Abstract
Background
Effective pain management is paramount for outpatient surgical success. This study aims to report a case series of patients undergoing cervical disc replacement (CDR) in an ambulatory surgery center (ASC) with the use of an enhanced multimodal analgesic (MMA) protocol.
Methods
Primary, single-/2-level CDR procedures at an ASC with an enhanced MMA protocol were included. ASC patients were discharged day of surgery. Patient-reported outcome measures (PROMs) were administered at preoperative/6-week/12-week/6-month/1-year/2-year timepoints and included Visual Analogue Scale (VAS) neck, VAS arm, Neck Disability Index (NDI), Patient-Reported Outcomes Measurement Information System-Physical Function (PROMIS-PF), and 12-Item Short-Form Physical and Mental Composite Score (SF-12 PCS/SF-12 MCS). A t-test assessed postoperative PROM improvement from baseline. MCID achievement was determined by comparing ΔPROM scores to previously established thresholds.
Results
106 patients were included, 76 single-level and 30 2-level. Most single-levels occurred at C5–C6, most 2-levels at C5–C7. One 2-level patient developed a hematoma 5 days postoperatively and underwent revision for evacuation. Five patients reported postoperative dysphagia; all were quickly resolved. One patient had an episode of seizure secondary to serotonin syndrome from concealed drug use. Patient was reintubated, transferred, and treated for serotonin syndrome. Two patients experienced postoperative nausea/vomiting. Cohort significantly improved from baseline for all PROMS at all timepoints except SF-12 MCS at 1-year/2-years and SF-12 PCS at 2 years (p < 0.047, all). Overall MCID achievement rates were: VAS arm (48.7%), VAS neck (69.1%), NDI (98.9%), SF-12 MCS (50.0%), SF-12 PCS (54.6%), and PROMIS-PF (73.4%).
Conclusion
Outpatient CDR, incorporating an enhanced MMA protocol, can be safely and effectively performed with proper patient selection and surgical technique. Patients saw timely discharge, well-controlled postoperative pain, and favorable long-term outcomes.
Keywords: Cervical disc replacement, Multimodal analgesia, MMA
1. Introduction
Surgery of the cervical spine is a treatment option for patients with symptoms of myelopathy or radiculopathy from cervical degenerative changes that have failed to respond to conservative measures. While anterior cervical discectomy and fusion (ACDF) has been a staple in anterior cervical surgery, cervical disc replacement (CDR) has gained popularity in recent years.1 Unlike fusion, CDR simulates physiological motion of the cervical spine, preserving flexion and extension in the patient.2 Indications for CDR include 1- or 2-level cervical disc disease between C3 and C7 with symptoms of myelopathy or radiculopathy without instability on flexion or extension.3
In the inpatient setting, patients undergoing CDR may expect to stay in the hospital for up to 3 days postoperatively. With recent advances in anesthesiology and the development of the Enhanced Recovery After Surgery (ERAS) protocol, many surgeries have transitioned to the outpatient setting. By conducting surgery at ambulatory surgery centers (ASCs), patients markedly reduce their length of stay, leading to increased patient satisfaction,4,5 while simultaneously reducing hospital-related costs and preserving healthcare resources.6 A recent systematic review and meta-analysis by Wang et al. reported that outpatient CDR can be performed safely.7 The study also noted significantly shorter operating times and reduced complication rates in outpatient CDR compared to inpatient surgery. However, prior to successful outpatient surgery, factors such as appropriate patient selection, distance to secondary medical care, and pain management must be taken into consideration. To effectively perform surgery at an ASC, it is critical to have a safe, efficacious, and reproducible multimodal analgesia protocol to reduce postoperative pain that may delay discharge, while also minimizing opioid use that could result in other complications.
While previous studies have documented the use of such protocols in cervical surgery, reports are scarce in documenting MMA protocols in relation to CDR specifically with long-term clinical outcome data.8, 9, 10 To address this relative paucity in the literature, the current study presents a clinical case series of patients undergoing outpatient CDR with an enhanced MMA protocol, describing their subsequent patient-reported outcome measures (PROMs) up to 2-years postoperatively. Evidence from this study may provide clinicians with a reproducible protocol for pain relief following outpatient CDR, reducing reliance on, and associated adverse effects of opioid-based analgesia.
2. Methods
2.1. Patient population
Prior to beginning this study approved patient consent for all enrolled subjects and Institutional Review Board approval (ORA #14051301) were obtained. Data was obtained from a prospectively maintained retrospective database of outcomes from a single surgeon at an academic institution. Patients undergoing elective, primary, single, or multi-level CDR procedures in an ASC with an enhanced MMA protocol between June 2017 and December 2021 were identified and included in this study. This MMA protocol was implemented in July 2013, and therefore, no patients in the present study received patient-controlled analgesia (PCA).
Observation greater than 23 hours was not permitted in the ASC, and all patients were discharged on the same day as surgery. Patients were excluded for etiologies of infectious, malignant, or traumatic pathology. Patients undergoing inpatient or revision surgery were also excluded from this analysis. Patient demographic data and perioperative characteristics were divided into two groups: single-level CDR and 2-level CDR.
