Abstract
A series of 1-oxa-4-azaspiro[4,5]deca-6,9-diene-3,8-dione derivatives containing structural fragments of conjugated dienone have been synthesized previously by our group, however the Michael addition reaction between conjugated dienone and nucleophilic groups in the body may generate harmful and adverse effects. To reduce harmful side effects, the authors started with p-aminophenol to make 1-oxo-4- azaspirodecanedione derivatives, then utilized the Michael addition and cyclopropanation to eliminate α, β unsaturated olefinic bond and lower the Michael reactivity of the compounds in vivo for optimization. At the same time, heteroatoms are put into the molecules in order to improve the hydrophilicity of the molecules and the binding sites of the molecules and the target molecules, establishing the groundwork for improved antitumor activity. The majority of the compounds had moderate to potent activity against A549 human lung cancer cells, MDA-MB-231 breast cancer cells, and Hela human cervical cancer cells. Among them, the compound 6d showed the strongest effect on A549 cell line with IC50 of 0.26 μM; the compound 8d showed the strongest cytotoxicity on MDA-MB-231 cell line with IC50 of 0.10 μM; and the compound 6b showed the strongest activity on Hela cell line with IC50 of 0.18 μM.
Keywords: 1-oxa-4-azaspironenone derivatives, Michael addition, Cyclopropanation, Antitumor activity
Spirodienone molecules have attracted much attention in medicinal chemistry in recent years due to their diverse biological activities including anti-inflammatory,1-2 antibacterial,3 and antiviral properties 4. More significantly, the spirodienone compounds showed good anticancer activity with potential therapeutic utility. Dat et al. 5 extracted the compound 1–1 from Amorpha fruticose that strongly suppressed nuclear transcription factor-κB (NF-κB) activation with IC50 values of 2.12 μM and 7.65 μM in human cervical carcinoma (HeLa) cells and murine macrophage RAW264.7 cells, respectively. Compounds 1–2 6 exhibit potent inhibitory effects on MCF-7 breast cancer, NCI-H460 non-small cell lung cancer, and CNS cancer (SF-268). Compounds 1–3 inhibited MDA-MB-231, HeLa, A549, and MCF-7, according to Gu et al.7. In cancer cells of the breast (MCF-7), lung (NCI-H460), CNS (SF268), nasopharyngeal (KB), and cervix (Hela) cells, the natural product hopeanol 8,9 shows antiproliferative properties. (Fig. 1).
Fig. 1.
Spirocyclic molecules with antitumor activity.
We previously reported on several spirodienone compounds, the majority of which displayed moderate to high anticancer activities on human lung cancer A549, human breast cancer MDA-MB-231, and human cervical cancer Hela cell lines 10-13. However, because these compounds include structural components of conjugated diketene, the Michael addition reaction of diketene and nucleophilic group can easily occur in vivo, resulting in significant adverse effects 14-16. For this reason, we tried to use Michael addition and cyclopropanation to remove an α, β-unsaturated olefinic bond of 1-oxo-4-azaspirodecadione derivatives and reduced reactivity of Michael receptor in vivo, as well as introduce heteroatom chains to improve the hydrophilicity of these derivatives and their binding sites to target proteins. (Fig. 2).
Fig. 2.
Design of novel spirodienone derivatives.
Synthesis of the Novel 1-Oxa-4-azaspironenone Derivatives.
The 1-oxa-4-azaspironenone derivatives were prepared according to the synthetic route of Scheme 1. 4-aminophenol, glycolic acid (1.2 eq) and DCC (2 eq) were refluxed in acetonitrile to give the compound 2 10. Next, the compound 2 (1 eq), PhI(OAc)2 (2 eq) and Cu[(CH3CN)4ClO4] (0.05 eq) were dissolved in dry DCM under nitrogen and reacted at room temperature to obtain the compound 3 10. The compound 3, DBU (1.5 eq) and halogenated hydrocarbons (1.5 eq) were stirred in dry THF at low temperature to obtain the compound 5 10. The compounds 6 and 7 were obtained by reacting the compound 5 with a nucleophilic reagent in the presence of water and ethanol (2:1, v/v), or DBU (1.2 eq) and acetonitrile17. Compound 8 could be obtained by the reaction of the compound 5 with trimethyl sulfoxide iodide (1.1 eq), sodium hydride (1.1 eq), DMSO as solvent and stirring at room temperature 18.
