Abstract
Objective:
Diffuse intrinsic pontine glioma (DIPG) is the most common and deadliest brainstem tumor in children. Focused ultrasound combined with microbubble-mediated BBB opening (FUS-BBBO) is a promising technique for overcoming the frequently intact blood-brain barrier (BBB) in DIPG to enhance therapeutic drug delivery to the brainstem. Since DIPG is highly diffusive, large-volume FUS-BBBO is needed to cover the entire tumor region. The objective of this study was to determine the optimal treatment strategy to achieve efficient and homogeneous large-volume BBBO at the brainstem for the delivery of an immune checkpoint inhibitor, anti-PD-L1 antibody (aPD-L1).
Methods:
Two critical parameters for large-volume FUS-BBBO, multi-point sonication pattern (interleaved vs. serial) and microbubble injection method (bolus vs. infusion), were evaluated by treating mice with four combinations of these two parameters. 2D Passive cavitation imaging (PCI) was performed for monitoring the large-volume sonication.
Results:
Interleaved sonication combined with bolus injection of microbubbles resulted in 1.29 to 2.06 folds higher efficiency than other strategies as evaluated by Evans blue extravasation. The average coefficient of variation of the Evans blue delivery was 0.66 for interleaved sonication with bolus injection, compared to 0.68–0.88 for all other strategies. Similar trend was also observed in the quantified total cavitation dose and coefficient of variance of the cavitation dose. This strategy was then applied to deliver fluorescently labeled aPD-L1 quantified using fluorescence imaging. A strong segmented linear correlation (R2=0.81) was found between the total cavitation dose and the total fluorescence intensity of aPD-L1 delivered at different sonication pressures (0.15 MPa, 0.30 MPa, and 0.45 MPa).
Significance:
Findings from this study suggest that efficient and homogeneous large-volume FUS-BBBO can be achieved by interleaved sonication combined with bolus injection of microbubbles, and the efficiency and homogeneity can be monitored by PCI.
Keywords: Focused ultrasound, Brainstem drug delivery, Blood-brain barrier, Passive cavitation imaging, Immune checkpoint inhibitor
I. Introduction
Diffuse intrinsic pontine glioma (DIPG) is a universally fatal brainstem tumor [1]. It is the leading cause of brain tumor-related death in children with no effective treatment options. Hundreds of chemotherapy trials have been conducted over the past several decades but have shown no survival benefit in this disease [2]. In recent decades, immune checkpoint inhibitor (ICI) therapy has revolutionized the paradigm of cancer treatment. The United States Food and Drug Administration (FDA) has approved ICIs that target programmed cell death-ligand 1 (PD-L1), programmed cell death-1 (PD-1), and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) for the treatment of a wide variety of cancers [3]. Several clinical trials have tested ICI therapy for the treatment of DIPG (e.g., NCT01952769 and NCT02359565) [4]. However, ICI therapy has demonstrated limited efficacy in patients with brain cancer [5]. Although the underlying mechanisms for the failure remain unclear, the blood-brain barrier (BBB) is considered to be one of the obstacles for ICI therapy of gliomas [6]. The BBB, which is responsible for maintaining a constant brain microenvironment, restricts the entry of ICIs into the brain and limits the efficacy of ICI therapy. The BBB is often intact in DIPG, making the treatment even more challenging than other brain tumors with relatively leaky BBB. The intrinsic diffusive nature leads to the proliferation and migration of DIPG tumor cells without affecting the BBB structures until the later stage of the disease [7].
Focused ultrasound combined with microbubble-mediated BBB opening (FUS-BBBO) can achieve noninvasive and localized brain drug delivery with clinical trials currently ongoing. FUS can noninvasively penetrate through the intact scalp and skull and focus the ultrasound energy deep into the brain within a focal region with a millimeter dimension. Microbubbles are often bolus injected or infused into the systemic blood circulation. When they reach the FUS targeted brain region, microbubbles undergo expansion and contraction (i.e., cavitation) inside the cerebral blood vessels, which leads to transient and reversible BBB opening [8][9]. Over the past decades, extensive research has proved the feasibility, efficacy, and safety of FUS-BBBO in animal models [10]-[19] and clinical trials [20]-[24].
Existing preclinical studies have demonstrated that FUS-BBBO is a promising technique for drug delivery to the brainstem. Ye et al. delivered radiolabeled nanoclusters to the brainstem in both wide-type mice [18] and genetically engineered DIPG mice [25] using FUS-BBBO. They confirmed successful trans-BBB delivery of the nanoclusters to the brainstem using positron emission tomography. Alli et al. demonstrated the feasibility and safety of FUS-BBBO in delivering doxorubicin to the brainstem of wild-type mice [26]. Recently, Ishida et al. showed that FUS-BBBO enhanced the antitumor effects of doxorubicin against DIPG in a xerograph mouse model [27]. Englander et al. showed that it was safe and feasible to perform repeated FUS-BBBO in a preclinical pontine glioma model [28]. A clinical trial is currently recruiting patients with diffuse midline glioma to evaluate the feasibility and safety of FUS-BBBO in assisting the delivery of panobinostat (NCT04804709).
The highly diffusive nature of DIPG requires large-volume drug delivery at the brainstem. The reported size of DIPG tumor ranges from 5 cm3 [29] to 40 cm3 [30] in patients. Efficient and homogeneous large-volume FUS-BBBO is therefore needed for effective drug delivery to DIPG tumor. Many studies have shown the feasibility and safety of large-volume FUS-BBBO using multiple point sonication in small animals [31]-[33], large animals [34]-[36], nonhuman primates [13], and recently in patients [20]-[22], [24]. Multiple point sonication was also used by Ishida et al. and Englander et al. in their DIPG drug delivery studies [27][28]. These studies achieved large-volume BBBO by sonicating multiple points within a defined grid using an interleaved or serial pattern combined with one-time bolus injection, multiple bolus injections or continuous infusion of microbubbles. Interleaved pattern refers to iterative FUS sonication through all points by repeating multiple rounds, and serial pattern refers to point-by-point FUS sonication without iterations. Although enhanced drug delivery was achieved using different combinations of sonication pattern and microbubble injection method, no study has been reported on comparing different large-volume sonication strategies to determine the optimal strategy. O’Reilly et al. reported the impact of bolus injection and infusion on single-point FUS-BBBO [37]. There is a need to compare delivery efficiency and spatial homogeneity using different sonication patterns and microbubble injection methods to determine the optimal strategy for large-volume BBBO.
Passive cavitation monitoring is often performed for real-time monitoring of cavitation activity during FUS-BBBO treatment. Several techniques have been developed for cavitation monitoring, including passive cavitation detection (PCD) using a single-element sensor [38]-[42], 2D passive cavitation imaging (PCI) using an ultrasound imaging array [43]-[45], and 3D passive cavitation imaging using customized phased arrays [33][46]. 3D PCI was proposed by Jones et al. to monitor the microbubble assisted, FUS-induced non-thermal brain ablation at multiple points [46]; however, it required a customized, expensive, and complicated hemispherical phased array. Recently, reconstruction of a cavitation energy map for large-volume FUS-BBBO has been proposed using PCD at multiple sonication points [24]. The cavitation dose was quantified based on each acquired PCD signal and assigned to the targeted brain location to form a 2D acoustic energy map. Although cavitation dose obtained from either PCD or PCI has been used to monitor and control FUS-BBBO, no study has evaluated the feasibility to use PCI to predict the efficiency and homogeneity of multi-point sonication for large-volume FUS-BBBO.
