/6 mice for 20 weeks | [83] |
| Chicken breast raw materials | Hydrolysates | Mitochondrial β-oxidation enhancement and anti-inflammation in HFD-fed mice | HFD (59% of energy as lard) + CPH-contained diet (12.5%, w/w) on male C57BL/6JBomTac mice for 12 weeks | [81] |
| Crude chalaza of egg | Hydrolysates | Lipolysis and bile-acid biosynthesis enhancement and cholesterol clearance ability upregulation in high-fat-diet-fed hamsters | HFD (12% lard and 0.2% cholesterol, w/w) + 240, 480, and 960 mg CCH kg−1 BW on male hamster for 10 weeks | [82] |
| Alaska Pollack fillets | Hydrolysates | Hypothalamic neuropeptide Y reduction White adipose tissue weight decreases Muscle hypertrophy attenuation |
AIN-93 control diet (7% fat, w/w) + 100 and 300 mg APP kg−1 BW on male Sprague–Dawley rats for 3 days | [79] |
| Yeast | Hydrolysates | Weight and body fat reduction in obese women | Asia–Pacific region women aged 20–60 years with BMI>25 kg m−2/0.25 g YH-500 twice a day for 8 weeks | [80] |
| Anti-diabetes | ||||
| Chicken liver | Hydrolysates | Insulin sensitivity enhancement in HFD-induced mice | HFD (46.5% energy as fat) + 170 and 510 mg CLH kg−1 BW on male C57BL/6 mice for 20 weeks | [83] |
| Camel milk | Hydrolysates | Hyperglycemic, hyperlipidemic, and antioxidative effects in STZ-induced rats | 100.500, and 1000 mg CMPH kg−1 BW in male STZ-induced diabetic rats for 8 weeks | [88] |
| Silver carp swim bladder | Hydrolysates | DPP-IV inhibition in vitro Insulin secretion improvement in INS-1 cells |
INS-1 cell treated with 4 mM bioactive peptides (IPGSPY or WGDEHIPGSPYH) for 60 min | [87] |
| Egg white | Hydrolysates | Glucose homeostasis improvement in vitro | Insulin secretion by isolated Zucker rat pancreas islets (Experimental groups: Zucker lean rats, control Zucker fatty rats, and Zucker fatty rat treated for 12 weeks [750 mg HEW1 kg−1 BW per day]) | [72] |
| Egg white | Hydrolysates | Insulin sensitivity improvement in skeletal muscle cells | L6 cell treated with 5 mg mL−1 EWH or 11 μM IRW for 4 h | [89] |
| Grey triggerfish muscle protein | Hydrolysates | Hypoglycemic and hypolipidemic activities in diabetic rats | 400 mg BPH kg−1 BW in male alloxan-induced diabetic Wistar rats for 21 days | [84] |
| Pasteurized liquid egg white | Hydrolysates | Insulin mimetic and insulin-sensitizing actions in 3T3-F442A cells | 3T3-F442A cell treated with 5 mg mL−1 EWH for 72 h | [78] |
| Pasteurized liquid egg white | Hydrolysates | Glucose homeostasis improvement in Zucker fatty rats | Plasma glucose and insulin (Experimental groups: Zucker lean rats, control Zucker fatty rats, and Zucker fatty rat treated for 12 weeks [750 mg HEW1 kg−1 BW per day]) | [72] |
| Liquid egg white | Hydrolysates | Insulin sensitivity enhancement in HFD-induced rats | HFD (20% fat, w/w) + EWH-contained diet (1, 2, and 4%, w/w) on male Sprague–Dawley rats for 6 weeks | [90] |
| Sea cucumber (Holothuria Nobilis) | Hydrolysates | Hypoglycemic, hypolipidemic, and insulin-sensitizing effects in STZ and HFD-induced diabetic rats | HFD (45% energy as fat) + 200 and 400 mg SCH kg−1 BW on STZ-induced male Sprague –Dawley rats for 8 weeks | [86] |
| Norwegian spring-spawning herring by-products | Hydrolysates | Hypolipidemic effect and glucose homeostasis improvement in obese Zucker rats | Fish protein (Herring or salmon) hydrolysate-contained diet (25%, w/w) in male obese Zucker fa/fa rats for 4 weeks | [85] |
| Cardiovascular protection | ||||
| Chicken blood | Hydrolysates | Anti-hypertensive effect in vivo ACE inhibition in vitro | 100, 300, and 600 mg BCH kg−1 BW in male spontaneous hypertension rats for 4 weeks | [94] |
| Chicken liver | Hydrolysates | Hypolipidemic effect in high-fat-diet-induced hamsters | HFD (12% lard and 0.2% cholesterol, w/w) + 100, 200, and 400 mg CLH kg−1 BW on male hamster for 8 weeks | [98] |
| Chicken liver | Hydrolysates | Cardiac muscle anti-fibrosis in high-fat-diet-induced mice | HFD (46.5% energy as fat) + 170 and 510 mg CLH kg−1 BW on male C57BL/6 mice for 20 weeks | [70] |
| Chicken skin protein | Hydrolysates | Renin and ACE activity inhibition in vitro | 1 mg mL−1 CTSH in ACE-inhibitory activity assay | [91] |
| Egg white | Peptides | Angiotensin II type I receptor downregulation in vitro | A7r5 cell treated with 5 mg mL−1 EWH or 100 μM synthetic peptides for 24 h | [92] |
| Egg white | Hydrolysates | The hypotensive effect in rats | EWP or EWH-contained diet (1%, w/w) in male spontaneous hypertension rats for 4 weeks | [93] |
3.1. Antioxidation, anti-inflammation, and antiapoptosis
