Abstract
Tumor necrosis factor alpha (TNF-α) is important in resistance to various microorganisms and provides signals to the target cells through two different receptors, TNF-α receptor I (TNFRI) (p55 receptor) and TNFRII (p75 receptor). To delineate the significance of the two different signaling pathways in resisting infections with extracellular bacteria, we examined the resistance of mice to Streptococcus pneumoniae (serotype 6B). TNF-α needs to be present early in infections, since one injection of wild-type mice with anti-TNF-α leads to an increased susceptibility of these mice to S. pneumoniae. TNF-α signaling through the p55 receptor (but not the p75 receptor) is crucial in resisting S. pneumoniae infections, because intraperitoneal injection of 100 CFU/mouse killed p55-deficient mice by day 2 of infection, whereas 1,000,000 CFU/mouse was needed to kill half of the control mice. p55-deficient mice do not show evidence of a deficient acute-phase response. All three types of mice (p55 deficient, p75 deficient, and normal) showed comparable rises in the levels of two acute-phase proteins (serum amyloid P and C3) at 24, 48, and 72 h after the experimental infections, and all of the mice showed comparable influxes of neutrophils to the site of infection. Finally, it was demonstrated that p55-deficient mice can be protected from the lethal effects of S. pneumoniae infection by injection of antibodies specific for S. pneumoniae polysaccharide capsule.
Tumor necrosis factor alpha (TNF-α) is a pleiotropic cytokine with two active forms: one is a surface-bound 26-kDa protein, and the second is a 17-kDa secreted protein which is produced from the 26-kDa surface protein by the cleavage mediated by TNF-α-converting enzyme (3, 29). TNF-α mediates its biological effects through two receptors designated TNF-α receptor I (TNFRI) and TNFRII, with molecular mass of 55 and 75 kDa, respectively. TNFRI (p55 receptor) has an intracytoplasmic death domain to which the intracellular protein TRADD binds (18). Signaling through TNFRI (p55) has been shown to be important in many biological processes, including apoptosis, lethal shock, germinal center formation, and ICAM, VCAM-1, and E selectin expression, and it is involved in early acute graft-versus-host disease (24, 26, 30, 33, 34, 38, 39, 47). TNFRII (p75 receptor) has intracytoplasmic domains to which TRAF-1 and TRAF-2 proteins bind (35). TNFRII (p75 receptor) plays an important role in apoptosis, lymphocyte proliferation, and dermal necrosis (9, 10, 16, 45, 47, 51). The p55 and p75 TNFRs lack intracellular homology, indicating that they probably use different intracellular signaling pathways when stimulated.
Studies of TNF-α function found it to be at the head of the proinflammatory cytokine cascade and to have both beneficial and detrimental effects. Among the beneficial effects is the critical importance of TNF-α in the host defense against various microorganisms. In particular, TNF-α is important in the defense against fungi (Candida albicans and Cryptococcus neoformans) (5, 41), intracellular bacteria (Listeria monocytogenes and BCG) (17, 20), and a parasite (Trypanosoma cruzi) (23). Among the detrimental effects are the roles that TNF-α plays in septic shock and autoimmune diseases. Administration of TNF-α can mimic the changes observed with septic shock, and mice can tolerate the lethal dose of lipopolysaccharide when endogenously produced TNF-α is neutralized (2, 46). Abnormal expression of TNF-α leads to autoimmune diseases, and autoimmune diseases such as rheumatoid arthritis (RA) may ameliorate following the neutralization of TNF-α function. In the case of RA, various agents that can block the function of TNF-α are being investigated as therapeutic measures for treatment (8, 27). However, it is unknown whether these treatments could increase the susceptibility to infection.
TNF-α is known to be important in inducing the acute-phase response, which induces a wide range of physiological changes beneficial in eliminating the infecting organism, limiting tissue damage, and activating the repair process. For instance, TNF-α, along with interleukin-1 (IL-1), can greatly increase the production of many acute-phase response molecules (type 1 acute-phase proteins) in the mouse, including serum amyloid P (SAP) (28) and C3 (43). In humans, SAP is not an acute-phase protein but C-reactive protein is. Both C-reactive protein and C3 are known to play important roles in the defense against Streptococcus pneumoniae (50). In addition to leading to production of acute-phase proteins, TNF-α has two important effects on neutrophils which are essential in the phagocytic killing of pneumococci. TNF-α potentiates the bactericidal properties of neutrophils (21, 37), and it also upregulates vascular and neutrophil adhesion molecules, which facilitates neutrophil influx to the site of infection (14, 24, 30).
