Abstract
Background
Fear of cancer recurrence (FCR) and sleep disturbance are common in cancer survivors. Yet, little research has examined their relationship, and even less is known about what links may exist between these variables among the intimate partners of cancer survivors.
Purpose
This study examines the relationship between FCR and sleep disturbance in breast cancer survivors and their partners. Using daily sleep data collected at two distinct periods early in survivorship—the completion of adjuvant treatment and the first post-treatment mammogram—higher survivor and partner FCR was hypothesized to predict greater sleep disturbance.
Methods
Breast cancer survivors and intimate partners (N = 76 couples; 152 individuals) each reported sleep duration, sleep quality, sleep onset latency, and wake after sleep onset each morning of two 21-day sleep diary bursts during the first year post-diagnosis. Three validated measures formed latent FCR factors for survivors and partners, which were used to predict average daily sleep.
Results
Across both sleep diary bursts, survivor FCR was associated with their own reduced sleep duration, reduced sleep quality, and greater sleep onset latency. Survivor FCR was also associated with their partners’ reduced sleep quality and greater sleep onset latency. Partner FCR was associated with their own reduced sleep duration, reduced sleep quality, and greater sleep onset latency. Partner FCR was also associated with survivors’ reduced sleep quality.
Conclusions
Findings revealed intrapersonal and interpersonal associations between FCR and sleep disturbance, addressing gaps in knowledge on FCR and an outcome with known short- and long-term implications for health and mortality.
Keywords: Fear of cancer recurrence, Sleep, Breast cancer, Couples, Mammogram
Breast cancer survivors and intimate partners who reported more fear of cancer recurrence had higher rates of daily sleep disturbance at two distinct times points in early survivorship.
Since 1975, breast cancer mortality has decreased by 40% [1], resulting in a population of 3.8 million breast cancer survivors in the USA today [2]. As many as 40% of these survivors report clinically significant levels of fear of cancer recurrence (FCR) [3], or the “fear, worry, or concern relating to the possibility that cancer will come back or progress” [4]. Theoretical models of FCR outline consequences including excessive body checking for cancer and avoidance of cancer-related triggers [5]. However, models have neglected sleep disturbance as a potential consequence of pathological FCR [5].
Sleep in Breast Cancer Survivorship
Sleep disturbance is an umbrella term referring to insufficient sleep duration, poor perceived sleep quality, difficulty falling asleep, and/or difficulty staying asleep. Insomnia disorder is diagnosed when one or more sleep disturbance facets occur ≥3 days per week, for ≥3 months, and cause distress and impairment [6]. Breast cancer survivors have a two-fold risk for insomnia relative to the general population [7]. Sixty-nine percent of breast cancer survivors report sleep disturbance at surgery, and as many as 42% experience sleep disturbance 18 months later. Interestingly, insomnia rates are higher in breast cancer than in other cancers, and this effect is not fully explained by gender differences [8]. Sleep has known implications for mental and physical health in the general population and cancer survivors. Regarding mental health implications, short-term sleep deprivation/loss is associated with impairments in emotional reactivity, recognition, and expression and sleep disturbance is both causally and bidirectionally related to mood and anxiety disorders [9]. Physical health implications of sleep disturbance include heightened risk for cardiovascular disease [10], a particularly relevant outcome for cancer survivors who already demonstrate elevated rates of cardiovascular disease-related mortality [11]. Among breast cancer survivors, sleep disturbance also has been implicated in compromised recovery from adjuvant treatment [12] and both all-cause and breast cancer mortality [13, 14].
Most research on sleep in cancer survivors has focused on treatment side effects and associated physiological changes [15]. FCR may be another cancer-specific contributor to sleep disturbance in survivorship. Indeed, accepted psychosocial conceptualizations of insomnia, including the 3 Ps [16–18] and cognitive models [19], provide theoretical support for a relationship between FCR and sleep disturbance: these models propose that stressful life events and associated cognitions (e.g., worry, rumination) serve as precipitating factors for sleep disturbance. Acute sleep disturbance may become chronic as worries expand to include those about sleep itself and as individuals develop wake-bed associations and maladaptive compensatory sleep behaviors (e.g., spending large amounts of time in bed). Drawing on these well-established theoretical frameworks, it is possible that cancer (including diagnosis, treatment, and follow-up mammograms) and associated FCR (particularly intrusive thoughts and worry) may contribute to the elevated rates of insomnia observed in breast cancer survivors.
In support of this hypothesis, cancer-related intrusive thoughts—a core component of FCR [20]—predict current and future sleep disturbance in breast cancer survivors [21, 22]. A small literature has directly examined the relationship between FCR and sleep disturbance. In separate studies of ovarian and prostate cancer survivors, FCR showed cross-sectional associations with global sleep disturbance [23, 24]. Another study found that cancer survivors with mixed diagnoses who reported frequent problems falling/staying asleep on a single item were more likely to report FCR than those with better sleep [25]. In another mixed sample of cancer survivors, FCR and global sleep quality, both measured cross-sectionally with single items, were positively associated [26]. Lastly, in a qualitative study, six of ten mixed-diagnosis cancer survivors described as having clinical FCR also reported difficulty sleeping [27].
These studies provide preliminary support for an association between FCR and sleep disturbance in cancer survivors. However, several questions remain. First, no studies have examined FCR and sleep disturbance specifically in breast cancer survivors, despite evidence that this population experiences increased sleep disturbance relative to other cancer survivors [7]. Second, evidence for associations between FCR and sleep disturbance is based solely on global, retrospective sleep measures, which correlate only moderately with daily sleep logs [28]. Global assessments are susceptible to recall bias, which may be magnified in individuals with insomnia who misperceive their sleep [29]. Moreover, FCR and sleep disturbance have often been measured with single items, overlooking the multi-faceted nature of both constructs. For example, sleep disturbance was often measured with a single sleep quality item, which neglects the number of other and unique ways (insufficient sleep duration, difficulty falling asleep, etc.) that sleep can be disturbed.
In addition to survivors, intimate partners of cancer survivors also report FCR [30, 31] and survivor and partner FCR are significantly associated [32, 33]. Sleep disturbance in intimate partners of cancer survivors has received relatively little empirical attention, but some data suggest that partners in fact demonstrate rates of sleep disturbance comparable to those seen in survivors [34, 35]. More generally, sleep is a shared activity for most couples, and thus, partners’ sleep, wake, and movements are interdependent [36]. Furthermore, dyadic effects of one partner’s psychological distress impacting the other’s sleep have been demonstrated in both the general population [37] and in couples coping with cancer [38, 39]. Taken together, there is value in studying the association between FCR and sleep in both survivors and their partners.
The Current Study
This study builds on this prior work by examining links between global FCR and daily sleep disturbance in breast cancer survivors and their partners during two important periods in the first year of survivorship: (a) the completion of adjuvant treatment and (b) the first post-treatment mammogram. Both periods are clinically significant in the FCR trajectory, but in different ways—the completion of adjuvant treatment marks the typical onset of FCR, as this is when survivors are declared “cancer-free,” lose regular contact with oncology providers, and often report a drop in other cancer-related supports [40]. In contrast, the post-treatment mammogram is conceptualized as a real-world, short-term trigger of FCR [30, 41, 42]. Given the different characteristics of these two periods and about a 6-month gap between them, questions concerning links with sleep were examined independently. A global, one-time measure of FCR was obtained immediately before each of two 21-day sleep diary bursts, during which participants reported on four aspects of sleep: sleep duration, sleep quality, difficulty falling asleep (sleep onset latency), and difficulty staying asleep (wake after sleep onset).