Antioxidation, anti-inflammation, and antiapoptosis were proven to be highly correlated in organisms; thus, those bioactivities were summarized jointly [64–66]. According to a report from Chou et al. [67], the antioxidant activities of chicken-liver hydrolysates have been successfully developed. Continuously, the in vivo antioxidant activity of chicken-liver hydrolysates was displayed in a D-galactose-induced mouse model in which chicken-liver-hydrolysate supplementation performed the universal antioxidant activities, especially in the brain and liver (Table 4). This is a good example linking the in vitro and in vivo antioxidative effects, and it further indicates why in vitro antioxidative capacity analysis is still included in many studies of protein hydrolysates or bioactive peptides. Furthermore, the food-origin protein hydrolysates or bioactive peptides were proven to be tissue-specific protective effects in recent in vivo studies (Table 3), such as hepatic [68] and cardiac tissues [69,70]. Incidentally, chicken egg-derived peptides for their biological multi-activities, especially antioxidation, anti-inflammation, and hypoglycemic effects have been attracted much attention [71–74]. It was demonstrated that trypsin-digested ovalbumin hydrolysates show anti-inflammatory activity in LPS-treated RAW 264.7 cells [75]. In addition, the antioxidative peptides, VYLPR, derived from egg-white protein, protected HEK-239 cells from H2O2 exposure [76]. Finally, some cases showed both biofunctional and processing capabilities. For example, Wang et al. proclaimed that alcalase-hydrolyzed scallop protein hydrolysate exhibited high antioxidative activity in the PC-12 cell model as well as good foaming and emulsifying properties [77]. In addition, it effectively inhibited lipid oxidation in the emulsifying system. This means that food-origin bio-active hydrolysates or peptides could incorporate various functional products that fulfill both bio-functionalities and product quality control requirements.
3.2. Anti-obesity
An anti-obesity property of food-origin hydrolysates or peptides was well reported as well, but the underlying mechanisms are various and inconclusive. Many researchers are still striving to clarify bioactive compounds and trying to connect structures and physiological outcomes. Jahandideh et al. revealed that bioactive peptides from egg white hydrolysate had an adipogenic-differentiating effect on the 3T3-F422A pre-adipocyte model [78]. Although the results are opposite and doubtful for its physiological meaning, they indicated the trend of structure–function studies of bio-functional food-origin peptides.
Table 4 contains the recent representative studies. Mizushige et al. found that Alaska-pollock-protein-hydrolysate supplementation decreases an energy intake in rats by reducing the mRNA expression of hypothalamic neuropeptide Y, which may reduce the appetite [79]. In another clinical case, low-dose yeast-hydrolysate supplementation was used as an obesity and weight-loss treatment among obese Korean women [80]. Although a further study for its mechanism is still needed, the anti-obesity effect was presented. The anti-obesity effects of poultry hydrolysates (i.e., egg chalaza, breast meat, and liver) were also reported, and the common mechanism was enhancing the lipolysis, fatty-acid βoxidation, and energy expenditure in mitochondria [81–83]. Overall, the formulation of multiple biohydrolysates or peptides may be another exploration for future scientists.
3.3. Anti-diabetes
Diabetes is a dread for human beings because it directly damages patients’ life quality. Certainly, there is a craving for anti-diabetic peptides. In Table 4, aquatic, egg white, chicken liver, and milk-derived hydrolysates or peptides are listed. It was indicated that Grey triggerfish (Balistes capricious) muscle protein hydrolysates can alleviate hyperglycemia and reduce HbA1c levels in diabetic rats [84]. Drotningsvik et al. obtained similar outcomes in their study of salmon hydrolysates [85]. Sea cucumber (Holothuria Nobilis) hydrolysates showed insulin-sensitizing effects in streptozotocin (STZ) and high-fat diet (HFD)-induced diabetic rats [86] while silver carp swim bladder hydrolysates inhibited dipeptidyl peptidase IV (DPP-IV) activity and enhanced insulin secretion in vitro [87]. In addition, the hyperglycemic effects of chicken-liver hydrolysates [83] and camel-milk hydrolysates are illustrated by a glucose tolerance test [88].
Remarkably, Garcés-Rimón et al. indicated that egg-white hydrolysates are a potential supplement to control complications associated with metabolic syndrome due to their DPP IV-inhibitory activity [72]. Moreover, egg white hydrolysates showed insulin-mimetic and sensitizing effects in the 3T3-F442A pre-adipocyte and skeletal muscle cell model [78,89]. Furthermore, the in vitro findings were verified in the in vivo studies. Egg-white-hydrolysate supplementation improved glucose metabolism and attenuated insulin resistance in diabetic rats via Akt activation [72,90]. All results indicated that egg-white hydrolysates had potential as a therapeutic diabetic agent.