It is important to understand how TNF-α and its receptors are involved in the host defense against microbes. To date few studies have addressed the TNFRs necessary for the host defense against microorganisms (40). No studies have examined the mechanism for resistance to infections by extracellular bacteria such as S. pneumoniae, which is a significant pathogen for the elderly, who are prone to RA and who would be the target population for the pharmaceutical agents neutralizing TNF-α function. We have investigated the timing of TNF-α neutralization as well as the importance of the two TNFRs (p55 and p75) by using S. pneumoniae infection as a model infection. Furthermore, we have determined whether the acute-phase response is altered in p55-deficient mice infected with S. pneumoniae. In these studies S. pneumoniae provides a model of an extracellular pathogen.
MATERIALS AND METHODS
Mice.
The p55- and p75-deficient mice both have the C57BL/6 background and have been previously described (32). p55-deficient mice were bred locally, whereas p75-deficient mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) (9). C57BL/6 mice were purchased from the Jackson Laboratory and used as controls. Mice were used at 6 to 10 weeks of age, and all groups contained both male and female mice.
Infection with S. pneumoniae serotype 6B.
S. pneumoniae serotype 6B strain BG9163 (4) was grown in 10 ml of Todd-Hewitt broth with 0.5% yeast extract until the optical density was 0.5 to 0.6 at 405 nm. The bacteria were spun down and resuspended in 3 ml of normal saline. Bacteria were then diluted 1/600, frozen with 15% glycerol, and stored in aliquots at −70°C. Frozen aliquots from the same batch of bacteria were used in all studies. Mice were injected intraperitoneally (i.p.) with 200 μl of the appropriately diluted bacteria in normal saline. In some cases mice were also injected i.p. with antibodies to TNF-α or to the polysaccharide capsule of S. pneumoniae serotype 6B. Polyclonal rabbit anti-mouse TNF-α antibody was purchased from Genzyme (IP-400), and 200 μl containing 2.5 × 104 U (neutralizing activity was 105 U/ml) was given to mice 2 h before the infection. In some cases mice were injected i.p. with 200 μl containing 42.4 μg of Hyp6BM1, an immunoglobulin M monoclonal antibody to 6B polysaccharide, 2 h before the infection with the bacteria. As a negative control, mice were injected with the same amount of immunoglobulin M monoclonal anti-group A streptococcus carbohydrate (HGAC82). After the infection, blood was collected from the orbital veins of mice at 24, 48, and 72 h. Bacteremia was quantitated by plating the serial dilutions of the mouse blood on blood agar plates and counting bacterial colonies after an overnight incubation at 37°C. The lower limit of detection was 100 CFU/ml. Infected mice were observed three times daily, and any deaths were recorded.
Peritoneal lavage and neutrophil count.
Twenty-four hours after infection with S. pneumoniae, mice were sacrificed and 2 ml of normal saline was injected into the peritoneal cavity. The peritoneum was massaged, and then the exudate was extracted. The total number of cells isolated from each peritoneum was counted on a hemacytometer. To identify the cell types, 100 μl of the collected peritoneal exudate was transferred to a microscope slide by cytospinning and stained with Wright-Giemsa stain. Neutrophils were identified morphologically. At least 100 cells were examined, and the total number of neutrophils was determined by multiplying the percentage of neutrophils obtained from the differential count by the total number of cells recovered.
Serum amyloid protein assay.
Enzyme-linked immunosorbent assay plates were coated overnight with 100 μl of sheep anti-mouse SAP antibody (Calbiochem, San Diego, Calif.) diluted 1/2,500 in diluent buffer. The diluent buffer was PBSE (0.0005 M KCl, 0.011 M Na2EDTA, 0.027 M NaCl, 0.003 M KH2PO4, 0.0016 M Na2HPO4, pH 7.5) with 1% bovine serum albumin and 0.05% Tween 20. The next day the coating solution was removed, and the plate was blocked with 100 μl of B-block buffer (1% bovine serum albumin in PBSE) for 1 h at room temperature. After the plates were washed, 50 μl of appropriately diluted samples was loaded into each well. Serum samples with unknown concentrations were diluted 1/25,000 in diluent buffer, and a SAP standard was serially diluted, with the highest concentration being 100 ng/ml. All samples and standards were analyzed in duplicate. The plate was incubated for 2.5 h at room temperature with shaking. After the plates were washed, 50 μl of rabbit anti-mouse SAP antibody (Calbiochem) diluted 1/2,500 in diluent buffer containing 2% normal sheep serum was added to each well. After a 1.5-h incubation at room temperature with shaking, the plate was washed three times and 50 μl of goat anti-rabbit peroxidase-conjugated antibody diluted 1/5,000 in diluent buffer containing 2% normal sheep serum was added to each well. After an incubation at 37°C for 45 min, the plates were washed again and azinobis(ethylbenzthiazolinesulfonic acid) (ABTS) (15 mg of ABTS powder per ml of distilled water) was added for color development. The optical density was read at 405 nm with a reader (Labsystems Multiscan MS) and was converted to the concentration.
