Summary
Innate visually guided behaviors are thought to promote survival by guiding organisms to sources of food and safety and away from harm without requiring learning. Historically, innate behaviors have been considered hard-wired and invariable, but emerging evidence shows that many innate behaviors are flexible and complex due to modulation. Here, we investigate the modulation of the innate preference for light displayed by the Xenopus laevis tadpole, an exceptionally invasive and well-studied organism that is known to display several different innate visually guided behaviors. We found that tadpoles display a circadian-regulated oscillation in their preference for light over dark which can be altered by experimentally increasing or decreasing levels of serotonin transmission. We also found that endogenous levels of serotonin transmission during the day maintain a consistently moderate preference for light. Theoretically, a moderate preference for light, as opposed to a strong preference, optimizes survival by rendering tadpoles’ behavior less predictable.
Subject areas: Behavioral neuroscience, Molecular neuroscience
Graphical abstract
Highlights
-
•
Xenopus tadpoles display an innate preference for light over dark
-
•
The strength of the preference oscillates over the day/night cycle
-
•
Serotonin modulates the strength of the preference for light
Behavioral neuroscience; Molecular neuroscience
Introduction
Light waves are reflected and absorbed by objects in the environment, creating a wide and dynamic range of colors and luminance, or light intensity, levels. In this way, light shapes the visual landscape and provides important visual cues for animals. Many animals, especially while in their larval or immature stages of development, are known to display innate preferences for specific wavelengths (“colors”) or intensities of light (Asirim et al., 2020; Shiratori et al., 2017; Moriya et al., 1996; Park et al., 2016; Mrosovsky and Shettleworth, 1968). Innate visually guided behaviors are thought to promote survival by guiding organisms to sources of food and safety and away from harm without requiring learning. Historically, innate behaviors have been considered hard-wired and invariable, but emerging evidence suggests that many innate behaviors are flexible and complex due to modulation (Gorostiza, 2018). Thus, modulation can add plasticity to an otherwise one-dimensional behavior. Given that one, non-variable behavior is unlikely appropriate for all situations (Gorostiza et al., 2016), the flexibility provided by modulation suggests another feature of innate behaviors that optimizes survival.
Here, we investigate the modulation of the innate preference for light displayed by Xenopus laevis tadpoles. The visual system of the Xenopus tadpole has been useful for studying how neural circuits form and give rise to behaviors. As a result, several innate visually guided behaviors displayed by these tadpoles have already been described. These behaviors include a visual avoidance response (Liu et al., 2018; Shen et al., 2014; Dong et al., 2009), an optomotor response—the tendency to follow moving bars of light projected onto the floor of the test dish (Dong et al., 2009; Pronych et al., 1996; Wassersug, 1973), an innate preference for midspectrum (green) wavelengths of light, (Hunt et al., 2020; Jaeger and Hailman, 1976), and a preference for light over dark (Moriya et al., 1996; Jaeger and Hailman, 1976). In addition, it has been shown that preferences for specific colors and intensities of light can be experimentally induced through an associative learning paradigm (Rothman et al., 2016; Blackiston et al., 2010). While these behaviors have been described and measured mostly in the context of visual system function and learning and memory, little focus has been placed on inherent variations or nuances associated with them or whether they are subject to modulation. In our previous study (Hunt et al., 2020), we found that pharmacologically enhancing serotonin transmission by exposing tadpoles to a selective serotonin reuptake inhibitor (SSRI) shifted an innate preference for the color green over light (which is brighter than green), to a preference for light over green, suggesting that serotonin may modulate these innate visual preferences. Here, we further explore the innate preference for light displayed by Xenopus tadpoles and how it is modulated by serotonin. For this, we used a novel open-field, dark versus light test paradigm to identify long-term luminance preferences of freely swimming tadpoles (Hunt et al., 2020). We measured the preference for light or dark at different points across the twenty-four hour day/night cycle. We found that the strength of the preference oscillates over the day/night cycle, strongest during the day and weakest at night, and that the oscillation can be exaggerated or dampened by experimentally increasing or decreasing serotonergic signaling, respectively. Our data suggest an ethological model in which circadian regulation of endogenous serotonin levels promote survival by generating a moderate preference for light.
Results
To measure luminance preferences displayed by Xenopus tadpoles, we used an established experimental paradigm designed to study long-term visual preferences of freely swimming tadpoles (Hunt et al., 2020). For this, ten tadpoles were placed into a Steinberg’s solution-filled Petri dish in which one quadrant of the floor was light and the remaining 3 quadrants of the floor were dark (Figure 1A). Tadpoles were videotaped for 30 min as they swam freely around the dish. Videos were analyzed by counting the number of tadpoles in the light quadrant every minute for the entire 30-min trial. An overall preference-for-light score for a given group was calculated by averaging the 30 data points. Given that there are 10 tadpoles per group, an average score of 2.5 tadpoles (25%) in the light quadrant at any given point in time indicates no preference for light or dark (i.e. indifference to luminance level). A score greater than 25% indicates a preference for light; below 25%, an avoidance of light (or preference for dark). We previously established that tadpoles display an even distribution across an all-white test dish, reflecting a lack of schooling behavior that could otherwise confound or interfere with the display of each individual’s preference (Hunt et al., 2020).