Scheme 1.
The synthesis route of 1-oxa-4-azaspironenone derivatives.
The Cytotoxicity Assay and Antitumor Activity of the Novel 1-Oxa-4-aza- spironenone Derivatives.
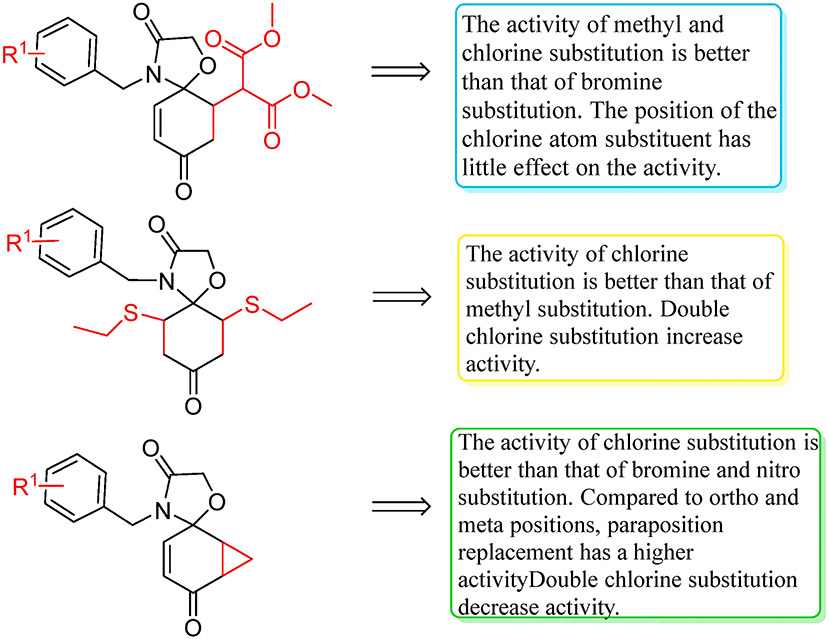
A number of 1-oxa-4-azaspironenone derivatives were synthesized according to our predicted design and scheme (Scheme 1). The anticancer effects of these synthesized compounds were then studied in vitro using bendamustine and vorinostat as positive pharmacological controls against lung cancer cell line A549, breast cancer cell line MDA-MB-231, and cervical cancer cell line HeLa (Table 1). After structural modifications, the activities of most compounds decreased. The activity of the compound 8b against MDA-MB-231 cells increased. The compound 6d’s inhibitory effect on A549 cells improved, and the compound 7c had a good inhibitory effect on 231 and Hela cells. The compound 6d was the most potent against the A549 cell line, with an IC50 of 0.26 μM; the compounds 8b showed the most potent cytotoxicity against the MDA-MB-231 cell line, with an IC50 of 0.10 μM; and the compounds 6b against the Hela cell line, with an IC50 of 0.18 μM. The inhibitory activities of most compounds on behalf of 6a-8f against MDA-MB-231 cell lines were generally stronger than other two cell lines, exhibiting an obvious selectivity to some degree. The structure–activity relationship of 1-Oxa-4-aza- spironenone derivatives is summarized in Scheme 2.
Table 1.
In vitro antitumor activities of active molecules against cancer cell lines.
| Compounds | IC50 (μM) | ||
|---|---|---|---|
| A549 | MDA-MB-231 | Hela | |
| 5a | 0.26 | 0.10 | 0.20 |
| 5b | 1.29 | 0.11 | 0.41 |
| 5c | 0.19 | 0.08 | 0.15 |
| 5d | 0.81 | 0.21 | 0.27 |
| 5e | 1.05 | 0.07 | 0.36 |
| 5f | 3.01 | 0.11 | 0.37 |
| 5 g | 4.95 | 0.12 | 0.29 |
| 6a | 9.25 | 0.34 | 0.41 |
| 6b | 9.98 | 0.38 | 0.18 |
| 6c | 2.55 | 1.23 | 1.06 |
| 6d | 0.26 | 0.25 | 0.34 |
| 7a | 2.30 | 1.36 | 2.41 |
| 7b | >10 | 0.26 | 3.92 |
| 7c | 9.27 | 0.12 | 0.21 |
| 8a | 8.83 | 5.52 | 1.76 |
| 8b | 6.06 | 0.10 | >10 |
| 8c | >10 | 0.46 | 1.73 |
| 8d | 0.66 | 0.42 | >10 |
| 8e | 6.09 | 1.12 | >10 |
| 8f | >10 | 1.20 | 3.86 |
| SAHA | – | 3.52 | 4.52 |
| Bendamustiune | – | 13.28 | >20 |
Scheme 2.