The objective of this study was to compare different strategies for large-volume FUS-BBBO and determine the optimal strategy for achieving efficient, homogeneous, and safe drug delivery to the whole brainstem. This objective was achieved in two steps. The first step determined the optimal strategy for large-volume FUS-BBBO by comparing different combinations of sonication patterns (interleaved vs. serial) and microbubble injection methods (bolus vs. infusion) using Evans blue as a model agent. In the second step, the selected optimal strategy was applied to deliver anti-PD-L1 antibody (aPD-L1) under different sonication pressures. PCI was performed to measure the target-wise cavitation dose for monitoring the efficiency and homogeneity of large-volume FUS-BBBO. The safety of FUS-BBBO was evaluated by monitoring heart rate and respiration rate, which are two critical functionalities regulated by the brainstem. The safety was also assessed by ex vivo histological analysis of the FUS-sonicated brainstem.
II. Materials and methods
A. Animal study design
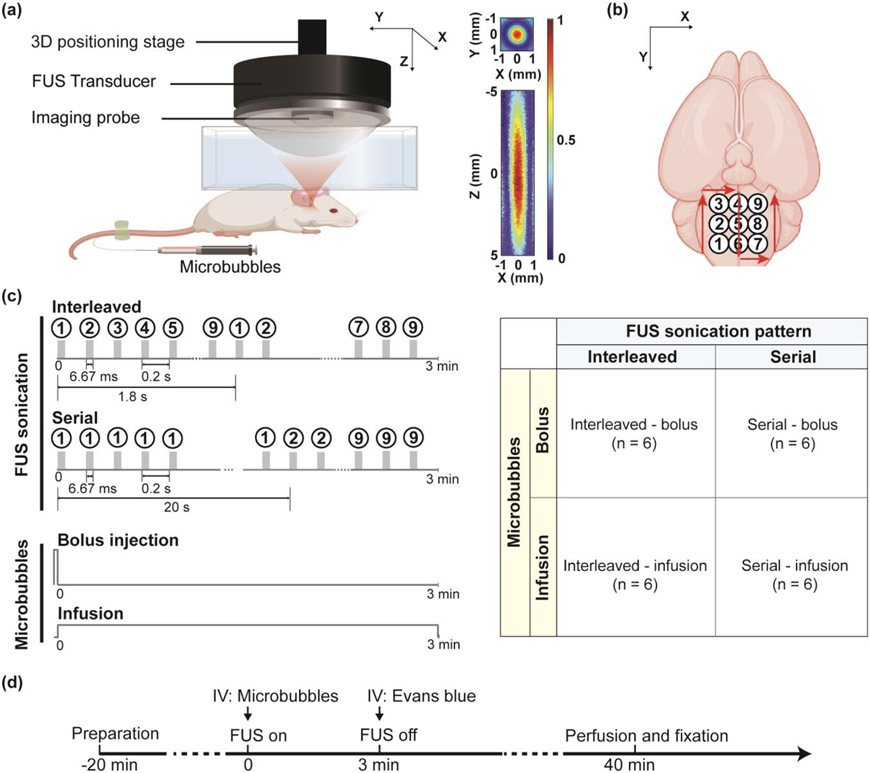
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Washington University in St. Louis in accordance with the National Institutes of Health Guidelines for animal research. A total of 39 adult mice (IACUC protocol number: 21-0187, Swiss, 6–8 week old, female, Charles River Laboratory, Wilmington, MA, USA) were used. In the first study, 24 mice were divided into four groups to compare different strategies for Evans blue delivery by FUS-BBBO at the brainstem (Fig. 1a, b). The four strategies were described as follows (Fig. 1c): interleaved sonication combined with bolus injection of microbubbles (interleaved-bolus, n=6), interleaved sonication combined with the infusion of microbubbles (interleaved-infusion, n=6), serial sonication combined with bolus injection of microbubbles (serial-bolus, n=6), and serial sonication combined with the infusion of microbubbles (serial-infusion, n=6). The acoustic pressure was kept the same at 0.45 MPa among all four groups. The strategy that achieved the most efficient and homogeneous delivery of Evans blue at the brainstem was selected. In the second study, 15 mice were divided into three groups to evaluate the delivery outcome of fluorescently labeled aPD-L1 using the selected optimal strategy at different sonication pressures: 0.15 MPa (n = 5), 0.30 MPa (n = 5), and 0.45 MPa (n = 5). One mouse in the 0.30 MPa group was eliminated due to unsuccessful perfusion.
Fig. 1.
Experimental methods for comparing different strategies for large-volume FUS-BBBO. (a) FUS treatment setup. 2D pressure maps of the FUS beam in the transverse plane (XY) and axial plane (XZ) are presented to show the beam dimension. (b) FUS sonication was performed using a 3×3 grid with a spacing of 1 mm to cover the mouse brainstem. (c) Multi-point FUS sonication was performed using strategies that combined different FUS sonication patterns (interleaved vs. serial) with different microbubble injection methods (bolus vs. infusion). In interleaved sonication, each point received one pulse (10,000 cycles, 6.67 ms) per iteration of 1.8 s, leading to a total of 100 pulses during 3-min sonication. In serial sonication, each point received 100 pulses within 20 s. (d) Mouse experimental timeline. After the mouse was prepared ready, microbubbles were bolus injected or infused followed by FUS sonication using different sonication patterns. Immediately after FUS sonication, a model drug, Evans blue was injected intravenously (IV). At 40 min after FUS sonication, the animal was sacrificed for quantification of the drug delivery outcome.
B. Near-infrared dye-labeled aPD-L1
IRDye 800CW with NHS ester (LI-COR Biotechnology, Lincoln, NE, USA) was conjugated to aPD-L1 (Bio X Cell, West Lebanon, NH, USA) following the manufacturer’s instruction with details provided in our previous publication [5][47]. The final concentration of the labeled aPD-L1 (800CW-aPD-L1) was 2.14 mg/ml measured by a spectrophotometer (NanoDrop, Thermo Scientific, Waltham, MA, USA). The fluorescence intensity of 800CW-aPD-L1 was calibrated by serial dilution in PBS and imaged by the Pearl Trilogy Image System (LI-COR Biotechnology). The fluorescence of 800CW-aPD-L1 was verified to be linearly correlated with the aPD-L1 concentration, which was consistent with our previous finding in the labeling of albumin using the same method [47].
C. FUS treatment
As illustrated in Fig. 1a, FUS sonication was performed using a preclinical ultrasound image-guided FUS system (VIFU 2000, Alpinion US Inc. Bothell, WA, USA). The FUS transducer was a single-element spherical-focused transducer with a center frequency of 1.5 MHz, a radius of curvature of 60 mm, an aperture of 60 mm, and a center opening with a diameter of 38 mm. The transducer was driven by a function generator (33500B Series, Keysight, Santa Rosa, CA, USA) followed by a 53 dB power amplifier (1020L, Electronics & Innovation Ltd., Rochester, NY, USA). Acoustic pressure fields generated by the FUS transducer were calibrated in a water tank filled with degassed water using a needle hydrophone (Precision Acoustic, Dorchester, Dorset, UK). Reported pressure levels were the derated values with 18% mouse skull insertion loss [11]. The full width at half maximum (FWHM) of the FUS transducer was 0.9 mm × 8.2 mm in the lateral direction (X) and axial direction (Z), respectively (Fig. 1a). A 128-element linear array (L8-17, Alpinion US Inc. Bothell, WA, USA) was co-aligned at the center opening of the FUS transducer to provide B-mode image guidance as well as passive cavitation imaging for sonication monitoring. The FUS focus was aligned with lambda on the skull under the guidance of B-mode imaging with the assistance of a metal grid [18].
Mice were anesthetized and placed in the prone position on a heating pad with their heads immobilized by a stereotaxic frame. The fur on the mouse head was removed while the scalp and the skull remaining intact. A water container filled with degassed water was placed on the mouse head and coupled with degassed ultrasound gel. A 3D positioning system (Xslide, Velmex Inc., Bloomfield, NY, USA) was programmed to move the FUS transducer within a 3×3 grid with 1 mm spacing to cover the whole brainstem (Fig. 1b). The center of the treatment grid was selected to be 0 mm lateral, 1 mm posterior, and 4 mm ventral from the lambda according to the mouse brain atlas. A catheter was inserted at the tail vein for intravenous injection of microbubbles, Evans blue, and 800CW-aPD-L1.