2.2. Data collection
Selected baseline demographic data were collected, including age, gender, body mass index (BMI), comorbidities (smoking, diabetic, and hypertensive status), comorbidity burden determined by the Charlson Comorbidity Index, American Anesthesia Society (ASA) classification, and insurance status. Perioperative characteristics indicating the spinal pathology, type of neuropathy, operative levels, operative duration, estimated blood loss and narcotic consumption were collected as well. A range of data was compiled regarding postoperative complications, pain scores, and revision and rehospitalization rates. Patient-reported outcome measures (PROMs) were recorded preoperatively and postoperatively at 6 weeks, 12 weeks, 6 months, 1 year, and 2 years. PROMs assessed included the Visual Analog Scale (VAS) arm, VAS neck, Neck Disability Index (NDI), 12-Item Short Form (SF-12) for Mental Component Score (MCS), SF-12 for Physical Component score (PCS), and the Patient-Reported Outcomes Measurement Information System for Physical Function (PROMIS-PF).
2.3. MMA protocol
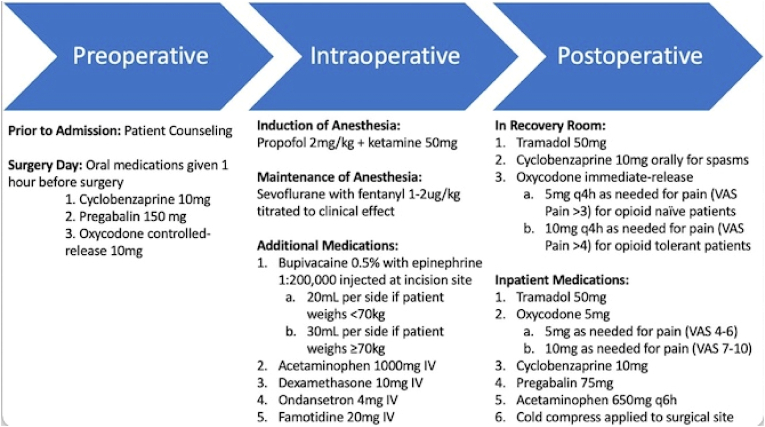
A standardized protocol for analgesia was initiated for all procedures and modified depending on individual patient pain scores (Fig. 1). Preoperatively, patients are given cyclobenzaprine, pregabalin, and oxycodone. Prior to beginning surgery, induction of anesthesia is achieved with propofol and ketamine; maintenance with sevoflurane and fentanyl. Additional intraoperative medications included bupivacaine with epinephrine, acetaminophen, dexamethasone, ondansetron, and famotidine. Postoperatively, patients received tramadol, cyclobenzaprine, and varying oxycodone doses dependent on VAS pain score.
Fig. 1.
Multimodal Analgesia protocol.
2.4. Statistical analysis
Data analysis was conducted using Stata 16.0 (StataCorp LP, College Station, TX). Descriptive statistics were conducted for patient demographic data, perioperative characteristics, and postoperative complications. PROMs were compared at each postoperative temporal interval to the preoperative scores with a paired sample t-test to determine significance. MCID achievement was assessed with descriptive statistics and was determined by previous literature guidelines as the following: VAS neck = 2.6,11 VAS arm = 4.1,11 NDI = 8.5,12 and SF-12 MCS = 4.7,11 and PROMIS-PF = 4.5.13
3. Results
3.1. Descriptive analysis
A total of 106 patients were included in this study, 76 of which underwent single-level CDR, while 30 had multilevel surgery. The mean cohort age was 46.4 years, most participants were male (64.2%), and had a BMI <30 kg/m2 (65.1%). The majority of patients were also non-diabetic, non-smokers, and non-hypertensive (97.2%, 90.6%, and 87.7%, respectively). Nearly all patients were ASA classification <3 (94.0%) and reported a mean CCI score of 0.37 (Table 1).
Table 1.
Patient demographics.
