Abstract
Hyperuricemia is a common disease caused by a high level of uric acid. Urate transporter 1 (URAT1) is an important protein and mediates approximately 90% of uric acid reabsorption. Therefore, the URAT1 inhibitor is a class of uricosuric medicines widely used in the clinic for the treatment of hyperuricemia. To find the new medicine with stronger URAT1 inhibition and lower toxicity, researchers have been exploring natural products. This study systematically summarizes the natural products with URAT1 inhibition. The results show that many natural products are potential URAT1 inhibitors, such as flavonoids, terpenoids, alkaloids, coumarins, stilbenes, and steroids, among which flavonoids are the most promising source of URAT1 inhibitors. It is worth noting that most studies have focused on finding natural products with inhibition of URAT1 and have not explored their activities and mechanisms toward URAT1. By reviewing the few existing studies of the structure-activity relationship and analyzing common features of natural products with URAT1 inhibition, we speculate that the rigid ring structure and negative charge may be the keys for natural products to produce URAT1 inhibition. In conclusion, natural products are potential URAT1 inhibitors, and exploring the mechanism of action and structure-activity relationship will be an important research direction in the future.
1. Introduction
Uric acid is the end metabolite derived from the oxidation of purine compounds [1]. Hyperuricemia is a chronic metabolic disease caused by a high level of uric acid. Excessive intake of purine-containing foods and insufficient uric acid excretion are the keys to causing hyperuricemia [2]. In recent years, the incidence of hyperuricemia has continued to increase worldwide, which may be related to changes in lifestyle, such as the prevalence of a high-purine diet, fructose beverages, and alcohol consumption [3, 4]. In China, the overall prevalence of hyperuricemia is 13.3%, and the prevalence in men is higher than in women [5]. In the United States, the prevalence of hyperuricemia is 21.2% in men and 21.6% in women [6]. Hyperuricemia is related to the occurrence of many diseases, such as cardiovascular disease, metabolic syndrome, and acute kidney injury [7]. Therefore, patients have an urgent need for efficient and safe therapeutic methods or drugs [8].
Reducing purine intake, inhibiting uric acid production, and promoting uric acid excretion are effective ways to treat or improve hyperuricemia [9]. URAT1 inhibitors are a widely used class of uricosuric drugs by inhibiting the reabsorption of uric acid, such as probenecid, sulfinpyrazone, and benzbromarone [10]. Although these drugs have good uric acid lowering effects, they all have varying degrees of side effects [11]. Currently, sulfinpyrazone has been withdrawn from most countries due to its severe gastrointestinal toxicity [12]. Benzbromarone has severe hepatotoxicity and is currently approved for use in only a few countries [13]. Even the newly approved lesinurad has renal toxicity and cardiovascular toxicity [14]. Therefore, scholars have been exploring new URAT1 inhibitors with low toxicity [15].
Natural products refer to components or metabolites from animals, plants, insects, and microorganisms, such as proteins, peptides, polysaccharides, and alkaloids [16–18]. Natural products have been used as medicines for thousands of years. Moreover, the importance of natural products is increasing day by day and has become an important source of drug development [19]. At present, long-term clinical practice has demonstrated that traditional Chinese medicine (one of the important sources of natural products) has exact efficacy in lowering serum uric acid without serious adverse effects [20]. With the deepening of research, scholars have found that natural products are expected to be the source of new URAT1 inhibitors. This study systematically summarizes natural products with URAT1 inhibition. The results showed that flavonoids, terpenoids, alkaloids, coumarins, stilbenes, steroids, organic acids, and polysaccharides show inhibitory effects of URAT1, which can inhibit URAT1 activity and promote uric acid excretion. However, most studies have focused on finding natural products with inhibition of URAT1 and have not explored their activities and mechanisms towards URAT1. By reviewing the few existing studies on the structure-activity relationship studies and analyzing common features of natural products with URAT1 inhibition, we speculate that the rigid ring structure and negative charge may be the keys for natural products to produce URAT1 inhibition. In conclusion, natural products are valuable sources of URAT1 inhibitor, and exploring the mechanism of action and structure-activity relationship will be an important research direction in the future.
2. Pathological Processes of Hyperuricemia and the Role of URAT1
Uric acid, also known as 2,6,8-trihydroxypurine, is a heterocyclic carbonyl compound with a relative molecular weight of 168 [21]. Uric acid is mainly produced by the metabolism of endogenous and dietary purine compounds under the action of xanthine oxidase in the liver (Figure 1) [22]. Hyperuricemia refers to an excessively high concentration of uric acid in the blood. That is, uric acid concentration <7.0 mg/dl in men or <6.0 mg/dl in women [23]. As a metabolic disease, hyperuricemia is closely related to the occurrence and development of many diseases, such as gout, hypertension, heart disease, and diabetes [24]. The appearance of gout is most closely related to hyperuricemia. This is because an excessively high concentration of uric acid is easily deposited in the articular cavity in body tissue, causing pain, edema, and inflammation in the joints, finally inducing gout [25].
Figure 1.

The production pathway of uric acid.
The metabolic disorder of uric acid includes excessive uric acid production and decreased uric acid excretion [26]. Causes of excess uric acid production include the intake of purine-rich foods, such as seafood and meat, and the increased concentrations or activities of intermediate metabolic enzymes of uric acid, such as xanthine oxidase [27]. Since more than 70% of uric acid in the human body is produced by metabolism, inhibiting the activities of metabolic enzymes can effectively inhibit the production of uric acid [28]. Therefore, xanthine oxidase inhibitors such as allopurinol, febuxostat, and topiroxostat are the drugs of choice for the clinical treatment of hyperuricemia and gout [29]. In addition, the main reason for the decrease in uric acid excretion is closely related to the insufficient renal excretion capacity. This is because the kidney is the main excretory organ of uric acid, and more than 2/3 of uric acid is excreted from the kidney [30]. Therefore, promoting the excretion of uric acid by the kidney by regulating the activities of uric acid transporters is an effective method to treat hyperuricemia and gout. Current studies have found that uric acid transport-related proteins mainly include uric acid reabsorption-related proteins and uric acid secretion-related proteins (Figure 2). Proteins related to uric acid reabsorption include URAT1, glucose transporter 9 (GLUT9), organic anion transporter 4 (OAT4), and organic anion transporter 10 [31]. Proteins related to uric acid secretion include organic anion transporter 1, organic anion transporter 2, organic anion transporter 3, sodium-dependent phosphate transport protein 1 (NPT1), sodium-dependent phosphate transport protein 4, ATP-binding cassette superfamily G2 (ABCG2), multidrug resistance protein 4 (MRP4), and urate transporter (UAT) [32]. Among these proteins, URAT1 is a highly valuable potential therapeutic target.
Figure 2.
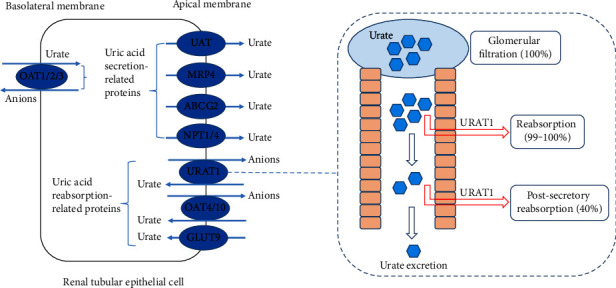
The distribution of uric acid transporters and the effects of URAT1 on uric acid metabolism.
URAT1 is encoded by the SLC22A12 gene, which is located on chromosome 11q13, contains 10 exons and 9 introns, encodes 555 amino acids, and has 12 transmembrane domains [33]. URAT1, originally called the renal-specific transporter, is a member of the organic anion transporter family and the first protein to be involved in renal uric acid transport [34]. Figure 2 shows that URAT1 is located in the renal tubule epithelial cell apical membrane and mediates the exchange of uric acid in the lumen with inorganic and organic anions in the proximal tubular epithelial cells, thus reabsorbing uric acid from the lumen into epithelial cells [35]. Although URAT1 is not the only protein that mediates uric acid re-absorption, the importance of URAT1 is reflected in its strong transport capacity: approximately 90% of uric acid re-absorption is mediated by URAT1 [36]. Therefore, considering the important role of URAT1 in uric acid re-absorption, URAT1 inhibitors are considered highly effective and promising drugs for the treatment of hyperuricemia. As early as 2002, related studies explored the possibility and value of URAT1 as a target for reducing uric acid and first proposed the development of URAT1 inhibitors [37]. So far, researchers have developed a variety of URAT1 inhibitors, such as probenecid, benzbromarone, lesinurad, and dotinurad [12]. These drugs can effectively inhibit the reabsorption of uric acid by URAT1 and promote the excretion of uric acid, thus exerting a uric acid-lowering effect.