In separate analyses for the two periods, we examined links between global FCR and the four indices of daily sleep averaged across each diary burst. FCR and sleep were assessed in survivors and partners, allowing for the examination of both actor and cross-partner effects. We hypothesized that survivors and partners with more FCR would report more daily sleep disturbance themselves (actor effects), as indicated by lower sleep duration, poorer subjective sleep quality, and greater sleep onset latency and wake after sleep onset. Given prior evidence for cross-partner effects of distress and sleep, we hypothesized that survivors with more FCR would have partners (and vice versa) with more daily sleep disturbance (cross-partner effects).
Methods
Participants
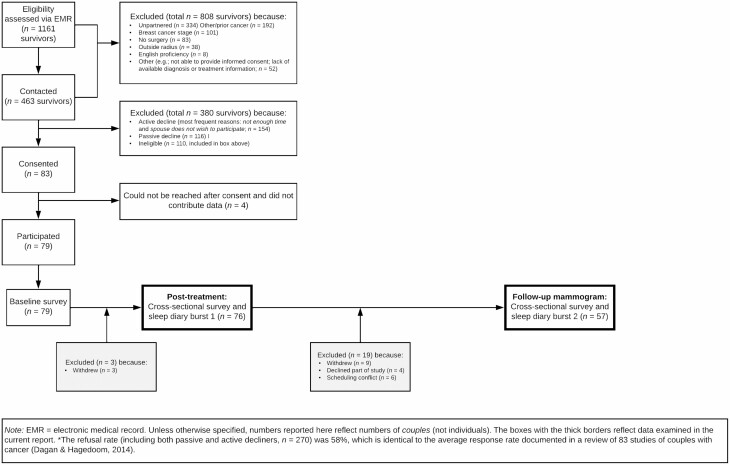
Data came from a larger longitudinal study (IRB approval; FWA00006557; CCC# 33026), whose purpose was to examine the interpersonal context of FCR [30, 31, 42–45]. Seventy-nine couples provided informed consent for the larger study. Eligibility criteria for survivors included: (a) Stage 0-IIIA breast cancer treated by surgery; (b) female; (c) in a committed, cohabiting relationship with a partner also willing to participate; (d) lived <1 hr from recruiting site; (e) no prior cancers; and (f) English-speaking. See Fig. 1 for a detailed participant flow diagram. Seventy-six couples completed sleep diary burst 1, which took place at the end of adjuvant treatment, and 57 couples also completed sleep diary burst 2, which overlapped with the first post-treatment mammogram (M = 6 months later). Between bursts, nine couples withdrew, four declined part of the study, and six had a scheduling conflict.
Fig. 1.
Participant flow diagram.
The average survivor age was 57.2 years (range = 35–75; SD = 9.5) and partner age was 59.3 years (range = 36–79; SD = 10.5). All but two partners were men. The mean relationship length was 28.7 years (range = 1–57; SD = 14.1). Most survivors (88%) and partners (84%) identified as non-Hispanic White. The modal family income was >$80,000. About 14% of survivors had Stage 0 cancer, 47% I, 37% II, and 2% IIIA. All had breast cancer surgery (77% breast-conserving, 23% mastectomy). About 35% received chemotherapy, 70% radiation, and 77% hormonal therapy; 59% and 19% received two and three of these modalities, respectively.
Procedure
The parent study followed couples starting shortly after initial breast cancer surgery until the first annual post-treatment mammogram (months post-diagnosis: M = 11, SD = 1.7). The present analyses focused on two important periods during this first year of survivorship: the completion of adjuvant treatment and the first post-treatment mammogram. Sleep diary burst 1 occurred as soon as possible after survivors completed adjuvant treatment (with exception of long-term hormonal treatment). Survivors and their partners independently completed an online panel survey once before beginning a 21-day sleep diary period. This online panel survey included several validated global FCR measures. Immediately after couples completed this panel survey, they began the 21-day sleep diary period (M = 5 months post-surgery, 20 days post-treatment), during which they independently completed four sleep disturbance items online daily within 1 hr of waking (diary completion rates: survivors 86%; partners 83%).
Sleep diary burst 2 overlapped with each survivor’s first post-treatment mammogram (typically the first mammogram since their diagnostic mammogram). As in sleep diary burst 1, a one-time panel survey including validated global FCR measures was immediately followed by a 21-day sleep diary period. For burst 2, this 21-day sleep diary period was scheduled to start approximately 2 weeks before each survivor’s mammogram appointment (M = 12 days pre-mammogram) and conclude 1 week after. As in the first diary burst, participants independently completed four online sleep disturbance items within 1 hr of waking (diary completion rates: survivors 92%; partners 87%). All but one survivor received negative results on the same day as their mammogram (excluding this couple’s data did not affect the results reported here).
Measures
Fear of cancer recurrence
Immediately before each sleep diary period, global FCR was assessed with three validated scales. Survivors and partners both reported concerns regarding the possibility of the survivor’s cancer recurrence. The 9-item Fear of Cancer Recurrence Inventory (FCRI) Severity subscale [46] assessed intrusive thoughts and perceived risk of cancer recurrence over the past month. The 4-item FCRI Distress subscale assessed emotional reactivity to thoughts about recurrence over the past month. The 4-item Concerns about Recurrence Scale (CARS) Overall Fear subscale [47] assessed the frequency and intensity of FCR and associated distress. All items were rated on a Likert-type scale and summed, with higher scores indicating more FCR. Coefficient alpha for each FCR scale reflected acceptable to good internal consistency reliability for survivors and partners at both burst 1 (α’s =.78–.91) and burst 2 (α’s =.71–.91).
Sleep disturbance
Within 1 hr of waking each morning during each of the sleep diary bursts, participants reported on sleep duration, subjective sleep quality, sleep onset latency, and wake after sleep onset. The sleep duration, subjective sleep quality, sleep onset latency, and wake after sleep onset (sleep disturbance) items were modeled after subscales of the Pittsburgh Sleep Quality Index (PSQI) [48]. For sleep duration, participants reported the number of hours and minutes they slept the previous night. For sleep onset latency and wake after sleep onset, participants indicated whether (0 = No, 1 = Yes) they had “difficulty getting to sleep” and “woke up in the middle of the night or early morning when they did not mean to,” respectively. Subjective sleep quality was defined as “feeling refreshed or rested in the morning,” a characterization of good sleep quality used in prior research [49]. This item (“How refreshed or rested do you feel right now after last night’s sleep?”) used a Likert-type response scale ranging from 0 = Not at all to 4 = Extremely. The four items were examined as separate outcomes due to (a) the unique characteristics of each measure (e.g., scalar vs. binary) and (b) each measure tapping unique sleep disturbances.
Statistical methods
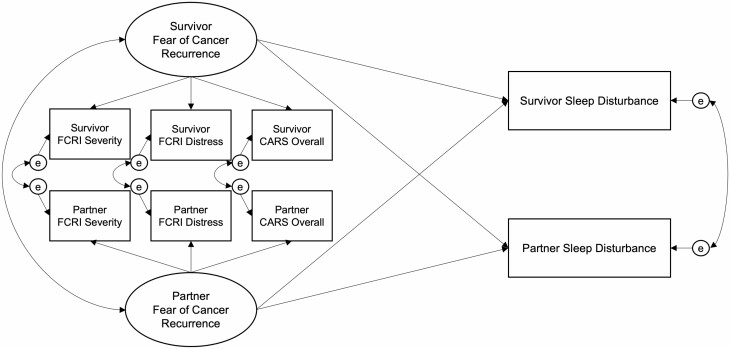
Primary analyses consisted of dyadic path models using structural equation modeling (SEM) with maximum likelihood estimation, which provides valid inferences assuming that data are missing at random. SEM was used to examine the relationship between an underlying FCR construct and each of the sleep outcomes at the two sleep diary bursts. The three manifest indicators (FCRI-Severity, FCRI-Distress, and CARS-Overall) were used to create latent FCR factors free of measurement error for both survivors and partners. To achieve identification and scale the latent factors, the FCRI-Severity subscale served as the scaling variable (i.e., its loading was fixed to one). The latent FCR factors for survivors and partners were allowed to covary. Actor-partner interdependence modeling [50] was used to capture the interdependence of sleep outcomes—associations between one participant’s FCR and their own sleep (actor effects) and with their partner’s sleep (cross-partner effects) were estimated simultaneously in each model (depicted in Fig. 2). For several models, the actor and/or cross-partner effects for survivors and partners were similar in magnitude. When supported by chi-square difference tests, these effects were constrained to be equal across survivors and partners and the results of the more parsimonious, constrained models are reported.