Microbubbles (Definity, Lantheus Medical Imaging, N. Billerica, MA, USA) were activated according to manufacturer instruction prior to the experiment. The concentration of the activated microbubbles was measured by a cell counter (Countless 3, Thermo Fisher Scientific, Waltham, MA, USA). The microbubbles were diluted with saline. The dilution fold ranged from 2.00 to 3.13 in order to reach a final concentration of 8×108 number of microbubbles/mL with a total volume of 30 μL [17][18]. The diluted microbubbles were injected through either bolus injection or infusion using a syringe pump (NE-1600, New Era Pump Systems Inc., Farmingdale, NY, USA). The flow rate for bolus injection was 240 μ L/min so that all microbubbles were injected within 7~8 seconds prior to the onset of FUS sonication. Infusion at a flow rate of 10 μL/min was started at the onset of FUS sonication and continued throughout the treatment. FUS sonication was performed with a peak negative pressure of 0.45 MPa, cycle number of 10,000, pulse length of 6.67 ms, pulse repetition frequency (PRF) of 5 Hz, and total treatment of 3 minutes [17][18]. PRF of 5 Hz has been used in previous studies to allow sufficient time for microbubbles to be replenished between pulses by heart pumping [48]. Interleaved sonication (1 pulse per point, 100 iterations) was compared with serial sonication (100 pulses per point, 1 iteration), where each point received the same total amount of pulses. The optimal strategy selected from the first sub-study was applied in the second sub-study to deliver 800CW-aPD-L1. Three pressure levels (0.15 MPa, 0.30 MPa, and 0.45 MPa) were compared to find the lowest pressure for efficient and safe 800CW-aPD-L1 delivery.
D. Ex-vivo fluorescence imaging and quantification
Immediately after the FUS treatment, 4% Evans blue or 800CW-aPD-L1 (10 mg/kg, [49]) was delivered intravenously and quantified via ex vivo fluorescence imaging (Fig. 1d). All mice were sacrificed 40 mins after FUS sonication by transcardial perfusion. Brains were harvested and fixed in 4% paraformaldehyde for 24 hours. The brains were then transversely cut into 1 mm slices using a brain matrix (RBM-4000C, ASI Instruments Inc., MI, USA) and imaged using Pearl Trilogy Image System. The delivery outcome of both Evans blue and 800CW-aPD-L1 was quantified using the system built-in software (Image Studio Lite, LI-COR, NE, USA). The last three brain slices containing the brainstem were used for quantification. The delivery efficiency to the brainstem was quantified by the sum of the fluorescence intensity within the last three slices, then normalized by the sum of the background fluorescence of the nontreated cerebrum.
E. Homogeneity analysis
Spatial homogeneity of large-volume FUS-BBBO was quantified based on Evans blue distribution within the brainstem. Coefficient of variation, the standard deviation normalized by the mean of fluorescence pixel intensity in the brainstem, was computed to characterize the spatial homogeneity of the delivery [50]. A lower coefficient of variation of pixel intensity indicates higher spatial homogeneity. The coefficient of variation within the brainstem was computed at each of the last three brain slices and averaged among them to obtain the coefficient of variation for each mouse.
F. PCI monitoring
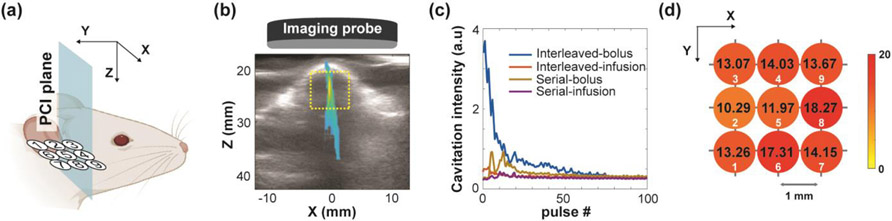
During FUS sonication, 2D PCI was performed to monitor the large-volume FUS-BBBO (Fig. 2a). The ultrasound imaging probe with an aperture of 25.4 mm (L8-17) was inserted in the center opening of the FUS transducer. It was programmed to passively receive signals without transmission. The imaging data acquisition at a frame rate of 40 fps was started when the FUS sonication was on and throughout the whole treatment. Eight acquisitions per pulse were evenly distributed within the pulse length of 6.67 ms, resulting in a total capture length of 0.31 ms and a 4.65% sampling of the acoustic emissions. The acquired PCI images at each sonicated point were processed offline in MATLAB 2019a (Mathworks Inc., Natick, MA, USA) using the frequency-domain algorithm in XZ plane (Fig. 2b) [51]. The frequency band for stable cavitation computation was from 8.86 MHz to 9.13 MHz, centered at 9 MHz which was the 6th harmonic of FUS center frequency and within the 8–17 MHz bandwidth of imaging probe. A region of interest (ROI) with dimension of 6 mm × 6 mm was selected to cover the mouse brain (Fig. 2b). The cavitation intensity within the ROI was summed for each timestamp and integrated over the sonication time to obtain the cavitation dose (Fig. 2c). Target-wise cavitation measurement for the whole treatment grid was performed by assigning the calculated dose value to each corresponding sonicated point (Fig. 2d). Total cavitation dose was computed by the summation of the cavitation dose for the nine points. Coefficient of variation among the doses of the nine points was computed to quantify the spatial homogeneity of the target-wise cavitation dose values. Broadband emissions were captured at inharmonic frequencies (0.3-MHz window between adjacent harmonics and ultra-harmonics [44]) for inertial cavitation dose calculation.
Fig. 2.
Target-wise cavitation dose measured from PCI. (a) Illustration of PCI acquisition plane (XZ) perpendicular to the treatment grid. (b) The acquired PCI in the XZ plane during FUS sonication of one point within the 3×3 grid overlaid on the B-mode image of the mouse head. An ROI was selected to cover the mouse brain. (c) Representative cavitation intensity summation with arbitrary unit (a.u.) within the ROI in time domain for each treatment strategy. The cavitation dose at each sonication location was calculated by the time integral of the cavitation intensity. (d) Target-wise cavitation dose values for the 9 points on the 3×3 grid. Each circle represents a sonication location. The calculated cavitation dose for each location is labeled on the circle. The color of the circle represents the amplitude of the cavitation dose.
G. Physiological monitoring
Heart rate and respiration rate monitoring started once the mice went under anesthesia so that the rates became steady during the preparation before FUS treatment. The PowerLab data acquisition system (PL3508, Model ML138, ADInstruments, Sydney, Australia) was used to record electrocardiogram (ECG) signals and nasopharynx temperature. Data analysis was performed using LabChart8 software (ADInstruments, Sydney, Australia). Heart rate was measured based on the RR-interval of ECG from the subdermal electrodes. Respiration rate was measured by the temperature changes at the mouse’s nasal cavity via a thermocouple probe [52]. The thermal fluctuation due to breathing was converted to the respiration rate in the software. A 3-minute time window was selected before FUS was on and after FUS was off to match the FUS treatment duration. Averaged heart rate and respiration rate were compared among the time windows before, during, and after FUS to evaluate any changes induced by the treatment.
H. Histological analysis
Histological analysis was performed using hematoxylin and eosin (H&E) staining to evaluate potential tissue damage under different sonication pressures. The brain slices used in ex vivo fluorescence imaging were fixed in 4% paraformaldehyde, dehydrated in 30% sucrose, and then embedded in optimum cutting temperature (OCT) at −20 °C. Slices were transversely sectioned into 10 μm using Leica CM1860 cryostat (Leica Biosystems Inc., Buffalo Grove, IL, USA), stained with H&E, and imaged using BZ-X800 microscope system (Keyence, Osaka, Japan). The red blood cells were detected based on the pixel hue threshold using ImageJ [53]. The hemorrhage density was quantified as the percentage of the area covered by red blood cells over the entire brainstem area (%).