C3 assay.
Enzyme-linked immunosorbent assay plates were coated overnight with 100 μl of PBSE containing 25 μg of goat anti-mouse C3 per ml. The plates were then blocked with 100 μl of B-block buffer for 1 h. After the blocking solution was discarded, 50 μl of mouse serum at a 1/4,000 dilution was added to each well. Mouse C3 controls (Calbiochem) were used to generate the standard curve. After an overnight incubation at 4°C, the plates were washed three times in wash buffer (PBSE with 0.05% Tween 20), and then 50 μl of a 1/5,000 dilution of goat anti-mouse C3–peroxidase conjugate was added to each well. After 1 h, the plates were washed two times and 100 μl of ABTS substrate was added to each well. The optical density was read and converted to concentration as described above.
RESULTS
Neutralization of TNF-α at the onset of infection increases susceptibility of mice to S. pneumoniae infection.
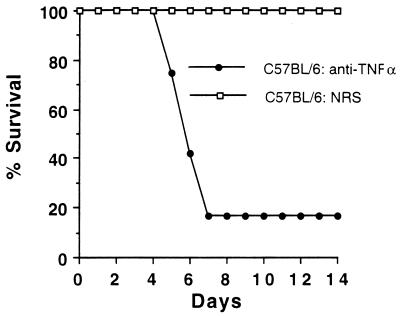
To begin examining the role of TNF-α in S. pneumoniae infection, C57BL/6 mice were treated with 2.5 × 104 U of anti-TNF-α 2 h before infection. It is known that 4 × 104 U of this antibody neutralizes 100 ng of TNF-α per ml of blood in mice (13). As shown in Fig. 1, 10 of 12 C57BL/6 mice treated with anti-TNF-α antibody died at 6 or 7 days postinfection, whereas control mice treated with normal rabbit serum survived the length of the experiment (21 days) (P = 0.00002 by the Fisher’s exact test on day 7 or later). Thus, the neutralization of TNF-α at the beginning of the infection was sufficient to disrupt the host defense against S. pneumoniae.
FIG. 1.
Effect of anti-TNF-α treatment on C57BL/6 mice infected with S. pneumoniae. C57BL/6 mice were given either 2.5 × 104 U of anti-TNF-α antibody or normal rabbit serum (NRS) 2 h before infection with 105 CFU of S. pneumoniae 6B. Twelve mice were treated with antibody to TNF-α, and 13 mice were treated with normal rabbit serum.
p55-deficient but not p75-deficient mice are susceptible to S. pneumoniae infection.
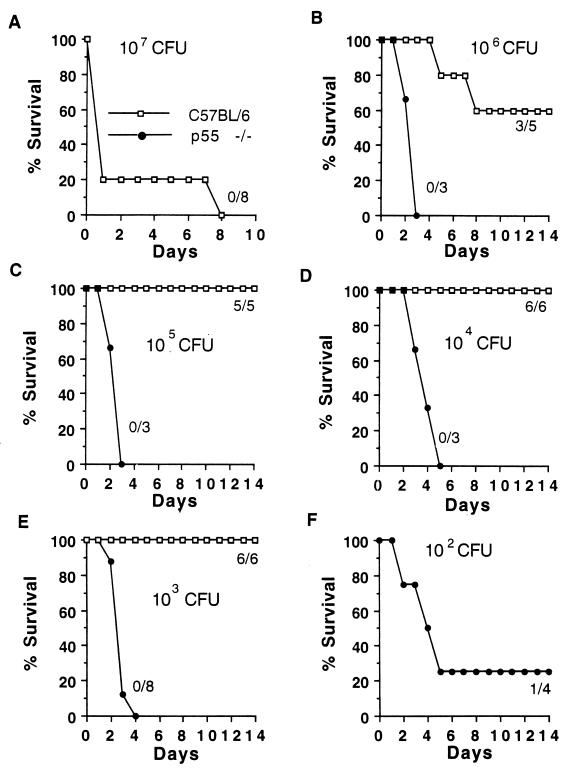
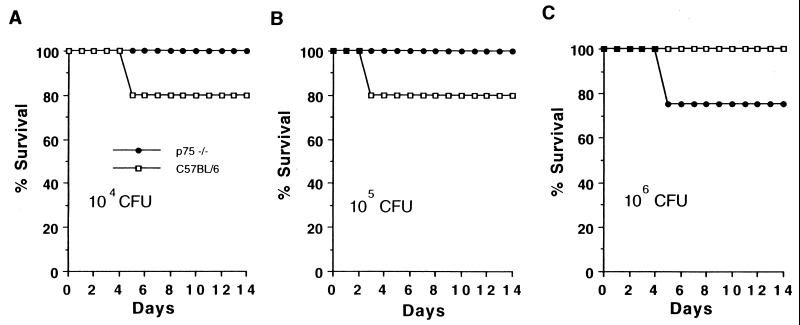
To determine the relative importance of TNF-α signaling through the p55 versus the p75 TNFR in resistance to S. pneumoniae infection, p55(−/−), p75(−/−), and wild-type (C57BL/6) mice were infected with different amounts of bacteria and observed for 3 weeks. All control mice died after an infection with 107 CFU (Fig. 2A), 40% died after an infection with 106 CFU of bacteria (Fig. 2B), and none died after an infection with 105, 104, or 103 CFU. In contrast, all p55-deficient mice died even when the doses of infecting bacteria were titrated down to 1,000 CFU, and 100 CFU killed most of the p55-deficient mice (Fig. 2). p55-deficient mice did survive the infections with 10 CFU of bacteria (data not shown). Thus, the 50% lethal dose for the p55-deficient mice was 10,000 times lower than that for the control mice. In contrast to the case for p55-deficient mice, there was no difference in survival of p75-deficient mice compared to control mice with infections with 104, 105, or 106 CFU of S. pneumoniae (Fig. 3). Thus, signaling through the p55 receptor but not the p75 receptor was found to be important in resistance to S. pneumoniae infection.