Fig. 2.
Full dyadic structural model estimated at both sleep diary bursts. FCRI Fear of Cancer Recurrence Inventory; CARS Concerns about Recurrence Scale.
We present results for sleep burst 1 followed by burst 2. The main results reflect links between latent FCR and each of the four sleep outcomes averaged across all days within each burst. For all models, actor and cross-partner effects were estimated for survivors and partners.
Results
Table 1 contains descriptive statistics and bivariate correlations for all variables. The observed range for the FCR indicators at burst 1 were as follows (shown as survivor/partner): FCRI Severity 2-20/1-17; FCRI Distress 0-8/0-10; CARS Overall 4-22/4-22. At burst 2, they were as follows: FCRI Severity 1-17/1-14; FCRI Distress 0-6/0-8; CARS Overall 4-19/4-18. Additionally, we examined bivariate correlations and mean differences between variables measured at diary burst 1 versus diary burst 2. Each of the four sleep variables showed significant bivariate correlations from diary burst 1 to diary burst 2, for survivors and partners (r’s range:.68–.89, all p’s < .001). The same was true for each of the three FCR indicator variables (r’s range:.50–.72, all p’s < .001). Paired samples t-tests failed to reveal statistically significant differences between mean levels of any of the sleep variables at diary burst 1 versus diary burst 2, for survivors or partners (all p’s > .09). However, all three FCR indicator variables, for both survivors and partners, were significantly higher at diary burst 1 compared to diary burst 2 (all p’s < .009; see Table 1), indicating consistent decreases in FCR for both partners over time.
Table 1.
Descriptive Statistics and Bivariate Correlations for Key Variables From Sleep Diary Bursts 1 and 2
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. FCRI-Severity | (.26)/(.28) | .65/.68 | .79/.79 | −.25/−.32 | −.31/−.22 | .11/.03 | .28/.05 |
| 2. FCRI-Distress | .71/.62 | (.15)/(.11) | .68/.56 | −.26/−.36 | −.16/−.16 | .06/.19 | .14/.22 |
| 3. CARS | .76/.71 | .60/.45 | (.22)/(.35) | −.22/−.35 | −.26/−.21 | .20/.10 | .25/.12 |
| 4. Sleep duration | −.38/−.25 | −.30/−.26 | −.21/−.12 | (.26)/(.36) | .43/.55 | −.34/−.36 | −.25/−.43 |
| 5. Sleep quality | −.39/−.56 | −.31/−.47 | −.17/−.44 | .52/.52 | (.23)/(.37) | −.45/−.25 | −.31/−.27 |
| 6. Sleep onset latencya | .37/.08 | .31/.16 | .41/.22 | −.49/−.43 | −.34/−.56 | (−.17)/(−.01) | .24/.29 |
| 7. Wake after sleep onseta | .15/.16 | .17/.24 | −.01/−.07 | −.33/−.18 | −.43/−.43 | .34/−.04 | (.13)/(.36) |
| M Burst 1 (Survivor/Partner) | 8.08/6.64 | 1.36/1.60 | 10.17/10.35 | 7.45/6.97 | 2.16/2.24 | 0.22/0.17 | 0.59/0.52 |
| SD Burst 1 (Survivor/Partner) | 4.72/4.37 | 1.85/2.53 | 3.88/4.66 | 0.79/1.01 | 0.71/0.73 | 0.25/0.23 | 0.31/0.33 |
| M Burst 2 (Survivor/Partner) | 7.19/4.91 | 1.04/0.54 | 9.51/8.49 | 7.48/7.07 | 2.24/2.28 | 0.27/0.15 | 0.63/0.58 |
| SD Burst 2 (Survivor/Partner) | 4.16/3.59 | 1.57/1.43 | 3.58/3.84 | 1.47/1.37 | 0.97/0.99 | 0.28/0.21 | 0.32/0.34 |
FCRI Fear of Cancer Recurrence Inventory; CARS Concerns about Recurrence Scale.
aReflects the proportion of days on which participants responded affirmatively. Sleep variables were derived by averaging each participant’s daily sleep. Top panel: Bivariate correlations among variables, with inter-correlations among Burst 1 variables shown before the slash and inter-correlations among Burst 2 variables shown after the slash (i.e., Burst 1/Burst 2). Correlations among survivors’ variables are below the diagonal (shaded light gray), correlations among partners’ variables are above the diagonal (shaded dark gray), and correlations between survivors’ and partners’ variables are in parentheses along the diagonal. Bottom panel: Means and SDs for survivors’ and partners’ variables are shown before and after the slash, respectively (i.e., survivor/partner). For Burst 1, all r> .23, p < .05. For Burst 2, all r > .28, p < .05. Burst 1 N = 76 couples. Burst 2 N = 57 couples. Correlations and mean differences between variables measured at burst 1 vs. burst 2 are not shown above but described in the main text.
Attrition Analysis
We tested whether the 57 couples who completed both sleep diary bursts differed from the 19 who only completed burst 1 in terms of the following: age, relationship length, income, survivor breast cancer stage, survivor adjuvant treatment type, FCR, and averaged daily sleep disturbance. Only one significant difference emerged: survivors who completed both diary bursts reported significantly greater sleep onset latency (p = .031) as compared to survivors who did not complete burst 2. Otherwise, there were no significant differences between these groups.
Estimated Levels of Clinical Dysfunction
Using burst 1 data, we estimated the extent of sleep disturbance and clinical FCR observed in this sample. On average, 27% of survivors and 52% of partners slept less than the consensus-recommended 7 hr for healthy adults [51]. To approximate the extent of clinical sleep disturbance (symptoms consistent with insomnia), we examined how many participants had difficulty falling/staying asleep (answering “yes” to sleep onset latency/wake after sleep onset) on ≥3 nights/week on average (diagnostic criterion for insomnia disorder; American Psychiatric Association et al., 2013). Thirteen percent of survivors and 10% of partners met this threshold for sleep onset latency; 62% and 54% for wake after sleep onset, respectively. Taken together, 10%–50% of the sample may have had clinically significant sleep disturbance potentially indicative of insomnia. Using an empirically supported cutoff of FCRI-Severity ≥13 [3], 20% of survivors and 18% of partners had clinical FCR. Of all sleep variables, only duration significantly differed between survivors and partners (M difference = 0.48 hr, t(71) = 3.14, p = .002), with survivors sleeping longer. FCR levels did not differ between survivors and partners (p’s > .05).
Sleep Diary Burst 1 (Completion of Adjuvant Treatment)
Latent FCR measurement model
The measurement model specifying latent survivor and partner FCR factors had good fit [χ 2(5) = 10.78, p = .056]. The three factor loadings were then constrained to be equal across survivors and partners but revealed somewhat poorer fit [Δχ 2(2) = 6.35, p = .042]. A model with two of three loadings constrained (the exception being the CARS-Overall) had acceptable fit, supporting partial metric invariance [Δχ 2(1) = 3.81, p = .051]. All standardized factor loadings were above 0.66 and statistically significant (p’s < .001). Coefficient omega indicated strong internal consistency of the latent variables for survivors and partners (both ω = .90). Survivor and partner factors were moderately positively correlated (r = .30, p = .030).