I. Statistical analysis
Statistical analysis was performed using GraphPad Prism (Version 9.0, La Jolla, CA, USA). Differences in efficiency, homogeneity and histology among groups were analyzed using Brown-Forsythe and Welch ANOVA and corrected by Dunnett’s post-hoc test for multiple comparisons. Changes in heart rate and respiration rate under different pressure levels were analyzed using two-way ANOVA corrected by Dunnett’s post-hoc test. A significant difference was determined by a p-value lower than 0.05.
III. Results
A. Efficiency and homogeneity of large-volume FUS-BBBO in the brainstem
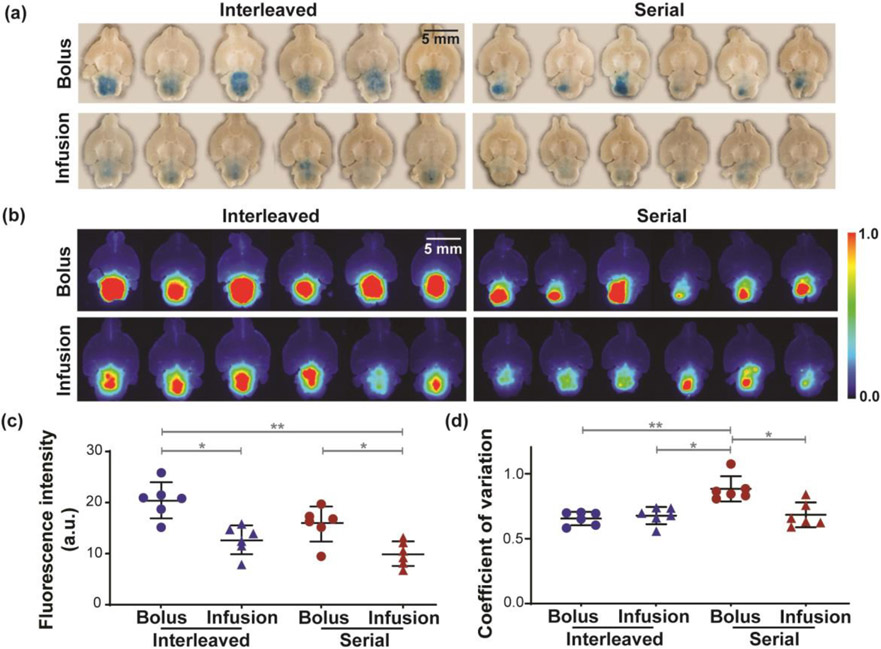
Fig. 3 shows Evans blue delivery outcome by FUS-BBBO at the brainstem using the four strategies. Evans blue was successfully delivered to the brainstem, as shown by the photographs (Fig. 3a) and fluorescence images (Fig. 3b) of ex vivo brain slices. Interleaved sonication combined with bolus injection yielded the highest delivery efficiency (Fig. 3c). The average fluorescence intensity of the interleaved-bolus group was 1.61-folds higher than interleaved-infusion (p < 0.05), 1.29-folds higher than serial-bolus (p > 0.05), and 2.06-folds higher than serial-infusion (p < 0.01). Serial-bolus also showed significantly higher delivery than serial-infusion (1.59-folds, p < 0.05). In terms of spatial homogeneity, no significant difference (p > 0.05, Fig. 3d) was found among the coefficient of variation for interleaved-bolus (0.66 ± 0.05), interleaved-infusion (0.68 ± 0.07) and serial-infusion (0.68 ± 0.10). However, the coefficient of variation for serial-bolus (0.88 ± 0.10) was significantly higher than interleaved-bolus (p < 0.01), interleaved-infusion (p < 0.05) and serial-infusion (p < 0.05).
Fig. 3.
Large-volume FUS-BBBO delivery outcome using different strategies. (a) Photographs of the ex vivo brain slices containing the brainstem from all mice treated by interleaved-bolus (upper left), interleaved-infusion (bottom left), serial-bolus (upper right), and serial-infusion (bottom right). (b) Corresponding fluorescence images of the brain slices shown in (a). Scale bar = 5 mm. (c) Group analysis of the fluorescence intensities for different strategies. (d) Quantification of the spatial homogeneity of Evans blue distribution. A lower coefficient of variation indicates higher homogeneity. (Multiple comparisons with Brown-Forsythe and Welch ANOVA corrected by Dunnett’s post-hoc test: * p < 0.05, ** p < 0.01). FUS parameters: center frequency =1.5 MHz, peak negative pressure = 0.45 MPa, pulse repetition frequency = 5 Hz, cycle number = 10,000, pulse length = 6.67 ms, total sonication duration = 3 minutes.
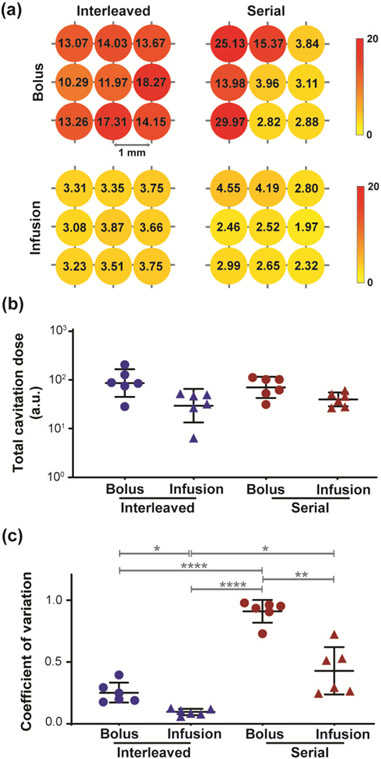
Fig. 4 displays the quantified cavitation monitoring results for large-volume FUS-BBBO under different strategies. Representative target-wise cavitation dose measurement for each strategy is shown in Fig. 4a. Consistent with the fluorescence quantification, interleaved-bolus yielded the highest total cavitation dose (Fig. 4b), which was 2.88-folds higher than interleaved-infusion, 1.23-folds higher than serial-bolus, and 2.14-folds higher than serial-infusion. Serial-bolus also resulted in 1.74-folds higher total cavitation dose than serial-infusion. No statistical difference in total cavitation dose was detected between any groups (p > 0.05).
Fig. 4.
Cavitation monitoring of FUS-BBBO with different strategies. (a) Representative target-wise cavitation dose for each strategy. (b) Total cavitation dose under each strategy. (c) Quantification of spatial homogeneity of the dose under each strategy. A lower coefficient of variation indicates higher homogeneity. (Multiple comparisons with Brown-Forsythe and Welch ANOVA corrected by Dunnett’s post-hoc test: * p < 0.05. ** p < 0.01. **** p < 0.0001)
In terms of spatial homogeneity, serial-bolus yielded the least homogeneous dose values with significantly higher coefficient of variation (0.91 ± 0.09, Fig. 4c) than interleaved-bolus (0.25 ± 0.08, p < 0.0001), interleaved-infusion (0.10 ± 0.03, p < 0.0001) and serial-infusion (0.43 ± 0.19, p < 0.01). Interleaved-infusion also showed significantly lower coefficient of variation than interleaved-bolus (p < 0.05) and serial-infusion (p < 0.05).