| Total |
1-Levels |
2-Levels |
|
|---|---|---|---|
| (n = 106) | (n = 76) | (n = 30) | |
| Age (Mean ± SD) | 46.4 ± 10.3 | 45.7 ± 10.2 | 47.8 ± 10.7 |
| Gender | |||
| Female | 35.9% (38) | 39.5% (30) | 26.7% (8) |
| Male | 64.2% (68) | 60.5% (46) | 73.3% (22) |
| Body Mass Index Category (BMI) | |||
| <30 kg/m2 | 65.1% (69) | 68.4% (52) | 56.7% (17) |
| ≥30 kg/m2 | 34.9% (37) | 31.6% (24) | 43.3% (13) |
| Body Mass Index (Mean ± SD) | |||
| 28.2 ± 5.3 | 28.0 ± 5.4 | 28.5 ± 5.2 | |
| Ethnicity | |||
| Caucasian | 81.7% (85) | 83.8% (62) | 76.7% (23) |
| African American | 7.7% (8) | 8.1% (6) | 6.7% (2) |
| Hispanic | 7.7% (8) | 5.4% (4) | 13.3% (4) |
| Asian | 1.9% (2) | 1.4% (1) | 3.3% (1) |
| Other | 0.9% (1) | 1.1% (1) | 0.0% (0) |
| Diabetes | |||
| Non-Diabetic | 97.2% (103) | 98.7% (75) | 93.3% (28) |
| Diabetic | 2.8% (3) | 1.3% (1) | 6.7% (2) |
| Smoking Status | |||
| Non-Smoker | 90.6% (96) | 88.2% (67) | 96.7% (29) |
| Smoker | 9.4% (10) | 11.8% (9) | 3.3% (1) |
| Hypertensive Status | |||
| Non-Hypertensive | 87.7% (93) | 89.5% (68) | 83.3% (25) |
| Hypertensive | 12.3% (13) | 10.5% (8) | 16.7% (5) |
| ASA Classification | |||
| <3 | 88.7% (94) | 92.1% (70) | 88.9% (24) |
| ≥3 | 11.3% (12) | 7.9% (6) | 20.0% (6) |
| CCI Score (Mean ± SD) | 0.37 ± 0.68 | 0.38 ± 0.70 | 0.36 ± 0.67 |
| Insurance | |||
| Medicare/Medicaid | 4.7% (5) | 2.6% (2) | 10.0% (3) |
| Workers' Compensation | 26.4% (28) | 23.7% (18) | 33.3% (10) |
| Private | 68.9% (73) | 73.7% (56) | 57.7% (17) |
ASA = American Society of Anesthesiologists; CCI = Charlson Comorbidity Index; SD = Standard Deviation.
3.2. Perioperative and postoperative outcomes
The most prominent spinal pathology in the enrolled patients was herniated nucleus pulposus (98.1%), followed by central stenosis (49.1%), and foraminal stenosis (27.4%), while 86.5% of patients also reported myeloradiculopathic symptoms related to their pathology. The most common operative levels in this cohort ranged from C5–C7, with an average operative duration of 58.6 min and estimated blood loss of 25.8 mL. While one patient required rehospitalization and revision surgery, the average total length of stay was only 6.3 hours. Reported inpatient pain scores on postoperative day 0 were 3.6 for the single-level cohort and 5.5 in the multi-level CDR group, however, their postoperative oral morphine equivalents (OME) to assess narcotic consumption were similar, at 16.9 and 18.5 OME, respectively (Table 2). Postoperative complications were rare in this cohort. Only 1.9% of patients reported nausea or vomiting, a single patient experienced a seizure secondary to serotonin syndrome, another developed a hematoma requiring readmission at 5-days postoperatively, and 5 patients reported mild to moderate dysphagia on postoperative day 1 with fully resolved symptoms at 6 weeks (Table 3). PROMs for VAS arm and neck, NDI, and PROMIS PF reported significantly improved pain scores at all time intervals up to 2-years compared to preoperative values. SF-12 MCS and PCS scores demonstrated significant improvement up to 6-months and 1-year, respectively (Table 4). Overall MCID achievement was noted in 98.9% of patients for NDI, 73.4% for PROMIS PF, and 69.1% for VAS neck. MCID achievement in the VAS arm, SF-12 MCS, and SF-12 PCS was met by about half of the participants (Table 5).
Table 2.
Perioperative characteristics.
| Total | 1-Levels | 2-Levels | |
| (n = 106) | (n = 76) | (n = 30) | |
| Spinal Pathology | |||
| Central Stenosis | 49.1% (52) | 43.4% (33) | 63.3% (19) |
| Foraminal Stenosis | 27.4% (29) | 25.0% (19) | 33.3% (10) |
| Herniated Nucleus Pulposus | 98.1% (104) | 98.7% (75) | 96.7% (29) |
| Neuropathy | |||
| None | 1.9% (2) | 2.7% (2) | 0.0% (0) |
| Radiculopathy | 7.5% (8) | 6.6% (5) | 10.0% (3) |
| Myeloradiculopathy | 86.5% (90) | 86.5% (64) | 86.7% (26) |
| Myelopathy | 3.8% (4) | 3.9% (3) | 3.3% (1) |
| Operative Levels | |||
| C3–C4 | 2.8% (3) | 3.9% (3) | 0.0% (0) |
| C3–C5 | 1.9% (2) | 0.0% (0) | 6.7% (2) |
| C4–C5 | 3.8% (4) | 5.3% (4) | 0.0% (0) |
| C4–C6 | 4.7% (5) | 0.0% (0) | 16.7% (5) |
| C5–C6 | 39.6% (42) | 55.3% (42) | 0.0% (0) |
| C5–C7 | 23.6% (25) | 0.0% (0) | 76.7% (23) |
| C6–C7 | 23.6% (25) | 32.9% (25) | 0.0% (0) |
| Operative Time (Mean ± SD; min) | 58.6 ± 60.9 | 57.9 ± 74.0 | 62.0 ± 9.5 |
| Estimated Blood Loss (Mean ± SD; mL) | 25.8 ± 4.4 | 26.2 ± 5.4 | 25.0 ± 4.5 |
| Rehospitalization | 0.9% (1) | 0.0% (0) | 3.3% (1) |
| Revision | 0.9% (1) | 0.0% (0) | 3.3% (1) |
| Hospital Length of Stay (Mean ± SD; hours) | 8.5 ± 3.3 | 8.1 ± 2.1 | 9.4 ± 4.9 |
| Postoperative Length of Stay (Mean ± SD; hours) | 6.3 ± 3.7 | 6.7 ± 4.3 | 5.4 ± 2.1 |
| Inpatient Pain Score | |||
| POD 0 | 4.1 ± 1.8 | 3.6 ± 1.8 | 5.5 ± 1.2 |
| Inpatient Narcotic Consumption (OME) | |||
| POD 0 | 17.4 ± 14.7 | 16.9 ± 15.5 | 18.5 ± 12.9 |
OME = Oral Morphine Equivalents; POD = Postoperative Day; SD = Standard Deviation; mL = mililitersRe-hospitalization = Defined as returning to hospital within 6-weeks of surgery with a surgical related complaint.