Full structural equation models
Across all four models, fit was similar after constraining actor and cross-partner effects to be equal for survivors and partners [sleep duration: Δχ 2(2) = 0.31; subjective sleep quality: Δχ 2(2) = 2.43; sleep onset latency: Δχ 2(2) = 2.26; wake after sleep onset: Δχ 2(2) = 1.33; all p’s >.05]. These constraints were maintained in the final models, results of which are shown below and in Table 2. Sleep duration model fit was acceptable: χ 2(16) = 20.02, p = .219; RMSEA = 0.01; CFI = 0.98; SRMR = 0.07. The actor effect—the effect of a participant’s FCR on their own sleep duration—was significant and negative (unstandardized b = −0.06, p = .001): a one-unit increase in latent FCR (scaled to range ~1–20) was associated with about 4 min less sleep each night, corresponding to a moderate-sized effect (β ≈ −0.30, see Table 2 for β’s for survivors and partners). The cross-partner effect—the effect of a person’s FCR on their partner’s sleep duration—did not differ from zero.
Table 2.
Sleep Diary Burst 1: Results of Structural Regression of Survivor and Partner Average Daily Sleep Disturbance Outcomes on FCR Factors
| Outcome/Effect | Estimate | SE | p | 95% CI | Standardized β | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Survivors | Partners | ||||
| Sleep duration (hours) | |||||||
| Actor effect | −0.064 | 0.019 | .001 | −0.100 | −0.027 | −0.343 | −0.264 |
| Cross-partner effect | −0.003 | 0.019 | .882 | −0.040 | 0.035 | −0.015 | −0.012 |
| Subjective sleep quality | |||||||
| Actor effect | −0.041 | 0.014 | .004 | −0.069 | −0.013 | −0.250 | −0.285 |
| Cross-partner effect | −0.051 | 0.015 | .001 | −0.080 | −0.022 | −0.239 | −0.320 |
| Sleep onset latency | |||||||
| Actor effect | 0.017 | 0.006 | .003 | 0.006 | 0.029 | 0.305 | 0.295 |
| Cross-partner effect | −0.002 | 0.006 | .702 | −0.013 | 0.009 | −0.036 | −0.039 |
| Wake after sleep onset | |||||||
| Actor effect | 0.011 | 0.007 | .123 | −0.003 | 0.025 | 0.150 | 0.140 |
| Cross-partner effect | 0.009 | 0.007 | .223 | −0.005 | 0.023 | 0.116 | 0.113 |
FCR fear of recurrence. Because all actor and cross-partner effects were constrained to be equal across survivors and partners, a single set of effects is shown for each sleep outcome (note that the standardized estimates remain unique). N = 76 couples.
Subjective sleep quality model fit was adequate: χ 2(16) = 30.93, p = 014; RMSEA = 0.02; CFI = 0.95; SRMR = 0.08. The actor effect of FCR on subjective sleep quality was significant, negative, and moderately sized (b = −0.04, p = .004). A one-unit increase in FCR was associated with a 0.04-unit decrease in sleep quality each night on average. A one-standard deviation (SD) increase in FCR predicted about a 0.25-SD decrease in subjective sleep quality each night. Here, the cross-partner effect was significant and negative (b = −0.05, p = .001) and corresponded to a moderate-sized effect (β ≈ −0.28). Compared to participants partnered with someone with lower FCR, participants partnered with someone with higher FCR reported poorer sleep quality each morning on average (controlling for effects of their own FCR).
Sleep onset latency model fit was adequate: χ 2(16) = 23.27, p = .107; RMSEA = 0.02; CFI = 0.97; SRMR = 0.09. There was a significant and positive effect of FCR on sleep onset latency (b = 0.02, p = .003), corresponding to a moderate-sized effect (β ≈ −.30). Participants with higher FCR had difficulty falling asleep a greater proportion of nights than participants with lower FCR. The cross-partner effect of FCR on sleep onset latency was not significant.
Wake after sleep onset model fit was acceptable: χ 2(16) = 22.47, p = .129; RMSEA = 0.02; CFI = 0.97; SRMR = 0.08. Neither actor nor cross-partner effects were significant.
Sleep Diary Burst 2 (First Post-Treatment Mammogram)
Latent FCR measurement model
To start, the measurement model was examined in which the factor loadings for the latent FCR factors for survivors and partners were freely estimated [χ 2(8) = 5.38, p = .716]. The three factor loadings were then constrained to be equal across survivors and partners [χ 2(10) = 7.48, p = .680]. The deviances of the model with constrained factor loadings did not significantly differ from the model where loadings were unconstrained [Δχ 2(2) = 2.17, p = .338], supporting partial metric invariance. All standardized factor loadings were above 0.65 and statistically significant (p’s < .001). Omega revealed good reliability for both survivors (ω = .86) and partners (ω = .89). Survivor and partner factors were moderately positively correlated (r = .33, p = .018).
Full structural equation models
Results of the final models are shown in Table 3. For sleep duration, the survivor and partner actor effects were similar in magnitude and so were constrained to be equal. The fit of the constrained model was similar to that of the unconstrained model [Δχ 2(1) = 0.025, p = .874] and model fit was adequate, χ 2(19) = 11.74, p = .896; RMSEA = 0.00; CFI = 1.00; SRMR = 0.06. The actor effect was significant and negative (b = −0.07, p = .004). Specifically, participants with a one-unit greater latent FCR score slept an average of about 4 min less per night, which corresponds to a moderate-sized effect (β ≈ −0.30, see Table 3 for β’s for survivors and partners). None of the cross-partner effects of FCR on sleep duration were statistically significant.
Table 3.
Sleep Diary Burst 2: Structural Regression of Survivor and Partner Average Daily Sleep Disturbance Outcomes on FCR Factors
| Outcome | Estimate | SE | p | 95% CI | ||
|---|---|---|---|---|---|---|
| Lower | Upper | Standardized β | ||||
| Survivors | ||||||
| Sleep duration | ||||||
| FCR actor effect | −0.072b | 0.025 | .004 | −0.121 | −0.024 | −0.30 |
| FCR cross-partner effect | 0.036 | 0.034 | .280 | -0.030 | 0.103 | 0.15 |
| Subjective sleep quality | ||||||
| FCR actor effect | −0.103 | 0.023 | <.001 | −0.149 | −0.057 | −0.64 |
| FCR cross-partner effect | 0.020 | 0.022 | .360 | −0.023 | 0.062 | 0.12 |
| Onset latencya | ||||||
| FCR actor effect | 0.139 | 0.112 | .214 | −0.080 | 0.359 | 0.24 |
| FCR cross-partner effect | −0.025 | 0.098 | .801 | −0.217 | 0.167 | −0.04 |
| Wake after sleep onseta | ||||||
| FCR actor effect | 0.056 | 0.116 | .629 | −0.172 | 0.284 | 0.09 |
| FCR cross-partner effect | 0.068 | 0.102 | .505 | −0.132 | 0.269 | 0.11 |
| Partners | ||||||
| Sleep duration | ||||||
| FCR actor effect | −0.072b | 0.025 | .004 | −0.121 | −0.024 | −0.31 |
| FCR cross-partner effect | −0.032 | 0.026 | .207 | −0.083 | 0.018 | −0.14 |
| Subjective sleep quality | ||||||
| FCR actor effect | −0.017 | 0.027 | .514 | −0.070 | 0.035 | −0.09 |
| FCR cross-partner effect | −0.085 | 0.027 | .001 | −0.137 | −0.033 | −0.45 |
| Onset latencya | ||||||
| FCR actor effect | 0.019 | 0.094 | .843 | −0.165 | 0.202 | 0.03 |
| FCR cross-partner effect | 0.230 | 0.100 | .022 | 0.033 | 0.427 | 0.37 |
| Wake after sleep onseta | ||||||
| FCR actor effect | −0.002 | 0.094 | .980 | −0.187 | 0.182 | −0.01 |
| FCR cross-partner effect | 0.099 | 0.123 | .421 | −0.143 | 0.341 | 0.14 |
FCR fear of recurrence.
aBinary outcome.
bCoefficients constrained to be equal for survivors and partners. N = 57 couples.
