Abstract
The COVID-19 pandemic affects the transplant recipients since March 2020. Transplant centers quickly organized themselves to optimize the management of the immunocompromised patients and to progress in the knowledge of this new disease. To this end, a French Registry was created, which includes all solid organ transplant patients who have developed a SARS Cov2 infection. Numerous studies have been carried out using these data to describe this new disease in transplant patients, to characterize its clinical and biological risk factors and to define its prognosis. The 60 days-mortality of transplant patients hospitalized for COVID-19 was evaluated at 23% and renal failure plays a major role in the poor prognosis in addition to the classical risk factors described in the general population. The advent of vaccination has been a great relief but transplanted patients have shown a poor vaccine response keeping them at risk of severe disease even after an adapted vaccination scheme. Specific strategies was proposed in this particularly fragile population like increasing vaccine doses or using anti SARS Cov-2 monoclonal antibodies.
Keywords: COVID-19, Mortality, Kidney transplant patients, Vaccination
1. Introduction
The COVID-19 epidemic hit the France in January 2020, and the first transplant centers were affected in the beginning of March in Grand Est and in the Paris regions. Very quickly, the first wave reached many transplant centers, and the infection concerned more and more transplant patients. Transplant teams were particularly concerned about the potential seriousness of this new viral infection occurring in organ transplant patients, who are more fragile because of their immunosuppression and frequent comorbidities. In order to rapidly establish a mapping and a clinico-biological description of COVID-19 in the solid organ transplant population, a French registry was set up in March 2020 with the support of the Société Francophone de Transplantation (box 1). This registry has recorded a large number of COVID-19 cases occurring in solid organ transplant recipients in France in renal, liver, heart and lung transplantation until December 31, 2020.
Box 1. List of contributors to the French Organ Transplant Registry COVID-19.
Sophie Caillard, Bruno Moulin, Service de Néphrologie et Transplantation, Hôpitaux Universitaires de Strasbourg, Strasbourg; Samira Fafi-Kremer, Laboratoire de Virologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Marc Hazzan, Service de Néphrologie, Hôpital Huriez, Lille; Dany Anglicheau, Service de Néphrologie et Transplantation Adultes, AP-HP, Hôpital Necker, Paris; Alexandre Hertig, Jérôme Tourret, Benoit Barrou, Service de Néphrologie, AP-HP, Hôpital La Pitié Salpétrière, Paris; Emmanuel Morelon, Olivier Thaunat, Service de Néphrologie, Hôpital Edouard Herriot, Lyon; Lionel Couzi, Pierre Merville, Service de Néphrologie–Transplantation–Dialyse, Hôpital Pellegrin, Bordeaux; Valérie Moal, Tristan Legris, Service de Néphrologie et Transplantation, AP-HM, Hôpital de la Conception, Marseille; Pierre-François Westeel, Maïté Jaureguy, Service de Néphrologie, CHU Amiens Picardie, Amiens; Luc Frimat, Service de Néphrologie, CHRU Nancy, Vandoeuvre; Didier Ducloux, Jamal Bamoulid, Service de Néphrologie, Hôpital Jean-Minjoz, Besancon; Dominique Bertrand, Service de Néphrologie, CHU de Rouen, Rouen; Michel Tsimaratos, Florentine Garaix-Gilardo, Service de Pédiatrie Multidisciplinaire, Hôpital La Timone, Marseille; Jérôme Dumortier, Service d'Hépato-Gastroentérologie, Hôpital Edouard Herriot, Lyon; Sacha Mussot, Antoine Roux, center Chirurgical Marie