Table 3.
Postoperative complications.
| Complication | Total |
1-Levels |
2-Levels |
|---|---|---|---|
| (n = 106) | (n = 76) | (n = 30) | |
| Reintubation | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Urinary Retention | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Urinary Tract Infection | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Acute Renal Failure | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Postoperative Anemia | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Altered Mental Status | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Venous Thromboembolism | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Pulmonary Embolism | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Pneumothorax | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Atelectasis | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Pleural Effusion | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Arrhythmia | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Ileus | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Nausea and Vomiting | 1.9% (2) | 1.3% (1) | 3.6% (1) |
| Seizure∗ | 0.9% (1) | 1.3% (1) | 0.0% (0) |
| Hematoma∗ | 0.9% (1) | 1.3% (1) | 0.0% (0) |
| Transient Dysphagia∗ | 4.7% (5) | 1.3% (1) | 13.3% (4) |
| Fever of Unknown Origin | 0.0% (0) | 0.0% (0) | 0.0% (0) |
| Overall | 8.5% (9) | 5.3% (4) | 16.7% (5) |
| ∗Summary of Complications | |||
| Seizure: 1 patient who had single-level CDR at C6–C7 was noted having elevated temperatures in the recovery room, and subsequently had an episode of a seizure secondary to serotonin syndrome. Patient was reintubated and emergently transferred to the nearest hospital. Patient was treated for serotonin syndrome and extubated and discharged the following day. | |||
| Hematoma: 1 patient who underwent two-level CDR at C5–C7 developed hematoma 5 days following surgery. Patient was readmitted to hospital for revision surgery for placement of cervical drain. Patient was discharged 2 days following placement of the drain. | |||
| Transient Dysphagia: 5 patients reported mild to moderate dysphagia on POD 1. 4 of these reports were for two-level cases. At the 6-week follow-up timepoint all reports of dysphagia were completely resolved. | |||
Table 4.
Patient reported outcome measures.
| Mean ± SD | Postoperative PROM Improvement | |
|---|---|---|
| VAS Arm | ||
| Preoperative | 5.7 ± 2.8 | – |
| 6-weeks | 2.2 ± 2.8 | < 0.001 |
| 12-weeks | 2.0 ± 2.7 | < 0.001 |
| 6-months | 2.7 ± 2.9 | < 0.001 |
| 1-year | 2.1 ± 2.3 | < 0.001 |
| 2-year | 1.1 ± 0.9 | 0.005 |
| VAS Neck | ||
| Preoperative | 6.4 ± 2.3 | – |
| 6-weeks | 2.9 ± 2.6 | < 0.001 |
| 12-weeks | 1.9 ± 2.2 | < 0.001 |
| 6-months | 2.1 ± 2.3 | < 0.001 |
| 1-year | 2.9 ± 3.2 | < 0.001 |
| 2-year | 2.5 ± 1.9 | 0.018 |
| NDI | ||
| Preoperative | 40.0 ± 18.7 | – |
| 6-weeks | 27.5 ± 19.8 | < 0.001 |
| 12-weeks | 17.1 ± 16.8 | < 0.001 |
| 6-months | 18.5 ± 16.1 | < 0.001 |
| 1-year | 16.0 ± 15.6 | < 0.001 |
| 2-year | 17.0 ± 9.6 | 0.010 |
| SF-12 MCS | ||
| Preoperative | 48.9 ± 9.9 | – |
| 6-weeks | 52.5 ± 10.4 | 0.002 |
| 12-weeks | 53.9 ± 9.8 | 0.001 |
| 6-months | 52.1 ± 9.4 | 0.047 |
| 1-year | 46.9 ± 14.8 | 0.888 |
| 2-year | 47.2 ± 12.2 | 0.485 |
| SF-12 PCS | ||
| Preoperative | 35.4 ± 8.4 | – |
| 6-weeks | 40.1 ± 10.4 | < 0.001 |
| 12-weeks | 46.7 ± 11.2 | < 0.001 |
| 6-months | 41.1 ± 10.1 | < 0.001 |
| 1-year | 40.1 ± 10.8 | 0.009 |
| 2-year | 42.3 ± 12.9 | 0.376 |
| PROMIS PF | ||
| Preoperative | 41.2 ± 7.5 | – |
| 6-weeks | 45.6 ± 9.9 | 0.019 |
| 12-weeks | 48.9 ± 10.5 | < 0.001 |
| 6-months | 53.3 ± 12.3 | < 0.001 |
| 1-year | 51.7 ± 11.4 | 0.001 |
| 2-year | 47.8 ± 9.7 | 0.009 |
∗p-values calculated using paired samples t-test to determine postoperative improvement. Boldface indicates statistical significance.