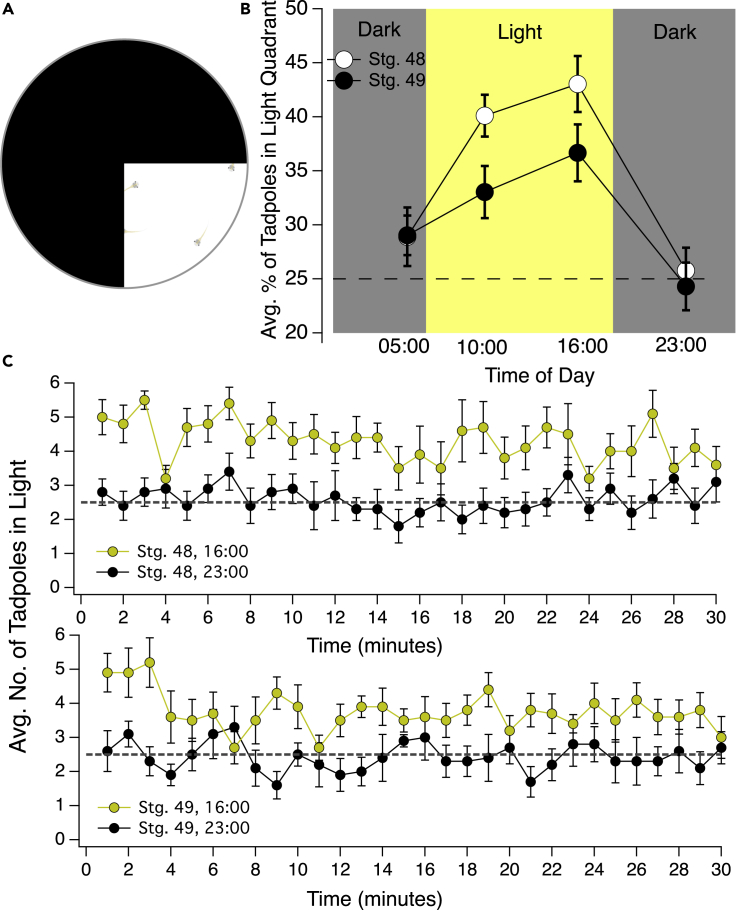
Figure 1.
Strength of preference for light displayed by developmental stage 48/49 Xenopus tadpoles oscillates over the 24 h day/night cycle
(A) Schematic of the light/dark test dish. One quadrant of the test dish is light and the remaining 3/4ths is dark. Tadpoles, in groups of ten, are video-taped using a Go-Pro as they swim freely around the dish for 30 min. Preference for light is quantified by counting the number of tadpoles in the light quadrant every minute for 30 min.
(B) Preference for light was tested at two time points during the day (10:00 and 16:00) and two time points during subjective night (23:00 and 05:00). Both stage 48 and 49 tadpoles displayed a stronger preference for light during the subjective day compared to night. The preference for light peaked at 16:00. Dashed line represents the % of tadpoles in the light quadrant that would be expected if no preference for, or avoidance of, light.
(C) Average number of tadpoles in the light quadrant at each minute of the 30-min trial, showing the preference at 16:00 (time of day when preference for light is at its peak) and at 23:00 (when the preference for light was lowest) test times. This plot shows that the strength for the preference for light is consistent across the 30-min trial. Dashed line represents the average number of tadpoles in the light quadrant that would be expected if no preference for, or avoidance of, light. Error bars represent SEM.
Xenopus tadpoles display a circadian-dependent preference for light
Tadpoles were raised on a 12 h:12 h light/dark schedule with lights turning on at 06:00 and turning off at 18:00. Preference for light was tested at 4 time points across the 24-h day/night cycle: two times during their subjective day (10:00 and 16:00) and two times during their subjective night (05:00 and 23:00). Both developmental stage 48 and 49 tadpoles displayed a persistent preference for light during their subjective day, the preference peaking at 16:00 (Figure 1B). During their subjective night, the preference for light approached indifference (Figure 1B). Non-parametric statistical analyses indicated that all of the average day-time strengths of the preference for light were significantly greater than those displayed at night, except for the developmental stage 49 05:00 (night) versus 10:00 (day) average data point (Table 1). In addition, we observed that stage 48 tadpoles consistently displayed a stronger preference for light during the daytime compared to stage 49. This is in accordance with a previous study by Moriya et al. (1996) showing that the preference for light displayed by Xenopus tadpoles declines over development. Figure 1C shows the overall average number of tadpoles in the light quadrant of the test dish at each minute of the 30-min trial at both 16:00, the time of day when the innate preference for light is at its peak, and at 23:00, the time of night when the innate preference for light is at its trough. These plots show that the strength of the preference for light stabilizes early in the trial and is fairly consistent over the duration of the trial.
