Abstract
Traditional Chinese medicine (TCM) has been applied to improve human health for millennia. In the TCM system, “medicinal property” ( yao xing; hot and cold properties) is a core concept used to describe the influences of medicinal materials on human physiological conditions, and metabolites are believed to be one of the major ingredients of TCMs that affect their medicinal property. However, due to a lack of comprehensive analyses of TCM metabolomes, information about the relationships between TCM metabolite composition and medicinal property remains limited. In this pilot study, a mass spectral molecular networking-based platform was established and applied to systematically profile the metabolome of 24 TCMs with various medicinal properties. The molecular networks were built based on the liquid chromatography-tandem mass spectrometry (LC-MS/MS) data from 50% EtOH extracts of 24 TCMs. The results showed that various classes of metabolites were clustered in the molecular networks, and the potential medicinal property-associated molecular families were filtered by screening the medicinal property and the diversity of TCM sources. For example, some specific types of flavonoids were identified in the representative cold-property (han xing) molecular families. In contrast, due to the limited sample size, the representative and universal hot-property (re xing) molecular family has not been well revealed. In summary, this study provides methodology and information on the potential relationships between the metabolite composition and the concept of medicinal property in TCM. Furthermore, the results can serve as a foundation for mass spectral molecular networking-based analysis of TCM metabolomes, facilitating TCM research and development.
Keywords: LC-MS/MS, Metabolome mining, Molecular networking, Medicinal property, Traditional Chinese medicine
1. Introduction
Traditional Chinese medicine (TCM) has been widely applied to prevent and treat various human diseases for millennia. The development of TCM is based on thousands of years of empirical practice, and the principles and diverse usages of TCM are recorded in detail in ancient Chinese medicinal books, forming a unique therapeutic system. Currently, TCM has become a mainstay of health maintenance and promotion in several Asian countries. In Taiwan, for example, TCM is included in the National Health Insurance system and is widely recognized and utilized by the locals.
In TCM theory, “medicinal property” ( yao xing; also known as “medicinal nature”), is one of the core concepts used to describe the functions of medicinal materials, and the medicinal properties of different TCM materials are recorded in various TCM books such as Shen Nong’s Classic of the Materia Medica (shen nong ben cao jing; the earliest existing TCM book). TCM materials can generally be categorized into four medicinal properties, also known as the “four qi” (si qi; cold, hot, warm, and cool) based on their influences on human physiological conditions and esthesia. TCM materials with “cold property” (han xing) or “cool property” (liang xing) can alleviate heat symptoms such as fever, thirst, swelling, and sore throat [1]. For example, Scutellariae Radix (huang qin; the root of Scutellaria baicalensis) is a popular TCM with cold property, and it possesses the functions of “clearing heat” (qing re) and “detoxifying” ( jie du), which are effective in treating inflammation and respiratory infections [2]. In contrast, TCM materials with “hot property” (re xing) or “warm property” (wen xing) can relieve cold symptoms such as cold limbs, loss of appetite, diarrhea, and vomiting [1]. For instance, a TCM with hot property, Euodiae Fructus (wu zhu yu; the fruit of Tetradium ruticarpum), is known for its functions of “warming up interior” (wen zhong), “expelling cold” (san han), “arresting vomiting” (zhi ou), and “arresting diarrhea” (zhi xie) [3]. Clinically, the medicinal property is one of the fundamental concepts that TCM practitioners use to prescribe appropriate TCM formulas for treating different syndromes (categorized patterns of symptoms and signs in a patient at a specific stage of disease) [4].
Among the different ingredients, metabolites appear to be one of the significant components determining the pharmacological effects of TCM, implying that metabolite composition may affect the medicinal property of TCM [5]. Previous studies have investigated the chemical structure differences in cold-property and hot-property TCMs using computational approaches and principal component analysis [6–8]; the compounds associated with cold property generally possess a higher polarity and more aliphatic rings or long chain alkenes [6,8], while the compounds associated with hot property typically have a lower average molecular weight and more aromatic rings [6,7]. Although the general comparative view of the chemical properties of known compounds in the cold- and hot-property TCMs has been proposed, understanding on the relationships between TCM medicinal property and metabolite composition is still limited. In addition, TCM is often used in the form of crude extracts or raw materials with limited processing, but conventional bioactivity-guided approaches for TCM metabolite analysis provide little holistic information about the TCM metabolome profile. Therefore, the systematic identification and characterization of the metabolites in complex TCM mixtures remains a significant challenge in TCM research and development [9].
With advancements in analytical techniques, especially high-resolution mass spectrometry and computational methodology, significant progress has been made in metabolite analysis. Liquid chromatography-mass spectrometry (LC-MS) is currently the most versatile and commonly used method for the metabolite profiling of TCM extracts and biological matrices [10,11]. However, compound identification and structure elucidation for complex mixtures remains a common challenge in MS data analysis. The mass spectral molecular networking (MN) approach has recently emerged and provided a new perspective for metabolite discovery and identification in natural products research [12,13]. MN is a computational strategy for organizing tandem mass spectrometry (MS/MS) data; it allows the visualization of the structural relationships among molecules based on comparing their MS/MS spectral similarity so that the molecules with similar fragmentation patterns (potential structural analogs) are clustered to form molecular families [14]. Along with mass spectral library matching and cutting-edge computational tools in network annotation, MN has played an influential role in large-scale compound identification, structure prediction, and chemical classification [15].
The employment of the MN approach can enormously facilitate systematic metabolome analysis and comparison of complex TCM samples with accurate MS/MS data. Furthermore, by integrating the information about TCM material name and medicinal property into the molecular network, MN provides a practical way to decipher the metabolome differences between cold-property and hot-property TCM. In this pilot study, 24 commonly-used TCMs with various medicinal properties were extracted by an automated extraction system and analyzed by LC-MS/MS under standardized conditions; the potential relationships between TCM metabolite composition and medicinal property were then explored using the MN strategy.
2. Materials and methods
2.1. Materials
Twenty-four TCMs were selected based on their medicinal properties and clinical applications. The powders of 24 TCMs were provided by Sheng Chang Pharmaceutical (Taoyuan, Taiwan), and these TCM materials were authenticated by morphology, microscopy, and chemical assays according to the Taiwan Herbal Pharmacopeia [16]. The names, abbreviations, and medicinal properties of the 24 selected TCMs are listed in Table 1.
Table 1.
The 24 TCMs analyzed in this study.
| English name | Chinese name | Abbreviation | Medicinal propertya |
|---|---|---|---|
| Oldenlandiae Diffusae Herba | Bai hua she she cao | BHSSC | Cold |
| Isatidis Radix | Ban lan gen | BLG | Cold |
| Scutellariae Barbatae Herba | Ban zhi lian | BZL | Cold |
| Andrographis Herba | Chuan xin lian | CXL | Cold |
| Rhei Radix et Rhizoma | Da huang | DH | Cold |
| Phellodendri Cortex | Huang bo | HB | Cold |
| Scutellariae Radix | Huang qin | HQ | Cold |
| Coptidis Rhizoma | Huang lian | HL | Cold |
| Lonicerae Japonicae Flos | Jin yin hua | JYH | Cold |
| Sophorae Flavescentis Radix | Ku shen | KS | Cold |
| Toraxaci Herba | Pu gong ying | PGY | Cold |
| Gardeniae Fructus | Zhi zi | ZZ | Cold |
| Origani Vulgaris Herba | Bei yin chen | BYC | Mild cold |
| Chrysanthemi Flos | Ju hua | JH | Mild cold |
| Forsythiae Fructus | Lian qiao | LQ | Mild cold |
| Houttuyniae Herba | Yu xing cao | YXC | Mild cold |
| Zingiberis Rhizoma | Gan jiang | GJ | Hot |
| Aconiti Lateralis Radix Praeparata | Pao fu zi | PFZ | Hot |
| Cinnamomi Cortex | Rou gui | RG | Hot |
| Processed Euodiae Fructus | Zhi wu zhu yu | ZWZY | Hot |
| Sinapis Albae Semen | Bai jie zi | BJZ | Warm |
| Caryophylli Flos | Ding xiang | DX | Warm |
| Morindae Officinalis Radix | Ba ji tian | BJT | Mild warm |
| Patriniae Herba | Bai jiang cao | BJC | Neutral |
The medicinal property of each TCM is classified according to the Taiwan Herbal Pharmacopeia [16].