3.4. Cardiovascular protection
In Table 4, Onuh et al. indicated that chicken-skin protein hydrolysates own an inhibitory ability on angiotensin-converting enzyme (ACE) activities in vitro [91]. In the cases of egg-white hydrolysates, Chen et al. successfully purified and identified the angiotensin receptor downregulating peptide, which was proven in the A7r5 cell model [92]. Moreover, the hypotensive effect of egg-white hydrolysates was confirmed in spontaneously hypertensive rats [93]. Besides, an in vivo anti-hypertensive property of chicken-blood hydrolysates was also demonstrated in the study of Wongngam et al. [94]. For bio-peptides, the lipid-lowering and hypolipemic properties may be concurrent with the anti-obesity property, and the details of lipid metabolic modulating hydrolysates or peptides were mentioned in the former paragraph. Wu et al. investigated the cardioprotective effects of chicken-liver hydrolysates in a long-term high-fat dietary habit [83]. Their study indicated that the cardioprotective effect of chicken-liver hydrolysates could be attributed to its synergistic hepatic lipid-lowering effect and systemic antioxidation. In the histological analysis, chicken-liver hydrolysate supplementation could attenuate cardiac pathological progression under long-term HFD induction. Meanwhile, the hypolipidemic, anti-obesity, and renal protective effects of chicken-liver hydrolysates against HFD were summarized. In addition, the anti-inflammatory and anti-fibrotic effects of chicken-liver hydrolysates on cardiac muscular tissues were confirmed. Those effects may be related to the early blockade of the autophagy pathway to prevent HFD-induced autophagosome accumulation. All works of evidence showed that the protective outcomes of the chicken-liver hydrolysates are due to systemic and synergistic effects. This study revealed the multi-activities and synergistic effects of hydrolysates, and a further application of bio-active hydrolysates and peptides need a comprehensive investigation.
Although several biofuncionalities of protein hydrolysates and peptides have been listed in this report, the delivery and stability of these benefits is still not clearly understood. In 2016, Rao et al. [99] also reported that the biofunctional availability and stability of the bioactive hydrolysates/peptides during postproduction still is a need to verify in vivo. They suggested two aspects on these two points: 1) the quality changes in different food protein hydrolysates during storage; (2) the resulting changes in the structure and texture of three food matrices. Hence, it is worthy for further investigation for the future commercial application. Meanwhile, those who possesses the key technology in following decades will get the ticket for global nutraceutical market, which is one of rapid growth industries nowadays.
4. Conclusion
Food preservation and nutraceutical ingredients are recent research targets of food protein-origin bioactive hydrolysates or peptides. However, the approaching method should be dynamic. Multi-activities, compound formulation, comprehensive evaluation, and the added value of by-products are possible strategies. Within the past few decades, there have been great results and findings regarding the functionalities of food protein-origin bioactive hydrolysates or peptides, but there are still efforts that are required to bridge laboratory studies with practical applications. Besides, there is a great need for the delivery and storage ability during the storage. Understandings and solutions for these two questions could benefit for future commercial application in the global nutraceutical market. Perhaps it is time to deal with these outcomes from different points of view, approaching the goal more comprehensively, creatively and with more novelty.
Acknowledgements
We acknowledge funding of this research from the Great Billion Biotech, Co., Ltd. (New Taipei City, Taiwan), and partially from Ministry of Science and Technology, Taiwan (Project: NSC 102-2313-B-002-039-MY3 and MOST 109-2313-B-002-007-MY3), Council of Agriculture, Executive Yuan, Taiwan (Project: 106AS-1.3.2-AD-U4, 109AS-12.1.1-ST-a1, 110AS-2.2.2-AD-U1(1), and 110AS-17.1.4-ST-a1).
Funding Statement
We acknowledge funding of this research from the Great Billion Biotech, Co., Ltd. (New Taipei City, Taiwan), and partially from Ministry of Science and Technology, Taiwan (Project: NSC 102-2313-B-002-039-MY3 and MOST 109-2313-B-002-007-MY3), Council of Agriculture, Executive Yuan, Taiwan (Project: 106AS-1.3.2-AD-U4, 109AS-12.1.1-ST-a1, 110AS-2.2.2-AD-U1(1), and 110AS-17.1.4-ST-a1).
Footnotes
Conflict of interest
There are no conflicts of interest to declare.