Table 1.
Statistical comparisons of preference for light across day and night
| P-values | ||||||
|---|---|---|---|---|---|---|
| Time | 23:00 v. 16:00 | 23:00 v. 10:00 | 23:00 v. 05:00 | 05:00 v. 10:00 | 05:00 v. 16:00 | 10:00 v. 16:00 |
| St. 48 | p = 0.001b (n = 10) |
p = 0.001b (n = 10) |
p = 0.385 (n = 10) |
p = 0.005b (n = 10) |
p = 0.005b (n = 10) |
p = 0.241 (n = 10) |
| St. 49 | p = 0.007b (n = 10) |
p = 0.023a (n = 10) |
p = 0.089 (n = 10) |
p = 0.174 (n = 10) |
p = 0.038a (n = 10) |
p = 0.326 (n = 10) |
Significant at p < 0.05.
Significant at p < 0.01.
In summary, stage 48 and 49 tadpoles displayed a modest yet persistent preference for light during the day (lights on). The strength of the preference appeared to ramp up across the day, peaking in the subjective late afternoon at 16:00, and then declined during the night. The oscillating, circadian-dependent nature of the preference for light suggests that this innate hard-wired phototactic behavior is modulated.
We next asked what may be the modulator producing the observed oscillations in the preference for light. Based on our previous finding that exposing tadpoles to the SSRI trazodone switched the preference displayed by tadpoles from color-based to luminance-based (Hunt et al., 2020), we hypothesized that serotonin may play a role.
SSRI-exposed tadpoles display an enhanced preference for light
If serotonin modulates preference for light, then experimentally enhancing serotonin transmission would alter this innate visually guided behavior. To test this, tadpoles were exposed to the SSRI fluoxetine (1 μM) for 24 h (Figure 2A) and then their preference for light was measured using the same test paradigm as described above (Figure 1A). SSRIs such as fluoxetine work by inhibiting the reuptake of endogenous serotonin once it has been released. Hence, for this experiment, fluoxetine was used as a pharmacological tool to boost endogenous serotonin transmission. Tadpoles were tested at approximately 16:00, the time of day when their preference for light peaks (Figure 1B). We observed a significant increase in the average number of tadpoles in the light region of the test dish for the fluoxetine-treated group compared to that of controls, indicating a stronger preference for the light Figure 2B; stage 48 control vs. fluoxetine: p < 0.001; stage 49 control vs. fluoxetine: p < 0.001). Although exposure to fluoxetine increased the preference for light to similar degrees across stage 48 and 49, the effect was more pronounced for stage 49 tadpoles due to a lower baseline preference displayed by controls at this older stage (Figures 2B and 2C). That developmental stage 49 fluoxetine-exposed tadpoles exhibit an equally robust increase in preference for light as stage 48 fluoxetine-exposed tadpoles suggest that the naturally occurring decrease in preference for light displayed by stage 49 tadpoles is not due to a decrease in endogenous serotonin release, because if it were, then fluoxetine would not be as effective. Plotting the average number of tadpoles in the light quadrant at each minute of the trial shows that the enhanced preference for light displayed by fluoxetine-exposed tadpoles persists across the entire 30-min time trial (Figure 2C). These data indicate that a 24-h exposure to fluoxetine significantly enhanced the tadpoles’ preference for light over dark, suggesting that bolstering normal serotonin transmission modulates the strength of the preference for light displayed by developmental stage 48/49 tadpoles.