2.2. TCM sample preparation
Accelerated Solvent Extractor (ASE 350) (Thermo Scientific, Waltham, MA, USA) was used to prepare the TCM extracts under standardized extraction conditions. ASE is an automated system for extracting organic compounds, and it uses elevated temperature and high pressure during the extraction process to enhance extraction efficiency. Extraction was performed using 50% EtOH as extraction solvent. Briefly, 5 g of TCM powder was added into a 66 mL stainless steel extraction cell. After loading the cell into the ASE system, the cell was automatically filled with extraction solvent, pressurized (1500 psi), and heated (50 °C), with a static extraction time of 15 min. At the end of each extraction procedure, the solvent rinse volume and the nitrogen purge time were set at 100% and 1 min, respectively. The resulting products obtained after the automated extraction were collected, concentrated, and lyophilized to give 50% EtOH extracts of TCM.
2.3. Analysis of TCM metabolites by LC-MS/MS
LC-MS/MS was used to analyze the metabolites in the 50% EtOH extracts of all selected TCMs. Briefly, dried extracts were re-dissolved in 50% EtOH at a concentration of 10 mg/mL and analyzed using an Agilent 1290 Infinity II LC system coupled with a 6545XT AdvanceBio LC/Q-TOF (quadrupole time-of-flight) mass spectrometer (Agilent Technologies, Santa Clara, CA, USA). The chromatographic separation was carried out on an ACQUITY BEH C18 UPLC column (2.1 × 100 mm, 1.7 μm; Waters, Milford, MA, USA) with a 0.4 mL/min flow rate and a column temperature of 40 °C. The mobile phase was composed of water (A) and acetonitrile (B) (both contained 0.1% formic acid). The gradient elution conditions were set as follows: 0–1 min, 5% B; 1–16 min, 5–99.5% B; and 16–26 min, 99.5% B. The column was re-equilibrated at 5% B for 3 min between each run. The sample injection volume was 10 μL.
The Q-TOF mass spectrometer was equipped with an electrospray ionization (ESI) source, and the analyses were performed separately in positive ion mode and negative ion mode for each sample. Source parameters were set as follows: capillary voltage at 3000 V, nebulizer pressure at 35 psi, sheath gas flow rate at 12 L/min with a temperature of 350 °C, drying gas flow rate at 9 L/min with a temperature of 250 °C, fragmentor voltage at 130 V, skimmer voltage at 65 V, and nozzle voltage at 250 V (in positive ion mode) or 1250 V (in negative ion mode). Agilent MassHunter Workstation software (version B.09.00) was used for LC-MS/MS data acquisition. Data was collected in centroid mode with the m/z range of 100–1500 and the scan rate of 5 spectra/s. To increase the coverage of compounds targeted for MS/MS fragmentation, iterative data-dependent acquisition (DDA) (five consecutive injections of the same sample) with active exclusion was applied in MS/MS scans, and the top five highest-intensity precursor ions per MS1 scan were selected and fragmented with collision energy set at 40 V.
The LC-MS/MS data of the 50% EtOH extracts of all selected TCMs are publicly available on the MassIVE website (https://massive.ucsd.edu), and the dataset accession numbers are summarized in Table S1 (Supporting Information; https://www.jfda-online.com/cgi/editor.cgi?article=3425&window=additional_files&context=journal).
2.4. Molecular network construction
2.4.1. Data preprocessing
The LC-MS/MS data were converted from Agilent MassHunter data format to mzML format using MSConvert software (part of the ProteoWizard package) [17], followed by performing a sequence of data processing steps in MZmine software (version 2.53) [18]. Because the signal intensity in negative ion data is generally weaker than in positive ion data, some parameters were set at lower values for negative ion data processing. The mass detection was realized with the noise level set at 1000 (500 for negative ion data) for MS1 and 100 (50 for negative ion data) for MS2. The chromatogram building was performed using the Automated Data Analysis Pipeline (ADAP) chromatogram builder with a minimum group size of scans of 5, a group intensity threshold of 1000 (500 for negative ion data), a minimum highest intensity of 5000 (2500 for negative ion data), and an m/z tolerance of 0.006 m/z (or 25 ppm). The ADAP wavelets algorithm was used for chromatographic deconvolution with the following settings: S/N threshold = 5, minimum feature height = 50000 (20000 for negative ion data), coefficient/area threshold = 50, peak duration range 0.05–2 min (0.05–1 min for negative ion data), and retention time (RT) wavelet range 0.03–0.15 min (0.03–0.12 min for negative ion data). The MS/MS scan pairing was carried out with an m/z range of 0.02 Da and an RT range of 0.1 min. The chromatograms were deisotoped using the isotopic peaks grouper with an m/z tolerance of 0.006 m/z (or 25 ppm) and an RT tolerance of 0.1 min. Peak alignment was performed using the join aligner with an m/z tolerance of 0.006 m/z (or 25 ppm), an RT tolerance of 0.3 min, a weight for m/z of 1, a weight for RT of 1, and a minimum cos similarity of 0.5 for MS2 spectral similarity comparison. The processed results were exported as two files using the “Export/ Submit to GNPS-FBMN” built-in option: an .mgf file containing MS/MS spectral information and a .csv file containing a feature list with MS1 m/z, peak retention time, and peak area information.
2.4.2. GNPS molecular networking
Global Natural Products Social Molecular Networking (GNPS; https://gnps.ucsd.edu) was used to generate molecular networks [19]. First, the preprocessed LC-MS/MS data were uploaded to the GNPS web-based platform, and a feature-based molecular networking workflow (version 28.2) was applied for molecular network building [20]. The major parameter settings were set as follows: precursor ion mass tolerance of 0.02 Da, fragment ion mass tolerance of 0.02 Da, minimum pair cosine score of 0.7, and minimum matched fragment ions of 6. After running the molecular networking algorithm on the GNPS server, the generated molecular networks were downloaded from the job status pages as Cytoscape data and then imported to Cytoscape software (version 3.8.2) for molecular network visualization and editing [21].
2.4.3. Compound identification
Compound identification was first performed by comparing the mass spectra against different mass spectral libraries/sources, including (1) Traditional Chinese Medicine Personal Compound Database and Library (TCM PCDL; Agilent-NatureStandard) [22]; (2) public spectral libraries on the GNPS website (such as MassBank and NIST) [19]; and (3) the mass spectra from the literature. Then, in silico tools, including Network Annotation Propagation (NAP), MS2LDA, and SIRIUS 4, were further used to predict the structure or improve the structural annotation of other unidentified compounds after spectral library matching [23–25]. Finally, ClassyFire and MolNetEnhancer were applied for the structural classification of identified compounds [26,27].