FIG. 2.
Infection of p55-deficient and control mice with various amounts of S. pneumoniae 6B. The numbers of CFU of bacteria injected i.p. into mice and the numbers of surviving mice/total numbers of mice are shown.
FIG. 3.
Infection of p75-deficient and control mice with S. pneumoniae. (A) Six C57BL/6 and six p75-deficient mice were infected with 104 CFU of S. pneumoniae serotype 6B per mouse. (B and C) Five C57BL/6 and four p75-deficient mice were infected with 105 CFU (B) or 106 CFU (C) of S. pneumoniae per mouse. One mouse died in each of the experiments for which the results are shown.
p55-deficient mice die of massive pneumococcal septicemia.
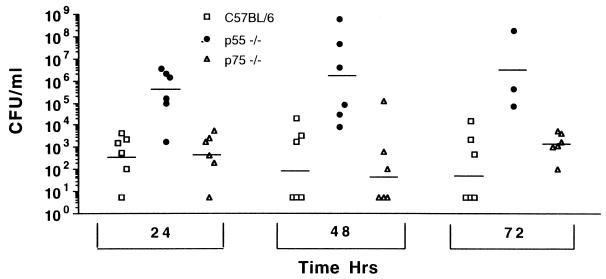
p55-deficient mice may die because they fail to normally control infection with pneumococci or because they are more sensitive to toxic mediators associated with the infection. To determine whether p55-deficient mice are overly sensitive to pneumococci bacteremia, p55- and p75-deficient mice and C57BL/6 mice were infected i.p. with 5 × 104 CFU of S. pneumoniae, and the degree of bacteremia was monitored at 24, 48, and 72 h (Fig. 4). By 24 h, the bacteremia in p55-deficient mice was 106 CFU/ml of blood. At 48 and 72 h, bacteremia in some p55-deficient mice became more severe, and it reached 107 to 109 CFU/ml when the mice began to die of sepsis. The numbers of CFU in the p55-deficient mice were greater than those in the C57BL/6 controls at all three time points. In contrast, the levels of bacteremia in p75-deficient and normal control mice were about 1,000 times lower than those in p55-deficient mice (Fig. 4). The level of infection in p75-deficient mice was not significantly different from that seen in C57BL/6 mice. Thus, it is evident that p55-deficient mice suffer a severe bacteremia after infection, which leads to death of the mice.
FIG. 4.
Numbers of bacteria in the blood of mice infected i.p. with S. pneumoniae at 24 h, 48 h, and 72 h postinfection. p55 knockout mice, p75 knockout mice, and C57BL/6 mice were used. The horizontal bars represents the average for each group. The numbers of CFU in the p55-deficient mice were statistically greater than those in the controls (P < 0.009, P < 0.005, and P < 0.023 at the 24-, 48-, and 72-h, time points, respectively, by the Wilcoxon two-sample rank test).
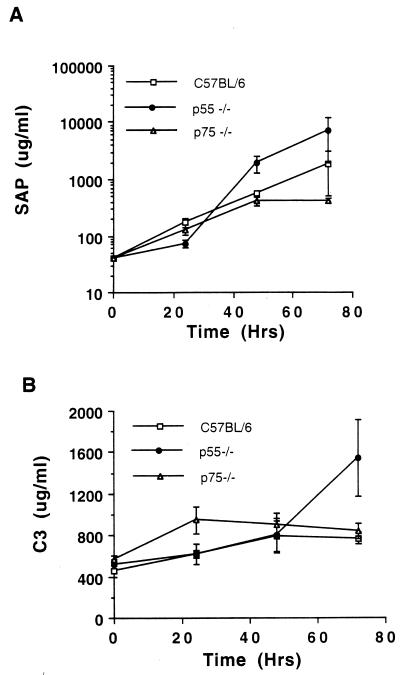
The acute-phase response is normal in p55-deficient mice.