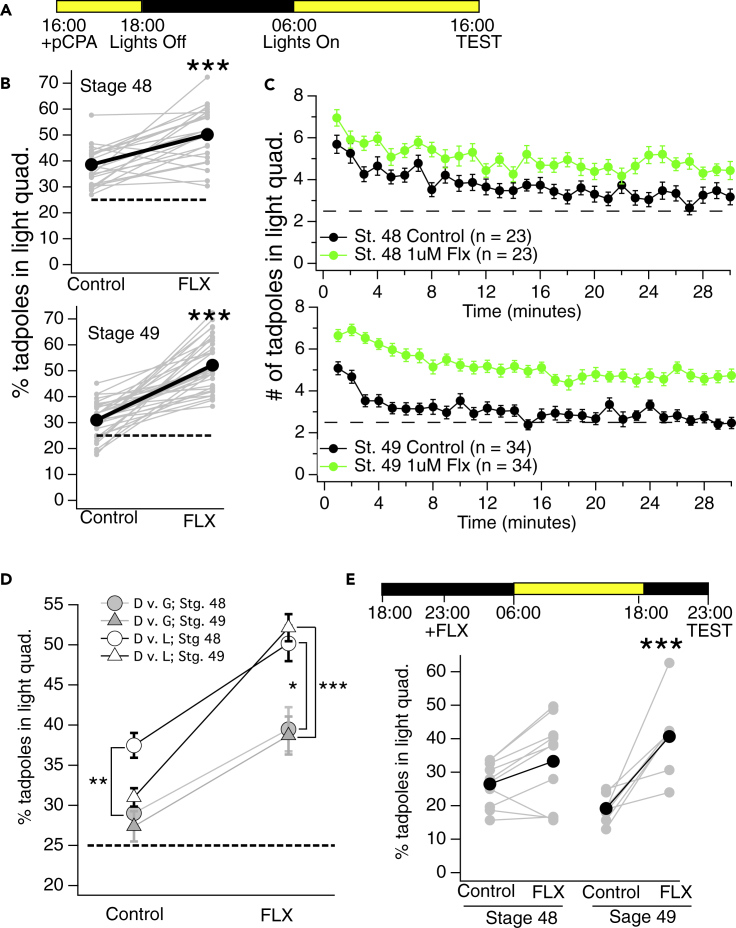
Figure 2.
Pretreating tadpoles with the SSRI fluoxetine enhances their preference for light over dark
(A) Experimental timeline for experiments pertaining to panels B–D: Fluoxetine (Flx) is added to tadpoles rearing solution at 16:00 and tadpoles are returned to their incubator. Tadpoles are tested 24 h later, at 16:00 on the following day.
(B) Plots showing the average number of control and fluoxetine-exposed developmental stage 48 (top), and stage 49 (bottom) tadpoles that resided in the light quadrant of the test dish. Each set of connected gray data points represents the average % of tadpoles in the light quadrant for batch-matched control and fluoxetine-treated groups, and the set of black connected data points represents the overall average of all the individual experiments (∗∗∗p < 0.001 as determined by Mann-Whitney test, top: control, n = 23; Flx, n = 23. Bottom: control, n = 34; Flx, n = 34).
(C) Plots showing the average number of control and fluoxetine-exposed developmental stage 48 (top) and stage 49 (bottom) tadpoles in the light quadrant at each minute of the 30-min trial.
(D) Plot showing the average preferences for gray displayed by control and fluoxetine-exposed tadpoles when gray is pitted against dark. For comparison, the average preferences for light (obtained from the dark vs light test) which are shown in panels B and C are super-imposed onto this graph. Notice that the preference for gray displayed by control and fluoxetine-exposed tadpoles is weaker than the preference for light, suggesting that the strength of the preference is light intensity-dependent (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by Mann-Whitney test. Light vs. dark: Stage 48: control, n = 23; Flx, n = 23; stage 49: control, n = 34; Flx, n = 34. Gray vs. dark: stage 48: control, n = 9; Flx, n = 9; stage 49: control, n = 12; Flx, n = 12.
(E, top) Experimental timeline used to test the effect of 24-h fluoxetine pre-treatment on the preference for light at 23:00: Fluoxetine (Flx) is added to tadpoles’ rearing solution at 23:00 and tadpoles are returned to their incubator. Tadpoles were tested 24 h later, at 23:00 on the following day. (Bottom) plots showing the average % of control and fluoxetine-exposed tadpoles in the light quadrant when tested at 23:00. The 16:00 and 23:00 data combined show that fluoxetine pre-treatment enhances the preference for light during the day and night (∗∗∗p < 0.001 as determined by Mann-Whitney test: stage 48, n = 10; stage 49, n = 8). All error bars represent SEM.
To determine whether these results reflect a preference for light or an avoidance of dark, we pitted dark against gray (instead of light). We reasoned that if tadpoles were avoiding the dark, then they should prefer gray as strongly as they prefer light. In other words, if they are only avoiding the dark, then they should equally prefer any option that is lighter than dark. Conversely, if residing in the light quadrant is due to a preference for light, then the average number in the gray quadrant should be less than the average number in the light quadrant. The data support the latter scenario: the average number of tadpoles in the gray quadrant (in the gray vs. dark test) was consistently less than the average in the light quadrant (in the light vs. dark test) for both control and fluoxetine-exposed tadpoles Figure 2D), suggesting that the behavior is driven more by a preference for light rather than purely an avoidance of dark.