3. Results and discussion
3.1. Overview of the molecular networks of the 24 selected TCMs
The LC-MS/MS data from the 50%EtOHextracts of 24 TCMs were used for molecular network construction. Because the positive and negative ion data were independent data sets, their corresponding molecular networks were constructed separately. Fig. 1A shows the molecular network built with the positive ion data from50%EtOHextracts of 24 TCMs; it contains 16269 nodes and 1265 molecular families. The TCM source of each node (molecule) was visualized by assigning different colors to different TCMs, and the TCMs with “cold” or “mild cold” (wei han) medicinal property were labeled with cool colors such as blue and green, while the TCMs with “hot”, “warm” or “mild warm” (wei wen) medicinal property was labeled with warm colors such as red and orange. Based on the compound identification results of spectral library matching and in silico tool-aided structure prediction, the chemical classes of several major molecular families were putatively classified as flavonoids, flavonoid glycosides, phenolic acid derivatives, alkaloids, benzenoids, phenols, and so on (Fig. 1A). The representative metabolites in these classified molecular families are summarized in Fig. S1A (Supporting Information; https://www.jfdaonline.com/cgi/editor.cgi?article=3425&window=additional_files&context=journal). Of note, the nodes in some of the major molecular families were derived from various TCMs (such as the flavonoid-containing molecular family), whereas some of the major molecular families were mainly derived from a single TCM (such as the diarylheptanoid-containing molecular family from gan jiang (GJ), and the neo-clerodane diterpenoid-containing molecular family from ban zhi lian (BZL)).
Fig. 1.
Molecular networks of the 50% EtOH extracts of the 24 selected TCMs and the putative chemical classes of major molecular families. (A) Positive ion data and (B) negative ion data. The number in the parentheses is the sequence number of the molecular family. The node size is proportional to the sum of the chromatographic peak area. The edge width is proportional to the MS/MS spectral similarity between two connected molecules. The threshold of library match is cosine score ≥0.8.
On the other hand, the molecular network constructed with the negative ion data comprises 13219 nodes and 931 molecular families (Fig. 1B). Various metabolites such as phenolic acid derivatives, flavonoids, flavonoid glycosides, and triterpenoids were clustered as major molecular families, and the corresponding representative metabolites are illustrated in Fig. S1B (Supporting Information; https://www.jfda-online.com/cgi/editor.cgi?article=3425&window=additional_files&context=journal). However, the molecules in some major molecular families did not share particular structural features, and therefore these families were roughly classified as glycosides or lipids and terpenoids. Most of the major molecular families were derived from several TCMs, such as the molecular families containing flavonoid glycosides and the molecular families containing phenolic acid derivatives. Nevertheless, the molecular family composed of iridoid glycosides was derived from a single TCM because these specific types of compounds were the characteristic metabolites of zhi zi (ZZ).
These results demonstrated that the molecular networking approach could reveal the metabolic diversity of TCM metabolomes and visualize the commonality and uniqueness of different classes of metabolites among the 24 selected TCMs.
3.2. Representative molecular families of cold-property and hot-property TCMs
To simplify the screening process for discovering the representative molecular families associated with medicinal properties, the TCMs with cold and mild cold medicinal properties were categorized as cold-property TCMs, and the TCMs with hot, warm, and mild warm medicinal properties were classified as hot-property TCMs.
The representative cold-property TCM molecular families were screened based on three criteria: (1) the number of TCM sources (≥7 cold-property TCMs and ≤2 hot-property TCMs); (2) the majority of nodes in the molecular family being derived from cold-property TCMs; and (3) containing identified metabolites with a library match score ≥0.8 (a perfect match score is 1). Table 2 shows a summary of representative cold-property TCM molecular families. After screening, six molecular families in the molecular network built based on positive ion data were representative cold-property TCM molecular families. The molecules within these molecular families were mainly derived from 13, 11, 11, 8, 7, and 7 different cold-property TCMs, respectively. On the other hand, for the molecular network built from negative ion data, five molecular families, with 10, 8, 8, 8, and 7 cold-property TCMs, were filtered to be representative cold-property TCM molecular families. The chemical classes of the TCM molecular families associated with cold-property mostly belonged to flavonoids, flavonoid glycosides, and phenolic acid derivatives, out of which more flavonoid compounds were clustered as large molecular families in the positive ion data, and more phenolic acid compounds were found in the negative ion data.
Table 2.
Summary of the representative cold-property TCM molecular families in the molecular networks of 50% EtOH extracts of the 24 selected TCMs.
| Chemical class and sequence number of molecular family | Node number | Number and name of TCM source categorized according to medicinal propertya |
|---|---|---|
| Positive ion data | ||
| Flavonoids (17) | 47 | 13 Cold (BHSSC, BYC, BZL, CXL, DH, HQ, JH, JYH, KS, LQ, PGY, YXC, ZZ) 2 Hot (DX, ZWZY) 1 Neutral (BJC) |
| Flavonoid glycosides (7) | 79 | 11 Cold (BHSSC, BYC, BZL, CXL, HL, HQ, JH, JYH, KS, PGY, ZZ) 2 Hot (DX, ZWZY) 1 Neutral (BJC) |
| Phenolic acids derivatives (44)b | 26 | 11 Cold (BHSSC, BZL, CXL, HB, HQ, JH, JYH, LQ, PGY, YXC, ZZ) 2 Hot (DX, ZWZY) 1 Neutral (BJC) |
| Flavonoid glycosides (45)b | 20 | 8 Cold (BYC, BZL, CXL, HL, HQ, JH, JYH, ZZ) 1 Hot (DX) |
| Flavonoids (46)b | 31 | 7 Cold (BYC, BZL, DH, HL, HQ, JYH, ZZ) |
| Carbohydrates (47)b | 6 | 7 Cold (BHSSC, BLG, DH, HB, HQ, JYH, KS) 2 Hot (BJZ, PFZ) |
| Negative ion data | ||
| Phenolic acids derivatives (51)b | 15 | 10 Cold (BHSSC, BYC, CXL, HB, HL, JH, JYH, PGY, YXC, ZZ) 1 Hot (ZWZY) 1 Neutral (BJC) |
| Stilbenes (52)b | 9 | 8 Cold (BYC, BZL, CXL, DH, JH, JYH, KS, PGY) 1 Hot (ZWZY) |
| Phenolic acids derivatives (53)b | 25 | 8 Cold (BYC, CXL, HB, JH, JYH, PGY, YXC, ZZ) 1 Hot (ZWZY) 1 Neutral (BJC) |
| Phenolic acids (54)b | 3 | 8 Cold (BYC, BZL, DH, JH, JYH, LQ, PGY, YXC) 1 Hot (ZWZY) 1 Neutral (BJC) |
| Flavonoid glycosides (41) | 31 | 7 Cold (BYC, BZL, CXL, DH, HQ, JH, KS) 1 Hot (ZWZY) |
Cold medicinal property includes cold and mild cold; hot medicinal property includes hot, warm, and mild warm.
The molecular families are shown in Fig. S2 (Supporting Information).
The screening criteria for hot-property TCM molecular families were similar to that of the cold-property TCMs, but with a lower required number of TCM sources (≥3 hot-property TCMs and ≤2 cold-property TCMs) as fewer hot-property TCMs were included in the 24 selected TCMs. As a result, four molecular families were found, and all were discovered in the positive ion data (Table 3), with the main sources being from 4, 3, 3, and 3 different hot-property TCMs, respectively. The significant cluster of molecular families was classified as quinolones, while the others were classified as sinapine derivatives, flavonoids, and beta-carboline alkaloids.