3. Natural Products with URAT1 Inhibitory Effects
Due to the great potential of URAT1 inhibitors in the treatment of hyperuricemia and gout, researchers have been exploring new URAT1 inhibitors [38]. As an important source of new drugs, natural products have received more and more attention for their inhibitory effects on URAT1 [39]. Table 1 summarizes the species, main sources, and inhibitory effects of URAT1 of these natural products. It can be seen that many of the natural products with URAT1 inhibition are flavonoids [45, 52, 53]. In addition, some terpenoids, alkaloids, coumarins, stilbenes, and steroids also show a URAT1 inhibitory effect [60, 66, 68, 74, 77]. However, most studies have focused on finding natural products with inhibition of URAT1 and have not explored their mechanisms toward URAT1 [63, 64]. Therefore, exploring the mechanism of action will be an important research direction in the future.
Table 1.
Natural products with an URAT1 inhibitory effect.
| Category | Name | Common or primary source | Cell lines/model | Dosage | Ref. |
|---|---|---|---|---|---|
| Flavones | Nobiletin | Citrus fruits | URAT1-expressing 293A cells | IC50 = 17.6 μM/l | [40] |
| Baicalein | Scutellaria baicalensis | URAT1 and potassium oxonate-induced hyperuricemia mice | IC50 = 31.56 μM/l and 200 mg/kg, respectively | [41] | |
| Apigenin | The leafy herbs parsley and dried chamomile flowers | URAT1, URAT1-expressing HK-2 cells and hyperuricemia nephropathy mice | IC50 = 0.64 μM/l, 3.125–100 μM/l and 100 mg/kg | [42, 43] | |
| Chrysin | Propolis, blue passion flower, and honey | High fructose corn syrup-induced hyperuricemia rats | 50–100 mg/kg | [44] | |
| Luteolin | Fruits and vegetable | Potassium oxonate-induced hyperuricemia mice | 3–10 mg/kg | [45] | |
| Luteolin-4′-O-glucoside | Fruits and vegetable | Potassium oxonate-induced hyperuricemia mice | 20–100 mg/kg | [45] | |
|
| |||||
| Flavonols | Fisetin | Vegetables and fruits | Potassium oxonate-induced hyperuricemia mice | 50–100 mg/kg | [46] |
| Morin | Plants and fruits of the Moraceae family | Potassium oxonate-induced hyperuricemia mice | 10–40 mg/kg | [47, 48] | |
| Rutin | Vegetables and fruits | Potassium oxonate-induced hyperuricemia mice | 25–100 mg/kg | [49] | |
| Gossypetin | Flowers of Hibiscus sabdariffa | URAT1-expressing 293A cells | IC50 = 31.3 μM/l | [50] | |
| Quercetagetin | Tagetes flowers | URAT1-expressing 293A cells | IC50 = 18.4 μM/l | [50] | |
| Quercetin | Vegetables and fruits | URAT1-expressing 293A cells | IC50 = 12.6 μM/l | [50] | |
|
| |||||
| Flavonones | Naringenin | Citrus fruits | URAT1-expressing 293A cells | IC50 = 16.1 μM/l | [51] |
| Hesperetin | Citrus fruits | URAT1-expressing 293A cells | IC50 = 17.6 μM/l | [51] | |
| Isobavachin | Psoralea corylifolia L. | URAT1-expressing HEK293 cells and hyperuricemia mice | IC50 = 0.39 μM/l and 10 mg/kg, respectively | [52] | |
| Flavanols | Epigallocatechin-3-gallate | Green tea | Potassium oxonate-induced hyperuricemia mice | 10–50 mg/kg | [53] |
| Flavanonols | Astilbin | Smilax glabra | Potassium oxonate-induced hyperuricemia mice | 5–20 mg/kg | [54, 55] |
| Isoflavones | Genistein | Leguminoseae plants | Potassium oxonate-induced hyperuricemia mice | 10–20 mg/kg | [56]. |
|
| |||||
| Xanthones | Mangiferin | Mangifera indica L. | Potassium oxonate-induced hyperuricemia mice | 1.5–24.0 mg/kg | [57] |
| Mangiferin aglycon | Mangifera indica L. | Potassium oxonate-induced hyperuricemia mice | 10–30 mg/kg | [58] | |
|
| |||||
| Coumarins | Psoralen | Cullen corylifolium | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [59] |
| Isopsoralen | Cullen corylifolium | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [59] | |
| Imperatorin | Angelica dahurica and Angelica sinensis | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [59] | |
| Isoimperatorin | Angelica dahurica and Angelica sinensis | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [59] | |
| Xanthotoxin | Zanthoxylum bungeanum | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [59] | |
| Fraxetin | Fraxinus chinensis | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [60] | |
| Fraxin | Fraxinus chinensis | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [60] | |
| Osthol | Clinopodium megalanthum | URAT1 and potassium oxonate-induced hyperuricemia mice | IC50 = 78.8 μM/l and 20–40 mg/kg | [61] | |
|
| |||||
| Stilbenes | Resveratrol | Grapes, soybeans, berries, pomegranate, and peanuts | Potassium oxonate-induced hyperuricemia mice | 10–40 mg/kg | [62] |
| Polydatin | Polygonum cuspidatum | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [63] | |
| Mulberroside A | Morus alba L. | Potassium oxonate-induced hyperuricemia mice | 10–40 mg/kg | [64] | |
|
| |||||
| Terpenes | Loganin | Cornus officinalis | Potassium oxonate-induced hyperuricemia mice | 20–40 mg/kg | [63] |
| Geniposide | Gardenia jasminoides | Potassium oxonate-induced hyperuricemia mice | 100–200 mg/kg | [65] | |
| 13β, 18-dihydroeurycomanol | Eurycoma longifolia | URAT1-expressing HEK293T cells | 50 μM/l | [66] | |
| Δ4,5,14-hydroxyglaucarubol | Eurycoma longifolia | URAT1-expressing HEK293T cells | 50 μM/l | [66] | |
| 13β, 21-dihydroxyeurycomanol | Eurycoma longifolia | URAT1-expressing HEK293T cells | 50 μM/l | [66] | |
| Eurycomanol | Eurycoma longifolia | URAT1-expressing HEK293T cells | 50 μM/l | [66] | |
| 13β, 21-dihydroxyeurycomanone | Eurycoma longifolia | URAT1-expressing HEK293T cells | 50 μM/l | [66] | |
| 13α21-epoxyeurycomanone | Eurycoma longifolia | URAT1-expressing HEK293T cells | 50 μM/l | [66] | |
| Emodinol | Elaeagus pungens | Potassium oxonate-induced hyperuricemia mice | 25–100 mg/kg | [67] | |
|
| |||||
| Alkaloids | Betaine | Beet | Potassium oxonate-induced hyperuricemia mice | 5–40 mg/kg | [68] |
| Nuciferine | Nelumbo nucifera | Potassium oxonate-induced hyperuricemia mice | 5–40 mg/kg | [69] | |
| Berberine | Coptis chinensis and Phellodendron chinense | Potassium oxonate-induced hyperuricemia mice | 6.25–25.0 mg/kg | [70] | |
| Dihydroberberine | Coptis chinensis and Phellodendron chinense | Potassium oxonate-induced hyperuricemia mice | 25–50 mg/kg | [71] | |
|
| |||||
| Steroids | Dioscin | Fenugreek plant | Potassium oxonate-induced hyperuricemia mice | 319.22–1276.86 mg/kg | [72] |
| Tigogenin | Agave sisalana | URAT1-expressing HCT116 cells | 10–100 μM/l | [73] | |
| Withaferin A | Withania somnifera | Potassium oxonate-induced hyperuricemia mice | 3–10 mg/kg | [74] | |
| Phenolic acids | Chlorogenic acid | Honeysuckle | Potassium oxonate-induced hyperuricemia mice | 0.75 mmol/l | [75] |
| Acetophenone | 2,5-Dihydroxyacetophenone | Ganoderma applanatum | Potassium oxonate-induced hyperuricemia mice | 20–80 mg/kg | [76] |
3.1. Flavonoids
Flavonoids are a class of secondary plant metabolites widely present in a variety of plants and are the active components of many Chinese herbal medicines. Chemical structure generally refers to the connection of two benzene rings (ring A and ring B) through three carbon atoms to form the structure C6-C3-C6 [78]. Flavonoids contain many subclasses based on the connection position of the B and C rings as well as the degree of saturation, oxidation, and hydroxylation of the C ring [79]. Currently, studies have shown that many natural products with URAT1 inhibitory effects belong to flavonoids, and the subclasses include flavones, flavonols, flavanols, flavonones, flavanonols, isoflavones, and xanthones. Figure 3 further summarizes the structural formulas of these flavonoids.
Figure 3.

Structural formula of flavonoids with URAT1 inhibitory effect.