The data that show that fluoxetine is enhancing the preference for light were all obtained between 15:00 and 17:00, the time of day when the natural preference for light was determined to be at its peak (Figure 1). To test whether fluoxetine may enhance the preference for light at 23:00, the time when tadpoles display the least preference for light, the same 24-h fluoxetine pre-treatment was carried out, and tadpoles were tested on the light/dark test at 23:00. Our data indicate that the fluoxetine-treated tadpoles display a stronger preference for light at 23:00 compared to controls, indicating that serotonin also enhances the preference for light during the night (Figure 2E).
Because a 24-h fluoxetine pre-treatment time was used in these experiments, we carried out a set of experiments to further characterize the action of this drug, specifically whether it was acting acutely or chronically, and the duration of its effect. First, to determine whether fluoxetine was acting acutely or non-acutely, we carried out a fluoxetine pre-treatment time course. We observed a gradual increase in fluoxetine’s effect on the preference for light as the pre-treatment time increased. A pre-treatment time of 6 h elicited almost the maximal (24-h pre-treatment) effect (Figure S1A). This indicates that fluoxetine is eliciting its effect via a non-acute mechanism that ramps up slowly over many hours. Next, we addressed the duration of the fluoxetine effect by testing the tadpoles’ preference for light at different time points after the 24-h exposure to the drug. We found that while the effect slowly decreased over time, the strength in the preference for light was still greater than control after 3 days, the latest time point tested (Figure S1B). These observations indicate that fluoxetine modulates visual behavior via a non-acute mechanism, and somehow induces a long-lasting change (strengthening) in the circuitry underlying the preference for light. However, because it is not possible to measure how long it takes for fluoxetine to reach the CNS or how quickly it washes out, it is not possible to interpret the action of fluoxetine beyond the kinetics on behavior.
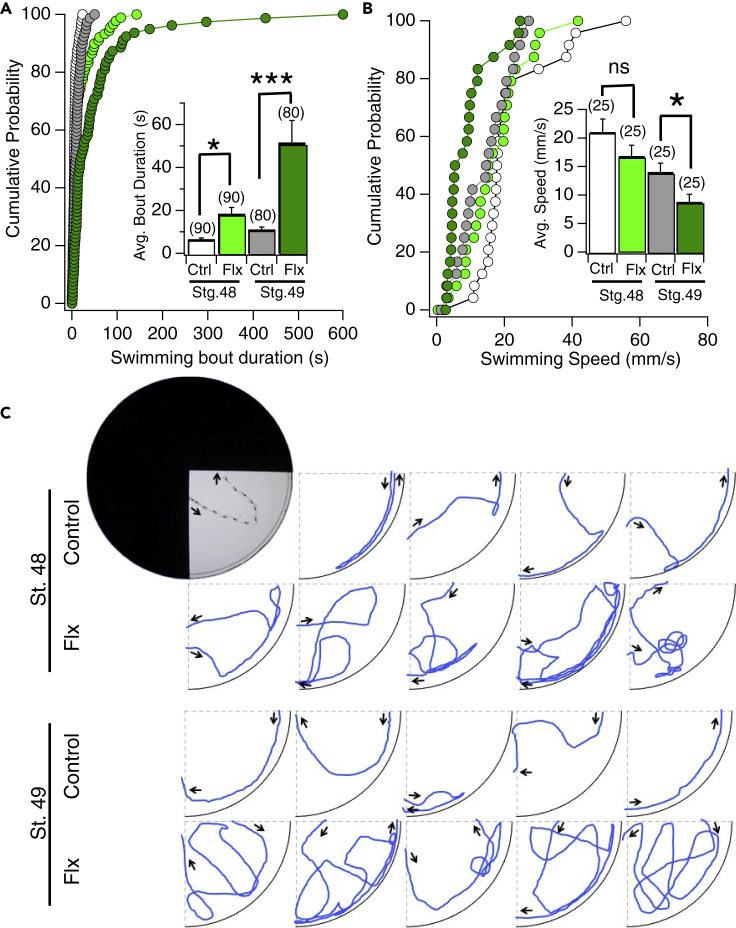
Next, we asked precisely how the enhanced preference for light is manifested. For this, we measured swimming speeds while in the light quadrant, duration of swimming bouts (defined as the amount of time a given tadpole spends in the light quadrant between entering and exiting the quadrant) and the frequency of entrances made into the light quadrant displayed by individual control and fluoxetine-exposed tadpoles. We found that both developmental stage 48 and 49 fluoxetine-exposed tadpoles exhibited significantly longer individual swimming bouts in the light quadrant compared to controls (Figures 3A and 3C). The examples in Figure 3 show that the longer swimming bouts typically involve a multitude of U-turns at the border between the light and dark region of the dish which prevent the tadpole from moving into the dark region. Notably, we observed robust U-turns displayed by fluoxetine-exposed tadpoles when they were swimming toward or along the border such that one eye overlapped with the dark side, and one eye overlapped with the light side. In addition, compared to controls, developmental stage 49 fluoxetine-exposed tadpoles displayed a significant decrease in speed while in the light quadrant, which would also contribute to the observed enhanced preference for light (Figure 3B; stage 49 control average speed in light: 14.06 ± 1.53 mm/s, n = 25 tadpoles; stage 49 fluoxetine-exposed tadpoles average speed in light: 8.83 ± 1.31 mm/s, n = 25 tadpoles; stage 49 control vs. fluoxetine: p < 0.05). Hence, overall, the enhanced preference for light displayed by the fluoxetine-exposed tadpoles is manifested mainly via longer swimming bouts in the light quadrant, which in turn is brought about via a higher frequency of U-turns at the dark/light border to avoid moving into the dark region.
