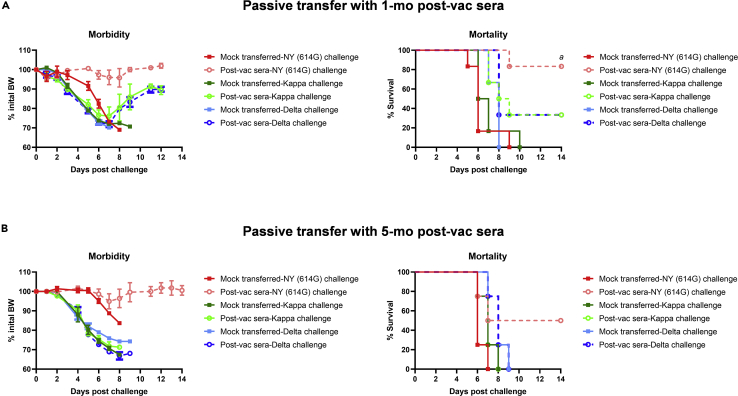
Figure 6.
Morbidity and mortality of K18-hACE2 mice receiving human post-vaccination sera after lethal challenges of SARS-CoV-2 and variants
K18-hACE2 mice of both sexes (1:1 ratio) were injected intraperitoneally with 200 μL/mouse of PBS (mock-transferred) or pooled human post-vaccination (post-vac) sera collected at 1 month (mo) or 5 months after the 2nd dose of COVID-19 mRNA vaccines (post-vac sera transferred). Recipient mice were then challenged intranasally with 103 TCID50/mouse of New York-PV09158/2020 (NY (614G)), or 102 TCID50/mouse of Kappa and Delta variants. Morbidity and mortality of infected mice were monitored for up to 14 days post-challenge. % body weight drops (mean ± s.e.m.) and % cumulative survivals were determined.
(A) Morbidity and mortality of mice receiving 1-mo post-vac sera (n = 6 mice/group).
(B) Morbidity and mortality of mice receiving 5-month post-vac sera (n = 4 mice/group). a indicates p < 0.05 vs mock-transferred group with NY (614G) challenge by Log rank (Mantel-Cox) survival test.