When modeling subjective sleep quality, the survivor and partner actor and cross-partner effects were freely estimated. Model fit was adequate χ 2(18) = 14.71, p = .682; RMSEA = 0.00; CFI = 1.00; SRMR = 0.06. FCR reported by survivors was significantly and negatively associated with their own sleep quality (b = −0.10, p < .001). A one-unit increase in survivor FCR was associated with a 0.10-unit decrease in their own subjective sleep quality each night. A one-SD increase in FCR predicted a −0.65-SD decrease in sleep quality each night on average, a moderate-to-large effect. Partners’ FCR was not significantly associated with their own sleep quality. A significant (b = −0.85, p = .001) cross-partner effect emerged such that survivors’ FCR was negatively associated with partners’ sleep quality (controlling for effects of their own FCR). This cross-partner effect corresponds to a moderately-sized effect (β = −0.45). The association between partners’ FCR and survivors’ sleep quality was not statistically significant.
When modeling sleep onset latency, the actor and cross-partner effects for survivors and partners were left unconstrained. Model fit was adequate, χ 2(18) = 14.38, p = .704.; RMSEA = 1.00; CFI = 1.00; SRMR = 0.06. There was a significant and positive cross-partner effect−survivors’ FCR was associated with partners’ sleep onset latency (b = 0.23, p = .022), a moderate-sized effect (β = 0.37). Survivors with higher FCR had partners who had difficulty falling asleep a greater proportion of nights than did survivors with lower FCR. Neither the actor effects nor links between partners’ FCR and survivors’ sleep onset latency were significant.
For wake after sleep onset, the actor and cross-partner effects for survivors and partners were freely estimated. Model fit was acceptable χ 2(18) = 24.76, p = .132; RMSEA = 0.02; CFI = 0.95; SRMR = 0.08. There were no significant actor or cross-partner effects of FCR found.
Discussion
Despite reports of elevated FCR and sleep disturbance in cancer survivors, this is the first known study of links between FCR and daily reports of sleep disturbance in cancer survivors. Furthermore, no prior studies have examined these links in cancer survivors’ intimate partners or the reciprocal effects of one partner’s FCR on the other’s sleep. The current study focused on two important periods during the first year of breast cancer survivorship: the completion of adjuvant treatment (typical onset of FCR) [40] and the first post-treatment mammogram (typical trigger of FCR) [41, 42, 46, 52]. Results from both periods supported the hypothesis that FCR may contribute to survivor and partner sleep disturbance.
In the first sleep diary burst, actor effects of FCR on sleep (i.e., effect of a person’s FCR on their own sleep) were significant and in the expected direction for three of the four sleep outcomes (wake after sleep onset being the exception). There was also a significant cross-partner effect such that participants’ FCR was associated with their partner’s reduced sleep quality. For sleep diary burst 1, actor and cross-partner effects for survivors and partners did not differ.
Results from the second sleep diary burst, which overlapped with survivors’ first post-treatment mammogram, also supported the hypothesis that FCR is associated with greater sleep disturbance. Survivors’ FCR was significantly associated with their own reduced sleep duration and sleep quality. Partners’ FCR was significantly associated with their own reduced sleep duration. There were also significant cross-partner effects, such that survivors’ FCR was associated with partners’ reduced sleep quality and greater sleep onset latency.
The standardized coefficients (see Tables 2 and 3) for the FCR-sleep links reported here were moderate to large in magnitude, highlighting the potential clinical significance of these findings. For example, among the most interesting findings, a four-unit increase in FCR—roughly equal to one SD in the FCRI-Severity scale—predicted about 15 min of sleep loss each night (1.75 hr less/week) and 17 min of sleep loss each night (~2 hr less/week) during sleep diary burst 1 and 2, respectively. Effects for survivors and partners did not differ.
Across both bursts, there were more robust actor than cross-partner effects, suggesting that the experience of FCR is more closely linked with one’s own versus one’s partner’s compromised sleep. Among the four significant cross-partner effects observed, three were only found for partners, not survivors, such that survivors’ FCR may have had a stronger impact on partners’ sleep than vice versa. This pattern of results is somewhat inconsistent with a few prior studies that have shown significant cross-partner effects of psychological distress on sleep for both survivors and their partners [38, 39]. Although speculative, it is possible that while in the stressful cancer survivorship trajectory, partners may be more emotionally and physiologically responsive to the survivor’s experience rather than vice versa. While beyond the scope of the current study, future research should examine potential mechanisms by which survivor FCR impacts partner sleep (and vice versa). Relationship functioning has been hypothesized as one such mechanism [38].
Of the four sleep outcomes examined, FCR was most consistently related to subjective sleep quality and these effect sizes were often large. For example, the model for sleep diary burst 2 predicted that survivors with a one-SD higher FCR score would have a 0.65-SD lower average daily sleep quality score. Poor perceived sleep quality is one of the main diagnostic criteria for insomnia [6]. Poor sleep quality can co-occur with other insomnia symptoms or be a standalone complaint, even among those reporting typical sleep duration [53]. Furthermore, sleep quality has a stable course [54] and is uniquely associated with important health outcomes, including cancer [55]. Therefore, each of the sleep outcomes examined here are independently clinically relevant for health.
Despite 62% of survivors reporting significant wake after sleep onset, no associations emerged between FCR and this dimension. Given the uniqueness of the sleep disturbance facets measured, it is possible that FCR impacts sleep duration, quality, and onset latency, but not wake after sleep onset. The wake after sleep onset item was binary and assessed whether or not participants unintentionally woke up in the middle of the night or early morning. This operationalization does not probe whether nocturnal awakenings were prolonged (difficulty falling back to sleep), which is an important feature of insomnia, particularly for older adults who experience increased awakenings as their sleep changes with age [56]. Therefore, it is also possible that this operationalization may have obscured relationships between FCR and wake after sleep onset because it is worry about recurrence during these awakenings that would be expected to result in prolonged awakenings and thereby, impaired sleep for this population. Future studies should explore other methods for brief, daily assessment of this aspect of sleep disturbance.
Taken together, the current findings highlight critical gaps in existing theoretical models of the antecedents and consequences of FCR, none of which have commented on the potential role of sleep. While a causal relation between FCR and sleep disturbance cannot be ascertained from this observational study, results suggest that further attention to sleep’s role in the etiology, maintenance, and/or consequences of FCR is warranted. The role of stressful life events and associated worry in insomnia is well-established [16–19]. Indeed, negative health-related events, including medical illness, are among the most common precipitating factors for insomnia [57]. Therefore, stressful cancer-related events such as the completion of adjuvant treatment and the first post-treatment mammogram and associated FCR (particularly pre- and mid-sleep) may result in acute sleep disturbance. This sleep disturbance may become chronic for those who begin to worry about sleep itself and/or develop wake-bed associations and maladaptive compensatory sleep behaviors. Therefore, this mechanism may in part explain the elevated insomnia rates in cancer survivors and partners compared to the general population. While this hypothesis is based on well-established theory and supported by empirical data pointing to similar mechanisms operating with other forms of psychological distress, it cannot be directly tested in the context of the current observational study.