B. Efficient and homogeneous 800CW-aPD-L1 delivery using the optimal strategy
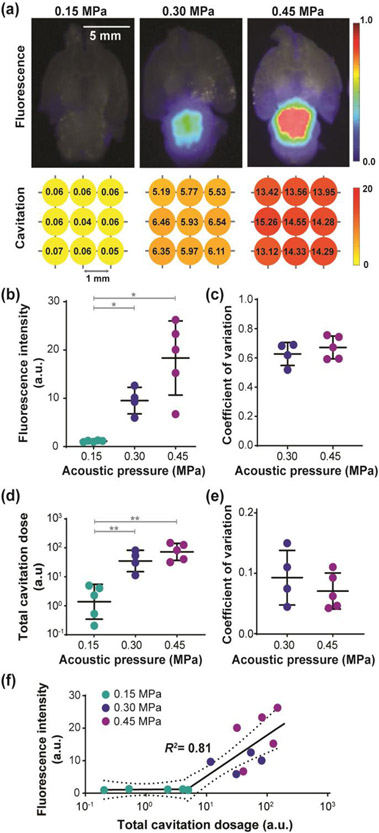
The optimal strategy, interleaved-bolus, was applied for 800CW-aPD-L1 delivery to the whole brainstem. Successful delivery was achieved under both 0.30 MPa and 0.45 MPa, while 0.15 MPa did not result in detectable delivery. Representative fluorescence images of the mouse brain sections are shown in Fig. 5a. Both 0.30 MPa and 0.45 MPa yielded significantly higher delivery efficiency than 0.15 MPa (Fig. 5b), with 0.30 MPa 8.44-folds (p < 0.05) and 0.45 MPa 16.40-folds higher than 0.15 MPa (p < 0.05). The delivery efficiency under 0.45 MPa was also 1.94-folds higher than 0.30 MPa (1.94-fold, p > 0.05). The optimal strategy also achieved the homogenous 800CW-aPD-L1 delivery (Fig. 5c) under 0.30 MPa (coefficient of variation = 0.67 ± 0.09) and 0.45 MPa (coefficient of variation = 0.72 ± 0.08), comparable to that of the Evans blue using the same strategy (0.66 ± 0.05).
Fig. 5.
800CW-aPD-L1 delivery by the selected large-volume FUS-BBBO strategy. (a) Representative fluorescence images of 800CW-aPD-L1 and target-wise cavitation dose values under three pressure levels. Bright-field images of the brain are displayed in the background of the fluorescence images to show the brain structure. Bar = 5 mm. (b) Fluorescence intensity under three pressure levels. (c) Quantification of spatial homogeneity for 800CW-aPD-L1 delivery under 0.30 MPa and 0.45 MPa. A lower coefficient of variation indicates higher homogeneity. (d) Total cavitation dose under three pressure levels. (e) Quantification of spatial homogeneity for the target-wise dose under 0.30 MPa and 0.45 MPa. (f) Segmented linear correlation (R2 = 0.81) between the fluorescence intensity and total cavitation dose. (Multiple comparisons with Brown-Forsythe and Welch ANOVA corrected by Dunnett’s post-hoc test: * p < 0.05; ** p < 0.01)
The representative cavitation dose values for the three representative cases are shown in the lower panel of Fig. 5a. Both 0.30 MPa and 0.45 MPa resulted in significantly higher total cavitation dose than 0.15 MPa (p < 0.01 for both, Fig. 5d). No significant difference was found between 0.30 MPa and 0.45 MPa (p > 0.05). The coefficient of variation of the doses was comparable (p > 0.05, Fig. 5e) between 0.30 MPa (0.10 ± 0.04) and 0.45 MPa (0.07 ± 0.03). A high segmented linear correlation was found between the total cavitation dose and the fluorescence intensity (R2 = 0.81, Fig. 5f).
C. Large-volume FUS-BBBO was safe at lower pressures
The heart rate and respiration rate before FUS treatment for all the mice were 492 ± 54 bpm and 86 ± 15 bpm, respectively. The statistical comparison found no significant changes of either heart rate or respiration rate both during and after FUS treatment (p > 0.05, Fig. 6a), where all the values stayed within the reasonable range for mice under anesthesia [48]. H&E staining to the ex vivo tissue found no hemorrhage in any subjects using 0.15 MPa or 0.30 MPa (Fig. 6b). Microhemorrhage at the bottom of the brainstem was observed in 4 out of 5 mice treated at 0.45 MPa (Fig. 6b). The hemorrhage density for 0.45 MPa was 0.010940 ± 0.006868 %, which was 16.22-folds higher than 0.15 MPa (0.000674 ± 0.000463 %) and 24.61-folds higher than 0.30 MPa (0.000445 ± 0.000591 %), although no statistical differences were detected between each groups (p > 0.05, Fig. 6c). The inertial cavitation dose for 0.45 MPa was found to be significantly higher than both 0.15 MPa (p < 0.05, Fig. 6d) and 0.30 MPa (p < 0.05, Fig. 6d), while there was no significant difference between 0.15 MPa and 0.30 MPa (p > 0.05, Fig. 6d).
Fig. 6.
Large-volume FUS-BBO safety evaluation. (a) Heart rate and respiration rate monitoring before, during, and after FUS sonication for all three pressure levels. Measurements from the same subject are connected by gray lines. No significant changes were detected throughout FUS treatment and among different groups (Multiple comparisons with two-way ANOVA corrected by Dunnett’s post-hoc test: p > 0.05). (b) Representative H&E-stained whole brainstem of mice treated at 0.15 MPa, 0.30 MPa, and 0.45 MPa, respectively. The displayed slices were selected at the same level close to the cranial base to show microhemorrhage at 0.45 MPa. Higher magnification images within the region highlighted by the black box are also shown. Arrows point to microhemorrhage observed at 0.45 MPa (c) Hemorrhage density quantification for all mice treated under 0.15 MPa (0.000674 ± 0.000463 %), 0.30 MPa (0.000445 ± 0.000591 %), and 0.45 MPa (0.010940 ± 0.006868 %). (d) Inertial cavitation dose under three pressure levels. (Multiple comparisons with Brown-Forsythe and Welch ANOVA corrected by Dunnett’s post-hoc test: * p < 0.05)
IV. Discussion
This study compared the delivery efficiency and homogeneity of large-volume FUS-BBBO using four strategies with interleaved or serial sonication combined with bolus or infusion of microbubbles. We first found under the acoustic pressure of 0.45 MPa that interleaved sonication combined with microbubble bolus injection was optimal among these four combined strategies for efficient and homogeneous FUS-BBBO at the whole brainstem. We then demonstrated that this identified strategy achieved efficient, homogeneous, and safe large volume delivery of 800CW-aPD-L1, where safety was achieved by a lower pressure of 0.30 MPa to avoid tissue damage. In addition, we showed the capability of PCI to monitor the efficiency and spatial homogeneity of the large-volume FUS sonication.
Although many studies have performed large-volume FUS-BBBO using different strategies, our study was the first to compare exiting strategies and determine the optimal strategy for efficient and homogeneous large-volume FUS-BBBO at the mouse brainstem. Based on our results, the optimal strategy was to combine interleaved sonication with microbubble bolus injection, which aligned with the approach used by the majority of reported studies in large-volume FUS-BBBO [20]-[22], [24], [27], [31], [32], [34], [36]. Our study provided solid evidence that this strategy has the combined advantages of high delivery efficiency and spatial homogeneity. In terms of efficiency, bolus injection achieved higher efficiency than infusion regardless of interleaved sonication or serial sonication (Fig. 3c). This was consistent with an earlier report by O’Reilly et al., who showed that the BBB permeability enhancement was higher using bolus injection versus infusion [37]. With bolus injection, microbubble concentration in the systemic circulation reached the maximum at the beginning and later decreased due to its short lifetime. On the contrary, the concentration was lower when microbubbles were constantly infused into the systemic circulation. With the same total dose of microbubbles, the peak concentration was higher using bolus injection than the prolonged infusion, thus yielding higher efficiency of FUS-BBBO. Bolus injection of microbubbles was used in most of the published clinical studies on FUS-BBBO treatment. Recently some clinical studies have started infusion administration of microbubbles using a saline bag; however, as Meng et al. pointed out, this approach has the potential disadvantages of microbubble settling over time, agent waste and a lower temporal-peak microbubble concentration compared to bolus injection [54].