The structure activity relationship (SAR) of novel 1-oxa-4-azaspironenone derivatives.
After that, a cytotoxicity test was carried out. The human umbilical vein endothelial cells (HUVEC) were treated with volinostat as a positive control. As shown in Table 2, compared with the positive drug and the compound 5b before optimization, the compound 8b had the least harm to HUVEC cells.
Table 2.
In vitro cytotoxicity assay.
| Compounds | IC50 (μM) |
|---|---|
| HUVEC | |
| 8b | 83.65 |
| 5b | 44.61 |
| SAHA | 26.40 |
In vitro cell cycle analysis.
Flow cytometry was used to investigate the effect of the compound 8b in influencing cell cycle distribution. The positive control was Combretastatin A4 (CA4). As shown in Fig. 3, treatment with 10 μM of the compound 8b led to decrease in the percentage of MDA-MB-231 cells in G1 phase from 61.33% to 53.57%, and to increase the cells in G2 phase from 16.61% to 27.16%, indicating that the compound 8b could induce cell cycle arrest in the G2 phase in MDA-MB-231 cells.
Fig. 3.
MDA-MB-231 cell cycle. A: DMSO; B: CA4 (10 μM); C: 8b (10 μM).
In vitro cell apoptosis analysis.
Annexin V-FITC and PI labeling were employed to investigate the probable mechanisms of the compound 8b’s antiproliferative action, and flow cytometry (FCM) was utilized to measure cell death (Fig. 4). The compound 8b was used to cultivate MDA-MB-231 cells at a concentration of 10 μM. As a positive control, combretastatin A4 (CA4) was employed. Compared to DMSO, treatment with the compound 8b had a certain effect on the amount of apoptotic cells. As shown in Fig 4, treatment with 10 μM of the compound 8b had a certain effect on the amount of apoptotic cells, increased the early-stage apoptosis percentage from 4.66% to 10.9% and late-stage apoptosis percentage from 1.02% to 3.00%. The results showed compound 8b inhibits the proliferation of tumor cells by blocking the cell cycle.
Fig. 4.
The cell apoptosis of MDA-MB-231. A: DMSO; B: CA4 (10 μM); C: 8b (10 μM).
In conclusion, using 4-aminophenol and glycolic acid as starting materials, a series of 1-oxa-4-azaspiro[4,5]deca-6,9-diene-3,8-dione derivatives were synthesized by four steps reaction. The design idea of reducing biotoxicity of the compounds in vivo by reducing the reactivity of the Michael receptor was realized. Most of the derivatives were active against the A549, MDA-MB-231, and HeLa cell lines with moderate potency. The compound 8b showed the strongest anti-proliferation effect on MDA-MB-231 cells, and the damage to HUVEC cells was less than that of the positive drug SAHA and the dual Michael receptor compound 5b.
Supplementary Material
Acknowledgments
This work was funded by Sichuan University-Lu Zhou Strategic Cooperation Projects (2017 CDLZ-S34), China (L. He) and NIH RCMI program at Xavier University of Louisiana through Grant (2U54MD007595) USA (G. Wang).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmcl.2022.128925.
Data availability
Data will be made available on request.