Published FCR interventions trials have not monitored or targeted sleep disturbance [58]. Given the current findings, FCR interventions may provide an opportunity to address sleep disturbance in this population known to be at elevated risk for insomnia, thereby also offering short- and long-term health benefits. Given the current evidence for cross-partner effects, couple-focused interventions for FCR should be considered. To the authors’ knowledge, neither couple- nor partner-focused interventions of FCR have been developed or tested.
Limitations of this study may inform future work on FCR and sleep. As previously mentioned, sleep disturbance is more prevalent in breast cancer than other cancers and this effect is not fully explained by gender [8]. Menopausal vasomotor symptoms (e.g., hot flashes) resulting from adjuvant treatment and/or the discontinuation of hormone replacement therapy may be unique contributors for this population [59–61]. Nevertheless, treatment side effects are also implicated theoretically as antecedents and consequences of FCR [5]. Survivors may erroneously interpret somatic symptoms as evidence of their cancer recurring, triggering FCR. Conversely, survivors with elevated FCR may become hypervigilant, excessively checking for and becoming preoccupied with somatic symptoms. Given the role of somatic symptoms in FCR, removing the variance associated with side effects may inadvertently remove part of the phenomenon of interest [62]; therefore, treatment side effects were not covariates in this study.
Additionally, due the observational nature of this study, the direction of effects cannot be determined. Thus, it is possible that, contrary to hypotheses, sleep disturbance causally influences FCR and future research should examine these within-person links. Like other studies of couples (vs. patient-only) coping with cancer, the response rate for the parent study was modest. This response rate is reflective of two challenges unique to studies of couples coping with cancer: couples were lost if either partner was unwilling to participate (indeed one of the most frequent reasons for declining) and indirect recruitment of partners—researchers were initially unable to contact partners directly [63]. Therefore, the modestly sized sample, which was also homogeneous regarding sociodemographic characteristics, limits generalizability and the ability to detect smaller effects. Future studies should attempt to recruit not only larger but also more diverse or distressed samples. Finally, sleep was measured via self-report. Future work should replicate and extend these results using well-powered designs with objective measures of sleep (e.g., actigraphy or polysomnography).
In conclusion, relationships between FCR and sleep are a critical gap in theoretical models and interventions for FCR. The present findings indicate that FCR experienced by both early-stage breast cancer survivors and their intimate partners is associated with their own as well as their partner’s sleep disturbance. These links highlight the need for more research on FCR and other health-related behaviors (e.g., adherence to adjuvant hormone therapy, physical activity) that are modifiable and have known associations with health and mortality. Such research could inform interventions for improving FCR and thereby health outcomes for the growing population of breast cancer survivors and their partners.
Contributor Information
Christine Perndorfer, Department of Psychological & Brain Sciences, University of Delaware, Newark, DE 19716, USA.
Emily C Soriano, Department of Psychological & Brain Sciences, University of Delaware, Newark, DE 19716, USA.
Scott D Siegel, Value Institute, Helen F. Graham Cancer Center and Research Institute, Newark, DE 19713, USA.
Rebecca M C Spencer, Department of Psychological & Brain Sciences, University of Massachusetts Amherst, Amherst, MA 01003, USA.
Amy K Otto, Department of Public Health Sciences, University of Miami Miller School of Medicine, Miami, FL 33136, USA.
Jean-Philippe Laurenceau, Department of Psychological & Brain Sciences, University of Delaware, Newark, DE 19716, USA.
Funding
The research was supported by the U.S. National Cancer Institute (CA171921).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Christine Perndorfer, Emily C. Soriano, Scott D. Siegel, Rebecca M. C. Spencer, Amy K. Otto, and Jean-Philippe Laurenceau declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. American Cancer Society. (2019). Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc. 2019. [Google Scholar]
- 2. Miller, K. D., Nogueira, L., Mariotto, A. B., Rowland, J. H., Yabroff, K. R., Alfano, C. M., Jemal, A., Kramer, J. L., & Siegel, R. L. (2019). Cancer treatment and survivorship statistics, 2019. CA: A Cancer Journal for Clinicians, 69(5), 363–385. 10.3322/caac.21565@10.3322/(ISSN)1542-4863.statistics [DOI] [PubMed] [Google Scholar]
- 3. Simard, S., & Savard, J. (2015). Screening and comorbidity of clinical levels of fear of cancer recurrence. Journal of Cancer Survivorship, 9(3), 481–491. 10.1007/s11764-015-0424-4 [DOI] [PubMed] [Google Scholar]
- 4. Lebel, S., Ozakinci, G., Humphris, G., Mutsaers, B., Thewes, B., Prins, J., Dinkel, A., & Butow, P. (2016). From normal response to clinical problem: Definition and clinical features of fear of cancer recurrence. Supportive Care in Cancer, 24(8), 3265–3268. 10.1007/s00520-016-3272-5 [DOI] [PubMed] [Google Scholar]
- 5. Curran, L., Sharpe, L., & Butow, P. (2017). Anxiety in the context of cancer: A systematic review and development of an integrated model. Clinical Psychology Review, 56, 40–54. 10.1016/j.cpr.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 6. American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- 7. Savard, J., Ivers, H., Villa, J., Caplette-Gingras, A., & Morin, C. M. (2011). Natural Course of Insomnia Comorbid With Cancer: An 18-Month Longitudinal Study. Journal of Clinical Oncology, 29(26), 3580–3586. 10.1200/JCO.2010.33.2247 [DOI] [PubMed] [Google Scholar]
- 8. Savard, J., Villa, J., Ivers, H., Simard, S., & Morin, C. M. (2009). Prevalence, Natural Course, and Risk Factors of Insomnia Comorbid With Cancer Over a 2-Month Period. Journal of Clinical Oncology, 27(31), 5233–5239. 10.1200/JCO.2008.21.6333 [DOI] [PubMed] [Google Scholar]