Table 5.
MCID achievement.
| PROM | % (n) |
|---|---|
| VAS Arm | |
| 6-weeks | 35.6% (21) |
| 12-weeks | 43.4% (23) |
| 6-months | 45.2% (14) |
| 1-year | 52.4% (11) |
| 2-year | 100.0% (4) |
| Overall | 48.7% (36) |
| VAS Neck | |
| 6-weeks | 50.0% (31) |
| 12-weeks | 67.2% (39) |
| 6-months | 75.0% (30) |
| 1-year | 57.2% (12) |
| 2-year | 100.0% (4) |
| Overall | 69.1% (56) |
| NDI | |
| 6-weeks | 55.0% (33) |
| 12-weeks | 75.0% (42) |
| 6-months | 75.0% (30) |
| 1-year | 76.2% (16) |
| 2-year | 50.0% (2) |
| Overall | 98.9% (92) |
| SF-12 MCS | |
| 6-weeks | 42.9% (21) |
| 12-weeks | 43.5% (20) |
| 6-months | 40.9% (9) |
| 1-year | 37.5% (6) |
| 2-year | 22.2% (2) |
| Overall | 50.0% (33) |
| SF-12 PCS | |
| 6-weeks | 38.8% (19) |
| 12-weeks | 45.7% (21) |
| 6-months | 59.1% (13) |
| 1-year | 43.8% (7) |
| 2-year | 44.4% (4) |
| Overall | 54.6% (36) |
| PROMIS PF | |
| 6-weeks | 42.5% (17) |
| 12-weeks | 59.5% (25) |
| 6-months | 73.3% (22) |
| 1-year | 66.7% (12) |
| 2-year | 37.5% (3) |
| Overall | 73.4% (47) |
4. Discussion
The presented case series details the operative and clinical outcomes of 106 patients who received single-level or double-level CDR surgery in the outpatient ASC setting. We evaluated several parameters critical in performing outpatient CDR, including careful patient selection and the incorporation of an enhanced MMA protocol for pain management. Additionally, we provide clinical outcomes up to the 2-year time point for the patient cohort.
4.1. Patient selection
The vast majority of patients in our study cohort found great benefit from the enhanced MMA protocol. The average operative time seen was 58.6 min, and on average patients were discharged from the ASC facility after just 6.3 hours.
The demographic nature of our cohort, however, elucidates one critical aspect of effective outpatient CDR performance: patient selection. The average patient in this study was not obese, carried a low comorbidity rate, and was under 50 years old. A strong majority of patients did not smoke and did not have diabetes or hypertension. 88.7% of patients had an ASA classification under 3, indicating that they carried low operative risk. In addition, all procedures evaluated in this study were performed at a single- or double-level; large, multilevel procedures may limit the safety and efficacy of outpatient CDR. These demographic characteristics are consistent with previous outpatient CDR cohorts,8,14 supporting the notion that careful patient selection is paramount in performing safe outpatient anterior cervical surgery. Particularly in the ambulatory setting, where resources are limited relative to large inpatient hospitals, patients must be screened to ensure that they carry low risks of postoperative hospitalization.15
4.2. Enhanced MMA protocol
The enhanced MMA regimen incorporated into the care of the 106 patients assessed in this study utilizes several different medications to create a positive interactive effect on pain management (Fig. 1). Approximately 1 hour before surgery, patients are orally given one muscle relaxant (cyclobenzaprine, 10 mg), one anticonvulsant (pregabalin, 150 mg), and one narcotic medication (oxycodone controlled-release, 10 mg). This preemptive triad of administration is supported by prior literature suggesting that such medication can work synergistically with other analgesics to control postoperative pain. Perioperative administration of the anticonvulsant pregabalin in particular has been observed to significantly mitigate postoperative pain, as evidenced by reduced VAS pain scores, as well as assist in avoidance of postoperative opioid consumption.16,17
During surgery, anesthesia is administered, beginning with 2 mg/kg of propofol and 50 mg of ketamine. Intraoperative ketamine administration has been reported to mitigate postoperative pain and decrease postoperative opioid use.18 To maintain intraoperative anesthesia, 1–2 μg/kg of sevoflurane with fentanyl is titrated for efficacy. In addition to anesthesia, several medications are administered intraoperatively. Local anesthetic (bupivacaine, dosage based on patient weight) and an anti-inflammatory (dexamethasone) control operative site pain and inflammation. Seeking to avoid gastroesophageal reflux disease (GERD) and other acid-related complications, we also incorporated famotidine into the intraoperative analgesic regimen. To mitigate nausea and vomiting, a primary threat to the successful discharge of patients on the day of surgery, an antiemetic (ondansetron) is also injected intraoperatively. Finally, acetaminophen is utilized during surgery. Acetaminophen's effect on the central nervous system has led to positively observed effects on postoperative pain and pain management following spine surgery.19
Postoperatively, 10 mg of cyclobenzaprine was again administered to control muscle spasms in the recovery room. 50 mg of tramadol and 5/10 mg of oxycodone (pain-dependent) were also delivered orally to control immediate postoperative pain.