Lannelongue, Le Plessis Robinson; Laurent Sebbag, Service d'Insuffisance Cardiaque, Hôpital Louis Pradel, Bron; Yannick Le Meur, Service de Néphrologie, Hôpital de la Cavale Blanche, Brest; Gilles Blancho, Christophe Masset, Service de Néphrologie–Transplantation, Hôtel Dieu, Nantes; Nassim Kamar, Service de Néphrologie et Transplantation, Hôpital Rangueil, Toulouse; Hélène Francois, Eric Rondeau, Service de Néphrologie, Dialyse et Transplantation, AP-HP, Hôpital Tenon, Paris; Nicolas Bouvier, Service de Néphrologie, Dialyse, Transplantation Rénale, CHU, Caen; Christiane Mousson, Service de Néphrologie, Dijon; Matthias Buchler, Philippe Gatault, Service de Néphrologie, Tours; Jean-François Augusto, Agnès Duveau, Service de Néphrologie, Dialyse, Transplantation, CHU Angers, Angers; Cécile Vigneau, Marie-Christine Morin, Jonathan Chemouny, Leonard Golbin, Service de Néphrologie, CHU de Rennes, Rennes; Philippe Grimbert, Marie Matignon, Antoine Durrbach, Service de Néphrologie, Hôpital Henri-Mondor, Creteil; Clarisse Greze, Service de Néphrologie, AP-HP, Hôpital Bichat Claude Bernard, Paris; Renaud Snanoudj, Service de Néphrologie, Hôpital Foch, Service de Néphrologie et Transplantation Hôpital du Kremlin Bicêtre, Le Kremlin Bicetre; Charlotte Colosio, Betoul Schvartz, Service de Néphrologie, Hôpital Maison Blanche, Reims; Paolo Malvezzi, Service de Néphrologie, Hémodialyse, Transplantation Rénale, Hôpital La Tronche, Grenoble; Christophe Mariat, Service de Néphrologie, CHU de Saint Etienne, Saint Etienne; Antoine Thierry, Service de Néphrologie, Hémodialyse et Transplantation Rénale, Hôpital Jean Bernard, Poitiers; Moglie Le Quintrec, Service de Néphrologie−Transplantation−Dialyse, CHU Lapeyronie, Montpellier; Antoine Sicard, Service de Néphrologie, Hôpital Pasteur, Nice; Jean Philippe Rerolle, Service de Néphrologie, CHU Dupuytren, Limoges; Anne-Élisabeth Heng, Cyril Garrouste, Service de Néphrologie, CHU Gabriel Montpied, Clermont-Ferrand; Henri Vacher Coponat, Service de Néphrologie, CHU de La Réunion, Saint Denis; Éric Epailly, Service de Cardiologie, Hôpitaux Universitaires de Strasbourg, Strasbourg; Olivier Brugiere, Service d'Hépatologie, Hôpital Foch, Suresnes; Sébastien Dharancy, Service d'Hépatologie, Hôpital Huriez, Lille; Éphrem Salame, Service de Chirurgie Hépatique, Hôpital Universitaire de Tours, Tours; Faouzi Saliba, Service d'Hépatologie, center hépato-biliaire Paul Brousse, Villejuif, France
Alt-text: Unlabelled box
The first analyses of the registry have permitted to rapidly inform the national and international communities of the clinical presentation of SARS CoV-2 infection in transplant patients and its prognosis.
Thus, the study of the first 491 kidney transplant patients identified in the registry during the 1st wave of the pandemic provided several information [1].
2. Manuscript
During the 1st wave, the vast majority of transplant patients diagnosed with COVID-19 by PCR were hospitalized (77%). Some patients with less severe clinical forms could be maintained at home. These outpatients were younger, had fewer comorbidities and did not have dyspnea. This practice increased significantly during the 2nd wave, when the diagnosis of less severe forms was made possible by the greater availability of PCR tests. Thus, 46% of kidney transplant patients were managed at home during the 2nd wave.
The main symptoms of COVID-19 in transplanted patients are quite similar to those in the general population (with fever, cough, dyspnea being the most frequent) with a higher incidence of digestive symptoms (30% of patients presented diarrhea, sometimes the only symptom of the disease).