References
- 1.Granica S; Piwowarski JP; Randazzo A; Schneider P; Zidorn C Novel stilbenoids, including cannabispiradienone glycosides, from Tragopogon tommasinii (Asteraceae, Cichorieae) and their potential anti-inflammatory activity. Phytochemistry. 2015. 117. 254–266. DOI: 10.1016/j.phytochem.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Du WK, Hung HY, Kuo PC, et al. Dragonbloodin A1 and A2: Flavan Trimers and Anti-inflammatory Principles from Sanguis Draconis. Org Lett. 2016;18:3042. 10.1021/acs.orglett.6b00593. [DOI] [PubMed] [Google Scholar]
- 3.Tang Y, Yang H, Xue L, Li Q, Zhang J. Total synthesis and preliminary SAR study of (±)-merochlorins A and B. Org Biomol Chem. 2015;14:198–205. 10.1039/c5ob01946j. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, He L. Advances in research of spirodienone and its derivatives: Biological activities and synthesis methods. Eur J Med Chem. 2020;203, 112577. 10.1016/j.ejmech.2020.112577. [DOI] [PubMed] [Google Scholar]
- 5.Dat NT, Lee JH, Lee K, Hong YS, Kim YH, Lee JJ. Phenolic Constituents ofAmorpha fruticosaThat Inhibit NF-κB Activation and Related Gene Expression. J Nat Prod. 2008;71:1696–1700. 10.1021/np800383q. [DOI] [PubMed] [Google Scholar]
- 6.Pinto O, Sardinha JO, Vaz PD, et al. Synthesis of tetrahydronaphthalene lignan esters by intramolecular cyclization of ethyl p-azidophenyl-2-phenylalkanoates and evaluation of the growth inhibition of human tumor cell lines. J Med Chem. 2011;54: 3175–3187. 10.1021/jm101182s. [DOI] [PubMed] [Google Scholar]
- 7.Gu L, Wang P, Zhong Q, et al. Copper salt-catalyzed formation of a novel series of triazole–spirodienone conjugates with potent anticancer activity. RSC Adv. 2017;7: 9412–9416. 10.1039/C6RA24764D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder SA, Wright NE, Pflueger JJ, Breazzano SP. Total Syntheses of Heimiol A, Hopeahainol D, and Constrained Analogues. Angew Chem Int Ed. 2011;50: 8629–8633. 10.1002/anie.201103575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolaou KC, Wu TR, Kang Q, Chen DY. Total Synthesis of Hopeahainol A and Hopeanol. Angew Chem Int Ed. 2009;48:3440–3443. 10.1002/anie.200900438. [DOI] [PubMed] [Google Scholar]
- 10.Yang Z, Zhong Q, Zheng S, Wang G, He L. Synthesis and Antitumor Activity of a Series of Novel 1-Oxa-4-azaspiro[4,5]deca-6,9-diene-3,8-dione Derivatives. Molecules. 2019;24:936. 10.3390/molecules24050936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo L, Jia JJ, Zhong Q, Zhong X, He L. Synthesis and anticancer activity evaluation of naphthalene-substituted triazole spirodienones. Eur J Med Chem. 2020;213, 113039. 10.1016/j.ejmech.2020.113039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing N, Chen C, Zhong Q, Zheng S, He L. Design, Synthesis, and Antitumor Activity of a Series of Novel 4-(Aromatic Sulfonyl)-1-oxa-4-azaspiro[4.5]deca-6,9-dien-8-ones. Molecules. 2020;25:5459. 10.3390/molecules25225459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Yang L, Honglu Y, et al. Design, Synthesis, and Antitumor Activity Evaluation of Novel Acyl Sulfonamide Spirodienones. Bioorg Med Chem. 2022;60, 116626. 10.1016/j.bmc.2022.116626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith DJ, Anderson RC. Toxicity and Metabolism of Nitroalkanes and Substituted Nitroalkanes. J Agric Food Chem. 2013;61:763–779. 10.1021/jf3039583. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51–61. 10.1016/j.ejps.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Lin YR, Chan MH, Peng HL, Peng KC, Liu SY. Effect of pachybasin on general toxicity and developmental toxicity in vivo. J Agric Food Chem. 2017;65:10489–10494. 10.1021/acs.jafc.7b03879. [DOI] [PubMed] [Google Scholar]
- 17.Walker GN. Michael Addition Products from 2,3-Dimethoxyphenylpropiolic Acid Derivatives. J Am Chem Soc. 1954;76:309–310. 10.1021/ja01630a104. [DOI] [Google Scholar]
- 18.Wei Y, Lin S, Liang F, Zhang J. N-bromosuccinimide/1,8-diazabicyclo [5.4.1]undec-7-ene combination: β-amination of chalcones via a tandem bromoamination/debromination sequence. Org Lett. 2013;15:852–855. 10.1021/ol303539u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.