References
- 1. Sánchez A, Vázquez A. Bioactive peptides: A review. Food Qual Saf. 2017;1:29–46. [Google Scholar]
- 2. Peighambardoust SH, Karami Z, Pateiro M, Lorenzo JM. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules. 2021;11:163. doi: 10.3390/biom11050631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim J, Moon SH, Ahn DU, Paik HD, Park E. Antioxidant effects of ovotransferrin and its hydrolysates. Poultry Sci. 2012;91:2747–54. doi: 10.3382/ps.2012-02150. [DOI] [PubMed] [Google Scholar]
- 4. Sun Y, Pan D, Guo Y, Li J. Purification of chicken breast protein hydrolysate and analysis of its antioxidant activity. Food Chem Toxicol. 2012;50:3397–404. doi: 10.1016/j.fct.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 5. Kim SK, Wijesekara I. Development and biological activities of marine-derived bioactive peptides: A review. J Funct Foods. 2010;2:1–9. [Google Scholar]
- 6. de Castro RJS, Sato HH. A response surface approach on optimization of hydrolysis parameters for the production of egg white protein hydrolysates with antioxidant activities. Biocatal Agric Biotechnol. 2015;4:55–62. [Google Scholar]
- 7. Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharmaceut Des. 2003;9:1309–23. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- 8. Pappenheimer JR, Volpp K. Transmucosal impedance of small intestine: Correlation with transport of sugars and amino acids. Am J Physiol. 1992;263:C480–93. doi: 10.1152/ajpcell.1992.263.2.C480. [DOI] [PubMed] [Google Scholar]
- 9. Chen HM, Muramoto K, Yamauchi F. Structural analysis of antioxidative peptides from soybean β-conglycinin. J Agric Food Chem. 1995;43:574–8. [Google Scholar]
- 10.Ward OP. Production of protein hydrolysates. In: Murray MY, editor. Comprehensive biotechnology. 3rd ed. Oxford, United Kingdom: Pergamon Press; 2019. p. 612. [Google Scholar]
- 11.Ganapathy V. Protein digestion and absorption. In: Johnson LR, Ghishan FK, Kaunitz JD, Merchant JL, Said HM, Wood JD, editors. Physiology of the gastrointestinal tract. 5th ed. Amsterdam, Netherland: Elsevier; 2012. 2012. pp. 1595–662. [Google Scholar]
- 12. Freeman HJ, Sleisenger MH, Kim YS. Human protein digestion and absorption: Normal mechanisms and protein-energy malnutrition. Clin Gastroenterol. 1983;12:357–78. [PubMed] [Google Scholar]
- 13.Food and Agriculture Organization of the United Nations (FAO) Meat market review: Overview of global market developments in 2018. [Accessed date: 2019/03.]. Available https://www.fao.org/3/ca3880en/ca3880en.pdf.
- 14. Dong XB, Li X, Zhang CH, Wang JZ, Tang CH, Sun HM, et al. Development of a novel method for hot-pressure extraction of protein from chicken bone and the effect of enzymatic hydrolysis on the extracts. Food Chem. 2014;157:339–46. doi: 10.1016/j.foodchem.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 15. Lapena D, Vuoristo KS, Kosa G, Horn SJ, Eijsink VGH. Comparative assessment of enzymatic hydrolysis for valorization of different protein-rich industrial by-products. J Agric Food Chem. 2018;66:9738–49. doi: 10.1021/acs.jafc.8b02444. [DOI] [PubMed] [Google Scholar]
- 16. Rajapakse N, Mendis E, Jung W, Je J, Kim S. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38:175–82. [Google Scholar]
- 17. Lin CC, Liang JH. Effect of antioxidants on the oxidative stability of chicken breast meat in a dispersion system. J Food Sci. 2002;67:530–3. [Google Scholar]
- 18. Sila A, Bougatef A. Antioxidant peptides from marine by-products: Isolation, identification, and application in food system. A review. J Funct Foods. 2016;21:10–26. [Google Scholar]
- 19. Van Aardt M, Duncan SE, Long TE, O’Keefe SF, Marcy JE, Sims SR. Effect of antioxidants on oxidative stability of edible fats and oils: Thermogravimetric analysis. J Agric Food Chem. 2004;52:587–91. doi: 10.1021/jf030304f. [DOI] [PubMed] [Google Scholar]
- 20. Ito N, Hirose M, Fukushima S, Tsuda H, Shirai T, Tatematsu M. Studies on antioxidants: Their carcinogenic and modifying effects on chemical carcinogenic. Food Chem Toxicol. 1986;24:1099–102. doi: 10.1016/0278-6915(86)90291-7. [DOI] [PubMed] [Google Scholar]
- 21. Williams GM, Iatropoulos MJ, Whysner J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem Toxicol. 1999;37:1027–38. doi: 10.1016/s0278-6915(99)00085-x. [DOI] [PubMed] [Google Scholar]
- 22.US Food & Drug Administration. CFR - code of federal regulations title 21. Food additives permitted for direct addition to food for human consumption. [Accessed 16 November 2021]. Updated Oct. 1, 2021. Available: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172.