Figure 3.
The increased preference for light displayed by fluoxetine-treated tadpoles is manifested mainly via longer swimming bouts while in the light quadrant
(A) Cumulative probability plot displaying the amount of time in seconds individual stage 48 (white dots) and 49 control (gray dots) and stage 48 (light green dots) and 49 1 μM fluoxetine exposed tadpoles (dark green dots) stay in the light from the time they enter to when they exit. Inset is a bar graph of the same data showing the overall average amount of time in seconds of the respective groups (∗p < 0.05, ∗∗∗p < 0.001 as determined by Mann-Whitney U test, Stage 48: control, n = 90; Flx, n = 90. Stage 49: control, n = 80; Flx, n = 80).
(B) Cumulative probability plot displaying speed of individual stage 48 (white dots) and 49 control (gray dots) and stage 48 (light green dots) and stage 49 (dark green dots) 1 μM fluoxetine exposed tadpoles measured in mm/s from the time they enter the light to when they exit. Inset is a bar graph of the same data showing the overall average swimming speed in seconds of the respective groups (∗p < 0.05 as determined by Mann-Whitney U test, Stage 48: control, n = 25; Flx, n = 25. Stage 49: control, n = 25; Flx, n = 25). Error bars in (A) and (B) represent SEM.
(C) Examples of individual tadpole swimming bouts tracked from the time they enter to when they exit the light region. The longer swimming bouts of the 1μM fluoxetine exposed tadpoles typically involve multiple U-turns at the border between the light and dark region of the dish.
Carrying out fluoxetine exposure in the dark attenuates its effect
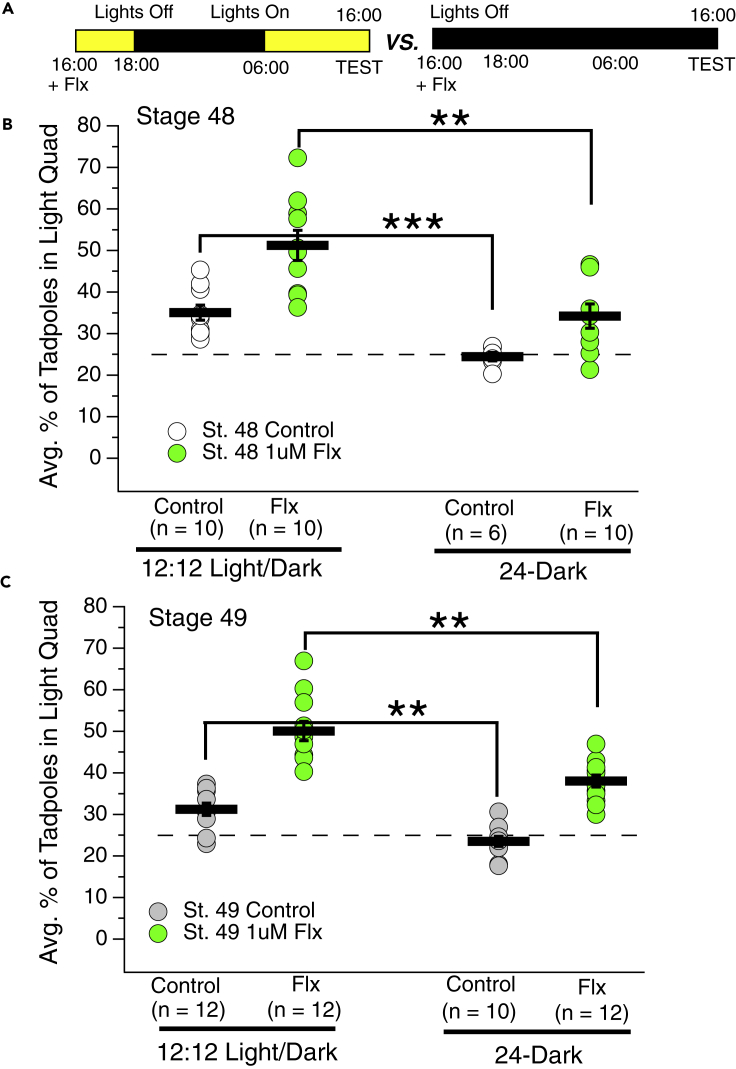
It has been previously reported in zebrafish that a subpopulation of serotonergic neurons in the dorsal raphe are activated by light (Cheng et al., 2016). Based on this study, and also our finding that the preference for light is stronger during the day (Figure 1) but can be further strengthened via enhancing serotonin transmission (Figure 2), we hypothesized that endogenous levels of serotonin released during the night are lower compared to during the day. If so, then the SSRI—which only enhances the action of endogenously released serotonin (i.e. it is not a serotonin agonist)—should be less effective in the absence of light. To test this, the 24-h fluoxetine exposure was carried out entirely in the dark, instead of the normal 12:12 light:dark schedule (Figure 4A). Consistent with our hypothesis, we found that the fluoxetine effect was attenuated when the 24-h exposure to the drug was carried out in the dark (Figures 4B and 4C). Hence, given that SSRIs rely on the release of serotonin suggests that serotonin release is higher in the presence of light (subjective day) compared to in the absence of light (subjective night). This finding also supports the possibility that the observed natural oscillations in the preference for light over the day and night cycle (stronger during the day, weaker at night), respectively, arises, at least in part from circadian-dependent oscillations in levels of serotonin release.