In the case of inpatient admission following surgery, our protocol calls for readministration of tramadol, oxycodone, cyclobenzaprine, pregabalin, and acetaminophen. Acetaminophen in particular has shown great utility in postoperative pain management, associated with reduced postoperative complications, opioid consumption, and length of hospital stay.20
A 2020 study by Ogura et al.21 followed 68 anterior lumbar fusion patients treated with a multimodal pain control regimen, finding postoperative opioid consumption to be dramatically reduced in these patients as compared with patients who did not receive a multimodal analgesic pain control regimen. Similar to our MMA protocol, the protocol used by Ogura and colleagues incorporated preoperative oral administration of cyclobenzaprine and gabapentin (an anticonvulsant analogous to pregabalin), intended for prophylactic mitigation of muscle pain and spasms following surgery. They also utilized acetaminophen in a similar manner to our protocol, citing its benefit in managing postoperative pain.
Akin to the aims of Ogura and colleagues, we sought through an MMA protocol to reduce postoperative opioid consumption following outpatient CDR surgery. A recent study by Lovecchio et al.22 evaluated the early postoperative opioid consumption of 57 anterior cervical spine surgery patients, finding that opioid consumption steadily decreased by postoperative day, though remained at a median of 10 oral morphine equivalents (OME) at six days postoperative, after seeing a median of 20 OME on postoperative day 1. In our study cohort, the mean narcotic consumption on the day of surgery was 17.4 OME, suggesting a good starting point if opioids are avoided after the day of surgery.
4.3. Clinical outcomes
The clinical outcomes of our study cohort were promising, with patients on average achieving statistically significant, lasting, and clinically meaningful improvement in VAS arm, VAS neck, NDI, SF-12 PCS, and PROMIS PF measures. Additionally, a majority of patients achieved MCID for overall VAS neck, NDI, SF-12 PCS, and PROMIS PF measures. 48.7% of patients achieved MCID for overall VAS arm, and 50% achieved MCID for overall SF-12 MCS.
Following assessment of 55 CDR procedures performed in the ASC setting, Chin et al.23 found that patients significantly improved in VAS neck, VAS arm, and NDI measures, and overall found that CDR could safely and effectively be performed in the outpatient ASC setting. Purger et al.24 evaluated 370 outpatient CDR procedures and also reported success for outpatient CDR. The outpatient CDR patients in their study demonstrated positive improvement in all clinical outcome measures, similar to those of the inpatient CDR patients assessed. In addition to this comparable improvement, Purger and colleagues noted that no outpatient CDR patients underwent a reoperation within 30 days, while 6 inpatient CDR patients did undergo such a reoperation.
Prior literature has supported the transition of CDR procedures to the outpatient setting for selected patients. Our study cohort's impressive postoperative course aligns well with the current literature. We evaluated clinical outcomes of arm pain, neck pain, neck disability, mental health, and physical function through two years following surgery. Statistically significant improvements from preoperative levels were observed at 6 weeks, 12 weeks, 6 months, 1 year, and 2 years for arm pain, neck pain, neck disability, and physical function in our study cohort. Such a robust clinical improvement profile supports prior literature that has suggested that CDR procedures can be performed safely and effectively in the outpatient ASC setting, without compromise given by way of clinical outcomes.
4.4. Complications
Imperative to the success of our MMA protocol and outpatient CDR surgery is the avoidance of postoperative complications. Wang et al.‘s 2021 systematic review revealed a 59% reduction in postoperative complication risk for outpatient CDR patients relative to inpatient CDR patients. A 2019 comparison of outpatient vs inpatient CDR patients by Bovonratwet et al.25 found that outpatient CDR patients did not demonstrate any significant differences from their inpatient counterparts by way of adverse events or readmissions. Both studies, therefore, judged outpatient CDR to be an appropriate treatment method in selected patients.
Similarly, we found complications and adverse events to be limited. Among the 106 total outpatients, CDR patients evaluated, just one two-level C5–C7 CDR patient was readmitted to the hospital for evacuation of a hematoma and discharged two days after. Including this patient, nine patients in our study cohort experienced postoperative complications. On the first day following surgery, five patients reported mild to moderate dysphagia, all of which were resolved by the time of the first follow-up visit six weeks after surgery. Two patients experienced postoperative nausea and/or vomiting. Postoperative nausea and vomiting have been associated with opioid consumption and may be tempered by increased administration of intraoperative local anesthesia and postoperative non-narcotic medication.26,27 In our protocol, appropriate treatment for PONV includes preoperative administration of anti-emetics such as ondansetron or metoclopramide and adequate hydration.
One patient who had single-level CDR at C6–C7 was noted to have elevated temperatures in the recovery room, and subsequently had an episode of a seizure secondary to serotonin syndrome. This episode was triggered by concealed drug use prior to surgery. The patient was reintubated, emergently transferred to the nearest hospital, treated for serotonin syndrome, and discharged the following day. Serotonin syndrome can result as sequelae to prior drug use, particularly with medications used to treat depressive disorders, such as selective serotonin reuptake inhibitors,28 as well as illicit drugs such as MDMA.29 With the increasing availability of agents with serotonergic activity, it is important for the care team to be aware of this rare condition in the postoperative patient so as respond quickly with treatment.