- 23. Xu X, Liu A, Hu S, Ares I, Martínez-Larrañaga MR, Wang X, et al. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021;353:129488. doi: 10.1016/j.foodchem.2021.129488. [DOI] [PubMed] [Google Scholar]
- 24. Wen C, Zhang J, Zhang H, Duan Y, Ma H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci Technol. 2020;105:308–22. [Google Scholar]
- 25. Sohaib M, Anjum FM, Sahar A, Arshad MS, Rahman UU, Imran A, et al. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int J Food Prop. 2017;20:2581–93. [Google Scholar]
- 26. Peña-Ramos EA, Xiong YL. Whey and soy protein hydrolysates inhibit lipid oxidation in cooked pork patties. Meat Sci. 2003;64:259–63. doi: 10.1016/S0309-1740(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 27. Zhao L, Wang S, Huang Y. Antioxidant function of tea dregs protein hydrolysates in liposome–meat system and its possible action mechanism. Int J Food Sci Technol. 2014;49:2299–306. [Google Scholar]
- 28. Ambigaipalan P, Shahidi F. Antioxidant potential of date (Phoenix dactylifera L.) seed protein hydrolysates and carnosine in food and biological systems. J Agric Food Chem. 2015;63:864–71. doi: 10.1021/jf505327b. [DOI] [PubMed] [Google Scholar]
- 29. Sarker A, Chakraborty S, Roy M. Dark red kidney bean (Phaseolus vulgaris L.) protein hydrolysates inhibit the growth of oxidizing substances in plain yogurt. J Agric Food Res. 2020;2:100062. [Google Scholar]
- 30. Gomes MHG, Kurozawa LE. Influence of rice protein hydrolysate on lipid oxidation stability and physico-chemical properties of linseed oil microparticles obtained through spray-drying. LWT - Food Sci Technol (Lebensmittel-Wissenschaft-Technol) 2021;139:110510. [Google Scholar]
- 31. Najafian L, Babji AS. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides. 2012;33:178–85. doi: 10.1016/j.peptides.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 32. Shahidi F, Han SQ, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53:285–93. [Google Scholar]
- 33. Nikoo M, Benjakul S, Ehsani A, Li J, Wu F, Yang N, et al. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J Funct Foods. 2014;7:609–20. [Google Scholar]
- 34. Kittiphattanabawon P, Benjakul S, Visessanguan W, Shahidi F. Gelatin hydrolysate from blacktip shark skin prepared using papaya latex enzyme: Antioxidant activity and its potential in model systems. Food Chem. 2012;135:1118–26. doi: 10.1016/j.foodchem.2012.05.080. [DOI] [PubMed] [Google Scholar]
- 35. Nasri R, Younes I, Jridi M, Trigui M, Bougatef A, Nedjar-Arroume N, et al. ACE inhibitory and antioxidative activities of Goby (Zosterissessor ophiocephalus) fish protein hydrolysates: Effect on meat lipid oxidation. Food Res Int. 2013;54:552–61. [Google Scholar]
- 36. Kang JH, Kim KS, Choi SY, Kwon HY, Won MH, Kang TC. Carnosine and related dipeptides protect human ceruloplasmin against peroxyl radical-mediated modification. Mol Cell. 2002;13:498–502. [PubMed] [Google Scholar]
- 37. Rossini K, Norena CP, Cladera-Olivera F, Brandelli A. Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat. LWT - Food Sci Technol (Lebensmittel-Wissenschaft-Technol) 2009;42:862–7. [Google Scholar]
- 38. Hogan S, Zhang L, Li J, Wang H, Zhou K. Development of antioxidant rich peptides from milk protein by microbial proteases and analysis of their effects on lipid peroxidation in cooked beef. Food Chem. 2009;117:438–43. [Google Scholar]
- 39. Al-Shamsi KA, Mudgil P, Hassan HM, Maqsood S. Camel milk protein hydrolysates with improved techno-functional properties and enhanced antioxidant potential in vitro and in vivo food model systems. J Dairy Sci. 2018;101:47–60. doi: 10.3168/jds.2017-13194. [DOI] [PubMed] [Google Scholar]
- 40. Sun W, Zhao M, Cui C, Zhao Q, Yang B. Effect of Maillard reaction products derived from the hydrolysate of mechanically deboned chicken residue on the antioxidant, textural and sensory properties of Cantonese sausages. Meat Sci. 2010;86:276–82. doi: 10.1016/j.meatsci.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 41. Verma AK, Chatli MK, Mehta N, Kumar P. Assessment of physicochemical, antioxidant, and antimicrobial activity of porcine blood protein hydrolysate in pork emulsion stored under aerobic packaging condition at 4+1°C. LWT - Food Sci Technol (Lebensmittel-Wissenschaft -Technol) 2018;88:71–9. [Google Scholar]
- 42. Tkaczewska J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings - a review. Trends Food Sci Technol. 2020;106:298–311. [Google Scholar]
- 43. Ning Y, Han P, Ma J, Liu Y, Fu Y, Wang Z, et al. Characterization of brevilaterins, multiple antimicrobial peptides simultaneously produced by Brevibacillus laterosporus S62–9, and their application in real food system. Food Biosci. 2021;42:101091. [Google Scholar]