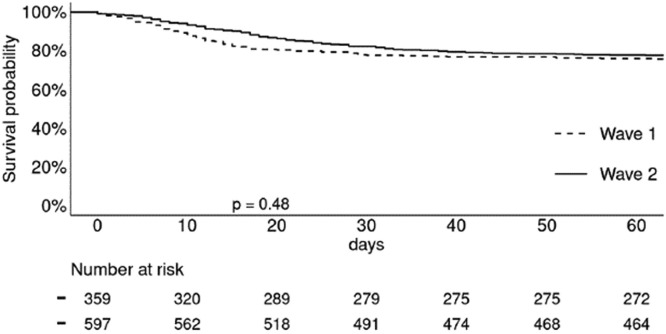
The prognosis of COVID-19 in transplant recipients is severe, since one third of patients were oxygeno-dependent, 22% of patients were immediately hospitalized or transferred to intensive care for mechanical ventilation, and 23% of patients died at D60. Frequently, patients hospitalized for COVID-19 worsened between D7 and D10, requiring transfer to intensive care. Causes of death were mostly directly related to respiratory distress syndrome secondary to SARS CoV-2 infection and rarely to bacterial surinfection or thromboembolic complications. Acute renal failure was frequent (around 40%), the need for dialysis was 11% and these 2 events were, as in the general population, major factors of poor prognosis. The other factors associated with poorer patient survival in multivariate analysis were age and cardiovascular disease. Survival analyses performed in transplant patients hospitalized for COVID-19 during the 2nd wave unfortunately showed the same mortality rate as these of the 1st wave (Fig. 1 ) [2]. Thus, there was no prognosis improvement despite a different treatment strategy in the 2nd wave, which was based on the recommendations in the general population (in particular the use of high dexamethasone doses). In a large French epidemiological series published in early 2021, it was confirmed that being transplanted increased the risk of dying from COVID-19 by a factor of 7 for kidney transplant recipients and by a factor of 6 for lung transplant recipients [3].
Fig. 1.
Survival of renal transplant patients hospitalized with COVID-19. No difference in mortality between patients hospitalized in Wave 1 (March to June 2020) and those hospitalized in Wave 2 (August to December 2020). 30-day mortality was 25.3% vs. 23.9% respectively; Log Rank, p = 0.48.
The management of transplant patients has its own specificities: reduction of immunosuppression in severe forms, the modalities of which are still poorly codified, management of acute renal failure, less frequent use of anti-viral drugs (less than 1% of patients), some of which interfering with immunosuppressants. The reduction of immunosuppression consisted most often in stopping anti-metabolites (50%) or mTor inhibitors, more rarely in stopping calcineurin inhibitors (reserved for severe forms). Steroids were maintained or increased. 44% of patients hospitalized in the second wave received anti-inflammatory treatments: dexamethasone or tocilizumab, although their efficacy cannot be precisely studied because of the retrospective nature of the data collection [1,2].
Biological parameters collected on admission of transplanted patients with COVID-19 provided information on the potential risk of progression to severe forms of the disease. Thus, CRP>50 mg/l, procalcitonin> 0.3 mg/l, troponin I-US > 20 ng/l, d-Dimers> 1500 IU/l and LDH> 280 IU/l were associated with severe forms of the infection and an unfavorable evolution [4]. On the other hand, parameters such as lymphopenia and thrombocytopenia were not discriminating in contrast to what has been described in the general population.
Finally, monocentric studies carried out on the 1st wave cohorts have revealed virological particularities. Indeed, transplanted patients have viral loads that remain positive in nasopharyngeal smear for a long time, sometimes up to several weeks, necessitating the maintenance of prolonged isolation of immunocompromised patients, in particular when they are in contact with other fragile patients within hospital structures [5]. A certain number of clusters of patients and caregivers have developed within transplantation teams, with sometimes dramatic consequences for certain patients and a suspension of transplantation activity in some centers.