With bolus injection, interleaved sonication contributed to higher spatial homogeneity than serial sonication (Fig. 3d). The interleaved sonication for multiple points FUS-BBBO was first introduced by Chopra et al., who utilized a computer-controlled three-axis positioning system to repeatedly translate the FUS exposure over six locations per second [55]. Their proposed approach, which was later commonly used for large-volume FUS-BBBO, avoided multiple injections of microbubbles which were usually needed in serial sonication at multiple targets [28]. The microbubble concentration changes over time with bolus injection. Interleaved sonication treated the nine points within 1.8 seconds for each round of sonication, which minimized the microbubble concentration differences among these points. Over the total sonication duration, all points achieved similar efficiency in FUS-BBBO by interleaved sonication, thus yielding a homogeneous distribution of large-volume FUS-BBBO. However, FUS sonication was performed in a point-by-point pattern in the serial-bolus group, where it stayed at each point for 20 seconds. In this case, the order in which different grid points were sonicated played an important role on which points received higher microbubble concentration. Points that were sonicated earlier resulted in more efficient BBBO when microbubble concentration was high, whereas the later sonicated points resulted in less efficiency when the microbubble concentration decreased. Such order-dependent difference was observed in both Evans blue extravasation (Fig. 3a-b) and cavitation dose measurement (Fig. 4a). This phenomenon was consistent with the result in Wu et al. [56], where they showed decreased Evans blue delivery along a 4-point serial sonication under one bolus injection with fixed pressure and exposure time. Therefore, the spatial distribution of BBBO using serial-bolus was less homogeneous than using interleaved-bolus. The coefficient of variation of the interleaved-bolus group (0.66 ± 0.05) was lower than that of the serial-bolus group (0.88 ± 0.10), indicating the former achieved more homogenous delivery than the latter. The coefficient of variation has been previously used by others to quantify the homogeneity of dextran delivery by FUS-BBBO [50]. Their reported coefficient of variation was around 0.8–1.3, which was comparable to our measurements.
This study also showed that PCI was capable of monitoring both efficiency and spatial homogeneity of large-volume FUS-BBBO. Previous studies have already demonstrated that target-wise cavitation dose can be measured from the PCD signals and correlated with the efficiency of BBBO over a multiple-point treatment grid [24][40]. In our study, we used PCI to extend the usage of the target-wise cavitation dose for evaluating not only efficiency but also spatial homogeneity of the large-volume BBBO outcome. The combined advantages using interleaved sonication with microbubble bolus injection to achieve efficient and homogeneous BBBO were supported by the cavitation monitoring (Fig. 4). Bolus injection led to higher total cavitation dose than infusion, corresponding to higher BBBO efficiency with bolus injection. Interleaved sonication was associated with lower coefficient of variation in the cavitation dose compared with serial sonication, corresponding to more homogeneous BBBO with interleaved sonication than serial sonication. By applying the identified optimal strategy (interleaved-bolus) to deliver 800CW-aPD-L1, the cavitation dose and coefficient of variation showed similar trend as the fluorescence intensity and coefficient of variation, respectively. Moreover, a high segmented linear correlation (R2 = 0.81, Fig. 5f) between the total cavitation dose and the total fluorescence intensity of the delivered aPD-L1 was found. Previous studies have assessed the correlation between cavitation dose and drug delivery outcome using segmented linear regression for single-point FUS-BBBO. Sun et al. reported a segmented linear correlation between stable cavitation dose measured by single-element PCD and the fluorescence intensity of a model agent, with a correlation coefficient R2 = 0.86 [38]. Yang et al. also performed segmented linear regression between stable cavitation dose measured by PCI and the delivery outcome of radiolabeled nanoclusters, with a correlation coefficient R2 = 0.61 [43]. In the current study, we found a comparable correlation coefficient (R = 0.81), suggesting that the cavitation dose quantified using PCI can be used to predict the drug delivery outcome by multi-point FUS-BBBO.
FUS-BBBO at the whole brainstem using interleaved sonication combined with bolus injection was safe at lower acoustic pressures based on the physiological monitoring and histological analysis. The H&E staining showed no hemorrhage except for 0.45 MPa, where only minimal hemorrhage was observed near the bottom of the brainstem. This observation was supported by the higher inertial cavitation dose under 0.45 MPa compared to both 0.15 MPa and 0.30 MPa (p < 0.05, Fig. 6d), where the correlation between inertial cavitation and tissue damage was demonstrated in our previous work [44]. The potential cause for the damage would be the reflection of acoustic energy from the cranial base where the targeted location was only ~2 mm above it. According to the beam calibration, the FWHM of the beam was 8.2 mm in the axial direction (Fig. 1a). When targeting the brainstem, acoustic energy could be reflected from the cranial base to cause tissue damage. Although the damage was very minimal compared to the whole sonicated area (0.010940 ± 0.006868 %), it remained a potential concern due to the critical role of the brainstem in regulating many vital functions. Lower pressure such as 0.30 MPa would be more preferred to ensure safe treatment, although it came with a trade-off with lower delivery efficiency than using 0.45 MPa.
This study has several limitations. First, this study was performed using wild-type mice. Future study is needed to validate whether the interleaved-bolus strategy can achieve efficient and homogeneous large-volume drug delivery in a DIPG model. The heterogeneous vasculature and the complicated tumor microenvironment would present challenges to achieve homogeneous large-volume drug delivery. Future work is needed to investigate the impact of tumor microenvironment on large-volume FUS-BBBO outcomes. Second, this study used a small animal model to evaluate different strategies for large-volume FUS-BBBO. Future study is needed to validate the performance of the interleaved-bolus strategy in drug delivery to the brainstem of large animal models. The choice of sonication order among the grid points would also be worth investigating as we scale the grid up for large animals. McDannold et al. [13] and Arvanitis et al. [40] proposed a 3 × 3 treatment grid in their monkey studies where their trajectory followed a different order from ours. Future study can compare different sonication trajectories to optimize large-volume FUS-BBBO in large animals. Last but not least, 2D PCI is able to capture not only intensity but also spatial distribution of the cavitation activity. Our approach of summarizing and collapsing the 2D information into a single dose value for each target was similar in principle to the approach of assigning the dose values for each target using single-element cavitation detectors [24][40]. Such target-wise cavitation dose value calculation is different from a tme cavitation dose map which has direct spatial information that correlated with spatial distribution of FUS-BBBO [43][45]. As Anastasiadis et al. demonstrated in their work, an acoustic energy map could be estimated by spatial interpolation of the target-wise cavitation dose values from PCD [24]. Our current setup with 2D PCI can potentially improve the estimation accuracy for the acoustic map with actual spatial measurements of the cavitation activity. Future work is needed to develop cavitation dose map reconstruction method based on 2D PCI and evaluate the spatial correlation between the reconstructed cavitation map and the large-volume FUS-BBBO outcome. Future studies can also explore the use of 2D PCI to reconstruct the 3D spatial distribution of the cavitation activity for multi-point FUS sonication.
V. Conclusion
This study revealed that interleaved sonication combined with microbubble bolus injection was the optimal strategy for efficient and homogeneous FUS-BBB opening at the whole brainstem compared to other combinations of sonication patterns and injection methods. Efficient and homogeneous delivery of aPD-L1 was achieved using this proposed strategy. PCI was applied for monitoring large-volume FUS-BBBO and showed a high correlation with both the efficiency and spatial homogeneity of the delivered drug. The safety of large-volume FUS-BBBO using the optimal strategy was confirmed by both physiological monitoring and histological staining. Overall, this study demonstrated that efficient, homogeneous, and safe FUS-BBBO in the whole brainstem could be achieved, which provides guidance for future clinical application of FUS-BBBO in DIPG treatment.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) grants R01EB027223, R01EB030102, and R01MH116981. C. Chien was partially supported by the Taiwan Washington University in St. Louis Scholarship.