Figure 4.
Carrying out the fluoxetine pre-treatment in the absence of light decreases its effectiveness
(A) Experimental timeline: Fluoxetine is added to tadpoles' rearing solution at 16:00 and then tadpoles are maintained either on the regular 12:12 light/dark schedule (left time-line) or on a 24 h dark schedule (right time-line) until testing. Tadpoles are tested 24 h later, at 16:00 on the following day.
(B and C) Dot plots showing the average percent of developmental stage 48 and (C) stage 49 control and fluoxetine-exposed tadpoles (green dots) when the fluoxetine exposure was carried out on a 12:12 light dark schedule and when the exposure was carried out entirely in darkness (24-h dark schedule). The total number of trials is shown in parentheses. Notice that the fluoxetine effect on preference for light is markedly attenuated when the pre-treatment is carried out in the dark, suggesting that endogenous levels of serotonin release are relatively lower in the dark. Also notice that the control tadpoles that were housed in the dark for 24 h before testing display a modest decrease in preference for light, suggesting that normal serotonin release during the day shapes the strength of preference for light (B and C, A Kruskal-Wallis non-parametric statistical test was used to determine if at least one experimental group was statistically different from the others. This was followed by a pairwise non-parametric Mann-Whitney test to compare specific groups; ∗∗p < 0.01, ∗∗∗p < 0.001 as determined by Mann-Whitney U test). Error bars represent SEM.
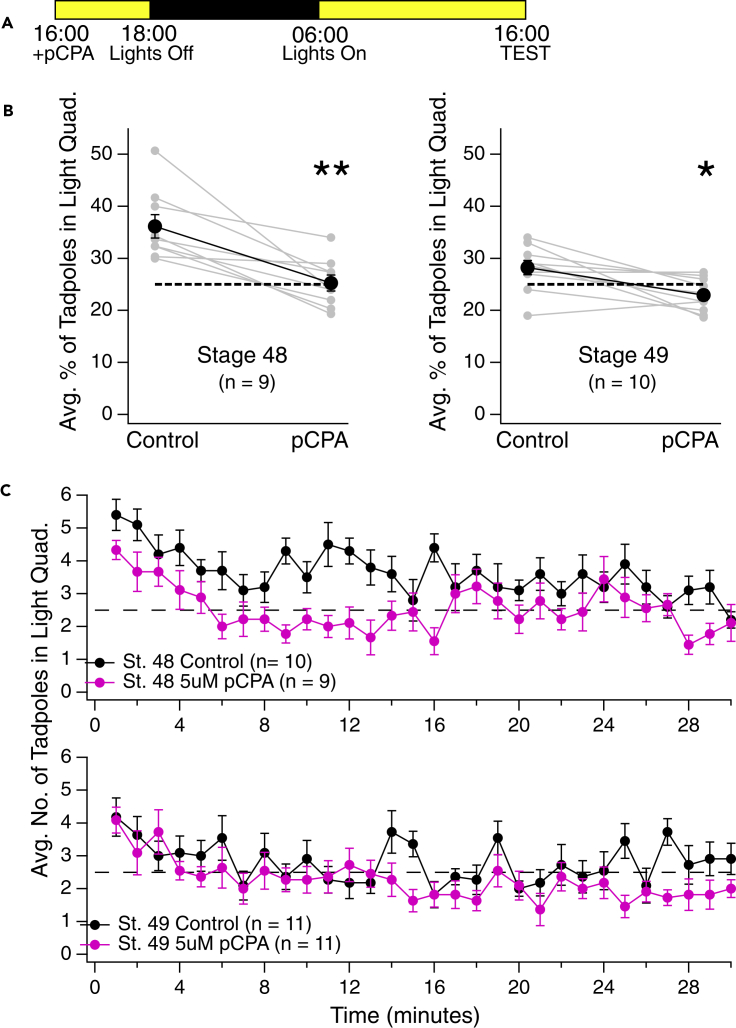
pCPA-exposed tadpoles display a dampened preference for light
If serotonin is responsible for the stronger preference for light during daytime hours, then inhibiting serotonin levels would be expected to reduce it. To test this, endogenous serotonin levels were inhibited by exposing tadpoles for 24 h to para-chlorophenylalanine (pCPA; 5 μM), a tryptophan hydroxylase inhibitor that is commonly used to inhibit serotonin production and therefore release (Cheng et al., 2016; Kawashima et al., 2016). We then tested tadpoles on the same light/dark test at 16:00 as described above for the fluoxetine study. We found that pCPA-exposed tadpoles displayed a significant reduction in their preference for light compared to controls (Figure 5; stage 48 control vs. pCPA: p < 0.01; stage 49 control vs. pCPA: p < 0.05). In fact, the preference for light displayed by the pCPA-exposed tadpoles was closer to that displayed by controls at night (Figure 1). The decreased preference for light displayed by the pCPA-exposed tadpoles was manifested mainly by a significant decrease in the frequency of tadpoles phototaxing into the light quadrant of the test dish (stage 48 control average frequency entering the light: 26.33 ± 2.13 tadpoles/minute, n = 9; stage 48 pCPA-exposed tadpoles average frequency entering the light: 19.44 ± 1.42 tadpoles/minute, n = 9; stage 48 control vs. pCPA: p < 0.05; stage 49 control average frequency entering the light: 17.45 ± 0.84 tadpoles/minute, n = 11; stage 49 pCPA-exposed tadpoles average frequency entering the light: 9.36 ± 1.24 tadpoles/minute, n = 11; stage 49 control vs. pCPA: p < 0.001; p values for both stage 48 and 49 determined by a pairwise