TNF-α is important in modulating the acute-phase response (28), which can help mice resist S. pneumoniae infections (42, 49). To monitor acute-phase responses in C57BL/6, p55-deficient, and p75-deficient mice, we measured two prominent acute-phase proteins (SAP and C3). As shown in Fig. 5, both proteins increased in concentration over 3 days in all three mouse strains. p55-deficient mice exhibited the largest increases in SAP and C3 levels, possibly as a result of their larger bacterial burden. Also, during this same time course, the numbers of total leukocytes, neutrophils, and lymphocytes in peripheral blood samples were measured. All three groups showed an increase in total leukocyte, neutrophil, and lymphocyte numbers over the time course measured, without any significant differences among the three groups (data not shown). Taken together, these results showed no evidence that the increased susceptibility of the p55-deficient mice was due to a defective acute-phase response.
FIG. 5.
The acute-phase protein response. The levels of SAP (A) and C3 (B) in p55-deficient mice, p75-deficient mice, and C57BL/6 mice were measured at 0, 24, 48, and 72 h. Data are means and standard errors for SAP and standard deviations for C3.
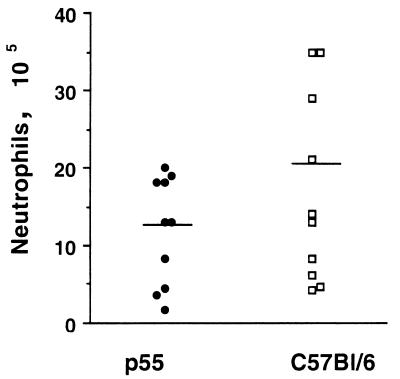
The number of granulocytes in the peritoneums of S. pneumoniae-infected p55-deficient mice is similar to that for control mice.
Signaling through p55 can modulate expression of the adhesion molecules important in the influx of leukocytes, which play a prominent role in S. pneumoniae phagocytosis (19). It was of interest to determine if there was an attenuation of the neutrophil influx in p55-deficient mice after infection. In this experiment, p55-deficient or control mice were injected with 105 CFU of S. pneumoniae, and 24 h later the infiltrating cells were recovered from the peritoneum and the number of neutrophils was determined. As shown in Fig. 6, there was a slight but not significant reduction (P = 0.133 by the Student t test) in the number of infiltrating neutrophils in p55-deficient mice compared to control mice.
FIG. 6.
Numbers of infiltrating peritoneal neutrophils in p55-deficient and normal mice after S. pneumoniae serotype 6B infection. Each mouse was infected with 105 CFU of S. pneumoniae serotype 6B, and peritoneal neutrophils were harvested 24 h later. Ten mice were used in each group. The horizontal line in each plot represents the average for the group.
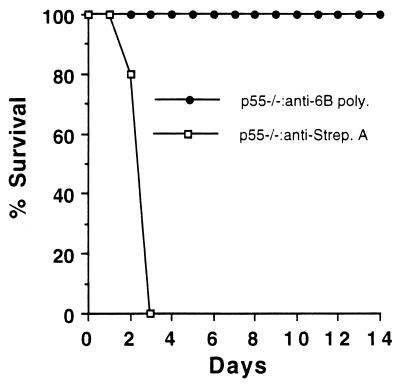
Antibody to S. pneumoniae (anti-6B) protects p55-deficient mice from S. pneumoniae infection.
To determine if antibody could protect the p55-deficient mice from the lethal effects of S. pneumoniae infection, the following experiment was performed. p55-deficient mice were injected, 2 h before S. pneumoniae infection, with either anti-6B antibody or a control antibody. As shown in Fig. 7, p55-deficient mice receiving the control antibody succumbed to infection as expected by day 3. However, p55-deficient mice treated with anti-6B antibody were resistant to infection with S. pneumoniae (serotype 6B) (P = 0.0079 by the Fisher exact test on day 3 or later). This shows that p55-deficient mice can be protected from infection by the presence of antibodies specific for the infecting bacteria.
FIG. 7.
Effect of antibody specific for S. pneumoniae on the survival of p55-deficient mice. S. pneumoniae serotype 6B (105 CFU) was given to mice 2 h after administration of antibody specific for pneumococcal capsular polysaccharide (6B poly.) or antibody specific for group A streptococcus carbohydrate (Strep. A). Five mice were in each group.