3.1.1. Flavones
It can be seen in Figure 3 that flavones are characterized by containing a double bond between positions 2 and 3 and a ketone in position 4 of the C ring [80]. Currently, flavones with the inhibitory effect of URAT1 include chrysin, apigenin, baicalein, nobiletin, and luteolin. The structures of these flavones are very similar, except for nobiletin (the substituents are all methoxy). They have hydroxyl groups at positions 5 and 7 of the A ring, and the differences are reflected in the number of hydroxyl groups at positions 3, 4, and 5 of the B ring. Chrysin is mainly derived from propolis, blue passion flower, and honey [81]. In rats induced by high fructose corn syrup hyperuricemia, chrysin (50–150 mg/kg) could inhibit the expression of URAT1 and promote uric acid excretion [44]. The main sources of apigenin are the leafy herbs parsley and dried chamomile flowers [82]. Cellular experiments showed that apigenin (3.125–100 μM/l) could inhibit cellular uptake of uric acid in HK-2 cells treated with uric acid by inhibiting URAT1 expression [42]. Li et al. found that apigenin (IC50 = 0.64 μM/l) not only competitively inhibited URAT1 activity in vitro, but also (100 mg/kg) promoted uric acid excretion by inhibiting URAT1 activity in potassium oxonate-induced hyperuricemic nephropathy mice [43]. The main source of baicalein is the root of Scutellaria baicalensis. Baicalein (IC50 = 31.56 μM/l) could non-competitively inhibit URAT1 activity in vitro and (200 mg/kg) improved renal urate excretion by inhibiting URAT1 expression in potassium oxonate-induced hyperuricemia mice. Protein docking analysis revealed that baicalein interacted with Ser35 and Phe241 of URAT1 [41]. Nobiletin is a highly methoxylated flavone compound, especially abundant in citrus [40]. Cell experiments showed that nobiletin (IC50 = 17.6 μM/l) could inhibit URAT1 expression and uric acid uptake in 293A cells expressing URAT1 treated with uric acid [51]. Luteolin is widely found in fruits and vegetables [83]. The animal experiment showed that both luteolin (3–10 mg/kg) and luteolin-4′-O-glucoside (20–100 mg/kg) could inhibit URAT1 expression and promote uric acid excretion in potassium oxonate-induced hyperuricemia mice [45].
3.1.2. Flavonols
It can be seen in Figure 3 that flavonols are characterized by containing a hydroxyl group at position 3 of the C ring [84]. Current studies show that six flavonols are promising as URAT1 inhibitors, which are gossypetin, quercetagetin, quercetin, fisetin, morin, and rutin. Cellular experiments showed that gossypetin (isolated from Hibiscus sabdariffa flowers), quercetagetin (isolated from tagetes flowers), and quercetin (widespread in vegetables and fruits) could inhibit URAT1 expression and uric acid uptake in 293A cells expressing URAT1, and the IC50 values were 31.3 μM/l, 18.4 μM/l, and 12.6 μM/l, respectively [50, 85–87]. Rutin, also called rutoside, quercetin-3-rutinoside, and sophorin, is abundant in vegetables and fruits, such as passion flower, tea, apple, asparagus, blackberry, quince, cherry, and red plum [88]. Fisetin is also widely found in vegetables and fruits, such as strawberry, blueberry, apple, grape, persimmon, kiwi, and cucumber [89]. The source of morin is mainly Moraceae plants [90]. Animal studies have shown that fisetin (50–100 mg/kg), morin (10–40 mg/kg), and rutin (25–100 mg/kg) could inhibit URAT1 activity and promote uric acid excretion in potassium oxonate-induced hyperuricemia mice [46–49].
3.1.3. Flavanols
Compared to flavonol, the structural characteristic of flavanol is that the C ring has no carbonyl group and the double bond at positions 2 and 3 is hydrogenated [91]. Flavanols are divided into flavan-3-ols and flavan-3,4-diols according to the position of the hydroxyl group in the C-ring. Current research has shown that only epigallocatechin-3-gallate, the main component of green tea polyphenols, has a URAT1 inhibitory effect [92]. As can be seen from the structure, epigallocatechin-3-gallate is an ester formed by epigallocatechin and gallic acid and belongs to the flavan-3-ols. The animal study showed that epigallocatechin-3-gallate (10–50 mg/kg) inhibited the expression of URAT1 and promoted uric acid excretion in hyperuricemia mice induced by potassium oxonate [53].
3.1.4. Flavonones
It can be seen in Figure 3 that the structural characteristic of flavonone is that the double bond at positions 2 and 3 of the C ring is hydrogenated [93]. Current research shows that flavonones with the inhibitory effect of URAT1 include hesperetin, naringenin, and isobavachin [94]. Both hesperetin and naringenin derive mainly from citrus fruits such as oranges and lemons [95]. Isobavachins are derived from the seeds of Psoralea corylifolia L. [96]. Hesperetin (IC50 = 17.6 μM/l) and naringenin (IC50 = 16.1 μM/l) could inhibit URAT1 expression and uric acid uptake in URAT1-expressing 293A cells [51]. Isobavachin could also inhibit URAT1 expression and uric acid uptake in URAT1-expressing HEK293 cells (IC50 = 0.39 μM/l) and promote uric acid excretion in potassium oxonate-induced hyperuricemia mice (10 mg/kg) [52].
3.1.5. Flavanonols
It can be seen in Figure 3 that flavanonol is produced by hydrogenation of the double bond at positions 2 and 3 of the C ring of flavonol [97]. The current study showed that only astilbin, a flavanonol glucoside of Smilax glabra, has the inhibitory effect of URAT1 [98]. In potassium oxonate-induced hyperuricemia mice, astilbin (5–20 mg/kg) inhibited URAT1 expression and promoted the excretion of uric acid [54, 55].
3.1.6. Isoflavones
Compared to flavone, the structural characteristic of isoflavone is that the B ring is attached to the 3-position of the C ring [99]. Current research has shown that only genistein derived from plants of Leguminoseae has the inhibitory effect of URAT1 [100]. In potassium oxonate-induced hyperuricemia mice, genistein (10–20 mg/kg) inhibited URAT1 expression and promoted uric acid excretion [56].
3.1.7. Xanthones
Xanthones (dibenzo-γ-pyrones) constitute an important class of oxygenated heterocycles and occur as secondary metabolites in plants and microorganisms. Xanthones do not conform to the basic skeleton of C6-C3-C6, but are also classified as flavonoids due to their benzo γ-pyranone structure [101]. Current research has shown that xanthones with the inhibitory effect of URAT1 include mangiferin and mangiferin aglycon, and they mainly derive from Mangifera indica L. [102]. The animal experiment showed that mangiferin (1.5–24.0 mg/kg) and mangiferin aglycon (10–30 mg/kg) inhibited URAT1 expression and promoted uric acid excretion in potassium oxonate-induced hyperuricemia mice [57, 58].
3.2. Terpenoids
Terpenoids consist of isoprene units and can be divided into hemiterpenes, monoterpenes, sesquiterpenes, diterpenes, disesquiterpenes, triterpenes, and polyterpenes according to the number of units containing isoprene [103]. The current study shows that terpenoids with an URAT1 inhibitory effect include monoterpenes and triterpenes (Figure 4).
Figure 4.

Structural formula of terpenes with URAT1 inhibitory effect.
Only two iridoids among monoterpenes show the URAT1 inhibitory effect, including loganin and geniposide. Loganin is a common iridoid glycoside derived from Cornus officinalis [104]. Geniposide is also an iridoid glycoside and an important active ingredient of Gardenia jasminoides [105]. Studies have shown that both loganin (20–40 mg/kg) and geniposide (100–200 mg/kg) could inhibit URAT1 activity and promote uric acid excretion in potassium oxonate-induced hyperuricemia mice [63, 65].
Triterpenoids with URAT1 inhibitory effect are mainly a series of quassinoids extracted from Eurycoma longifolia, including eurycomanol, eurycomanone, 13β,18-dihydroeurycomanol, Δ4,5,14-hydroxyglaucarubol, 13β,21-dihydroxyeurycomanol, 13β,21-dihydroxyeurycomanone, and 13α(21)-epoxyeurycomanone [66]. The structural differences of these quassinoids are reflected in the differences of the substituents of the 2 and 21 positions. The cellular experiment showed that these quassinoids (50 μM/l) decreased urate uptake in HEK293T cells expressing URAT1 by inhibiting URAT1 activity [66]. Furthermore, emodinol, a triterpenoid extracted from Elaeagus pungens, also had the inhibitory effect of URAT1, which (25–100 mg/kg) inhibited the expression of URAT1 and promoted uric acid excretion in potassium oxonate-induced hyperuricemia mice [67, 106].
3.3. Coumarins
Coumarin is a general term for a class of natural compounds with benzo-α-pyrone core, which can be regarded as lactones formed by the dehydration of cis-o-hydroxycinnamic acid [107]. Currently, studies have shown that a variety of coumarins have an inhibitory effect on URAT1 (Figure 5), including psoralen and isopsoralen (extracted from Cullen corylifolium) [108], imperatorin and isoimperatorin (extracted from Angelica dahurica and Angelica sinensis) [109], xanthotoxin (extracted from Zanthoxylum bungeanum) [110], fraxetin and fraxin (extracted from Fraxinus chinensis) [111], and osthol (extracted from Clinopodium megalanthum) [112]. Osthol, fraxetin, and fraxin are simple coumarins characterized by the 7-position hydroxyl group not forming a furan or pyran ring with the 6- or 8-position isopentenyl group. Studies have shown that osthol (IC50 = 78.8 μM/l) could non-competitively inhibit URAT1 activity in vitro, and both fraxetin and fraxin (20–40 mg/kg) could negatively regulate URAT1 expression in potassium oxonate-induced hyperuricemia mice [60, 61]. Xanthotoxin, psoralen, imperatorin, and isoimperatorin are linear furocoumarins formed by the condensation of the 7-position hydroxyl group with the 6-position isopentenyl group. Isopsoralen is an angular furocoumarin formed by condensation of the 7-position hydroxyl group with the 8-position isopentenyl group. Animal studies have shown that these furocoumarins (20–40 mg/kg) inhibited URAT1 activity and promoted uric acid excretion in mice with potassium oxonate-induced hyperuricemia nephropathy [59, 60].