- 44. Pane K, Durante L, Crescenzi O, Cafaro V, Pizzo E, Varcamonti M, et al. Antimicrobial potency of cationic antimicrobial peptides can be predicted from their amino acid composition: Application to the detection of “cryptic” antimicrobial peptides. J Theor Biol. 2017;419:254–65. doi: 10.1016/j.jtbi.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 45. Yang S, Li J, Aweya JJ, Yuan Z, Weng W, Zhang Y, et al. Antimicrobial mechanism of Larimichthys crocea whey acidic protein-derived peptide (LCWAP) against Staphylococcus aureus and its application in milk. Int J Food Microbiol. 2020;335:108891. doi: 10.1016/j.ijfoodmicro.2020.108891. [DOI] [PubMed] [Google Scholar]
- 46. Juneja VK, Dwivedi HP, Yan X. Novel natural food antimicrobials. Annu Rev Food Sci Technol. 2012;3:381–403. doi: 10.1146/annurev-food-022811-101241. [DOI] [PubMed] [Google Scholar]
- 47. Pan M, Liu K, Yang J, Liu S, Wang S, Wang S. Advances on food-derived peptidic antioxidants-a review. Antioxidants. 2020;9:799. doi: 10.3390/antiox9090799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rangaraj VM, Rambabu K, Banat F, Mittal V. Natural anti-oxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021;43:101251. [Google Scholar]
- 49. Otoni CG, Avena-Bustillos RJ, Azeredo HMC, Lorevice MV, Moura MR, Mattoso LHC, et al. Recent advances on edible films based on fruits and vegetables-A review. Compr Rev Food Sci Food Saf. 2017;16:1151–69. doi: 10.1111/1541-4337.12281. [DOI] [PubMed] [Google Scholar]
- 50. Mokrejs P, Langmaier F, Janacova D, Mladek M, Kolomaznik K, Vasek V. Thermal study and solubility tests of films based on amaranth flour starch–protein hydrolysate. J Therm Anal Calorim. 2009;98:299–307. [Google Scholar]
- 51. Galus S, Kadzińska J. Food applications of emulsion-based edible films and coatings. Trends Food Sci Technol. 2015;45:273–83. [Google Scholar]
- 52. Hemker AK, Nguyen LT, Karwe M, Salvi D. Effects of pressure-assisted enzymatic hydrolysis on functional and bioactive properties of tilapia (Oreochromis niloticus) by-product protein hydrolysates. LWT - Food Sci Technol (Lebensmittel-Wissenschaft-Technol) 2020;122:109003. [Google Scholar]
- 53. Jamróz E, Kulawik P, Tkaczewska J, Guzik P, Zając M, Juszczak L, et al. The effects of active double-layered furcellaran/gelatin hydrolysate film system with Ala-Tyr peptide on fresh Atlantic mackerel stored at −18°C. Food Chem. 2021;338:127867. doi: 10.1016/j.foodchem.2020.127867. [DOI] [PubMed] [Google Scholar]
- 54. Tkaczewska J, Kulawik P, Jamróz E, Guzik P, Zając M, Szymkowiak A, et al. One- and double-layered furcellaran/carp skin gelatin hydrolysate film system with antioxidant peptide as an innovative packaging for perishable foods products. Food Chem. 2021;351:129347. doi: 10.1016/j.foodchem.2021.129347. [DOI] [PubMed] [Google Scholar]
- 55. Giménez B, Gómez-Estaca J, Alemán A, Gómez-Guillén MC, Montero MP. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocolloids. 2009;23:1322–7. [Google Scholar]
- 56. Mukherjee D, Haque ZZ. Reduced protein carbonylation of cube steak and catfish fillet using antioxidative coatings containing cheddar whey, casein hydrolyzate, and oolong tea extract. Ann Food Sci Technol. 2016;17:529–36. [Google Scholar]
- 57. Haque ZZ, Zhang Y, Mukherjee D. Casein hydrolysate augments antimicrobial and antioxidative persistence of cheddar whey protein concentrate based edible coatings. Ann Food Sci Technol. 2016;17:468–77. [Google Scholar]
- 58. Kchaou H, Jridia M, Benbettaieb N, Debeaufort F, Nasri M. Bioactive films based on cuttlefish (Sepia officinalis) skin gelatin incorporated with cuttlefish protein hydrolysates: Physicochemical characterization and antioxidant properties. Food Pack Shelf Life. 2020;24:100477. [Google Scholar]
- 59. Mirzapour-Kouhdasht A, Moosavi-Nasab M. Shelf-life extension of whole shrimp using an active coating containing fish skin gelatin hydrolysates produced by a natural protease. Food Sci Nutr. 2020;8:214–23. doi: 10.1002/fsn3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khwaldia K, Perez C, Banon S, Desobry S, Hardy J. Milk proteins for edible films and coatings. Crit Rev Food Sci Nutr. 2004;44:239–51. doi: 10.1080/10408690490464906. [DOI] [PubMed] [Google Scholar]
- 61. Singh P, Singh TP, Gandhi N. Prevention of lipid oxidation in muscle foods by milk proteins and peptides: A review. Food Rev Int. 2018;34:226–47. [Google Scholar]
- 62.Grand View Research. Nutraceutical market size, share & trends analysis report by product (dietary supplements, functional foods, functional beverages), by region, and segment forecasts, 2020–2028. [Accessed 8 November 2021]. Available: https://www.grandviewresearch.com/industry-analysis/nutraceuticalsmarket.