Table 3.
Summary of the representative hot-property TCM molecular families in the molecular networks of 50% EtOH extracts of the 24 selected TCMs.
| Chemical class and sequence number of molecular family | Node number | Number and name of TCM source categorized according to medicinal propertya |
|---|---|---|
| Positive ion data | ||
| Quinolones (8) | 76 | 4 Hot (DX, PFZ, RG, ZWZY) 2 Cold (BLG, HB) |
| Sinapine derivatives (48)b | 5 | 3 Hot (BJZ, GJ, ZWZY) |
| Flavonoids (49)c | 11 | 3 Hot (DX, GJ, ZWZY) 1 Cold (CXL) |
| Beta-carboline alkaloids (50)b | 4 | 3 Hot (BJT, GJ, ZWZY) 2 Cold (CXL, HL) |
Cold medicinal property includes cold and mild cold; hot medicinal property includes hot, warm, and mild warm.
The molecular families are shown in Fig. 3B and Fig. S2 (Supporting Information).
The molecular family is shown in Fig. S2 (Supporting Information).
3.3. Metabolites identified in the major representative molecular families of cold-property TCMs
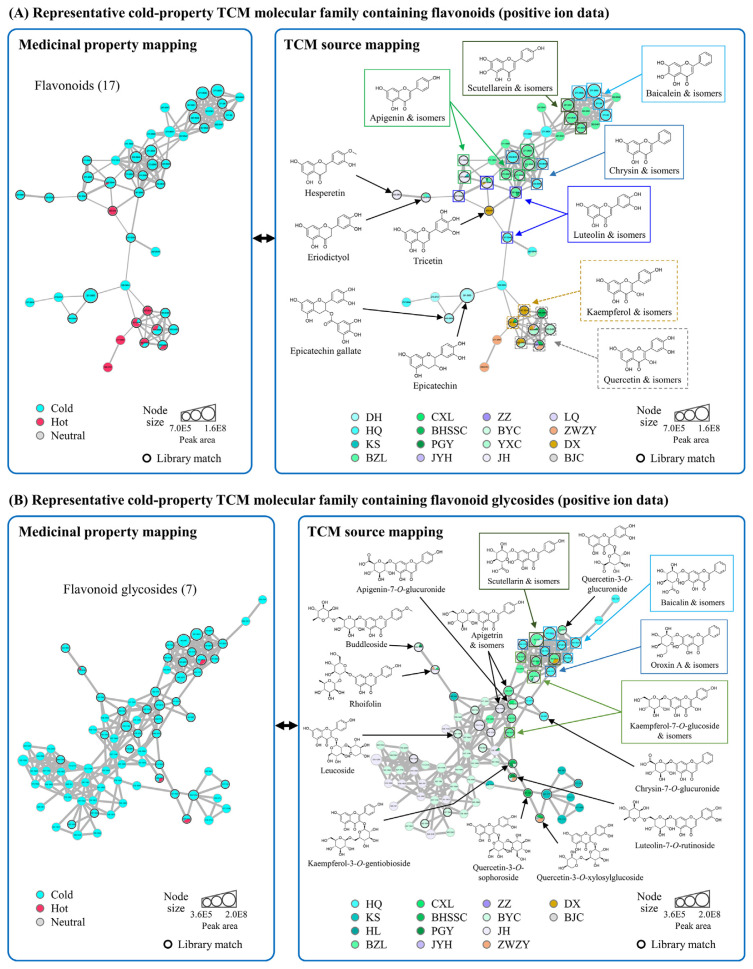
Two major representative cold-property TCM molecular families containing flavonoids (with 13 cold-property TCM sources and 47 nodes; Fig. 2A) and flavonoid glycosides (with 11 cold-property TCM sources and 79 nodes; Fig. 2B) were selected for investigation. A list of identified compounds in the representative cold-property TCM molecular families is presented in Table 4. Twelve flavonoids, including apigenin, baicalein, chrysin, luteolin, and scutellarein, were identified, as shown in Fig. 2A; fifteen flavonoid glycosides including apigetrin, baicalin, and scutellarin were identified, as shown in Fig. 2B. To further confirm the identity of these compounds, the identification points were calculated according to the Commission Implementing Regulation (EU) 2021/808 [28], which provides the confirmation and identification strategy for MS-based analytical methods. Based on this EU regulation, verifying the identity of compounds requires a minimum of four identification points; for high-resolution mass spectrometry such as Q-TOF, a precursor ion (monitored in full scan) yields 1.5 points, and a characteristic product ion yields 2.5 points. Therefore, the obtained identification points of the flavonoids and flavonoid glycosides were 6.5 (one precursor ion and two characteristic product ions) and 4 (one precursor ion and one characteristic product ion), respectively, suggesting that their identity was successfully confirmed.
Fig. 2.
Representative cold-property TCM molecular families. (A) Flavonoids and (B) flavonoid glycosides. The number in the parentheses is the sequence number of the molecular family. Values in the nodes are precursor m/z. The node size is proportional to the sum of the chromatographic peak area. The edge width is proportional to the MS/MS spectral similarity between two connected molecules. The threshold of library match is cosine score ≥0.8.
Table 4.
Summary of the identified metabolites in the major representative cold-property TCM molecular families.