Figure 5.

Structural formula of coumarins with the URAT1 inhibitory effect.
3.4. Stilbenes
Stilbenes refer to the general term of monomers with a 1,2-diphenylethylene skeleton and their polymers [113]. Current studies show that stilbenes with URAT1 inhibitory effects include resveratrol, polydatin, and mulberroside A (Figure 6). Resveratrol is a well-known stilbene compound present in grapes, soybeans, berries, pomegranate, and peanuts [114]. Animal experiments have shown that resveratrol (10–40 mg/kg) could promote uric acid excretion by inhibiting URAT1 activity in potassium oxonate-induced hyperuricemia mice. Furthermore, the researchers believed that this was related to inhibiting the activation of the inflammatory response, namely inhibiting the NLRP3 (NOD-like receptor family, pyrin domain-containing 3) inflammasome and TLR4 (toll-like receptor 4)/MyD88 (myeloid differentiation factor 88)/NF-κB (nuclear factor-κB) signaling pathway [62]. In addition to resveratrol, Chen et al. found that the resveratrol tetramer (20–60 mg/kg) could also inhibit URAT1 activity and promote uric acid excretion in mice with potassium oxonate induced hyperuricemia mice [77]. Polydatin and mulberroside A are two stilbene compounds with a very similar structure derived from Polygonum cuspidatum and Morus alba L., respectively [115, 116]. In potassium oxonate-induced hyperuricemia mice, polydatin (20–40 mg/kg) and mulberroside A (10–40 mg/kg) could down-regulate the expression of URAT1 in the kidney and promote uric acid excretion [63, 64].
Figure 6.

Structural formula of stilbenes, alkaloids, steroids, and other natural products with an URAT1 inhibitory effect.
3.5. Alkaloids
Alkaloids are a class of nitrogen-containing organic compounds derived primarily from plants [117]. Current studies show that alkaloids with URAT1 inhibition include betaine, nuciferine, berberine, and dihydroberberine (Figure 6). Betaine is a quaternary ammonium-type alkaloid derived from beet [118]. Nuciferine is an aporphine alkaloid extracted from Nelumbo nucifera [119]. Berberine and dihydroberberine are two isoquinoline alkaloids derived from Coptis chinensis and Phellodendron chinense [119, 120]. Animal experiments showed that betaine (5–40 mg/kg), nuciferine (5–40 mg/kg), berberine (6.25–25.0 mg/kg) and dihydroberberine (25–50 mg/kg) could inhibit URAT1 expression and promote the excretion of uric acid in potassium oxonate-induced hyperuricemia mice [68–71].
3.6. Steroids
Steroid is a general term for a large class of compounds with the basic skeleton structure of perhydrocyclopentano-phenanthrene [121]. Current studies have shown that steroids with an inhibitory effect of URAT1 include withaferin A, dioscin, and tigogenin (Figure 6). Dioscin is an isospirostane glycoside mainly extracted from the fenugreek plant [122]. Su et al. found that dioscin (319.22–1276.86 mg/kg) could negatively regulate URAT1 expression in potassium oxonate-induced hyperuricemia mice [72]. Tigogenin is a spirostane glycoside extracted from Agave sisalana [123]. Zhang et al. found that tigogenin (10–100 μM/l) could decrease uric acid uptake in URAT1-expressing HCT116 cells by inhibiting URAT1 activity [73]. Withaferin A extracted from Withania somnifera, is a steroidal lactone [124]. In potassium oxonate-induced hyperuricemia mice, withaferin A (3–10 mg/kg) negatively regulated URAT1 expression in the kidney and promoted uric acid excretion [74].
3.7. Other Natural Products
Chlorogenic acid is an organic acid derived from honeysuckle [125]. In vitro research showed that chlorogenic acid (0.75 mmol/l) could inhibit uric acid reuptake in URAT1 expressing-HEK293T cells by inhibiting URAT1 expression [75]. Liang et al. isolated 2,5-dihydroxyacetophenone from Ganoderma applanatum, which (20–80 mg/kg) could inhibit URAT1 activity in mice induced by potassium oxonate hyperuricemia [76]. Li et al. isolated a pure polysaccharide ULP from Ulva lactuca consisting of rhamnose, glucuronic acid, galactose and xylose at a molar ratio of 32.75 : 22.83 : 1.07 : 6.46 with a molecular weight of 2.24 × 105 Da. In potassium oxonate-induced hyperuricemia mice, ULP (10–50 mg/kg) inhibited the expression of URAT1 and promoted uric acid excretion [126].
4. Review and Speculation of the Structure-Activity Relationship
Currently, most studies have focused on finding natural products with inhibition of URAT1 and have not explored their activities and mechanisms toward URAT1. Therefore, in addition to exploring the mechanism of action, exploring the structure-activity relationship will be another important research direction in the future. By reviewing the few existing studies on the structure-activity relationship studies and analyzing common features of natural products with URAT1 inhibition, we speculate that the rigid ring structure and negative charge may be the keys for natural products to produce URAT1 inhibition. We hope that the analysis and speculation in this chapter can broaden the current understanding and trigger further interest in exploring the relationship between structure and URAT1 inhibition of natural products.
First, natural products may need to contain rigid structures. It can be found that among these natural products with URAT1 inhibition, almost all of them have a rigid ring structure. For example, one third of natural products with URAT1 inhibition are flavonoids. It can be seen from the structure of the flavonoid that two benzene rings connected to an oxygen-containing pyranyl group are a typical rigid plane molecular structure [127]. Polycyclic terpenoids, such as quassinoids, also contain unique rigid ring backbones [128]. In addition, other compounds also contain rigid ring structures, such as the benzene ring, the naphthalene ring, or the pyridine ring. Therefore, the presence of a rigid structure may be one of the elements that natural products must use to inhibit URAT1.
Second, natural products may require anions to act as URAT1 inhibitors. Wempe et al. evaluated the inhibitory effect of a series of (2-ethylbenzofuran-3-yl) (substituted-phenyl) methanone compounds on URAT1 activity in oocytes expressing hURAT1. The experimental data indicated that a potent hURAT1 inhibitor requires an anion (that is, a formal negative charge) to interact with the positively charged URAT1 binding pocket [129]. The C-ring of flavonoids is an electron-rich region with a strong negative charge. This partially explains the inhibitory activities of flavonoids in URAT1 [130]. Furthermore, most natural products with URAT1 inhibition also contain phenolic hydroxyl groups, so that these compounds can show acidity and generate anions [131]. However, alkaloids including betaine, nuciferine, berberine, and dihydroberberine also show inhibition of URAT1, so the presence of anions may not be a determinant of whether a natural product has inhibition of URAT1.
5. Conclusion and Prospects
In summary, current studies have shown that many natural products have a URAT1 inhibitory effect and are expected to be developed as URAT1 inhibitors, including flavonoids, terpenoids, alkaloids, coumarins, stilbenes, steroids, organic acids, and polysaccharides. The number of flavonoids is the largest among them, including many subtypes. Animal experiments have shown that these natural products can inhibit URAT1 activity in hyperuricemia mice and promote uric acid excretion. By reviewing the few existing studies on the structure-activity relationship studies and analyzing common features of natural products with URAT1 inhibition, we speculate that the rigid ring structure and negative charge may be the keys for natural products to produce URAT1 inhibition.
Although studies have confirmed that natural products are promising as URAT1 inhibitors, there are still some issues that need to be addressed in the future. First, the mechanism by which these natural products inhibit URAT1 is unclear. Therefore, more research is needed to explore the mechanism of action. Second, current research is still in the experimental study stage and it is necessary to carry out clinical research to further explore its therapeutic effects. Third, the relationship between structure and URAT1 inhibitory activity requires further investigation. In addition to the rigid ring structure and negative charge, what other structural features are essential for the URAT1 inhibitory effect of natural products? Fourth, structural modification is a common method to improve the therapeutic effect of drugs and reduce side effects. Therefore, structural modification based on clarifying the structure-activity relationship of natural products to improve the inhibitory activity of URAT1 may be a key research direction in the future.
Acknowledgments
This work was supported by the Sichuan Science and Technology Program (2019YJ0614), the Sichuan Administration of Traditional Chinese Medicine (2020JC0050), the Science and Technology Development Fund of the Hospital of Chengdu University of Traditional Chinese Medicine (18PY19), and the Development Fund of the Hospital of Chengdu University of Traditional Chinese Medicine (2012-D-YY-13).