DISCUSSION
Previously it was reported that repeated neutralization of TNF-α during the first 7 days is important for survival from pneumococcal infections (1, 44). In this study, we extend the previous reports by showing that brief TNF-α neutralization at the beginning of infection with S. pneumoniae is sufficient to make the mouse more susceptible to infection. Studies with Klebsiella pneumoniae showed that TNF-α released from mast cells early in the infection is critical for the resistance to K. pneumoniae infections (7, 25). Similarly, it is possible that mast cells, which are located at the portals of infection with presynthesized TNF-α, may be important in the resistance to S. pneumoniae as well.
We demonstrate here that TNF-α signaling through the p55 receptor, but not the p75 receptor, is critical in host resistance to S. pneumoniae infection. p55-deficient mice have rapid progression of bacteremia and are about 10,000-fold more susceptible than normal or p75-deficient mice. p55 is more important than p75 for resistance to fungal infection (40), and p55 is important in infection by intracellular bacteria (Listeria monocytogenes and Leishmania major) (33, 34, 48) or Mycobacterium tuberculosis (12). The host defense mechanism against the pathogens listed above is complex, and p55 may be important in any of several defensive steps. In contrast, the host defense against S. pneumoniae, an extracellular pathogen, is relatively simple and involves mainly acute-phase responses (42, 49), as well as antibody-mediated opsonophagocytosis. p55 (but not p75) may be critical for one of these types of host defense. Our finding could be relevant to humans, since signaling through the p55 and p75 TNFRs may be analogous in humans and mice. For instance, signaling through the p55 but not the p75 receptor upregulates adhesion molecule expression on endothelial cells in both species (24, 33).
We found that the two acute-phase proteins increased normally in p55-deficient mice, perhaps due to the compensating effects of other cytokines, such as IL-1 and IL-6, on the acute-phase response. At 72 h postinfection, the C3 level was actually higher in p55 knockout mice than in other mice (P = 0.02), perhaps due to the larger burden of bacteria. The presence of an acute-phase response even in p55/p75 double-deficient mice was shown recently by others (31). In these mice the acute-phase proteins serum amyloid A, α1 acid glycoprotein, and SAP were measurable at 24, 48, and 72 h in both normal and p55/p75 double-deficient mice after stimulation with lipopolysaccharide (32). Thus, the increased susceptibility of p55-deficient mice is probably not due to the lack of a protective acute-phase response protein.
An alternate interpretation is that p55-deficient mice actually do have a local decreased host (acute) response early in infection that permits the overgrowth of the pneumococci. The reason that this decreased activity may not be apparent in the present study is that at most time points measured in the present study, the bacterial burden of the p55-deficient mice was at least 1,000-fold higher than that of the normal mice. If the mice bearing these high levels of pneumococci had not been p55 deficient, they may have had much higher levels of acute-phase proteins or cellular infiltrate.
There are two reports demonstrating a modest to substantial reduction in neutrophil influx at the site of infection upon TNF-α neutralization (22, 25). Thus, we were surprised to find no significant differences in the number of neutrophils infiltrating into the peritoneums of p55-deficient S. pneumoniae-infected mice. However, our report is in agreement with another that demonstrated no difference in neutrophil influx to the lung after Pseudomonas aeruginosa infection and treatment with anti-TNF-α antibodies (15). Several in vitro studies have shown enhancement of neutrophil bactericidal properties upon TNF-α treatment. TNF-α can directly stimulate the respiratory burst, modulate lysosomal enzyme release, and induce nitric oxide synthesis in neutrophils (6, 15, 21). TNF-α may not be as critical in neutrophil influx in vivo as in activation of neutrophil phagocytosis. Further studies of the activation status of neutrophils from p55-deficient mice are needed.
p55 (but not p75)-deficient mice lack germinal centers, which are important in generating high-affinity antibodies as well as for memory B cells. Defective immune memory is unlikely to be responsible for the susceptibility of p55-deficient mice, because they die very soon after the infection, before B-cell immune memory could be reactivated. Also, affinity to polysaccharide antigens is generally low, and affinity maturation is relatively unimportant in the immune response to polysaccharide antigens such as pneumococcal capsular polysaccharide. However, it has been reported that lymphotoxin/TNF-α double-deficient mice cannot mount an immune response to T-independent antigens (36). Also, we found that the susceptibility to infection in p55-deficient mice can be compensated for by passive immunization with antibodies to S. pneumoniae. These considerations argue that a defective antigen-specific adaptive immune response may be partially responsible for the increased rapid death of p55-deficient mice from S. pneumoniae.
Our observation of passive protection is significant because TNF-α neutralization is being actively investigated as a therapeutic measure for many autoimmune diseases. For instance, approaches neutralizing the activity of the TNF-α molecule itself or the function of the p55 receptor are being actively investigated and have been found to provide a short-term means to alleviate RA in patients. These therapeutic measures, like the clinical use of corticosteroids, would put RA patients at risk for bacterial infection. Although the current regimen of neutralizing TNF-α activity has not led to an increased occurrence of infection (11), perhaps active immunization of RA patients before use of a potential TNF-α therapy would be beneficial.