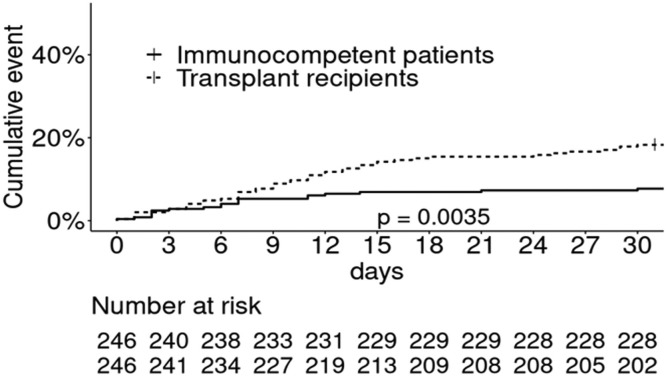
We also investigated whether SARS CoV-2 infection was more severe in transplant patients than in immunocompetent patients. Indeed, immunosuppression could be an aggravating factor when faced with a new infectious agent. On the other hand, we could also hypothesize that immunosuppression protects our patients from the cytokine release syndrome described in patients with severe forms of COVID-19. We therefore compared the cohort of kidney transplant patients hospitalized for COVID-19 reported in the French Registry to a single-center cohort of immunocompetent patients hospitalized in Strasbourg hospital at the same period (March-April 2020) [6]. We found that hospitalized transplant patients have more comorbidities than immunocompetent patients. After matching on risk factors for severe COVID-19, we established that transplant patients and immunocompetent patients have the same incidence of ICU admission. However, transplanted patients have poorer survival than immunocompetent patients matched on age, diabetes, BMI, a history of cancer or cardiovascular disease (Fig. 2 ). Finally, the poorer prognosis of transplant patients was associated with poorer renal function more than with being a renal transplant recipient (Table 1 ). This is supported by a study from the Necker team that showed comparable mortality between transplant recipients and immunocompetent patients when matched on glomerular filtration rate [7].
Fig. 2.
Cumulative 30-day incidence of death in non-transplant patients (solid line) and kidney transplant patients (dashed line) matched on risk factors for severe Covid: non-transplant patients: 11.4% vs. kidney transplant patients: 17.9%, p = 0.0035.
Table 1.
Risk factors in multivariate analysis for severe forms and death in matched* transplanted and non-transplanted patients hospitalized for COVID-19 (n = 546).
| A. Severe forms | HR | p | B. Death | HR | p |
|---|---|---|---|---|---|
| Cardio vascular diseases | 1.35 [1.03;1.76] | 0.028 | Cardio vascular diseases | 1.54 [0.96;2.46] | 0.071 |
| Cough | 0.61 [0.46;0.80] | <0.001 | Cough | 0.58 [0.36;0.92] | 0.022 |
| Dyspnea | 1.90 [1.43;2.53] | 0.004 | Dyspnea | 1.74 [1.08;2.78] | 0.022 |
| Fever | 1.70 [1.19;1.76] | 0.004 | Fever | 1.81 [1.00;3.28] | 0.050 |
| Creatinine > 115 µmol/L | 2.40 [1.48;3.87] | <0.001 | |||
| Age > 60 years | 3.47 [1.86; 6.47] | <0.001 |
Severe forms were defined as transfer to intensive care, mechanical ventilation, or death.
Patients were matched (1:1 ratio) for age, BMI, cardiovascular and respiratory diseases, cancer, and diabetes. HR, hazard ratio.