Contributor Information
Yan Gong, Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63105 USA..
Dezhuang Ye, Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63105 USA..
Chih-Yen Chien, Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63105 USA..
Yimei Yue, Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63105 USA..
Hong Chen, Department of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63105 USA; Department of Radiation Oncology, Washington University School of Medicine, St. Louis, MO 63110 USA..
Reference
- [1].Warren KE, “Diffuse intrinsic pontine glioma: poised for progress,” Front. Oncol, vol. 2, no. December, pp. 1–9, 2012, doi: 10.3389/fonc.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Williams JR et al. , “Progress in diffuse intrinsic pontine glioma: Advocating for stereotactic biopsy in the standard of care,” Neurosurg. Focus, vol. 48, no. 1, pp. 1–8, 2020, doi: 10.3171/2019.9.FOCUS19745. [DOI] [PubMed] [Google Scholar]
- [3].Topalian SL, Taube JM, and Pardoll DM, “Neoadjuvant checkpoint blockade for cancer immunotherapy,” Science (80-. )., vol. 367, no. 6477, 2020, doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kline C et al. , “Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience,” j. Neurooncol, vol. 140, no. 3, pp. 629–638, 2018, doi: 10.1007/s11060-018-2991-5. [DOI] [PubMed] [Google Scholar]
- [5].Ye D, Yuan J, Yue Y, Rubin JB, and Chen H, “Focused ultrasound-enhanced delivery of intranasally administered anti-programmed cell death-ligand 1 antibody to an intracranial murine glioma model,” Pharmaceutics, vol. 13, no. 2, pp. 1–12, Jan. 2021, doi: 10.3390/PHARMACEUTICS13020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bors LA and Erdö F, “Overcoming the blood-brain barrier. Challenges and tricks for CNS drug delivery,” Scientia Pharmaceutica, vol. 87, no. 1. 2019, doi: 10.3390/scipharm87010006. [DOI] [Google Scholar]
- [7].Chaves C et al. , “Characterization of the blood–brain barrier integrity and the brain transport of SN-38 in an orthotopic xenograft rat model of diffuse intrinsic pontine glioma,” Pharmaceutics, vol. 12, no. 5, 2020, doi: 10.3390/pharmaceutics12050399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, and Hynynen K, “Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles,” Ultrasound Med. Biol, vol. 30, no. 7, pp. 979–989, Jul. 2004, doi: 10.1016/J.ULTRASMEDBIO.2004.04.010. [DOI] [PubMed] [Google Scholar]
- [9].Hynynen K, McDannold N, Sheikov NA, Jolesz FA, and Vykhodtseva N, “Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications,” Neuroimage, vol. 24, no. 1, pp. 12–20, 2005, doi: 10.1016/j.neuroimage.2004.06.046. [DOI] [PubMed] [Google Scholar]
- [10].Burgess A and Hynynen K, “Noninvasive and Targeted Drug Delivery to the Brain Using Focused Ultrasound,” ACS Chem. Neurosci, vol. 4, no. 4, pp. 519–526, Apr. 2013, doi: 10.1021/cn300191b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choi JJ, Pemot M, Small SA, and Konofagou EE, “Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice,” Ultrasound Med. Biol, vol. 33, no. 1, pp. 95–104, Jan. 2007, doi: 10.1016/J.ULTRASMEDBIO.2006.07.018. [DOI] [PubMed] [Google Scholar]
- [12].Wei KC et al. , “Focused Ultrasound-Induced Blood-Brain Barrier Opening to Enhance Temozolomide Delivery for Glioblastoma Treatment: A Preclinical Study,” PLoS One, vol. 8, no. 3, pp. 1–10, 2013, doi: 10.1371/journal.pone.0058995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McDannold N, Arvanitis CD, Vykhodtseva N, and Livingstone MS, “Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques.,” Cancer Res, vol. 72, no. 14, pp. 3652–3663, Jul. 2012, doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ting CY et al. , “Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment,” Biomaterials, vol. 33, no. 2, pp. 704–712, Jan. 2012, doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- [15].Tsai HC, Tsai CH, Chen WS, Inserra C, Wei KC, and Liu HL, “Safety evaluation of frequent application of microbubble-enhanced focused ultrasound blood-brain-barrier opening,” Sci. Rep, vol. 8, no. 1, pp. 1–13, 2018, doi: 10.1038/s41598-018-35677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chu P-C, Chai W-Y, Tsai C-H, Kang S-T, Yeh C-K, and Liu H-L, “Focused Ultrasound-Induced Blood-Brain Barrier Opening: Association with Mechanical Index and Cavitation Index Analyzed by Dynamic Contrast-Enhanced Magnetic-Resonance Imaging,” Sci. Rep, vol. 6, no. 1, p. 33264, Dec. 2016, doi: 10.1038/srep33264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen H and Konofagou EE, “The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure,” j. Cereb. Blood Flow Metab, vol. 34, no. 7, pp. 1197–1204, 2014, doi: 10.1038/jcbfm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ye D et al. , “Focused ultrasound-enabled delivery of radiolabeled nanoclusters to the pons,” J. Control. Release, vol. 283, pp. 143–150, Aug. 2018, doi: 10.1016/j.jconrel.2018.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tran VL et al. , “Impact of blood-brain barrier permeabilization induced by ultrasound associated to microbubbles on the brain delivery and kinetics of cetuximab: An immunoPET study using 89Zr-cetuximab,” J. Control. Release, vol. 328, pp. 304–312, Dec. 2020, doi: 10.1016/j.jconrel.2020.08.047. [DOI] [PubMed] [Google Scholar]
- [20].Abrahao A et al. , “First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound,” Nat. Commun, vol. 10, no. 1, pp. 1–9, 2019, doi: 10.1038/s41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lipsman N et al. , “Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound,” Nat. Commun, vol. 9, no. 1, Dec. 2018, doi: 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Park SH et al. , “Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy,” J. Neurosurg, pp. 1–9, Jan. 2020, doi: 10.3171/2019.10.jns192206. [DOI] [PubMed] [Google Scholar]
- [23].Gasca-Salas C et al. , “Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia,” Nat. Commun, vol.12, no. 1, pp. 1–7, 2021, doi: 10.1038/s41467-021-21022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anastasiadis P et al. , “Localized blood–brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions–controlled focused ultrasound,” Proc. Natl. Acad Sci, vol. 118, no. 37, p. e2103280118, Sep. 2021, doi: 10.1073/pnas.2103280118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang X et al. , “Magnetic Resonance Imaging-Guided Focused Ultrasound-Based Delivery of Radiolabeled Copper Nanoclusters to Diffuse Intrinsic Pontine Glioma,” ACS Appl. Nano Mater, vol. 3, no. 11, pp. 11129–11134, 2020, doi: 10.1021/acsanm.0c02297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alli S et al. , “Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery,” j. Control. Release, vol. 281, pp. 29M1, Jul. 2018, doi: 10.1016/j.jconrel.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ishida J et al. , “MRI-guided focused ultrasound enhances drug delivery in experimental diffuse intrinsic pontine glioma,” J. Control. Release, vol. 330, no. July 2020, pp. 1034–1045, 2020, doi: 10.1016/j.jconrel.2020.11.010. [DOI] [PubMed] [Google Scholar]
- [28].Englander ZK et al. , “Focused ultrasound mediated blood – brain barrier opening is safe and feasible in a murine pontine glioma model,” Sci. Rep, pp. 1–10, 2021, doi: 10.1038/s41598-021-85180-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hall MD et al. , “First clinical experience with DRD2/3 antagonist ONC201 in H3 K27M–mutant pediatric diffuse intrinsic pontine glioma: A case report,” j Neurosurg. Pediatr, vol. 23, no. 6, pp. 719–725, 2019, doi: 10.3171/2019.2.PEDS18480. [DOI] [PubMed] [Google Scholar]