| No. | RT (min) | Name | Formula | Calculated m/z | Observed m/z | Error (ppm) | Adduct | Identification pointa | Fragment ions (relative abundance in %)b | TCM source | Ref.c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive ion data | |||||||||||
| Flavonoids | |||||||||||
| 1 | 2.84 | Epicatechin | C15H14O6 | 291.0863 | 291.0855 | −2.75 | [M+H]+ | 6.5 | 147.0431 (21), 139.0381 (65), 123.0436 (100), 119.0482 (18), 111.0433 (11) | DH | G; T |
| 2 | 3.63 | Quercetin and isomers | C15H10O7 | 303.0499 | 303.0498 | −0.33 | [M+H]+ | 6.5 | 229.0489 (46), 201.0538 (26), 183.0431 (13), 173.0596 (13), 165.0177 (13), 155.0488 (16), 153.0178 (100), 137.0225 (50), 121.0273 (14), 109.0282 (18) | BHSSC, BJC, DX, JYH, LQ, YXC, ZWZY, ZZ | G; T |
| 4.00 | 303.0495 | −1.32 | |||||||||
| 4.28 | 303.0494 | −1.65 | |||||||||
| 4.66 | 303.0495 | −1.32 | |||||||||
| 5.82 | 303.0493 | −1.98 | |||||||||
| 3 | 3.83 | Luteolin and isomers | C15H10O6 | 287.0550 | 287.0549 | −0.35 | [M+H]+ | 6.5 | 287.0540 (39), 241.0488 (8), 213.0538 (5), 161.0229 (15), 153.0176 (100), 139.0537 (5), 137.0224 (12), 135.0434 (34), 117.0326 (18), 107.0483 (6) | BHSSC, BYC, BZL, CXL, DH, DX, HQ, JH, JYH, PGY | G; T |
| 4.28 | 287.0542 | −2.79 | |||||||||
| 5.84 | 287.0544 | −2.09 | |||||||||
| 6.26 | 287.0544 | −2.09 | |||||||||
| 4 | 4.28 | Scutellarein and isomers | C15H10O6 | 287.0550 | 287.0549 | −0.35 | [M+H]+ | 6.5 | 287.0544 (21), 269.0436 (5), 195.0437 (6), 169.0126 (20), 167.0486 (5), 157.0640 (5), 123.0074 (100), 121.0278 (14), 119.0489 (41) | BZL, HQ | T |
| 4.52 | 287.0551 | 0.35 | |||||||||
| 5.32 | 287.0549 | −0.35 | |||||||||
| 5 | 4.34 | Epicatechin gallate | C22H18O10 | 443.0973 | 443.0954 | −4.29 | [M+H]+ | 6.5 | 153.0170 (25), 151.0381 (6), 147.0426 (7), 139.0380 (60), 125.0225 (5), 123.0436 (100) | DH | G; T |
| 6 | 4.38 | Apigenin and isomers | C15H10O5 | 271.0601 | 271.0595 | −2.21 | [M+H]+ | 6.5 | 271.0598 (31), 163.0385 (5), 153.0182 (100), 145.0278 (16), 141.0693 (5), 121.0279 (18), 119.0491 (44) | BYC, BZL, CXL, JH, KS | G; T |
| 4.80 | 271.0597 | −1.48 | |||||||||
| 5.29 | 271.0597 | −1.48 | |||||||||
| 5.66 | 271.0598 | −1.11 | |||||||||
| 6.48 | 271.0605 | 1.48 | |||||||||
| 6.52 | 271.0603 | 0.74 | |||||||||
| 7 | 4.67 | Kaempferol and isomers | C15H10O6 | 287.0550 | 287.0546 | −1.39 | [M+H]+ | 6.5 | 287.0541 (16), 213.0531 (21), 165.0166 (17), 157.0630 (18), 153.0164 (100), 147.0423 (11), 137.0207 (16), 121.0257 (59), 107.0463 (17), 105.0310 (12) | BHSSC, BJC, DX | G; T |
| 6.66 | 287.0544 | −2.09 | |||||||||
| 8 | 5.09 | Tricetin | C15H10O7 | 303.0499 | 303.0490 | −2.97 | [M+H]+ | 6.5 | 303.0483 (51), 257.0432 (13), 229.0487 (18), 203.0327 (47), 177.0165 (8), 155.0473 (6), 153.0165 (100), 151.0363 (27), 133.0264 (18), 105.0309 (15) | DX | G |
| 9 | 5.49 | Baicalein and isomers | C15H10O5 | 271.0601 | 271.0600 | −0.37 | [M+H]+ | 6.5 | 271.0595 (14), 169.0127 (10), 151.0538 (5), 141.0693 (5), 123.0079 (100), 105.0331 (8), 103.0542 (23) | BZL, HQ, ZZ | T |
| 6.00 | 271.0600 | −0.37 | |||||||||
| 7.16 | 271.0693 | 33.94 | |||||||||
| 7.18 | 271.0599 | −0.74 | |||||||||
| 10 | 5.69 | Eriodictyol | C15H12O6 | 289.0707 | 289.0706 | −0.35 | [M+H]+ | 6.5 | 163.0387 (5), 153.0179 (100), 145.0270 (7), 135.0433 (18), 123.0434 (6), 117.0327 (24) | BZL, HQ, JH | G; T |
| 11 | 5.92 | Chrysin and isomers | C15H10O4 | 255.0652 | 255.0649 | −1.18 | [M+H]+ | 6.5 | 255.0652 (40), 153.0697 (12), 153.0184 (100), 152.0619 (18), 147.0437 (9), 139.0540 (5), 129.0335 (26), 105.0333 (21), 103.0545 (60) | BZL, HQ | G; T |
| 6.10 | 255.0648 | −1.57 | |||||||||
| 8.37 | 255.0649 | −1.18 | |||||||||
| 12 | 6.76 | Hesperetin | C16H14O6 | 303.0863 | 303.0861 | −0.66 | [M+H]+ | 6.5 | 177.0537 (8), 153.0182 (100), 149.0594 (15), 145.0269 (9), 134.0353 (8), 117.0326 (26) | JH | T |
| Flavonoid glycosides | |||||||||||
| 13 | 3.63 | Quercetin-3-O-sophoroside | C27H30O17 | 627.1556 | 627.1562 | 0.96 | [M+H]+ | 4 | 303.0506 (100), 145.0487 (1), 127.0380 (2), 109.0278 (1) | BHSSC | T |
| 14 | 3.68 | Kaempferol-7-O-glucoside and isomers | C21H20O11 | 449.1078 | 449.1079 | 0.22 | [M+H]+ | 4 | 287.0557 (100), 269.0440 (2), 169.0127 (1), 123.0072 (2), 119.0487 (2) | BJC, BYC, BZL, JH, JYH, PGY | T |
| 3.92 | 449.1079 | 0.22 | |||||||||
| 4.38 | 449.1076 | −0.45 | |||||||||
| 15 | 3.79 | Quercetin-3-O-glucuronide | C21H18O13 | 479.0820 | 479.0814 | −1.25 | [M+H]+ | 4 | 303.0502 (100), 285.0389 (1), 169.0124 (1), 135.0435 (1), 123.0073 (1) | BYC, BZL, HQ | G |
| 16 | 3.89 | Quercetin-3-O-xylosylglucoside | C26H28O16 | 597.1450 | 597.1447 | −0.50 | [M+H]+ | 4 | 303.0505 (100), 127.0381 (1), 115.0379 (1) | BHSSC, JYH, ZWZY | G |
| 17 | 3.91 | Kaempferol-3-O-gentiobioside | C27H30O16 | 611.1607 | 611.1602 | −0.82 | [M+H]+ | 4 | 287.0522 (100), 145.0483 (1), 127.0380 (1) | BHSSC, BYC, PGY | G; T |
| 18 | 4.22 | Luteolin-7-O-rutinoside | C27H30O15 | 595.1658 | 595.1650 | −1.34 | [M+H]+ | 4 | 287.0550 (100) | CXL, JYH, PGY, ZWZY | G |
| 19 | 4.27 | Scutellarin and isomers | C21H18O12 | 463.0871 | 463.0870 | −0.22 | [M+H]+ | 4 | 287.0554 (100), 269.0435 (1), 169.0125 (1), 123.0073 (1), 119.0487 (1) | BYC, BZL, CXL, DX, HQ, JH | T |
| 4.62 | 463.0874 | 0.65 | |||||||||
| 5.31 | 463.0865 | −1.30 | |||||||||
| 20 | 4.29 | Baicalin and isomers | C21H18O11 | 447.0922 | 447.0922 | 0.00 | [M+H]+ | 4 | 271.0612 (100), 253.0492 (2), 169.0128 (1), 123.0077 (2), 103.0541 (1) | HQ, ZZ | G; T |
| 5.48 | 447.0923 | 0.22 | |||||||||
| 6.27 | 447.0924 | 0.45 | |||||||||
| 8.27 | 447.0916 | −1.34 | |||||||||
| 21 | 4.41 | Apigetrin and isomers | C21H20O10 | 433.1129 | 433.1130 | 0.23 | [M+H]+ | 4 | 271.0604 (100), 153.0181 (4), 119.0487 (1) | BZL, JH, JYH, KS | G; T |
| 4.80 | 433.1128 | −0.23 | |||||||||
| 22 | 4.57 | Leucoside | C26H28O15 | 581.1501 | 581.1491 | −1.72 | [M+H]+ | 4 | 287.0540 (100), 153.0167 (1) | BYC | G |
| 23 | 4.68 | Rhoifolin | C27H30O14 | 579.1708 | 579.1709 | 0.17 | [M+H]+ | 4 | 271.0599 (100) | CXL, JH, JYH, ZWZY | G; T |
| 24 | 4.85 | Apigenin-7-O-glucuronide | C21H18O11 | 447.0922 | 447.0918 | −0.89 | [M+H]+ | 4 | 271.0594 (100), 153.0169 (2), 119.0482 (1) | BYC, BZL, CXL | G |
| 25 | 5.53 | Oroxin A and isomers | C21H20O10 | 433.1129 | 433.1126 | −0.69 | [M+H]+ | 4 | 271.0602 (100), 253.0489 (2), 169.0129 (1), 123.0077 (3), 103.0541 (1) | HQ | T |
| 6.00 | 433.1126 | −0.69 | |||||||||
| 6.31 | 433.1127 | −0.46 | |||||||||
| 26 | 5.86 | Buddleoside | C28H32O14 | 593.1865 | 593.1862 | −0.51 | [M+H]+ | 4 | 285.0765 (100), 242.0568 (1) | BYC, HL, JH, PGY | G; T |
| 27 | 6.10 | Chrysin-7-O-glucuronide | C21H18O10 | 431.0973 | 431.0970 | −0.70 | [M+H]+ | 4 | 255.0652 (100), 153.0181 (2) | HQ | G |
RT: Retention time.