- 63. Jakubczyk A, Karaś M, Rybczynska-Tkaczyk K, Zielińska E, Zieliński D. Current trends of bioactive peptides-new sources and therapeutic effect. Foods. 2020;9:846. doi: 10.3390/foods9070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jian J, Xuan F, Qin F, Huang R. The antioxidant, anti-inflammatory, and anti-apoptotic activities of the Bauhinia Championii flavone are connected with protection against myocardial ischemia/reperfusion injury. Cell Physiol Biochem. 2016;38:1365–75. doi: 10.1159/000443080. [DOI] [PubMed] [Google Scholar]
- 65. Chen P, Chen F, Zhou B. Anti-oxidative, anti-inflammatory, and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci Rep. 2018;8:1465. doi: 10.1038/s41598-018-19732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guo J, Cao X, Hu X, Li S, Wang J. The anti-apoptotic, anti-oxidant, and anti-inflammatory effects of curcumin on acrylamide-induced neurotoxicity in rats. BMC Pharmacol Toxicol. 2020;21:62. doi: 10.1186/s40360-020-00440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chou CH, Wang SY, Lin YT, Chen YC. Antioxidant activities of chicken liver hydrolysates by pepsin treatment. Int J Food Sci Technol. 2014;49:1654–62. [Google Scholar]
- 68. Chen PJ, Tseng JK, Lin YL, Wu YHS, Hsiao YT, Chen JW, et al. Protective effects of functional chicken liver hydrolysates against liver fibrogenesis: Antioxidation, anti-inflammation, and antifibrosis. J Agric Food Chem. 2017;65:4961–9. doi: 10.1021/acs.jafc.7b01403. [DOI] [PubMed] [Google Scholar]
- 69. Huang CY, Chiang WD, Pai P, Lin WT. Potato protein hydrolysate attenuates high fat diet-induced cardiac apoptosis through SIRT1/PGC-1a/Akt signaling. J Funct Foods. 2015;12:389–98. [Google Scholar]
- 70. Wu YHS, Lin YL, Huang C, Chiu CH, Nakthong S, Chen YC. Cardiac protection of functional chicken-liver hydrolysates on the high-fat diet-induced cardio-renal damages via sustaining autophagy homeostasis. J Sci Food Agric. 2020;100:2443–52. doi: 10.1002/jsfa.10261. [DOI] [PubMed] [Google Scholar]
- 71. Garcés-Rimón M, López-Expósito I, López-Fandiño R, Miguel M. Egg white hydrolysates with in vitro biological multiactivities to control complications associated with the metabolic syndrome. Eur Food Res Tech. 2016;242:61–9. [Google Scholar]
- 72. Garcés-Rimón M, González C, Vera G, Uranga JA, López-Fandiño R, López-Miranda V, et al. Pepsin egg white hydrolysate improves glucose metabolism complications related to metabolic syndrome in Zucker fatty rats. Nutrients. 2018;10:441. doi: 10.3390/nu10040441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Benedé S, Molina E. Chicken egg proteins and derived peptides with antioxidant properties. Foods. 2020;9:735. doi: 10.3390/foods9060735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moreno-Fernández S, Garcés-Rimón M, Miguel M. Eggderived peptides and hydrolysates: A new bioactive treasure for cardiometabolic diseases. Trends Food Sci Technol. 2020;104:208–18. [Google Scholar]
- 75. Kim HS, Lee JH, Moon SH, Ahn DU, Paik HD. Ovalbumin hydrolysates inhibit nitric oxide production in LPS-induced RAW264.7 macrophages. Food Sci Anim Resour. 2020;40:274–85. doi: 10.5851/kosfa.2020.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhang B, Wang H, Wang Y, Yu Y, Liu J, Liu B, et al. Identification of antioxidant peptides derived from egg-white protein and its protective effects on H2O2-induced cell damage. Int J Food Sci Technol. 2019;54:2219–27. [Google Scholar]
- 77. Wang Z, Liu X, Xie H, Liu Z, Rakariyatham K, Yu C, et al. Antioxidant activity and functional properties of alcalase-hydrolyzed scallop protein hydrolysate and its role in the inhibition of cytotoxicity in vitro. Food Chem. 2021;344:128566. doi: 10.1016/j.foodchem.2020.128566. [DOI] [PubMed] [Google Scholar]
- 78. Jahandideh F, Chakrabarti S, Davidge ST, Wu J. Egg white hydrolysate shows insulin mimetic and sensitizing effects in 3T3-F442A pre-adipocytes. PLoS One. 2017;12:e0185653. doi: 10.1371/journal.pone.0185653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mizushige T, Komiya M, Onda M, Uchida K, Hayamizu K, Kabuyama Y. Fish protein hydrolysate exhibits anti-obesity activity and reduces hypothalamic neuropeptide Y and agouti-related protein mRNA expressions in rats. Biomed Res-Tokyo. 2017;38:351–7. doi: 10.2220/biomedres.38.351. [DOI] [PubMed] [Google Scholar]
- 80. Jung EY, Lee JW, Hong YH, Chang UJ, Suh HJ. Low dose yeast hydrolysate in treatment of obesity and weight loss. Prev Nutr Food Sci. 2017;22:45–9. doi: 10.3746/pnf.2017.22.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Aloysius TA, Carvajal AK, Slizyte R, Skorve J, Berge RK, Bjørndal B. Chicken protein hydrolysates have anti-inflammatory effects on high-fat diet induced obesity in mice. Medicine. 2019;6:5. doi: 10.3390/medicines6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen JW, Lin YL, Chou CH, Wu YHS, Wang SY. Antiobesity and hypolipidemic effects of protease A-digested crudechalaza hydrolysates in a high-fat diet. J Funct Foods. 2020;66:103788. [Google Scholar]