ACKNOWLEDGMENTS
We thank J. Peschon for providing p55 knockout mice and for careful reading of the manuscript.
This work was funded by NIH grant AI-31473 to M.H.N. M.H.N. is partially supported by NIAID contract NO1 AI-45248.
REFERENCES
- 1.Benton K A, VanCott J L, Briles D E. Role of tumor necrosis factor alpha in the host response of mice to bacteremia caused by pneumolysin-deficient Streptococcus pneumoniae. Infect Immun. 1998;66:839–842. doi: 10.1128/iai.66.2.839-842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beutler B, Milsark I W, Cerami A C. Passive immunization against cachectin/tumor necrosis factor (TNF) protects mice from the lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 3.Black R A, Rauch C T, Kozlosky C J, Peschon J J, Slack J L, Wolfson M F, Castner B J, Stocking K L, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley K A, Gerhart M, Davis R, Fitzner J N, Johnson R S, Paxton R J, March C J, Cerretti D P. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 4.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins H L, Bancroft G L. Cytokine enhancement of complement-dependent phagocytosis by macrophages: synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- 6.Dusi S, Della Bianca V, Donini M, Nadalini K A, Rossi F. Mechanisms of stimulation of the respiratory burst by TNF in nonadherent neutrophils: its independence of lipidic transmembrane signaling and dependence on protein tyrosine phosphorylation and cytoskeleton. J Immunol. 1996;157:4615–4623. [PubMed] [Google Scholar]
- 7.Echtenacher B, Mannel D N, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 8.Elliot M J, Maini R N, Feldman M, Long-Fox A, Charles P, Katsikis P, Brennan F M, Walker J, Bijl H, Ghrayeb J, Woody J N. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arth Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 9.Erickson S L, deSauvage F J, Kikly K, Carver-Moore K, Pitts-Meek S, Gillett N, Sheehan K C, Schreiber R D, Goeddel D V, Moore M W. Decreased sensitivity to tumour-necrosis factor but normal T-cell development in TNF receptor-2-deficient mice. Nature. 1994;372:560–563. doi: 10.1038/372560a0. [DOI] [PubMed] [Google Scholar]
- 10.Erikstein B K, Smeland E B, Blomhoff H K, Funderud S, Prydz K, Lesslauer W, Espevik T. Independent regulation of 55-kDa and 75-kDa tumour necrosis factor receptors during activation of human peripheral blood B lymphocytes. Eur J Immunol. 1991;21:1033–1037. doi: 10.1002/eji.1830210426. [DOI] [PubMed] [Google Scholar]
- 11.Firesetein G S, Zvaifler N J. Anticytokine therapy in rheumatoid arthritis. N Engl J Med. 1998;337:195–197. doi: 10.1056/NEJM199707173370310. [DOI] [PubMed] [Google Scholar]
- 12.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Franks A K, Kujawa K I, Yaffe L J. Experimental elimination of tumor necrosis factor in low-dose endotoxin models has variable effects on survival. Infect Immun. 1991;59:2609–2614. doi: 10.1128/iai.59.8.2609-2614.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamble J R, Harlan J M, Klebanoff S J, Vadas M A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci USA. 1985;82:8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gosselin D J, De Sanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grell M, Becke F M, Wajant H, Mannel D N, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28:257–263. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Havell E A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989;143:2894–2899. [PubMed] [Google Scholar]
- 18.Hsu H, Xiong J, Goeddel D V. The TNF receptor I-associated protein TRADD signals cell death and NF-kB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 19.Johnston R B., Jr The host response to invasion by Streptococcus pneumoniae: protection and the pathogenesis of tissue damage. Rev Infect Dis. 1981;3:282–288. doi: 10.1093/clinids/3.2.282. [DOI] [PubMed] [Google Scholar]
- 20.Kindler V, Sappino A P, Grau P E, Piguet P F, Vassalli P. The inducing role of tumor necrosis factor in development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 21.Klebanoff S J, Vadas M A, Harlan J M, Sparks L H, Gamble J R, Agosti J M, Waltersdorph A M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986;136:4220–4225. [PubMed] [Google Scholar]