The identification points were calculated according to the Commission Implementing Regulation (EU) 2021/808 [28]; the identification points of a precursor ion (monitored in full scan) and a product ion in high-resolution mass spectrometry are 1.5 and 2.5, respectively. The mass spectrum with the highest library match score was selected for identification point calculation if more than one molecule was identified as the same metabolite (potential structural isomer).
The mass spectrum with the highest library match score was selected to represent the fragment ions if more than one molecule was identified as the same metabolite (potential structural isomer). The fragment ions that are underlined were the characteristic product ions used for identification point calculation. The characteristic product ions were selected based on the information of characteristic product ions of flavonoids in the literature [42,43].
G: Public spectral libraries on the GNPS website; T: TCM PCDL.
Based on spectral library matching, several nodes with different retention times were annotated to be the same compound, indicating that there were structural isomers of some types of flavonoids with similar MS/MS fragmentation patterns. For example, the luteolin-type group (luteolin and isomers in Fig. 2A) consisted of four nodes with the retention time of 3.83, 4.28, 5.84, and 6.26 min; this suggested that there were three structural isomers of luteolin in the luteolin-type group. However, further analysis will be needed to confirm the exact node of luteolin by comparing the retention time with the standard compound.
Although there may be a few structural isomers in the representative cold-property TCM molecular families, the general structural features of the potentially cold property-related metabolites were revealed using the molecular networking strategy. For example, luteolin- and apigenin-type flavonoids were grouped in the representative cold-property TCM molecular family (Fig. 2A). They were predominantly derived from nine and five cold-property TCMs, respectively; this indicates that these specific types of flavonoids may be part of the consensus metabolites in cold-property TCM. Of note, baicalein-, chrysin-, and scutellarein-type flavonoids were mainly derived from only one or two cold-property TCMs. Still, they were clustered in the sub-family (Fig. 2A, upper right) with the majority of nodes from various cold-property TCM sources, suggesting that the structural features of these flavonoids may also be involved in the cold-property TCM-related metabolites. On the other hand, although kaempferol- and quercetin-type flavonoids were found in the cold-property TCM molecular family, most were derived from both cold- and hot-property TCMs; therefore, they may not serve as the consensus metabolites of cold-property TCMs.
Several corresponding glycosides of the flavonoids in Fig. 2A were identified in another cold-property TCM molecular family (Fig. 2B), including apigetrin-type flavonoid glycosides (apigenin-7-O-glucoside and isomers), apigenin-7-O-glucuronide, rhoifolin (apigenin-7-O-rutinoside), buddleoside (4′-methoxyapigenin-7-O-rutinoside), baicalin-type flavonoid glycosides (baicalein-7-O-glucuronide and isomers), oroxin A-type flavonoid glycosides (baicalein-7-O-glucoside and isomers), scutellarin-type flavonoid glycosides (scutellarein-7-O-glucuronide and isomers), chrysin-7-O-glucuronide, and luteolin-7-O-rutinoside. In addition, kaempferol- and quercetin-type flavonoid glycosides such as kaempferol-7-O-glucoside and quercetin-3-O-glucuronide were also present in this molecular family and mainly derived from cold-property TCMs, which is different from their aglycones that were derived from both cold- and hot-property TCMs; this observation implies that there may be differences in the glycosylation of these flavonoids between the selected cold- and hot-property TCMs.
Some specific types of flavonoids (apigenin, baicalein, chrysin, luteolin, and scutellarein) and their corresponding glycosides are mutually present in the representative cold-property molecular families, confirming their potential roles as part of the consensus metabolites in cold-property TCMs. The general structural features of the cold-property TCM-related flavonoids are proposed—flavones with two or three hydroxyl groups on ring A (at positions 5 and 7, or 5, 6, and 7) and zero, one, or two hydroxyl groups on ring B (at position 4′, or 3′ and 4′). At the same time, the predominant sugar moiety of their glycosides was glucose or glucuronic acid attached at the C-7 position on ring A. Compared to the chemical properties of cold property-associated compounds (found to have a higher polarity and more aliphatic rings) reported in the previous study [6], the specific types of flavonoids in the representative cold-property molecular families in this study may concur with respect to the property of higher polarity but not with respect to having more aliphatic rings. The difference is probably because numerous types of common metabolites were not included in the chemical property analysis in the previous study [6] (because a compound filtering process was performed before conducting the analysis), and the obtained chemical properties were general features that may not universally fit all specific classes of compounds.
Furthermore, the cold property-associated flavonoids investigated in this study (including apigenin, baicalein, chrysin, luteolin, scutellarein, and so on) have mostly been reported to possess various bioactivities, notably, anti-inflammation and anti-oxidation [29–34], which further connects their potential pharmacological roles to the roles ascribed to the cold property of TCM such as “clearing heat” (qing re).