- 9. Goldstein, A. N., & Walker, M. P. (2014). The Role of Sleep in Emotional Brain Function. Annual Review of Clinical Psychology, 10(1), 679–708. 10.1146/annurev-clinpsy-032813-153716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irwin, M. R. (2015). Why Sleep Is Important for Health: A Psychoneuroimmunology Perspective. Annual Review of Psychology, 66(1), 143–172. 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradshaw, P. T., Stevens, J., Khankari, N., Teitelbaum, S. L., Neugut, A. I., & Gammon, M. D. (2016). Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology, 27(1), 6–13. 10.1097/EDE.0000000000000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alfano, C. M., Lichstein, K. L., Vander Wal, G. S., Smith, A. W., Reeve, B. B., McTiernan, A., Bernstein, L., Baumgartner, K. B., & Ballard-Barbash, R. (2011). Sleep duration change across breast cancer survivorship: Associations with symptoms and health-related quality of life. Breast Cancer Research and Treatment, 130(1), 243–254. 10.1007/s10549-011-1530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palesh, O., Aldridge-Gerry, A., Zeitzer, J. M., Koopman, C., Neri, E., Giese-Davis, J., Jo, B., Kraemer, H., Nouriani, B., & Spiegel, D. (2014). Actigraphy-Measured Sleep Disruption as a Predictor of Survival among Women with Advanced Breast Cancer. Sleep, 37(5), 837–842. 10.5665/sleep.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trudel-Fitzgerald, C., Zhou, E. S., Poole, E. M., Zhang, X., Michels, K. B., Eliassen, A. H., Chen, W. Y., Holmes, M. D., Tworoger, S. S., & Schernhammer, E. S. (2017). Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. British Journal of Cancer, 116(9), 1239–1246. 10.1038/bjc.2017.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bower, J. E., Ganz, P. A., Irwin, M. R., Kwan, L., Breen, E. C., & Cole, S. W. (2011). Inflammation and Behavioral Symptoms After Breast Cancer Treatment: Do Fatigue, Depression, and Sleep Disturbance Share a Common Underlying Mechanism? Journal of Clinical Oncology, 29(26), 3517–3522. 10.1200/JCO.2011.36.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hauri, P. J. (Ed.). (1991). Case Studies in Insomnia. Springer; US. 10.1007/978-1-4757-9586-8 [DOI] [Google Scholar]
- 17. Morin, C. M. (1993). Insomnia: Psychological assessment and management (pp. xvii, 238). Guilford Press. [Google Scholar]
- 18. Spielman, A. J. (1986). Assessment of insomnia. Clinical Psychology Review, 6(1), 11–25. 10.1016/0272-7358(86)90015-2 [DOI] [Google Scholar]
- 19. Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002; 40:869–893. [DOI] [PubMed] [Google Scholar]
- 20. Curran, L., Sharpe, L., MacCann, C., & Butow, P. (2020). Testing a model of fear of cancer recurrence or progression: The central role of intrusions, death anxiety and threat appraisal. Journal of Behavioral Medicine, 43(2), 225–236. 10.1007/s10865-019-00129-x [DOI] [PubMed] [Google Scholar]
- 21. Dupont, A., Bower, J. E., Stanton, A. L., & Ganz, P. A. (2014). Cancer-related intrusive thoughts predict behavioral symptoms following breast cancer treatment. Health Psychology, 33(2), 155–163. 10.1037/a0031131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor, T. R., Huntley, E. D., Makambi, K., Sween, J., Adams-Campbell, L. L., Frederick, W., & Mellman, T. A. (2012). Understanding sleep disturbances in African-American breast cancer survivors: A pilot study: Sleep disturbances in African-American breast cancer survivors. Psycho-Oncology, 21(8), 896–902. 10.1002/pon.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matulonis, U. A., Kornblith, A., Lee, H., Bryan, J., Gibson, C., Wells, C., Lee, J., Sullivan, L., & Penson, R. (2008). Long-term adjustment of early-stage ovarian cancer survivors. International Journal of Gynecological Cancer, 18(6), 1183–1193. 10.1111/j.1525-1438.2007.01167.x [DOI] [PubMed] [Google Scholar]
- 24. Roth, A. J., Rosenfeld, B., Kornblith, A. B., Gibson, C., Scher, H. I., Curley-Smart, T., Holland, J. C., & Breitbart, W. (2003). The Memorial Anxiety Scale for Prostate Cancer. Cancer, 97(11), 2910–2918. 10.1002/cncr.11386 [DOI] [PubMed] [Google Scholar]
- 25. Peppercorn, J. M., Jimenez, R., Rabin, J., Quain, K., Chinn, G., McDonough, A., O’Donnell, E., Park, E. R., & Perez, G. K. (2017). Prevalence and predicators of insomnia among cancer survivors. Journal of Clinical Oncology, 35(15_suppl), e21603–e21603. 10.1200/JCO.2017.35.15_suppl.e21603 [DOI] [Google Scholar]
- 26. Berrett-Abebe, J., Cadet, T., Pirl, W., & Lennes, I. (2015). Exploring the Relationship Between Fear of Cancer Recurrence and Sleep Quality in Cancer Survivors. Journal of Psychosocial Oncology, 33(3), 297–309. 10.1080/07347332.2015.1020586 [DOI] [PubMed] [Google Scholar]
- 27. Mutsaers, B., Jones, G., Rutkowski, N., Tomei, C., Séguin Leclair, C., Petricone-Westwood, D., Simard, S., & Lebel, S. (2016). When fear of cancer recurrence becomes a clinical issue: A qualitative analysis of features associated with clinical fear of cancer recurrence. Supportive Care in Cancer, 24(10), 4207–4218. 10.1007/s00520-016-3248-5 [DOI] [PubMed] [Google Scholar]
- 28. Matthews, K. A., Patel, S. R., Pantesco, E. J., Buysse, D. J., Kamarck, T. W., Lee, L., & Hall, M. H. (2018). Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health, 4(1), 96–103. 10.1016/j.sleh.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harvey, A. G., & Tang, N. K. Y. (2012). ( Mis)perception of sleep in insomnia: A puzzle and a resolution. Psychological Bulletin, 138(1), 77–101. https://doi-org.udel.idm.oclc.org/10.1037/a0025730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soriano, E. C., Perndorfer, C., Siegel, S. D., & Laurenceau, J.-P. (2019). Threat sensitivity and fear of cancer recurrence: A daily diary study of reactivity and recovery as patients and spouses face the first mammogram post-diagnosis. Journal of Psychosocial Oncology, 37(2), 131–144. 10.1080/07347332.2018.1535532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soriano, E. C., Otto, A. K., LoSavio, S. T., Perndorfer, C., Siegel, S. D., & Laurenceau, J.-P. (2021). Fear of Cancer Recurrence and Inhibited Disclosure: Testing the Social-Cognitive Processing Model in Couples Coping With Breast Cancer. Annals of Behavioral Medicine, 55(3), 192–202. 10.1093/abm/kaaa043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim, Y., Carver, C. S., Spillers, R. L., Love-Ghaffari, M., & Kaw, C.-K. (2012). Dyadic effects of fear of recurrence on the quality of life of cancer survivors and their caregivers. Quality of Life Research, 21(3), 517–525. 10.1007/s11136-011-9953-0 [DOI] [PubMed] [Google Scholar]
- 33. Mellon, S., Kershaw, T. S., Northouse, L. L., & Freeman-Gibb, L. (2007). A family-based model to predict fear of recurrence for cancer survivors and their caregivers. Psycho-Oncology, 16(3), 214–223. 10.1002/pon.1074 [DOI] [PubMed] [Google Scholar]
- 34. Carney, S., Koetters, T., Cho, M., West, C., Paul, S. M., Dunn, L., Aouizerat, B. E., Dodd, M., Cooper, B., Lee, K., Wara, W., Swift, P., & Miaskowski, C. (2011). Differences in Sleep Disturbance Parameters Between Oncology Outpatients and Their Family Caregivers. Journal of Clinical Oncology, 29(8), 1001–1006. 10.1200/JCO.2010.30.9104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang, E. W.-C., Tsai, Y.-Y., Chang, T.-W., & Tsao, C.-J. (2007). Quality of sleep and quality of life in caregivers of breast cancer patient. Psycho-Oncology, 16(10), 950–955. 10.1002/pon.1167 [DOI] [PubMed] [Google Scholar]
- 36. Gunn, H. E., Buysse, D. J., Hasler, B. P., Begley, A., & Troxel, W. M. (2015). Sleep Concordance in Couples is Associated with Relationship Characteristics. SLEEP. 10.5665/sleep.4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Revenson, T. A., Marín-Chollom, A. M., Rundle, A. G., Wisnivesky, J., & Neugut, A. I. (2016). Hey Mr. Sandman: Dyadic effects of anxiety, depressive symptoms and sleep among married couples. Journal of Behavioral Medicine, 39(2), 225–232. 10.1007/s10865-015-9693-7 [DOI] [PubMed] [Google Scholar]