In December 2020, mRNA vaccines received their marketing authorization and vaccination of people over 75 years of age began in France on December 28. Very quickly, immunocompromised patients were given the opportunity to be vaccinated because of their great fragility. Our works then focused on the study of the vaccine response to the SARS CoV2 mRNA vaccine in transplanted patients, knowing that it was urgent to protect our patients but also knowing the flaws of the vaccine response to previous vaccines (hepatitis B, influenza) in this population. We quickly set up monocentric studies allowing us to answer these questions. We showed rapidly that the seroconversion rate was very low after the first dose of mRNA vaccine (around 10%) [8]. This rate improved after the second dose, reaching about 50% seroconversion in the Strasbourg and Toulouse cohorts [9,10]. Other teams have reported lower seroconversion rates (between 10 and 40%), partly because of heterogeneity in patient characteristics, the type of immunosuppressive drugs prescribed and the type of serological test used [11], [12], [13]. Data from the literature (as of September 1, 2021) are summarized in Table 2 . Factors associated with poorer vaccine response included age, shorter time from transplant, diabetes, poorer renal function, and especially the use of more intense immunosuppression. Among the immunosuppressive drugs most strongly associated with a decreased vaccine response are mycophenolate mofetil, belatacept and rituximab [9], [10], [11], [12], [13]. This poor response to mRNA vaccines was reflected in the occurrence of new cases of COVID-19 during the 3rd wave of the pandemic (April 21) in patients who had been vaccinated [14]. Some of these patients had severe forms of COVID-19 (25%) and 3 of the 55 patients described in this cohort died. On the basis of these data, the French Vaccine Strategy Steering Committee authorized a third dose of vaccine to be offered to transplant patients in order to strengthen their immunity. This strategy has been effective since half of the patients revaccinated with a 3rd dose have seroconverted, increasing the overall percentage of seroconverted patients in our cohorts (literature summary in Table 3 as of Sept 1, 2021) [15,16].
Table 2.
Summary of the literature (as of September 1, 2021) regarding vaccine response (seroconversion rate) after 2 doses of mRNA vaccine in kidney transplant patients.
| First Author, Country | Patients | Vaccine | Delay | Seroconversion rate |
|---|---|---|---|---|
| Benotmane et al., France | 204 KTR | 2 doses mRNA-1273 |
28 days | 48% |
| Boyarsky et al., US | 658 SOT 168 KTR |
2 doses mRNA-1273 or BNT162b2 |
29 days | 48% |
| Rozen-Zvi et al., Israel | 308 KTR | 2 doses BNT162b2 |
2–4 weeks | 38.4% |
| Grupper et al., Israel | 136 KTR | 2 doses BNT162b2 |
10–20 days | 37.5% |
| Marion et al., France | 367 SOT 271 KTR |
2 doses BNT162b2 |
28 days | 33% |
| Cucchiari et al., Spain | 117 KTR | 2 doses mRNA-1273 |
2 weeks | 29.9% |
| Husain et al., US | 28 KTR | 2 doses mRNA-1273 ou BNT162b2 |
2–6 weeks | 25% |
| Korth et al., Germany | 23 KTR 23 HC |
2 doses BNT162b2 |
16 days | 22% |
| Hall et al., Canada | 110 SOT 20 KTR |
2 doses mRNA-1273 |
4–6 weeks | 21.1% |
| Marinaki et al., Greece | 34 SOT 10 KTR |
2 doses BNT162b2 |
10 days | 20% |
| Midtvedt K, Norway | 141 KTR | 2 doses BNT162b2 |
25–89 days | 18% |
| Bertrand et al., France | 45 KTR 10 HD |
2 doses BNT162b2 |
30 days | 17.8% |
| Chavarot et al., France | 35 KTR | 2 doses BNT162b2 |
28 days | 5.7% |
| Danthu et al., France | 74 KTR 78 HD 7 HC |
2 doses BNT162b2 |
30 days | 4% |
| Sattler et al., Germany Rincon Arevalo et al |
39 KTR | 2 doses BNT162b2 |
8 days | 2.5% |
Abbreviations KTR: kidney transplant recipients, SOT: solid organ transplant recipients, HD: hemodialysis patients, HC: healthy control.
Table 3.