Data Availability
Data sharing are not applicable to this article, as no new data were created or analyzed in this study.
Disclosure
Qianghua yuan, Yuan cheng, Rong sheng and Mei hu should be regarded as the co first author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Qianghua yuan, Yuan cheng, Rong sheng and Mei hu contributed equally to this work.
References
- 1.Xie D., Zhao H., Lu J., et al. High uric acid induces liver fat accumulation via ROS/JNK/AP-1 signaling. American Journal of Physiology—Endocrinology And Metabolism . 2021;320(6):E1032–E1043. doi: 10.1152/ajpendo.00518.2020. [DOI] [PubMed] [Google Scholar]
- 2.Li C. G., Hsieh M. C., Chang S. J. Metabolic syndrome, diabetes, and hyperuricemia. Current Opinion in Rheumatology . 2013;25(2):210–216. doi: 10.1097/bor.0b013e32835d951e. [DOI] [PubMed] [Google Scholar]
- 3.Jakše B., Jakše B., Pajek M., Pajek J. Uric acid and plant-based nutrition. Nutrients . 2019;11:p. 1736. doi: 10.3390/nu11081736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokose C., McCormick N., Choi H. K. Dietary and lifestyle-centered approach in gout care and prevention. Current Rheumatology Reports . 2021;23(7):p. 51. doi: 10.1007/s11926-021-01020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo Y. F., Lu H., Gan J., Li D. D., Gao J. D., Zhang C. M. Efficacy of Chinese herbal medicine jiangniaosuan formula for treatment of hyperuricemia: study protocol for a double-blindednon-inferiority randomized controlled clinical trial. Trials . 2022;23(1):p. 1. doi: 10.1186/s13063-021-05959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P., Xu H. C., Shi Y. C., Deng L., Chen X. Y. Potential molecular mechanisms of plantain in the treatment of gout and hyperuricemia based on network pharmacology. Evidence-Based Complementary and Alternative Medicine . 2020;2020:20. doi: 10.1155/2020/3023127.3023127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abeles A. M., Pillinger M. H. Gout and cardiovascular disease: crystallized confusion. Current Opinion in Rheumatology . 2019;31(2):118–124. doi: 10.1097/bor.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z. L., Li Y. C., Liao W. H., et al. Gut microbiota remodeling: a promising therapeutic strategy to confront hyperuricemia and gout. Frontiers in Cellular and Infection Microbiology . 2022;12 doi: 10.3389/fcimb.2022.935723.935723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gliozzi M., Malara N., Muscoli S., Mollace V. The treatment of hyperuricemia. International Journal of Cardiology . 2016;213:23–27. doi: 10.1016/j.ijcard.2015.08.087. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., You R., Wang K., Wang Y. Recent updates of natural and synthetic URAT1 inhibitors and novel screening methods. Evidence-Based Complementary and Alternative Medicine . 2021;2021:12. doi: 10.1155/2021/5738900.5738900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strilchuk L., Fogacci F., Cicero A. F. Safety and tolerability of available urate-lowering drugs: a critical review. Expert Opinion on Drug Safety . 2019;18(4):261–271. doi: 10.1080/14740338.2019.1594771. [DOI] [PubMed] [Google Scholar]
- 12.Song D. N., Zhao X., Wang F. Q., Wang G. A brief review of urate transporter 1 (URAT1) inhibitors for the treatment of hyperuricemia and gout: current therapeutic options and potential applications. European Journal of Pharmacology . 2021;907 doi: 10.1016/j.ejphar.2021.174291.174291 [DOI] [PubMed] [Google Scholar]
- 13.Zhang W., Doherty M., Bardin T., et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT) Annals of the Rheumatic Diseases . 2006;65(10):1312–1324. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoy S. M. Lesinurad: first global approval. Drugs . 2016;76(4):509–516. doi: 10.1007/s40265-016-0550-y. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y., Zhao T., Ai W., et al. Novel urate transporter 1 (URAT1) inhibitors: a review of recent patent literature (2016–2019) Expert Opinion on Therapeutic Patents . 2019;29(11):871–879. doi: 10.1080/13543776.2019.1676727. [DOI] [PubMed] [Google Scholar]
- 16.Shakeri F., Bianconi V., Pirro M., Sahebkar A. Effects of plant and animal natural products on mitophagy. Oxidative Medicine and Cellular Longevity . 2020;2020:11. doi: 10.1155/2020/6969402.6969402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratcliffe N. A., Mello C. B., Garcia E. S., Butt T. M., Azambuja P. Insect natural products and processes: new treatments for human disease. Insect Biochemistry and Molecular Biology . 2011;41(10):747–769. doi: 10.1016/j.ibmb.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Chen L., Wang X. Y., Liu R. Z., Wang G. Y. Culturable microorganisms associated with sea cucumbers and microbial natural products. Marine Drugs . 2021;19(8):p. 461. doi: 10.3390/md19080461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo H. D., Nam S. J., Chin Y. W., Kim M. S. Misassigned natural products and their revised structures. Archives of Pharmacal Research . 2016;39(2):143–153. doi: 10.1007/s12272-015-0649-9. [DOI] [PubMed] [Google Scholar]
- 20.Lin J. P., Chen S. Q., Li S. Z., Lu M. L., Li Y. A., Su Y. X. Efficacy and safety of Chinese medicinal herbs for the treatment of hyperuricemia: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine . 2016;2016:12. doi: 10.1155/2016/2146204.2146204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X. D., Yang Y., Hu X. N., et al. Effects of sodium-glucose cotransporter 2 inhibitors on serum uric acid in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Diabetes, Obesity and Metabolism . 2022;24(2):228–238. doi: 10.1111/dom.14570. [DOI] [PubMed] [Google Scholar]
- 22.Cicero A. F. G., Fogacci F., Cincione R. I., Tocci G., Borghi C. Clinical effects of xanthine oxidase inhibitors in hyperuricemic patients. Medical Principles and Practice . 2021;30(2):122–130. doi: 10.1159/000512178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominguez-Zambrano E., Pedraza-Chaverri J., López-Santos A. L., et al. Association between serum uric acid levels, nutritional and antioxidant status in patients on hemodialysis. Nutrients . 2020;12(9):p. 2600. doi: 10.3390/nu12092600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S. S., Wang Y., Cheng J. S., et al. Hyperuricemia and cardiovascular disease. Current Pharmaceutical Design . 2019;25(6):700–709. doi: 10.2174/1381612825666190408122557. [DOI] [PubMed] [Google Scholar]
- 25.Danve A., Sehra S. T., Neogi T. Role of diet in hyperuricemia and gout. Best Practice & Research Clinical Rheumatology . 2021;35(4) doi: 10.1016/j.berh.2021.101723.101723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L. J., Zhang Y. P., Zeng C. C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. American Journal of Tourism Research . 2020;12(7):3167–3181. [PMC free article] [PubMed] [Google Scholar]
- 27.Kakutani-Hatayama M., Kadoya M., Okazaki H., et al. Nonpharmacological management of gout and hyperuricemia: hints for better lifestyle. American Journal of Lifestyle Medicine . 2017;11(4):321–329. doi: 10.1177/1559827615601973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liote F. Gout furonculosis. Joint Bone Spine . 2019;86(1):p. 103. doi: 10.1016/j.jbspin.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Wakabayashi T., Ueno S., Nakatsuji T., et al. Safety profiles of new xanthine oxidase inhibitors: a post-marketing study. International Journal of Clinical Pharmacology & Therapeutics . 2021;59(05):372–377. doi: 10.5414/cp203898. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama A., Nakaoka H., Yamamoto K., et al. GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Annals of the Rheumatic Diseases . 2017;76(5):869–877. doi: 10.1136/annrheumdis-2016-209632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandal A. K., Mercado A., Foster A., Zandi-Nejad K., Mount D. B. Uricosuric targets of tranilast. Pharmacology Research & Perspectives . 2017;5 doi: 10.1002/prp2.291.e00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nigam S. K., Bhatnagar V. The systems biology of uric acid transporters: the role of remote sensing and signaling. Current Opinion in Nephrology and Hypertension . 2018;27(4):305–313. doi: 10.1097/mnh.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan P. K., Liu S., Gunic E., Miner J. N. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Scientific Reports . 2017;7(1):p. 665. doi: 10.1038/s41598-017-00706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ljubojevic M., Balen D., Breljak D., et al. Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. American Journal of Physiology—Renal Physiology . 2007;292(1):F361–F372. doi: 10.1152/ajprenal.00207.2006. [DOI] [PubMed] [Google Scholar]