4.5. Limitations
All patients were obtained from a single-surgeon database, which while increasing homogeneity of the data and reducing incidence of confounding variables in surgeon technique, support staff, and facility offerings, leads to several strong limitations. Primarily, all patients included in this study underwent the MMA protocol described without a historical control cohort to compare to, as this protocol was implemented prior to any recorded data on CDR at this institution. As a retrospective study, we are unable to compare this cohort to a control and instead are submitting this as a case series. Additionally, use of a single-surgeon database limits the generalizability of these findings as different outpatient centers may have varying capabilities and practices, which may further vary by anesthesiology staff present. Further, as a retrospective study, there is not available data on the exact discharge medication quantities and durations for each patient. This limits our ability to report acute and sub-acute postoperative pain medication requirements after discharge. Additionally, immediate postoperative pain levels at 1-week were not recorded.
5. Conclusion
This is the largest clinical case series focused on CDR procedures within an ASC requiring no planned 23-h observation. This study demonstrates the efficacy of performing CDR surgery with an enhanced MMA protocol in an outpatient setting with proper patient selection and surgical technique. Patients in this cohort were discharged in a timely manner, had well-controlled postoperative pain, and demonstrated favorable long-term outcomes.
Author contribution
Michael C. Prabhu: Conceptualization, Methodology, Visualization, Formal Analysis, Software, Investigation, Writing – Original Draft, Writing – Review & Editing, Kevin C. Jacob: Conceptualization, Methodology, Visualization, Formal Analysis, Software, Investigation, Writing – Original Draft, Writing – Review & Editing, Madhav R. Patel: Conceptualization, Methodology, Visualization, Formal Analysis, Software, Investigation, Writing – Original Draft, Writing – Review & Editing, Timothy J. Hartman: Project Administration, Investigation, Writing – Review & Editing, James W. Nie: Project Administration, Investigation, Writing – Review & Editing, Kern Singh: Conceptualization, Methodology, Supervision, Resources, Investigation, Writing – Review & Editing.
Conflict of interest and disclosure of funding
None declared.
Disclosure-conflict of interest
No authors have any financial or personal relationships with people or organizations to disclose. No funds were received in support of this work. No benefits in any form have been or will be received from any commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Steinberger J., Qureshi S. Cervical disc replacement. Neurosurg Clin. 2020;31(1):73–79. doi: 10.1016/j.nec.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Puttlitz C.M., Rousseau M.A., Xu Z., Hu S., Tay B.K.-B., Lotz J.C. Intervertebral disc replacement maintains cervical spine kinetics. Spine. 2004;29(24):2809–2814. doi: 10.1097/01.brs.0000147739.42354.a9. [DOI] [PubMed] [Google Scholar]
- 3.Leven D., Meaike J., Radcliff K., Qureshi S. Cervical disc replacement surgery: indications, technique, and technical pearls. Curr Rev Musculoskelet Med. 2017;10(2):160–169. doi: 10.1007/s12178-017-9398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yerneni K., Burke J.F., Chunduru P., et al. Safety of outpatient Anterior cervical discectomy and fusion: a systematic review and meta-analysis. Neurosurgery. 2020;86(1):30–45. doi: 10.1093/neuros/nyy636. [DOI] [PubMed] [Google Scholar]
- 5.Sheperd C.S., Young W.F. Instrumented outpatient anterior cervical discectomy and fusion: is it safe? Int Surg. 2012;97(1):86–89. doi: 10.9738/CC35.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wohns R. Safety and cost-effectiveness of outpatient cervical disc arthroplasty. Surg Neurol Int. 2010;1:77. doi: 10.4103/2152-7806.73803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Meng Y., Liu H., et al. Comparison of the safety of outpatient cervical disc replacement with inpatient cervical disc replacement: a systematic review and meta-analysis. Global Spine J. 2021;11(7):1121–1133. doi: 10.1177/2192568220959265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins N.W., Parrish J.M., Nolte M.T., et al. Multimodal analgesic management for cervical spine surgery in the ambulatory setting. Internet J Spine Surg. 2021;15(2):219–227. doi: 10.14444/8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohl D.D., Louie P.K., Shah N., et al. Multimodal versus patient-controlled analgesia after an anterior cervical decompression and fusion. Spine. 2016;41(12):994–998. doi: 10.1097/BRS.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 10.Soffin E.M., Wetmore D.S., Barber L.A., et al. An enhanced recovery after surgery pathway: association with rapid discharge and minimal complications after anterior cervical spine surgery. Neurosurg Focus. 2019;46(4):E9. doi: 10.3171/2019.1.FOCUS18643. [DOI] [PubMed] [Google Scholar]