- 38. Chan, J. S. M., Yu, N. X., Chow, A. Y. M., Chan, C. L. W., Chung, K.-F., Ho, R. T. H., Ng, S., Yuen, L. P., & Chan, C. H. Y. (2017). Dyadic associations between psychological distress and sleep disturbance among Chinese patients with cancer and their spouses. Psycho-Oncology, 26(6), 856–861. 10.1002/pon.4240 [DOI] [PubMed] [Google Scholar]
- 39. Otto, A. K., Gonzalez, B. D., Heyman, R. E., Vadaparampil, S. T., Ellington, L., & Reblin, M. (2019). Dyadic effects of distress on sleep duration in advanced cancer patients and spouse caregivers. Psycho-Oncology, 28(12), 2358–2364. 10.1002/pon.5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stanton, A. L., Ganz, P. A., Rowland, J. H., Meyerowitz, B. E., Krupnick, J. L., & Sears, S. R. (2005). Promoting adjustment after treatment for cancer. Cancer, 104(S11), 2608–2613. 10.1002/cncr.21246 [DOI] [PubMed] [Google Scholar]
- 41. McGinty, H. L., Small, B. J., Laronga, C., & Jacobsen, P. B. (2016). Predictors and patterns of fear of cancer recurrence in breast cancer survivors. Health Psychology, 35(1), 1–9. 10.1037/hea0000238 [DOI] [PubMed] [Google Scholar]
- 42. Soriano, E. C., Perndorfer, C., Otto, A. K., Siegel, S. D., & Laurenceau, J.-P. (2018). Does sharing good news buffer fear of bad news? A daily diary study of fear of cancer recurrence in couples approaching the first mammogram post-diagnosis. Psycho-Oncology, 27(11), 2581–2586. 10.1002/pon.4813 [DOI] [PubMed] [Google Scholar]
- 43. Perndorfer, C., Soriano, E. C., Siegel, S. D., & Laurenceau, J.-P. (2019). Everyday protective buffering predicts intimacy and fear of cancer recurrence in couples coping with early-stage breast cancer. Psycho-Oncology, 28(2), 317–323. 10.1002/pon.4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soriano, E. C., Pasipanodya, E. C., LoSavio, S. T., Otto, A. K., Perndorfer, C., Siegel, S. D., & Laurenceau, J.-P. (2018). Social constraints and fear of recurrence in couples coping with early stage breast cancer. Health Psychology, 37(9), 874–884. 10.1037/hea0000649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soriano, E. C., Valera, R., Pasipanodya, E. C., Otto, A. K., Siegel, S. D., & Laurenceau, J.-P. (2019). Checking Behavior, Fear of Recurrence, and Daily Triggers in Breast Cancer Survivors. Annals of Behavioral Medicine, 53(3), 244–254. 10.1093/abm/kay033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simard, S., & Savard, J. (2009). Fear of Cancer Recurrence Inventory: Development and initial validation of a multidimensional measure of fear of cancer recurrence. Supportive Care in Cancer, 17(3), 241–251. 10.1007/s00520-008-0444-y [DOI] [PubMed] [Google Scholar]
- 47. Vickberg, S. M. J. (2003). The Concerns About Recurrence Scale (CARS): A systematic measure of women’s fears about the possibility of breast cancer recurrence. Annals of Behavioral Medicine, 25(1), 16–24. [DOI] [PubMed] [Google Scholar]
- 48. Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 49. Libman, E., Fichten, C., Creti, L., Conrod, K., Tran, D.-L., Grad, R., Jorgensen, M., Amsel, R., Rizzo, D., Baltzan, M., Pavilanis, A., & Bailes, S. (2016). Refreshing Sleep and Sleep Continuity Determine Perceived Sleep Quality. Sleep Disorders, 2016, 1–10. 10.1155/2016/7170610. Article ID: 7170610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kenny, D. A., Kashy, D. A., & Cook, W. L. (2006). Dyadic Data Analysis. Guilford Press. [Google Scholar]
- 51. Watson Nathaniel F., Badr M. Safwan, Belenky Gregory, Bliwise Donald L., Buxton Orfeu M., Buysse Daniel, Dinges David F., Gangwisch James, Grandner Michael A., Kushida Clete, Malhotra Raman K., Martin Jennifer L., Patel Sanjay R., Quan Stuart F., & Esra Tasali. (n.d.). Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Journal of Clinical Sleep Medicine, 11(06), 591–592. 10.5664/jcsm.4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gil, K. M., Mishel, M. H., Belyea, M., Germino, B., Porter, L. S., Carlton LaNey, I., & Stewart, J. (2004). Triggers of uncertainty about recurrence and long-term treatment side effects in older African American and Caucasian breast cancer survivors. Oncology Nursing Forum, 31(3), 633–639. 10.1188/04.ONF.633-639 [DOI] [PubMed] [Google Scholar]
- 53. Roth, T., Zammit, G., Lankford, A., Mayleben, D., Stern, T., Pitman, V., Clark, D., & Werth, J. L. (2010). Nonrestorative Sleep as a Distinct Component of Insomnia. Sleep, 33(4), 449–458. 10.1093/sleep/33.4.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang, J., Lam, S.-P., Li, S. X., Li, A. M., & Wing, Y.-K. (2012). The longitudinal course and impact of non-restorative sleep: A five-year community-based follow-up study. Sleep Medicine, 13(6), 570–576. 10.1016/j.sleep.2011.12.012 [DOI] [PubMed] [Google Scholar]
- 55. Zhang, J., Lamers, F., Hickie, I. B., He, J.-P., Feig, E., & Merikangas, K. R. (2013). Differentiating Nonrestorative Sleep from Nocturnal Insomnia Symptoms: Demographic, Clinical, Inflammatory, and Functional Correlates. Sleep, 36(5), 671–679. 10.5665/sleep.2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bootzin, R. R., & Epstein, D. R. (2011). Understanding and Treating Insomnia. Annual Review of Clinical Psychology, 7(1), 435–458. 10.1146/annurev.clinpsy.3.022806.091516 [DOI] [PubMed] [Google Scholar]
- 57. Bastien, C. H., Vallieres, A., & Morin, C. M. (2004). Precipitating Factors of Insomnia. Behavioral Sleep Medicine, 2(1), 50–62. 10.1207/s15402010bsm0201_5 [DOI] [PubMed] [Google Scholar]
- 58. Tauber, N. M., O’Toole, M. S., Dinkel, A., Galica, J., Humphris, G., Lebel, S., Maheu, C., Ozakinci, G., Prins, J., Sharpe, L., Smith, A.“Ben,” Thewes, B., Simard, S., & Zachariae, R. (2019). Effect of Psychological Intervention on Fear of Cancer Recurrence: A Systematic Review and Meta-Analysis. Journal of Clinical Oncology, JCO.19.00572. 10.1200/JCO.19.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carpenter, J. S., Elam, J. L., Ridner, S. H., Carney, P. H., Cherry, G. J., & Cucullu, H. L. (2004). Sleep, Fatigue, and Depressive Symptoms in Breast Cancer Survivors and Matched Healthy Women Experiencing Hot Flashes. Oncology Nursing Forum, 31(3), 591–598. 10.1188/04.ONF.591-598 [DOI] [PubMed] [Google Scholar]
- 60. Couzi, R. J., Helzlsouer, K. J., & Fetting, J. H. (1995). Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. Journal of Clinical Oncology, 13(11), 2737–2744. 10.1200/JCO.1995.13.11.2737 [DOI] [PubMed] [Google Scholar]
- 61. Savard, J., Davidson, J. R., Ivers, H., Quesnel, C., Rioux, D., Dupéré, V., Lasnier, M., Simard, S., & Morin, C. M. (2004). The association between nocturnal hot flashes and sleep in breast cancer survivors. Journal of Pain and Symptom Management, 27(6), 513–522. 10.1016/j.jpainsymman.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 62. Spector, P. E., & Brannick, M. T. (2011). Methodological Urban Legends: The Misuse of Statistical Control Variables. Organizational Research Methods, 14(2), 287–305. 10.1177/1094428110369842 [DOI] [Google Scholar]
- 63. Dagan, M., & Hagedoorn, M. (2014). Response rates in studies of couples coping with cancer: A systematic review. Health Psychology, 33(8), 845. 10.1037/hea0000013 [DOI] [PubMed] [Google Scholar]