3.4. Metabolites identified in the major representative molecular families of hot-property TCMs
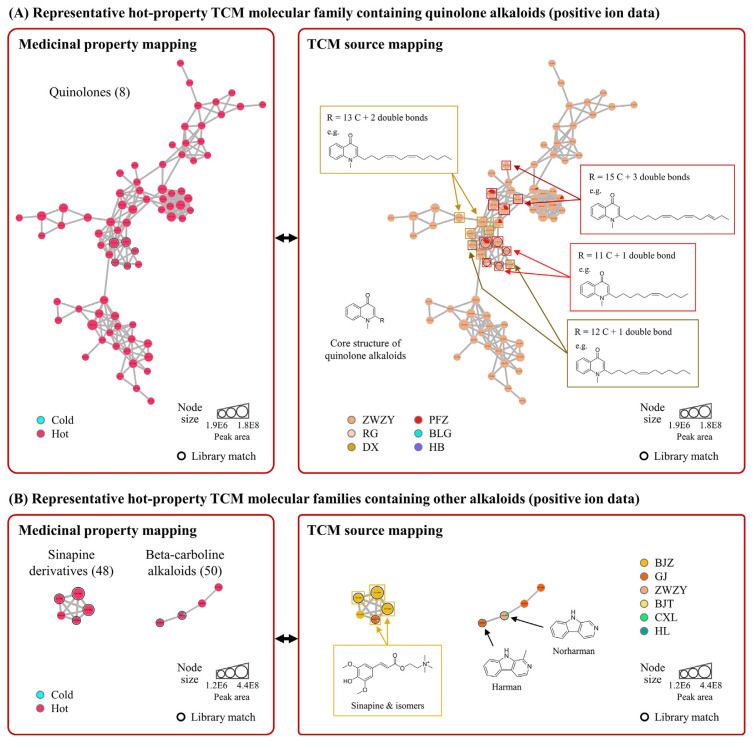
Most representative molecular families of hot-property TCMs were mainly composed of molecules from single hot-property TCM, probably because fewer hot-property TCMs in the samples were investigated. Therefore, based on the family size and the diversity of hot-property TCMs, the prominent molecular family containing 76 nodes with four hot-property TCM sources was selected for further study (Fig. 3A). Quinolone alkaloids with different side chains were identified in this molecular family (Table S2; Supporting Information; https://www.jfda-online.com/cgi/editor.cgi?article=3425&window=additional_files&context=journal), and several quinolone alkaloids were found to have potential structural isomers, which is probably due to the variety in the structure of side chains, such as the position of the double bond. In addition, it was notable that most nodes were predominantly derived from zhi wu zhu yu (ZWZY). This is reasonable because quinolone alkaloids are one of the major characteristic metabolites of ZWZY. However, the library-based compound identification rate of this molecular family was relatively low. Therefore, manual identification was performed to identify other known quinolone alkaloids of ZWZY by comparing the exact mass and MS/MS fragmentation patterns with those in the literature [35,36].
Fig. 3.
Representative hot-property TCM molecular families. (A) Quinolone alkaloids and (B) other alkaloids. The number in the parentheses is the sequence number of the molecular family. Values in the nodes are precursor m/z. The node size is proportional to the sum of the chromatographic peak area. The edge width is proportional to the MS/MS spectral similarity between two connected molecules. The threshold of library match is cosine score ≥0.8.
The sub-family in the middle part of the representative hot-property TCM molecular family was mainly derived from four different hot-property TCMs, suggesting that the compounds in this sub-family may be metabolites present in all four hot-property TCMs. Four types of quinolone alkaloids with different side chain structural features were identified in this sub-family. Their side chains comprised 11 carbons with one double bond, 12 carbons with one double bond, 13 carbons with two double bonds, and 15 carbons with three double bonds, respectively; the known quinolone alkaloids of ZWZY are shown as the example structure in each isomer group. Previous studies have reported that the quinolone alkaloids from ZWZY possess antibacterial and cytotoxic activities [36,37]. However, these bioactivities have no undeniable connection to the hot property of TCM.
Two smaller molecular families identified in three hot-property TCMs are shown in Fig. 3B. Sinapine and its potential isomers were identified in one of the molecular families, while two beta-carboline alkaloids (harman and norharman) were identified in the other molecular family (Table S2; Supporting Information; https://www.jfda-online.com/cgi/editor.cgi?article=3425&window=additional_files&context=journal). Sinapine is a major characteristic metabolite of bai jie zi (BJZ) with antioxidant and acetylcholinesterase inhibitory activities [38,39]. Harman and norharman have been reported to possess antimicrobial and monoamine oxidase inhibitory activities [40,41]. Nevertheless, there seems to be no direct association between these bioactivities and the medicinal properties of hot-property TCM. Further study using more hot-property TCM will be needed to discover more hot property-associated metabolites.
4. Conclusions
This pilot study successfully developed a mass spectral molecular networking-based platform for TCM metabolite analysis. This platform was utilized as a visualization approach to reveal and explore the metabolic diversity of the TCM metabolomes. In contrast to previous studies that applied in silico analysis to provide the comparative chemical properties of known metabolites in cold- and hot-property TCMs, the specific chemical classes of metabolites were characterized in the present study. By screening the medicinal property and the diversity of TCM sources of each molecular family, the potentially cold property-associated metabolites were predominantly flavonoids, flavonoid glycosides, and phenolic acid derivatives. It was further revealed that luteolin and apigenin could be the characteristic flavonoids of cold-property TCMs. Therefore, further investigating the roles of these types of flavonoids in the cold property of TCM would be worthwhile.
On the other hand, we could not reveal universal hot property-associated metabolites in this pilot study because the sample size of hot-property TCMs is small. Some alkaloids, such as quinolones, were identified in hot-property TCMs. However, the alkaloid structures are highly diverse and are found in cold- and hot-property TCMs. For example, leonurine and berberine were isolated from cold-property TCMs Leonurus artemisia and Coptis chinensis, respectively. In contrast, ephedrine and corydalis B were isolated from Chinese Ephedra Herb (Ma Huang) and Corydalis Rhizoma, respectively, and both of them are hot-property TCMs. Therefore, a comprehensive and detailed structural classification for alkaloids from more TCMs would be needed to investigate further or confirm the relationships between alkaloids and the medicinal properties of TCMs.
Supplementary Information
Acknowledgments
This work was supported by the Ministry of Science and Technology (MOST), Taiwan (Grant number: MOST109-2320-B001-022), the Research Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology (Grant numbers: ZRRPF3K0111 and ZZRPF3G0011), and Chang Gung Memorial Hospital (Grant number: CMRPF1J0053, CMRPF1M0101, CMRPF1M0131). In addition, the authors acknowledge the Metabolomics Core Facility, Agricultural Biotechnology Research Center (ABRC), Academia Sinica, for assistance in the LC-Q-TOF analysis of extracts. Finally, the authors thank Sheng Chang Pharmaceutical Co., Ltd. for providing TCM materials.