Summary of the literature (as of September 1, 2021) regarding vaccine response (seroconversion) after 3 doses of vaccine in kidney transplant patients.
| First Author, Country | Patients | Vaccine | Delay | Seroconversion rate |
|---|---|---|---|---|
| Hall et al., Canada Randomised |
120 SOT | 3 doses mRNA-1273 |
30 days | 55% |
| Benotmane et al., France | 159 KTR | 3 doses mRNA-1273 |
51 days | 49% |
| Werbel et al., US | 30 SOT 23 KTR |
3 doses mRNA-1273 or BNT162b2 or ad26.COV2.S |
14 days | 46.6% |
| Kamar et al., France | 101 SOT 78 KTR |
3 doses BNT162b2 |
30 days | 66% |
| Stumpf et al., Germany | 48 KTR | 3 doses BNT162b2 |
30 days | 40% |
| Masset et al., France | 136 KTR | 3 doses BNT162b2 |
30 days | 69% |
| Bertrand et al., France | 80 KTR | 3 doses BNT162b2 |
61% |
Abbreviations KTR: kidney transplant recipients, SOT: solid organ transplant recipients, HD: hemodialysis patients, HC: healthy control.
Nevertheless, 2 pitfalls should be highlighted despite this intensive vaccination strategy. First, a significant proportion (at least 30%) of patients still do not have a serological response after 3 doses of RNA vaccines; second, a large proportion of patients who seroconverted (about half), did not develop enough antibodies to protect them from severe forms of COVID-19.
Solutions does exist nevertheless to protect the immunocompromised patients, some of which are still being explored. One suggestion is to use higher doses of vaccine (double dose) as has been done for hepatitis B or influenza. This is supported by the finding of a good humoral response in transplanted patients with COVID-19 infection, much better than the post-vaccine response, suggesting a role for antigenic dose in stimulating the humoral response [17]. Modulation of immunosuppression could be proposed, but it exposes the risk of organ rejection or development of donor specific antibodies, and few centers are comfortable putting this strategy into practice. The use of heterologous vaccination could also be proposed but has not yet been evaluated. Strategies that have been implemented and evaluated included administration of a 4th vaccine dose and injection of SARS CoV2 monoclonal antibodies. The administration of a 4th dose is feasible without significant side effects but is not very effective in patients who did not seroconvert at all after the 3rd dose (less than 10% responders). In contrast, in patients who had a weak response after the third dose, administration of a fourth dose resulted in a significant increase in antibody levels in more than half of the patients, giving them protection against the disease [18]. In our experience, 50% of patients who received a fourth dose and had no neutralizing antibodies after the third dose developed neutralizing antibodies against the delta variant after the fourth dose [19]. For patients with no response after the third dose of vaccine, infusion or injection of anti-SARS CoV-2 monoclonal antibodies as pre-exposure prophylaxis seems to be an interesting approach, which is currently used in most French transplantation centers. However, this approach faces two major obstacles: the availability of products in some French centers and the cumbersome organization of infusions in already overburdened hospital facilities.
3. Conclusion
The work carried out in France since the beginning of the pandemic has generated interesting data for the community of physicians caring for transplant patients. Nevertheless, there are still many unresolved questions concerning COVID-19 and COVID-19 vaccination in our solid organ transplant population: the optimization of the management of transplant patients with COVID-19 in order to decrease the mortality rate, the duration of post-infectious and post-vaccination immunity, the best strategy to increase the post-vaccination response, and the optimal strategy for the administration of monoclonal antibodies in transplant patients.