- 35.Enomoto A., Endou H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clinical and Experimental Nephrology . 2005;9(3):195–205. doi: 10.1007/s10157-005-0368-5. [DOI] [PubMed] [Google Scholar]
- 36.Cai W. Q., Liu W., Liu C. Y., Wang J. W., Zhao G. L. A systematic review of uric acid transporter 1 (URAT1) inhibitors for the treatment of hyperuricemia and gout and an insight into the structure-activity relationship (SAR) Chinese Journal of Structural Chemistry . 2017;36(6):897–910. [Google Scholar]
- 37.Enomoto A., Kimura H., Chairoungdua A., et al. Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature . 2002;417(6887):447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 38.Sivera F., Andres M., Dalbeth N. A glance into the future of gout. Therapeutic Advances in Musculoskeletal Disease . 2022;14 doi: 10.1177/1759720x221114098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ling X., Bochu W. A review of phytotherapy of gout: perspective of new pharmacological treatments. Pharmazie . 2014;69(4):243–256. [PubMed] [Google Scholar]
- 40.Mizuno H., Yoshikawa H., Usuki T. Extraction of nobiletin and tangeretin from peels of shekwasha and ponkan using C(2)mim (MeO)(H)PO2 and centrifugation. Natural Product Communications . 2019;14(5) doi: 10.1177/1934578x19845816. [DOI] [Google Scholar]
- 41.Chen Y. Y., Zhao Z. A., Li Y. M., et al. Baicalein alleviates hyperuricemia by promoting uric acid excretion and inhibiting xanthine oxidase. Phytomedicine . 2021;80 doi: 10.1016/j.phymed.2020.153374.153374 [DOI] [PubMed] [Google Scholar]
- 42.Zhu J. X., Yang H. Y., Hu W. Q., et al. Active components from Lagotis brachystachya maintain uric acid homeostasis by inhibiting renal TLR4-NLRP3 signaling in hyperuricemic mice. Inflammopharmacology . 2021;29(4):1187–1200. doi: 10.1007/s10787-021-00844-5. [DOI] [PubMed] [Google Scholar]
- 43.Li Y. M., Zhao Z. A., Luo J., et al. Apigenin ameliorates hyperuricemic nephropathy by inhibiting URAT1 and GLUT9 and relieving renal fibrosis via the Wnt/β-catenin pathway. Phytomedicine . 2021;87 doi: 10.1016/j.phymed.2021.153585.153585 [DOI] [PubMed] [Google Scholar]
- 44.Chang Y. H., Chiang Y. F., Chen H. Y., et al. Anti-inflammatory and anti-hyperuricemic effects of chrysin on a high fructose corn syrup-induced hyperuricemia rat model via the amelioration of urate transporters and inhibition of NLRP3 inflammasome signaling pathway. Antioxidants . 2021;10(4):p. 564. doi: 10.3390/antiox10040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin Y., Liu P. G., Liang W. Q., et al. Luteolin-4′-O-glucoside and its aglycone, two major flavones of gnaphalium affine D. Don, resist hyperuricemia and acute gouty arthritis activity in animal models. Phytomedicine . 2018;41:54–61. doi: 10.1016/j.phymed.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Ren Q., Tao S. B., Guo F., et al. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling. Phytomedicine . 2021;87 doi: 10.1016/j.phymed.2021.153552.153552 [DOI] [PubMed] [Google Scholar]
- 47.Shi Y. W., Wang C. P., Wang X., et al. Uricosuric and nephroprotective properties of ramulus mori ethanol extract in hyperuricemic mice. Journal of Ethnopharmacology . 2012;143(3):896–904. doi: 10.1016/j.jep.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J. J., Shuai X., Li J. B., Xiang N. X., Gong T., Zhang Z. R. Biodistribution, hypouricemic efficacy and therapeutic mechanism of morin phospholipid complex loaded self-nanoemulsifying drug delivery systems in an experimental hyperuricemic model in rats. Journal of Pharmacy and Pharmacology . 2016;68(1):14–25. doi: 10.1111/jphp.12492. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y. S., Hu Q. H., Zhang X., Zhu Q., Kong L. D. Beneficial effect of rutin on oxonate-induced hyperuricemia and renal dysfunction in mice. Pharmacology . 2013;92(1-2):75–83. doi: 10.1159/000351703. [DOI] [PubMed] [Google Scholar]
- 50.Toyoda Y., Takada T., Saito H., et al. Identification of inhibitory activities of dietary flavonoids against URAT1, a renal urate Re-absorber: in vitro screening and fractional approach focused on rooibos leaves. Nutrients . 2022;14(3):p. 575. doi: 10.3390/nu14030575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toyoda Y., Takada T., Saito H., et al. Inhibitory effect of Citrus flavonoids on the in vitro transport activity of human urate transporter 1 (URAT1/SLC22A12), a renal re-absorber of urate. Npj Science of Food . 2020;4:p. 3. doi: 10.1038/s41538-020-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X., Zhao Z., Luo J., et al. Novel natural scaffold as hURAT1 inhibitor identified by 3D-shape-based, docking-based virtual screening approach and biological evaluation. Bioorganic Chemistry . 2021;117 doi: 10.1016/j.bioorg.2021.105444.105444 [DOI] [PubMed] [Google Scholar]
- 53.Zhu C., Xu Y., Liu Z. H., Wan X. C., Li D. X., Tai L. L. The anti-hyperuricemic effect of epigallocatechin-3-gallate (EGCG) on hyperuricemic mice. Biomedicine & Pharmacotherapy . 2018;97:168–173. doi: 10.1016/j.biopha.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Wang M., Zhao J., Zhang N., Chen J. H. Astilbin improves potassium oxonate-induced hyperuricemia and kidney injury through regulating oxidative stress and inflammation response in mice. Biomedicine & Pharmacotherapy . 2016;83:975–988. doi: 10.1016/j.biopha.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 55.Wang S. W., Fang Y. J., Yu X. F., Guo L., Zhang X. X., Xia D. Z. The flavonoid-rich fraction from rhizomes of smilax glabra Roxb. ameliorates renal oxidative stress and inflammation in uric acid nephropathy rats through promoting uric acid excretion. Biomedicine & Pharmacotherapy . 2019;111:162–168. doi: 10.1016/j.biopha.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 56.Bi W. Y., Zhu C. L. Genistein ameliorates hyperuricemia-associated nephropathy in hyperuricemic mice. Food and Agricultural Immunology . 2021;32(1):778–797. [Google Scholar]
- 57.Yang H., Gao L. H., Niu Y. F., et al. Mangiferin inhibits renal urate reabsorption by modulating urate transporters in experimental hyperuricemia. Biological and Pharmaceutical Bulletin . 2015;38(10):1591–1598. doi: 10.1248/bpb.b15-00402. [DOI] [PubMed] [Google Scholar]
- 58.Qin Z. Z., Wang S. B., Lin Y. H., et al. Antihyperuricemic effect of mangiferin aglycon derivative J99745 by inhibiting xanthine oxidase activity and urate transporter 1 expression in mice. Acta Pharmaceutica Sinica B . 2018;8(2):306–315. doi: 10.1016/j.apsb.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X., Lou Y. J., Wang M. X., Shi Y. W., Xu H. X., Kong L. D. Furocoumarins affect hepatic cytochrome P450 and renal organic ion transporters in mice. Toxicology Letters . 2012;209(1):67–77. doi: 10.1016/j.toxlet.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 60.Li J. M., Zhang X., Wang X., Xie Y. C., Kong L. D. Protective effects of cortex fraxini coumarines against oxonate-induced hyperuricemia and renal dysfunction in mice. European Journal of Pharmacology . 2011;666(1-3):196–204. doi: 10.1016/j.ejphar.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 61.Tashiro Y., Sakai R., Hirose-Sugiura T., et al. Effects of osthol isolated from cnidium monnieri fruit on urate transporter 1. Molecules . 2018;23(11):p. 2837. doi: 10.3390/molecules23112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X. M., Nie Q., Zhang Z. M., et al. Resveratrol affects the expression of uric acid transporter by improving inflammation. Molecular Medicine Reports . 2021;24(2):p. 564. doi: 10.3892/mmr.2021.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim O.-K., Yun J.-M., Lee M., Kim D., Lee J. Hypouricemic effects of Chrysanthemum indicum L. And Cornus officinalis on hyperuricemia-induced HepG2 cells, renal cells, and mice. Plants . 2021;10(8):p. 1668. doi: 10.3390/plants10081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C. P., Wang Y. M., Wang X., et al. Mulberroside A possesses potent uricosuric and nephroprotective effects in hyperuricemic mice. Planta Medica . 2011;77(8):786–794. doi: 10.1055/s-0030-1250599. [DOI] [PubMed] [Google Scholar]
- 65.Liu C., Zhou H. N., Zhang R. R., et al. Anti-hyperuricemic and nephroprotective effect of geniposide in chronic hyperuricemia mice. Journal of Functional Foods . 2019;61 doi: 10.1016/j.jff.2019.05.011.103355 [DOI] [Google Scholar]