Funding Statement
This work was supported by the Ministry of Science and Technology (MOST), Taiwan (Grant number: MOST109-2320-B001-022), the Research Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology (Grant numbers: ZRRPF3K0111 and ZZRPF3G0011), and Chang Gung Memorial Hospital (Grant number: CMRPF1J0053, CMRPF1M0101, CMRPF1M0131).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1. Liu J, Feng W, Peng C. A song of ice and fire: cold and hot properties of traditional Chinese medicines. Front Pharmacol. 2021;11:598744. doi: 10.3389/fphar.2020.598744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao T, Tang H, Xie L, Zheng Y, Ma Z, Sun Q, et al. Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmacol. 2019;71:1353–69. doi: 10.1111/jphp.13129. [DOI] [PubMed] [Google Scholar]
- 3. Sun Q, Xie L, Song J, Li X. Evodiamine: A review of its pharmacology, toxicity, pharmacokinetics and preparation researches. J Ethnopharmacol. 2020;262:113164. doi: 10.1016/j.jep.2020.113164. [DOI] [PubMed] [Google Scholar]
- 4. Cheng F, Wang X, Song W, Lu Y, Li X, Zhang H, et al. Biologic basis of TCM syndromes and the standardization of syndrome classification. J Tradit Chin Med Sci. 2014;1:92–7. [Google Scholar]
- 5. Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fu X, Mervin LH, Li X, Yu H, Li J, Mohamad Zobir SZ, et al. Toward understanding the cold, hot, and neutral nature of Chinese medicines using in silico mode-of-action analysis. J Chem Inf Model. 2017;57:468–83. doi: 10.1021/acs.jcim.6b00725. [DOI] [PubMed] [Google Scholar]
- 7. Huang Y, Yao P, Leung KW, Wang H, Kong XP, Wang L, et al. The Yin-Yang property of Chinese medicinal herbs relates to chemical composition but not anti-oxidative activity: an illustration using spleen-meridian herbs. Front Pharmacol. 2018;9:1304. doi: 10.3389/fphar.2018.01304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang F, Li L, Wang M, Niu X, Zhan J, He X, et al. Molecular network and chemical fragment-based characteristics of medicinal herbs with cold and hot properties from Chinese medicine. J Ethnopharmacol. 2013;148:770–9. doi: 10.1016/j.jep.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 9. Zhou X, Li C-G, Chang D, Bensoussan A. Current status and major challenges to the safety and efficacy presented by Chinese herbal medicine. Medicines. 2019;6:14. doi: 10.3390/medicines6010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu W, Guo X, Li Q, Xu G, Feng M, Guan T, et al. Mass spectrometry based molecular profile dissects the complexity of traditional Chinese medicine. Anal Methods. 2015;7:2902–12. [Google Scholar]
- 11. Wolfender J-L, Nuzillard J-M, van der Hooft JJJ, Renault J-H, Bertrand S. Accelerating metabolite identification in natural product research: toward an ideal combination of liquid chromatography–high-resolution tandem mass spectrometry and NMR profiling, in silico databases, and chemometrics. Anal Chem. 2019;91:704–42. doi: 10.1021/acs.analchem.8b05112. [DOI] [PubMed] [Google Scholar]
- 12. Allard P-M, Peresse T, Bisson J, Gindro K, Marcourt L, Pham VC, et al. Integration of molecular networking and insilico MS/MS fragmentation for natural products dereplication. Anal Chem. 2016;88:3317–23. doi: 10.1021/acs.analchem.5b04804. [DOI] [PubMed] [Google Scholar]
- 13. Fox Ramos AE, Evanno L, Poupon E, Champy P, Beniddir MA. Natural products targeting strategies involving molecular networking: different manners, one goal. Nat Prod Rep. 2019;36:960–80. doi: 10.1039/c9np00006b. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen DD, Wu C-H, Moree WJ, Lamsa A, Medema MH, Zhao X, et al. MS/MS networking guided analysis of molecule and gene cluster families. Proc Natl Acad Sci USA. 2013;110:E2611–20. doi: 10.1073/pnas.1303471110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang JY, Sanchez LM, Rath CM, Liu X, Boudreau PD, Bruns N, et al. Molecular networking as a dereplication strategy. J Nat Prod. 2013;76:1686–99. doi: 10.1021/np400413s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ministry of Health and Welfare. Taiwan Herbal Pharmacopeia. 4th ed. Taipei: Ministry of Health and Welfare; 2021. [Google Scholar]
- 17. Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30:918–20. doi: 10.1038/nbt.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinf. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat Biotechnol. 2016;34:828–37. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nothias L-F, Petras D, Schmid R, Dührkop K, Rainer J, Sarvepalli A, et al. Feature-based molecular networking in the GNPS analysis environment. Nat Methods. 2020;17:905–8. doi: 10.1038/s41592-020-0933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agilent-NatureStandard product PCDL. [Accessed 15 April 2022]. Available at, https://www.antpedia.com/attachments/antop/2017/1571244031925.pdf.
- 23. da Silva RR, Wang M, Nothias L-F, van der Hooft JJJ, Caraballo-Rodriguez AM, Fox E, et al. Propagating annotations of molecular networks using in silico fragmentation. PLoS Comput Biol. 2018;14:e1006089. doi: 10.1371/journal.pcbi.1006089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dührkop K, Fleischauer M, Ludwig M, Aksenov AA, Melnik AV, Meusel M, et al. SIRIUS 4: a rapid tool for turning tandem mass spectra into metabolite structure information. Nat Methods. 2019;16:299–302. doi: 10.1038/s41592-019-0344-8. [DOI] [PubMed] [Google Scholar]
- 25. van der Hooft JJJ, Wandy J, Barrett MP, Burgess KEV, Rogers S. Topic modeling for untargeted substructure exploration in metabolomics. Proc Natl Acad Sci USA. 2016;113:13738–43. doi: 10.1073/pnas.1608041113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Djoumbou Feunang Y, Eisner R, Knox C, Chepelev L, Hastings J, Owen G, et al. ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. J Cheminform. 2016;8:61. doi: 10.1186/s13321-016-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ernst M, Kang KB, Caraballo-Rodriguez AM, Nothias L-F, Wandy J, Chen C, et al. MolNetEnhancer: enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites. 2019;9:144. doi: 10.3390/metabo9070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/ 179/EC. Off J Eur Union. 2021;180:84–109. [Google Scholar]
- 29. Aziz N, Kim M-Y, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–58. doi: 10.1016/j.jep.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 30. Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 31. Liu Q, Li X, Ouyang X, Chen D. Dual effect of glucuronidation of a pyrogallol-type phytophenol antioxidant: a comparison between scutellarein and scutellarin. Molecules. 2018;23:3225. doi: 10.3390/molecules23123225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mani R, Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry. 2018;145:187–96. doi: 10.1016/j.phytochem.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 33. Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of apigenin. Int J Mol Sci. 2019;20:1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sung NY, Kim M-Y, Cho JY. Scutellarein reduces inflammatory responses by inhibiting Src kinase activity. Korean J Physiol Pharmacol. 2015;19:441–9. doi: 10.4196/kjpp.2015.19.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ling Y, Hu P, Zhang L, Jin H, Chen J, Tao Z, et al. Identification and structural characterization of acylgluconic acids, flavonol glycosides, limonoids and alkaloids from the fruits of Evodia rutaecarpa by high performance liquid chromatography coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry. J Chromatogr Sci. 2016;54:1593–604. doi: 10.1093/chromsci/bmw109. [DOI] [PubMed] [Google Scholar]
- 36. Wang X-X, Zan K, Shi S-P, Zeng K-W, Jiang Y, Guan Y, et al. Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia. 2013;89:1–7. doi: 10.1016/j.fitote.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 37. Huang X, Li W, Yang X-W. New cytotoxic quinolone alkaloids from fruits of Evodia rutaecarpa. Fitoterapia. 2012;83:709–14. doi: 10.1016/j.fitote.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 38. Martinović N, Polak T, Ulrih NP, Abramovič H. Mustard seed: phenolic composition and effects on lipid oxidation in oil, oil-in-water emulsion and oleogel. Ind Crop Prod. 2020;156:112851. [Google Scholar]
- 39. Nićiforović N, Abramovič H. Sinapic acid and its derivatives: natural sources and bioactivity. Compr Rev Food Sci Food Saf. 2014;13:34–51. doi: 10.1111/1541-4337.12041. [DOI] [PubMed] [Google Scholar]
- 40. Zheng L, Chen H, Han X, Lin W, Yan X. Antimicrobial screening and active compound isolation from marine bacterium NJ6-3-1 associated with the sponge Hymeniacidon perleve. World J Microbiol Biotechnol. 2005;21:201–6. [Google Scholar]
- 41. Truman P, Grounds P, Brennan KA. Monoamine oxidase inhibitory activity in tobacco particulate matter: are harman and norharman the only physiologically relevant inhibitors? Neurotoxicology. 2017;59:22–6. doi: 10.1016/j.neuro.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 42. Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kachlicki P, Piasecka A, Stobiecki M, Marczak Ł. Structural characterization of flavonoid glycoconjugates and their derivatives with mass spectrometric techniques. Molecules. 2016;21:1494. doi: 10.3390/molecules21111494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.