- [30].Sedlacik J, Winchell A, Kocak M, Loeffler RB, Broniscer A, and Hillenbrand CM, “MR imaging assessment of tumor perfusion and 3D segmented volume at baseline, during treatment, and at tumor progression in children with newly diagnosed diffuse intrinsic pontine glioma,” Am. J. Neuroradiol, vol. 34, no. 7, pp. 1450–1455, 2013, doi: 10.3174/ajnr.A3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aryal M, Vykhodtseva N, Zhang YZ, Park J, and McDannold N, “Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model,” J. Control. Release, vol. 169, no. 1–2, pp. 103–111, 2013, doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McDannold N et al. , “Acoustic feedback enables safe and reliable carboplatin delivery across the blood-brain barrier with a clinical focused ultrasound system and improves survival in a rat glioma model,” Theranostics, vol. 9, no. 21, pp. 6284–6299, 2019, doi: 10.7150/thno.35892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jones RM, Deng L, Leung K, McMahon D, O’Reilly MA, and Hynynen K, “Three-dimensional transcranial microbubble imaging for guiding volumetric ultrasound-mediated blood-brain barrier opening,” Theranostics, vol. 8, no. 11, pp. 2909–2926, 2018, doi: 10.7150/thno.24911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huang Y, Alkins R, Schwartz ML, and Hynynen K, “Opening the blood-brain barrier with MR imaging-guided focused ultrasound: Preclinical testing on a trans-human skull porcine model,” Radiology, vol. 282, no. 1, pp. 123–130, Jan. 2017, doi: 10.1148/radiol.2016152154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wei KC et al. , “Neuronavigation-guided focused ultrasound-induced blood-brain barrier opening: A preliminary study in swine,” Am. J. Neuroradiol, vol. 34, no. 1, pp. 115–120, Jan. 2013, doi: 10.3174/ajnr.A3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Reilly MA, Jones RM, Barrett E, Schwab A, Head E, and Hynynen K, “Investigation of the safety of focused ultrasound-induced blood-brain barrier opening in a natural canine model of aging,” Theranostics, vol. 7, no. 14, pp. 3573–3584, 2017, doi: 10.7150/thno.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].O’Reilly MA, Waspe AC, Ganguly M, and Hynynen K, “Focused-Ultrasound Disruption of the Blood-Brain Barrier Using Closely-Timed Short Pulses: Influence of Sonication Parameters and Injection Rate,” Ultrasound Med. Biol, vol. 37, no. 4, pp. 587–594, 2011, doi: 10.1016/j.ultrasmedbio.2011.01.008.Focused-Ultrasound. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sun T et al. , “Closed-loop control of targeted ultrasound drug delivery across the blood–brain/tumor barriers in a rat glioma model,” Proc. Natl. Acad Sci. U. S. A,vol. 114,no.48, pp. E10281–E10290, Nov. 2017, doi: 10.1073/pnas.1713328114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].O’Reilly MA, Hynynen K, O’Reilly MA, Hynynen K, O’Reilly MA, and Hynynen K, “Blood-brain barrier: Real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions–based controller,” Radiology, vol. 263, no. 1, pp. 96–106, Apr. 2012, doi: 10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arvanitis CD, Livingstone MS, Vykhodtseva N, and McDannold N, “Controlled Ultrasound-Induced Blood-Brain Barrier Disruption Using Passive Acoustic Emissions Monitoring,” PLoS One, vol. 7, no. 9, Sep. 2012, doi: 10.1371/journal.pone.0045783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Çavuşoǧlu M et al. , “Closed-loop cavitation control for focused ultrasound-mediated BBB opening by long-circulating microbubbles,” Rhys. Med. Biol, vol. 64, no. 4, 2019, [Online], Available: https://iopscience.iop.org/article/10.1088/1361-6560/aafaa5. [DOI] [PubMed] [Google Scholar]
- [42].Kamimura HAS et al. , “Feedback control of microbubble cavitation for ultrasound-mediated blood–brain barrier disruption in non-human primates under magnetic resonance guidance,” J. Cereb. Blood Flow Metab, vol. 39, no. 7, pp. 1191–1203, 2019, doi: 10.1177/0271678X17753514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yang Y et al. , “Cavitation dose painting for focused ultrasound-induced blood-brain barrier dismption,” Sci. Rep, vol. 9, no. 1, pp. 1–10, 2019, doi: 10.1038/s41598-019-39090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xu S et al. , “Correlation Between Brain Tissue Damage and Inertial Cavitation Dose Quantified Using Passive Cavitation Imaging,” Ultrasound Med. Biol, vol. 45, no. 10, pp. 2758–2766, 2019, doi: 10.1016/j.ultrasmedbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- [45].Wu SY et al. , “Efficient blood-brain barrier opening in primates with neuronavigation-guided ultrasound and real-time acoustic mapping,”Scz. Rep, vol. 8, no. 1, Dec. 2018, doi: 10.1038/s41598-018-25904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jones RM, McMahon D, and Hynynen K, “Ultrafast three-dimensional microbubble imaging in vivo predicts tissue damage volume distributions during nonthermal brain ablation,” Theranostics, vol. 10, no. 16, pp. 7211–7230, 2020, doi: 10.7150/thno.47281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ye D et al. , “Characterization of focused ultrasound-mediated brainstem delivery of intranasally administered agents,” J. Control. Release, vol. 328, no. August, pp. 276–285, 2020, doi: 10.1016/j.jconrel.2020.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ewald AJ, Werb Z, and Egeblad M, “Monitoring of vital signs for long-term survival of mice under anesthesia,” Cold Spring Harb. Protoc, vol. 6, no. 2, 2011, doi: 10.1101/pdb.prot5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Galstyan A et al. , “Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy,”Nat. Commun, vol. 10, no. 1, pp. 1–13, 2019, doi: 10.1038/s41467-019-11719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Morse SV et al. , “Rapid short-pulse ultrasound delivers drugs uniformly across the murine blood-brain barrier with negligible disruption,” Radiology, vol. 291, no. 2, pp. 459–466, May 2019, doi: 10.1148/radiol.2019181625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Haworth KJ, Bader KB, Rich KT, Holland CK, and Mast TD, “Quantitative frequency-domain passive cavitation imaging,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control, vol. 64, no. 1, pp. 177–191, 2017, doi: 10.1109/TUFFC.2016.2620492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Grimaud J and Murthy VN, “How to monitor breathing in laboratory rodents: A review of the current methods,” J. Neurophysiol, vol. 120, no. 2, pp. 624–632, 2018, doi: 10.1152/jn.00708.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Schindelin J et al. , “Fiji: An open-source platform for biological-image analysis,” Nat. Methods, vol. 9, no. 7, pp. 676–682, 2012, doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Meng Y et al. , “Technical Principles and Clinical Workflow of Transcranial MR-Guided Focused Ultrasound,” Stereotact. Funct. Neurosurg, vol. 99, no. 4, pp. 329–342, 2021, doi: 10.1159/000512111. [DOI] [PubMed] [Google Scholar]
- [55].Chopra R, Curiel L, Staruch R, Morrison L, and Hynynen K, “An MRI-compatible system for focused ultrasound experiments in small animal models,” Med. Phys, vol. 36, no. 5, pp. 1867–1874, 2009, doi: 10.1118/1.3115680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Wu SK et al. , “Characterization of different microbubbles in assisting focused ultrasound-induced blood-brain barrier opening,” Sci. Rep, vol. 7, no. March, pp. 1–11, 2017, doi: 10.1038/srep46689. [DOI] [PMC free article] [PubMed] [Google Scholar]