- 66.Bao R. X., Liu M. Y., Wang D., et al. Effect of eurycoma longifolia stem extract on uric acid excretion in hyperuricemia mice. Frontiers in Pharmacology . 2019;10:p. 1464. doi: 10.3389/fphar.2019.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu H., Yuan Y. L., Chen Y. D., et al. Hypouricemic and nephroprotective effects of emodinol in oxonate-induced hyperuricemic mice are mediated by organic ion transporters and OIT3. Planta Medica . 2016;82(4):289–297. doi: 10.1055/s-0035-1558212. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y. L., Pan Y., Wang X., et al. Betaine reduces serum uric acid levels and improves kidney function in hyperuricemic mice. Planta Medica . 2014;80(1):39–47. doi: 10.1055/s-0033-1360127. [DOI] [PubMed] [Google Scholar]
- 69.Wang M. X., Liu Y. L., Yang Y., Zhang D. M., Kong L. D. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. European Journal of Pharmacology . 2015;747:59–70. doi: 10.1016/j.ejphar.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 70.Lin G. S., Yu Q. X., Xu L. Q., et al. Berberrubine attenuates potassium oxonate- and hypoxanthine-induced hyperuricemia by regulating urate transporters and JAK2/STAT3 signaling pathway. European Journal of Pharmacology . 2021;912 doi: 10.1016/j.ejphar.2021.174592.174592 [DOI] [PubMed] [Google Scholar]
- 71.Xu L. Q., Lin G. S., Yu Q. X., et al. Anti-hyperuricemic and nephroprotective effects of dihydroberberine in potassium oxonate- and hypoxanthine-induced hyperuricemic mice. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.645879.645879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su J. X., Wei Y. H., Liu M. L., et al. Anti-hyperuricemic and nephroprotective effects of Rhizoma Dioscoreae septemlobae extracts and its main component dioscin via regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Archives of Pharmacal Research . 2014;37(10):1336–1344. doi: 10.1007/s12272-014-0413-6. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y., Jin L. J., Liu J., et al. Effect and mechanism of dioscin from dioscorea spongiosa on uric acid excretion in animal model of hyperuricemia. Journal of Ethnopharmacology . 2018;214:29–36. doi: 10.1016/j.jep.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Zhao X., Wang J., Tang L. Y., Li P., Ru J., Bai Y. Z. Withaferin A protects against hyperuricemia induced kidney injury and its possible mechanisms. Bioengineered . 2021;12(1):589–600. doi: 10.1080/21655979.2021.1882761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dai H. N., Lv S., Fu X. Q., Li W. N. Identification of scopoletin and chlorogenic acid as potential active components in sunflower calathide enzymatically hydrolyzed extract towards hyperuricemia. Applied Sciences . 2021;11(21) doi: 10.3390/app112110306.10306 [DOI] [Google Scholar]
- 76.Liang D. L., Yong T. Q., Chen S. D., et al. Hypouricemic effect of 2, 5-dihydroxyacetophenone, a computational screened bioactive compound from ganoderma applanatum, on hyperuricemic mice. International Journal of Molecular Sciences . 2018;19(5):p. 1394. doi: 10.3390/ijms19051394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Y. S., Chen C. J., Yan W., Ge H. M., Kong L. D. Anti-hyperuricemic and anti-inflammatory actions of vaticaffinol isolated from dipterocarpus alatus in hyperuricemic mice. Chinese Journal of Natural Medicines . 2017;15(5):330–340. doi: 10.1016/s1875-5364(17)30053-5. [DOI] [PubMed] [Google Scholar]
- 78.Sisa M., Bonnet S. L., Ferreira D., Van der Westhuizen J. H. Photochemistry of flavonoids. Molecules . 2010;15(8):5196–5245. doi: 10.3390/molecules15085196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kopustinskiene D. M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as anticancer agents. Nutrients . 2020;12(2):p. 457. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berim A., Gang D. R. Methoxylated flavones: occurrence, importance, biosynthesis. Phytochemistry Reviews . 2016;15(3):363–390. doi: 10.1007/s11101-015-9426-0. [DOI] [Google Scholar]
- 81.Mani R., Natesan V. Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry . 2018;145:187–196. doi: 10.1016/j.phytochem.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 82.Lefort E. C., Blay J. Apigenin and its impact on gastrointestinal cancers. Molecular Nutrition & Food Research . 2013;57(1):126–144. doi: 10.1002/mnfr.201200424. [DOI] [PubMed] [Google Scholar]
- 83.Cao J., Chen W., Zhang Y., Zhang Y. Q., Zhao X. J. Content of selected flavonoids in 100 edible vegetables and fruits. Food Science and Technology Research . 2010;16(5):395–402. doi: 10.3136/fstr.16.395. [DOI] [Google Scholar]
- 84.Pollastri S., Tattini M. Flavonols: old compounds for old roles. Annals of Botany . 2011;108(7):1225–1233. doi: 10.1093/aob/mcr234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mounnissamy V. M., Gopal V., Gunasegaran R., Saraswathy A. Antiinflammatory activity of gossypetin isolated from Hibiscus sabdariffa. Indian Journal of Heterocyclic Chemistry . 2002;12(1):85–86. [Google Scholar]
- 86.Alvarado-Sansininea J. J., Sánchez-Sánchez L., López-Muñoz H., et al. Quercetagetin and patuletin: antiproliferative, necrotic and apoptotic activity in tumor cell lines. Molecules . 2018;23(10):p. 2579. doi: 10.3390/molecules23102579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jan A. T., Kamli M. R., Murtaza I., Singh J. B., Ali A., Haq Q. M. R. Dietary flavonoid quercetin and associated health benefits an overview. Food Reviews International . 2010;26(3):302–317. doi: 10.1080/87559129.2010.484285. [DOI] [Google Scholar]
- 88.Damin F., Meinhart A., Caldeirao L., et al. Determination of rutin in fruits and vegetables in natura. Journal of Food and Nutrition Research . 2019;58(4):328–338. [Google Scholar]
- 89.Antika L. D., Dewi R. M. Pharmacological aspects of fisetin. Asian Pacific Journal of Tropical Biomedicine . 2021;11(1):1–9. doi: 10.4103/2221-1691.300726. [DOI] [Google Scholar]
- 90.Park C., Lee W. S., Go S. I., et al. Morin, a flavonoid from moraceae, induces apoptosis by induction of BAD protein in human leukemic cells. International Journal of Molecular Sciences . 2014;16(1):645–659. doi: 10.3390/ijms16010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martin M. Á., Ramos S. Impact of cocoa flavanols on human health. Food and Chemical Toxicology . 2021;151 doi: 10.1016/j.fct.2021.112121.112121 [DOI] [PubMed] [Google Scholar]
- 92.Hatasa Y., Chikazawa M., Furuhashi M., et al. Oxidative deamination of serum albumins by (-)-epigallocatechin-3-O-gallate: a potential mechanism for the formation of innate antigens by antioxidants. PLoS One . 2016;11 doi: 10.1371/journal.pone.0153002.e0153002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang L., Wang C. X., Wu J., et al. Metabolic profiling of mice plasma, bile, urine and feces after oral administration of two licorice flavonones. Journal of Ethnopharmacology . 2020;257 doi: 10.1016/j.jep.2020.112892.112892 [DOI] [PubMed] [Google Scholar]
- 94.Pingili R., Vemulapalli S., Mullapudi S. S., Nuthakki S., Pendyala S., Kilaru N. Pharmacokinetic interaction study between flavanones (hesperetin, naringenin) and rasagiline mesylate in wistar rats. Drug Development and Industrial Pharmacy . 2016;42(7):1110–1117. doi: 10.3109/03639045.2015.1115868. [DOI] [PubMed] [Google Scholar]
- 95.Khan A., Ikram M., Hahm J. R., Kim M. O. Antioxidant and anti-inflammatory effects of citrusflavonoid hesperetin: special focus on neurological disorders. Antioxidants . 2020;9(7):p. 609. doi: 10.3390/antiox9070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xing H., Yang J., Ren K. D., et al. Investigation on the metabolic characteristics of isobavachin inPsoralea corylifoliaL. (Bu-gu-zhi) and its potential inhibition against human cytochrome P450s and UDP-glucuronosyltransferases. Journal of Pharmacy and Pharmacology . 2020;72(12):1865–1878. doi: 10.1111/jphp.13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karimov A. M., Botirov E. K. Structural diversity and state of knowledge of flavonoids of the scutellaria L. genus. Russian Journal of Bioorganic Chemistry . 2017;43(7):691–711. doi: 10.1134/s1068162017070068. [DOI] [Google Scholar]
- 98.Chen X., Ge H. Z., Lei S. S., et al. Dendrobium officinalis six nostrum ameliorates urate under-excretion and protects renal dysfunction in lipid emulsion-induced hyperuricemic rats. Biomedicine & Pharmacotherapy . 2020;132 doi: 10.1016/j.biopha.2020.110765.110765 [DOI] [PubMed] [Google Scholar]