- 22.Laichalk L L, Kunkel S L, Streicter R M, Danforth J M, Bailie M B, Strandeford T J. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumoniae. Infect Immun. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima E C S, Garcia I, Vicentelli M-H, Vassalli P, Minoprio P. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect Immun. 1997;65:457–465. doi: 10.1128/iai.65.2.457-465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor α (TNF-α)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malaviya R, Ikeda T, Ross E, Abraham S N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNFα. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Mariathasan S, Nahm M H, Baranyay F, Peschon J J, Chaplin D D. Role of lymphotoxin and type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 27.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L, Ettlinger R E, Cohen S, Koopman W J, Mohler K, Widmer M B, Blosch C M. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:195–197. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 28.Mortenson R F, Shapiro J, Lin B-F, Douches S, Neta R. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988;140:2260–2266. [PubMed] [Google Scholar]
- 29.Moss M L, Jin S L C, Milla M E, Bickett D M, Burkhart H L, Carter W, Chen W J, Clay W C, Didsbury J R, Hassler D, Hoffman C R, Kost T A, Lambert M H, Leesnitzer M A, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton L K, Schoenen F, Seaton T, Su J L, Warner J, Willard D, Becherer J D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 30.Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak T W, Holzmann B, Kronke M. Crucial role of 55-kilodalton TNF receptor in TNF-induced adhesion molecule expression and leukocyte organ infiltration. J Immunol. 1996;156:1587–1593. [PubMed] [Google Scholar]
- 31.Paleolog E M, Delasalle S A, Buurman W A, Feldman M. Functional activities of receptors for tumor necrosis factor-alpha on human vascular endothelial cells. Blood. 1994;84:2578–2590. [PubMed] [Google Scholar]
- 32.Peschon J J, Torrance D S, Stocking K L, Glaccum M B, Otten C, Willis C R, Charrier K, Morrissey P J, Ware C B, Mohler K M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 33.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Mice deficient for the 55kd tumor necrosis factor receptor are resistant to endotoxic shock yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 34.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 35.Rothe M, Wong S C, Henzel W J, Goeddel D V. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75-kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 36.Ryffel B, DiPadova F, Schreier M H, Le Hir M, Eugster H P, Quesniaux V F. Lack of type 2 T cell-independent B cell responses and defect in isotype switching in TNF-lymphotoxin alpha-deficient mice. J Immunol. 1997;158:2126–2133. [PubMed] [Google Scholar]
- 37.Shalaby M R, Aggarwal B B, Rinderknecht E, Svedersky L P, Finkle B S, Palladino M A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factor. J Immunol. 1985;135:2069–2073. [PubMed] [Google Scholar]
- 38.Speiser D E, Bachmann M F, Frick T W, McKall-Faienza K, Griffiths E, Pfeffer K, Mak T W, Ohashi P S. TNF receptor p55 controls early acute graft-versus-host disease. J Immunol. 1997;158:5185–5190. [PubMed] [Google Scholar]
- 39.Speiser D E, Sebzda E, Ohteki T, Bachmann M F, Pfeffer K, Mak T W, Ohashi P S. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur J Immunol. 1996;26:3055–3060. doi: 10.1002/eji.1830261235. [DOI] [PubMed] [Google Scholar]
- 40.Steinshamn S, Bemelmans M H A, van Tits L J H, Bergh K, Buurman W A, Waage A. TNF receptors in murine Candida albicans infection. J Immunol. 1996;157:2155–2159. [PubMed] [Google Scholar]
- 41.Steinshamn S, Waage A. Tumor necrosis factor and interleukin-6 in Candida albicans infection in normal and granulocytopenic mice. Infect Immun. 1992;60:4003–4008. doi: 10.1128/iai.60.10.4003-4008.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szalai A J, Briles D E, Volanakis J E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J Immunol. 1995;155:2557–2563. [PubMed] [Google Scholar]
- 43.Szalai A J, Briles D E, Volanakis J E. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumoniae in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tartaglia L A, Goeddel D V, Reynolds C, Figari I S, Weber R F, Fendly B M, Palladino M A., Jr Stimulation of human T-cell proliferation by specific activation of the 75 kDa tumour necrosis factor receptor. J Immunol. 1993;151:4637–4641. [PubMed] [Google Scholar]
- 46.Tracey K J, Beutler B, Lowry S F, Merryweather J, Wolpe S, Milsark I W, Hariri R J, Fahey T J, Zentella A, Albert J D, Shires G T, Cerami A. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 47.Vandenabeele P, Declercq W, Vanhaesebroeck B, Grooten J, Fiers W. Both TNF receptors are required for TNF-mediated induction of apoptosis in PC60 cells. J Immunol. 1995;154:2904–2913. [PubMed] [Google Scholar]
- 48.Vieira L Q, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 49.Winkelstein J A. The role of complement in the host’s defense against Streptococcus pneumoniae. Rev Infect Dis. 1981;3:289–298. doi: 10.1093/clinids/3.2.289. [DOI] [PubMed] [Google Scholar]
- 50.Yother J, Volanakis J E, Briles D E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J Immunol. 1982;128:2374–2376. [PubMed] [Google Scholar]
- 51.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]