- 83. Wu YHS, Lin YL, Yang WY, Wang SY, Chen YC. Pepsin-digested chicken-liver hydrolysate attenuates hepatosteatosis by relieving hepatic and peripheral insulin resistance in long-term high-fat dietary habits. J Food Drug Anal. 2021;29:375–88. doi: 10.38212/2224-6614.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Siala R, Khabir A, Lassoued I, Abdelhedi O, Elfeki A, Vallaeys T, et al. Functional and antioxidant properties of protein hydrolysates from grey triggerfish muscle and in vivo evaluation of hypoglycemic and hypolipidemic activities. J Appl Environ Microbiol. 2016;4:105–19. [Google Scholar]
- 85. Drotningsvik A, Mjøs SA, Pampanin DM, Slizyte R, Carvajal A, Remman T, et al. Dietary fish protein hydrolysates containing bioactive motifs affect serum and adipose tissue fatty acid compositions, serum lipids, postprandial glucose regulation, and growth in obese Zucker fa/fa rats. Br J Nutr. 2016;116:1336–45. doi: 10.1017/S0007114516003548. [DOI] [PubMed] [Google Scholar]
- 86. Wang T, Zheng L, Zhao T, Zhang Q, Liu Z, Liu X, et al. Anti-diabetic effects of sea cucumber (Holothuria nobilis) hydrolysates in streptozotocin and high-fat-diet induced diabetic rats via activating the PI3K/Akt pathway. J Funct Foods. 2020;75:104224. [Google Scholar]
- 87. Hong H, Zheng Y, Song S, Zhang Y, Zhang C, Liu J, et al. Identification and characterization of DPP-IV inhibitory peptides from silver carp swim bladder hydrolysates. Food Biosci. 2020;38:100748. [Google Scholar]
- 88. Kilari BP, Mudgil P, Azimullah S, Bansal N, Ojha S, Maqaood S. Effect of camel milk protein hydrolysates against hyperglycemia, hyperlipidemia, and associated oxidative stress in streptozotocin (STZ)-induced diabetic rats. J Dairy Sci. 2021;104:1304–17. doi: 10.3168/jds.2020-19412. [DOI] [PubMed] [Google Scholar]
- 89. Son M, Wu J. Egg white hydrolysate and peptide reverse insulin resistance associated with tumor necrosis factor-α (TNF-α) stimulated mitogen-activated protein kinase (MAPK) pathway in skeletal muscle cells. Eur J Nutr. 2019;58:1961–9. doi: 10.1007/s00394-018-1753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jahandideh F, de Campos Zani SC, Son M, Proctor SD, Davidge ST, Chan CB, et al. Egg white hydrolysate enhances insulin sensitivity in high-fat diet-induced insulin-resistant rats via Akt activation. Br J Nutr. 2019;122:14–24. doi: 10.1017/S0007114519000837. [DOI] [PubMed] [Google Scholar]
- 91. Onuh JO, Girgih AT, Aluko RE, Aliani M. Inhibitions of renin and angiotensin converting enzyme activities by enzymatic chicken skin protein hydrolysates. Food Res Int. 2013;53:260–7. [Google Scholar]
- 92. Chen L, Liao W, Fang J, Qin S, Lu X, Wu J. Purification and identification of angiotensin II type I receptor down-regulating peptide from egg white hydrolysate. J Food Biochem. 2020;44:e13220. doi: 10.1111/jfbc.13220. [DOI] [PubMed] [Google Scholar]
- 93. Lee DE, Jung TH, Jo YN, Yun SS, Han KS. Enzymatic hydrolysis of egg white protein exerts a hypotensive effect in spontaneously hypertensive rats. Food Sci Anim Res. 2019;39:980–7. doi: 10.5851/kosfa.2019.e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wongngam W, Mitani T, Katayama S, Nakamura S, Yongsawatdigul J. Production and characterization of chicken blood hydrolysate with anti-hypertensive properties. Poultry Sci. 2020;99:5163–74. doi: 10.1016/j.psj.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li Y, Kong B, Liu Q, Xia X, Chen H. Improvement of the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions by addition of zein hydrolysates. Process Biochem. 2017;53:116–24. [Google Scholar]
- 96. Shwaiki LN, Arendt EK, Lynch KM. Study on the characterisation and application of synthetic peptide Snakin-1 derived from potato tubers-Action against food spoilage yeast. Food Control. 2020;118:107362. [Google Scholar]
- 97. Zhang C, Wang Z, Li Y, Yang Y, Ju X, He R. The preparation and physiochemical characterization of rapeseed protein hydrolysate-chitosan composite films. Food Chem. 2019;272:694–701. doi: 10.1016/j.foodchem.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 98. Yang KT, Lin C, Liu CW, Chen YC. Effects of chicken liver hydrolysates on lipid metabolism in a high-fat diet. Food Chem. 2014;160:148–56. doi: 10.1016/j.foodchem.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 99. Rao Q, Kamdar AK, Labuza TP. Storage stability of food protein hydrolysates-a review. Crit Rev Food Sci Nutr. 2016;56:1169–92. doi: 10.1080/10408398.2012.758085. [DOI] [PubMed] [Google Scholar]