- 99.Krizova L., Dadakova K., Kasparovska J., Kasparovsky T. Isoflavones. Molecules . 2019;24:p. 1076. doi: 10.3390/molecules24061076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sureda A., Sanches Silva A., Sanchez-Machado D. I., et al. Hypotensive effects of genistein: from chemistry to medicine. Chemico-Biological Interactions . 2017;268:37–46. doi: 10.1016/j.cbi.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 101.Elsaman T., Mohamed M. S., Eltayib E. M., Abdalla A. E., Mohamed M. A. Xanthone: a promising antimycobacterial scaffold. Medicinal Chemistry . 2021;17(4):310–331. doi: 10.2174/1573406416666200619114124. [DOI] [PubMed] [Google Scholar]
- 102.Selles A. J. N., Daglia M., Rastrelli L. The potential role of mangiferin in cancer treatment through its immunomodulatory, anti-angiogenic, apoptopic, and gene regulatory effects. BioFactors . 2016;42(5):475–491. doi: 10.1002/biof.1299. [DOI] [PubMed] [Google Scholar]
- 103.Proshkina E., Plyusnin S., Babak T., et al. Terpenoids as potential geroprotectors. Antioxidants . 2020;9(6):p. 529. doi: 10.3390/antiox9060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen L. G., Jiang Y. Y., Yu Z. Determining concentrations of loganin in plasma of rat by UPLC-MS/MS method: applications for a pharmacokinetic study. Latin American Journal of Pharmacy . 36(12):2374–2378. [Google Scholar]
- 105.Gao H. M., Chen J., Yu P., et al. Pharmacokinetic comparisons of five different combinations of Zhi-zi-chi Decoction among rats: competing mechanisms between geniposide and genistein. Chinese Journal of Analytical Chemistry . 2021;49(12):19–25. doi: 10.1016/j.cjac.2021.07.002. [DOI] [Google Scholar]
- 106.Wu H., Zhou M. Z., Lu G., Yang Z. L., Ji H., Hu Q. H. Emodinol ameliorates urate nephropathy by regulating renal organic ion transporters and inhibiting immune inflammatory responses in rats. Biomedicine & Pharmacotherapy . 2017;96:727–735. doi: 10.1016/j.biopha.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 107.Loncar M., Jakovljevic M., Subaric D., et al. Coumarins in food and methods of their determination. Foods . 2020;9(5):p. 645. doi: 10.3390/foods9050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jafernik K., Halina E., Ercisli S., Szopa A. Characteristics of bakuchiol-the compound with high biological activity and the main source of its acquisition-cullen corylifolium (L.) Medik. Natural Product Research . 2021;35(24):5828–5842. doi: 10.1080/14786419.2020.1837813. [DOI] [PubMed] [Google Scholar]
- 109.Zschocke S., Liu J. H., Stuppner H., Bauer R. Comparative study of roots of Angelica sinensis and related umbelliferous drugs by thin layer chromatography, high-performance liquid chromatography, and liquid chromatography mass spectrometry. Phytochemical Analysis . 1998;9(6):283–290. [Google Scholar]
- 110.Zhang M. M., Wang J. L., Zhu L., et al. Zanthoxylum bungeanum maxim. (Rutaceae): a systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. International Journal of Molecular Sciences . 2017;18(10):p. 2172. doi: 10.3390/ijms18102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kostova I., Iossifova T. Chemical components of fraxinus species. Fitoterapia . 2007;78(2):85–106. doi: 10.1016/j.fitote.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Sun M. N., Sun M. J., Zhang J. Y. Osthole: an overview of its sources, biological activities, and modification development. Medicinal Chemistry Research . 2021;30(10):1767–1794. doi: 10.1007/s00044-021-02775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen T., Wang X. N., Lou H. X. Natural stilbenes: an overview. Natural Product Reports . 2009;26(7):916–935. doi: 10.1039/b905960a. [DOI] [PubMed] [Google Scholar]
- 114.Arora D., Jaglan S. Therapeutic applications of resveratrol nanoformulations. Environmental Chemistry Letters . 2018;16(1):35–41. doi: 10.1007/s10311-017-0660-0. [DOI] [Google Scholar]
- 115.Komaikul J., Kitisripanya T., Tanaka H., Sritularak B., Putalun W. Enhanced mulberroside a production from cell suspension and root cultures of morus alba using elicitation. Natural Product Communications . 2015;10(7) doi: 10.1177/1934578x1501000730. [DOI] [PubMed] [Google Scholar]
- 116.Zhou L. F., Li S. H., Zhang T., Mu W. M., Jiang B. Properties of a novel polydatin-beta-D-glucosidase from aspergillus niger SK34.002 and its application in enzymatic preparation of resveratrol. Journal of the Science of Food and Agriculture . 2016;96(7):2588–2595. doi: 10.1002/jsfa.7465. [DOI] [PubMed] [Google Scholar]
- 117.Koleva I. I., van Beek T. A., Soffers A. E. M. F., Dusemund B., Rietjens I. M. C. M. Alkaloids in the human food chain—natural occurrence and possible adverse effects. Molecular Nutrition & Food Research . 2012;56(1):30–52. doi: 10.1002/mnfr.201100165. [DOI] [PubMed] [Google Scholar]
- 118.Cholewa J. M., Guimaraes-Ferreira L., Zanchi N. E. Effects of betaine on performance and body composition: a review of recent findings and potential mechanisms. Amino Acids . 2014;46(8):1785–1793. doi: 10.1007/s00726-014-1748-5. [DOI] [PubMed] [Google Scholar]
- 119.Wu M., Wang J., Liu L. T. Advance of studies on anti-atherosclerosis mechanism of berberine. Chinese Journal of Integrative Medicine . 2010;16(2):188–192. doi: 10.1007/s11655-010-0188-7. [DOI] [PubMed] [Google Scholar]
- 120.Sun Y., Lenon G. B., Yang A. W. H. Phellodendri cortex: a phytochemical, pharmacological, and pharmacokinetic review. Evidence-Based Complementary and Alternative Medicine . 2019;2019:45. doi: 10.1155/2019/7621929.7621929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mostafa Y. A., Taylor S. D. Steroid derivatives as inhibitors of steroid sulfatase. Journal of Steroid Biochemistry and Molecular Biology . 2013;137:183–198. doi: 10.1016/j.jsbmb.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 122.Zeng H. Q., Yang L. J., Zhang X. B., Chen Y., Cai J. H. Dioscin prevents LPS-induced acute lung injury through inhibiting the TLR4/MyD88 signaling pathway via upregulation of HSP70. Molecular Medicine Reports . 2018;17(5):6752–6758. doi: 10.3892/mmr.2018.8667. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y. C., Li X., Sun H., et al. Biotransformation of steroidal saponins in sisal (agave sisalana perrine) to tigogenin by a newly isolated strain from a karst area of guilin, China. Biotechnology & Biotechnological Equipment . 2014;28(6):1024–1033. doi: 10.1080/13102818.2014.978199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park J. W., Min K. J., Kim D. E., Kwon T. K. Withaferin a induces apoptosis through the generation of thiol oxidation in human head and neck cancer cells. International Journal of Molecular Medicine . 2015;35(1):247–252. doi: 10.3892/ijmm.2014.1983. [DOI] [PubMed] [Google Scholar]
- 125.Maalik A., Bukhari S. M., Zaidi A., Shah K. H., Khan F. A. Chlorogenic acid: a pharmacologically potent molecule. Acta Poloniae Pharmaceutica . 2016;73(4):851–854. [PubMed] [Google Scholar]
- 126.Li X. Q., Chen Y. H., Gao X. X., et al. Antihyperuricemic effect of green alga ulva lactuca ulvan through regulating urate transporters. Journal of Agricultural and Food Chemistry . 2021;69(38):11225–11235. doi: 10.1021/acs.jafc.1c03607. [DOI] [PubMed] [Google Scholar]
- 127.Zhang H., Zhai J., Zhang L. P., et al. In Vitro inhibition of glyoxalase I by flavonoids: new insights from crystallographic analysis. Current Topics in Medicinal Chemistry . 2015;16(4):460–466. doi: 10.2174/1568026615666150813150944. [DOI] [PubMed] [Google Scholar]
- 128.Duan Z. K., Zhang Z. J., Dong S. H., Wang Y. X., Song S. J., Huang X. X. Quassinoids: phytochemistry and antitumor prospect. Phytochemistry . 2021;187 doi: 10.1016/j.phytochem.2021.112769.112769 [DOI] [PubMed] [Google Scholar]
- 129.Wempe M. F., Quade B., Jutabha P., et al. Human uric acid transporter 1 (hurat1): an inhibitor structure-activity relationship (sar) study. Nucleosides, Nucleotides & Nucleic Acids . 2011;30(12):1312–1323. doi: 10.1080/15257770.2011.594031. [DOI] [PubMed] [Google Scholar]
- 130.Veiko A. G., Lapshina E. A., Zavodnik I. B. Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin. Molecular and Cellular Biochemistry . 2021;476(12):4287–4299. doi: 10.1007/s11010-021-04243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li X. J., Shang X. F., Liu L. L., Xi N. K., Zhang J. L., Xu X. F. Anion recognition based on phenolic hydroxyl group in competitive media. Journal of Inclusion Phenomena and Macrocyclic Chemistry . 2012;73(1-4):185–192. doi: 10.1007/s10847-011-0041-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing are not applicable to this article, as no new data were created or analyzed in this study.