- 11.Parker S.L., Godil S.S., Shau D.N., Mendenhall S.K., McGirt M.J. Assessment of the minimum clinically important difference in pain, disability, and quality of life after anterior cervical discectomy and fusion: clinical article. J Neurosurg Spine. 2013;18(2):154–160. doi: 10.3171/2012.10.SPINE12312. [DOI] [PubMed] [Google Scholar]
- 12.Palit M., Schofferman J., Goldthwaite N., et al. Anterior discectomy and fusion for the management of neck pain. Spine. 1999;24(21):2224–2228. doi: 10.1097/00007632-199911010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Steinhaus M.E., Iyer S., Lovecchio F., et al. Minimal clinically important difference and substantial clinical benefit using PROMIS CAT in cervical spine surgery. Clin Spine Surg. 2019;32(9):392–397. doi: 10.1097/BSD.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 14.Adamson T., Godil S.S., Mehrlich M., Mendenhall S., Asher A.L., McGirt M.J. Anterior cervical discectomy and fusion in the outpatient ambulatory surgery setting compared with the inpatient hospital setting: analysis of 1000 consecutive cases. J Neurosurg Spine. 2016;24(6):878–884. doi: 10.3171/2015.8.spine14284. [DOI] [PubMed] [Google Scholar]
- 15.Mohandas A., Summa C., Worthington W.B., et al. Best practices for outpatient Anterior cervical surgery: results from a delphi panel. Spine. 2017;42(11):E648–E659. doi: 10.1097/BRS.0000000000001925. [DOI] [PubMed] [Google Scholar]
- 16.Hurley R.W., Cohen S.P., Williams K.A., Rowlingson A.J., Wu C.L. The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med. 2006;31(3):237–247. doi: 10.1016/j.rapm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Khurana G., Jindal P., Sharma J.P., Bansal K.K. Postoperative pain and long-term functional outcome after administration of gabapentin and pregabalin in patients undergoing spinal surgery. Spine. 2014;39(6):E363–E368. doi: 10.1097/BRS.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 18.Maheshwari K., Avitsian R., Sessler D.I., et al. Multimodal analgesic regimen for spine surgery: a randomized placebo-controlled trial. Anesthesiology. 2020;132(5):992–1002. doi: 10.1097/ALN.0000000000003143. [DOI] [PubMed] [Google Scholar]
- 19.Nolte M.T., Parrish J.M., Jenkins N.W., et al. Multimodal analgesic management for lumbar decompression surgery in the ambulatory setting: clinical case series and review of the literature. World Neurosurg. 2021;154:e656–e664. doi: 10.1016/j.wneu.2021.07.105. [DOI] [PubMed] [Google Scholar]
- 20.Hansen R.N., Pham A.T., Böing E.A., Lovelace B., Wan G.J., Miller T.E. Comparative analysis of length of stay, hospitalization costs, opioid use, and discharge status among spine surgery patients with postoperative pain management including intravenous versus oral acetaminophen. Curr Med Res Opin. 2017;33(5):943–948. doi: 10.1080/03007995.2017.1297702. [DOI] [PubMed] [Google Scholar]
- 21.Ogura Y., Gum J.L., Steele P., et al. Multi-modal pain control regimen for anterior lumbar fusion drastically reduces in-hospital opioid consumption. J Spine Surg. 2020;6(4):681–687. doi: 10.21037/jss-20-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovecchio F, Premkumar A, Steinhaus M, et al. Early opioid consumption patterns after anterior cervical spine surgery. Clin Spine Surg. Published online March 29, 2021. doi:10.1097/BSD.0000000000001176. [DOI] [PubMed]
- 23.Chin K.R., Pencle F.J.R., Seale J.A., Pencle F.K. Clinical outcomes of outpatient cervical total disc replacement compared with outpatient Anterior cervical discectomy and fusion. Spine. 2017;42(10):E567–E574. doi: 10.1097/BRS.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 24.Purger D.A., Pendharkar A.V., Ho A.L., et al. Analysis of outcomes and cost of inpatient and ambulatory anterior cervical disk replacement using a state-level database. Clin Spine Surg. 2019;32(8):E372–E379. doi: 10.1097/BSD.0000000000000840. [DOI] [PubMed] [Google Scholar]
- 25.Bovonratwet P., Fu M.C., Tyagi V., Ondeck N.T., Albert T.J., Grauer J.N. Safety of outpatient single-level cervical total disc replacement: a propensity-matched multi-institutional study. Spine. 2019;44(9):E530–E538. doi: 10.1097/BRS.0000000000002884. [DOI] [PubMed] [Google Scholar]
- 26.Buvanendran A., Thillainathan V. Preoperative and postoperative anesthetic and analgesic techniques for minimally invasive surgery of the spine. Spine. 2010;35(26 Suppl):S274–S280. doi: 10.1097/BRS.0b013e31820240f8. [DOI] [PubMed] [Google Scholar]
- 27.Shaikh S.I., Nagarekha D., Hegade G., Marutheesh M. Postoperative nausea and vomiting: a simple yet complex problem. Anesth Essays Res. 2016;10(3):388–396. doi: 10.4103/0259-1162.179310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49–54. doi: 10.4037/ccn2017169. [DOI] [PubMed] [Google Scholar]
- 29.Silins E., Copeland J., Dillon P. Qualitative review of serotonin syndrome, ecstasy (MDMA) and the use of other serotonergic substances: hierarchy of risk. Aust N Z J Psychiatr. 2007;41(8):649–655. doi: 10.1080/00048670701449237. [DOI] [PubMed] [Google Scholar]