Declaration of Competing Interest
I have no confict of interest to declare
References
- 1.Caillard S., Anglicheau D., Matignon M., Durbach A., Greze C., Frimat L., et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020 Dec;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger B., Hazzan M., Kamar N., Francois H., Matignon M., et al. Changing of therapeutic trends between the 1st and 2nd wave did not reduce COVID-19 related mortality of renal transplant recipients. Kidney Int. 2021 soumis. [Google Scholar]
- 3.https://www.epi-phare.fr/rapports-detudes-et-publications/covid-19-facteurs-risques/. 9 Feb 2021
- 4.Caillard S., Chavarot N., Francois H., Matignon M., Snanoudj R., Tourret J., et al. Clinical utility of biochemical markers for the prediction of COVID-19-related mortality in kidney transplant recipients. Kidney Int Rep. 2021;6(10):2689–2693. doi: 10.1016/j.ekir.2021.06.034. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillard S., Benotmane I., Gautier Vargas G., Perrin P., Fafi-Kremer S. SARS-CoV-2 viral dynamics in immunocompromised patients. Am J Transplant. 2021 Apr;21(4):1667–1669. doi: 10.1111/ajt.16353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caillard S., Chavarot N., Francois H., Matignon M., Greze C., Kamar N., et al. Is COVID-19 infection more severe in kidney transplant recipients ? Am J Transpl. 2021;21(3):1295–1303. doi: 10.1111/ajt.16424. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavarot N., Gueguen J., Bonnet G., Jdidou M., Trimaille A., Burger C., et al. Critical COVID-19 France investigators. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21(3):1285–1294. doi: 10.1111/ajt.16416. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benotmane I., Gautier-Vargas G., Cognard N., Olagne J., Heibel F., Braun-Parvez L., et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021;99(6):1487–1489. doi: 10.1016/j.kint.2021.03.014. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benotmane I., Gautier-Vargas G., Cognard N., Olagne J., Heibel F., Braun-Parvez L., et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. doi: 10.1016/j.kint.2021.04.005. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marion O., Del Bello A., Abravanel F., Couat C., Faguer S., Esposito L., et al. Safety and Immunogenicity of Anti-SARS-CoV-2 Messenger RNA Vaccines in Recipients of Solid Organ Transplants. Ann Intern Med. 2021;174(9):1336–1338. doi: 10.7326/M21-1341. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavarot N., Ouedrani A., Marion O., Leruez Ville M., Vilain E., Baaziz M., et al. Poor anti-SARS-CoV-2 humoral and t-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105(9):e94–e95. doi: 10.1097/TP.0000000000003784. Sep 1. [DOI] [PubMed] [Google Scholar]
- 12.Danthu C., Hantz S., Dahlem A., Duval M., Ba B., Guibbert M., et al. Humoral response after SARS-CoV-2 mRNA vaccination in a cohort of hemodialysis patients and kidney transplant recipients. J Am Soc Nephrol. 2021;32(9):2153–2158. doi: 10.1681/ASN.2021040490. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertrand D., Hamzaoui M., Lemée V., Lamulle J., Hanoy M., Laurent C., et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021;32(9):2147–2152. doi: 10.1681/ASN.2021040480. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caillard S., Chavarot N., Bertrand D., Kamar N., Thaunat O., Moal V., et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021 doi: 10.1016/j.kint.2021.05.011. May 23:S0085-2538(21)00509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamar N., Abravanel F., Marion O., Coat C., Izopet J., Del Bello A. Three doses of an mRNA COVID-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benotmane I., Gautier G., Perrin P., Olagne J., Cognard N., Fafi Kremer S., et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063–1065. doi: 10.1001/jama.2021.12339. Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charmetant X., Espi M., Benotmane I., Heibel F., Burron F., Gautier Vargas G. et al. Comparison of infected and vaccinated transplant recipients highlights the role of Tfh and neutralizing IgG in COVID-19 protection. MedrXiv; 2021:2021.07.22.21260852. doi:10.1101/2021.07.22.21260852
- 18.Caillard S., Thaunat O., Benotmane I., Masset C., Blancho G. Antibody response to a fourth mRNA COVID-19 vaccine boost in weak responder kidney transplant recipients. medRxiv 2021.09.03.21262691; doi: 10.1101/2021.09.03.2126269
- 19.Benotmane I., Bruel T., Planas D., Fafi-Kremer S., Schwartz O., Caillard S. A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the delta variant in kidney transplant recipients. Kidney Int. 2022;101(5):1073–1076. doi: 10.1016/j.kint.2022.02.011. May. [DOI] [PMC free article] [PubMed] [Google Scholar]