Mann-Whitney U test), showing that the way in which these pCPA-exposed tadpoles avoid light is by not moving toward it. A caveat associated with the pCPA experiments was that the concentration of this drug used to inhibit serotonin in larvae zebrafish (25 μM; Cheng et al., 2016) was found to render tadpole larvae essentially immobile. We suspect that this general loss of mobility was most likely due to depressed serotonin levels at the level of the spinal cord where it has been shown to modulate the spinal cord central pattern generators that control swimming (Demarque and Spitzer, 2010; Sillar et al., 1992, 2006). Because the preference test requires tadpoles to be able to swim normally, we were restricted to using a concentration of 5 μM pCPA, the highest concentration that did not affect the ability to swim. So, while the decrease in preference for light was significant, it is possible that a more dramatic effect may have been observed if it were possible to more completely inhibit serotonin.
Figure 5.
Inhibiting serotonin release by pretreating tadpoles with the tryptophan hydroxylase inhibitor pCPA weakens the preference for light
(A) Experimental time line: pCPA (5μM) is added to tadpoles rearing solution at 16:00 and tadpoles are returned to their incubator. Tadpoles are tested 24 h later, at 16:00 on the following day.
(B) Pre-treatment with pCPA decreases the average number of both stage 48 (left) and stage 49 (right) tadpoles residing in the light quadrant at any given time, suggesting that decreasing serotonin release decreases the preference for light. Each set of connected gray data points represents the average % of tadpoles in the light quadrant for batch-matched control and pCPA-treated group. The set of black connected data points represents the overall average of all the individual experiments (∗p < 0.05, ∗∗p < 0.01 as determined by Mann-Whitney U test).
(C) Plots showing the average number of stage 48 (top) and stage 49 (bottom) control and pCPA-treated tadpoles in the light quadrant at each minute of the 30 min trial. Error bars represent SEM.
Discussion
Our data indicate that Xenopus tadpole larvae display an innate circadian-regulated preference for light over dark which ramps up across the day, peaking in the late afternoon at approximately 16:00. At night, the same tadpoles display essentially no preference for light. Developmental stage 49 tadpoles were found to display a significantly weaker preference for light during the day compared to the younger stage 48 tadpoles. This is in accordance with a previous study by Moriya and colleagues (Moriya et al., 1996) that report a gradual decline in preference for light across developmental stages 44-46 and 58-60 (metamorphic stage). Nevertheless, the significant decline in the preference for light that we observed between stages 48 and 49 was somewhat unexpected given that these two stages are separated by only five days and that there are no noticeable changes in overall morphology during this time. This underscores that developmental changes in behaviors can happen over relatively short time spans and without changes in morphology.
While this is the first report of a circadian-dependent visually guided behavior displayed by Xenopus tadpoles, circadian-dependent preferences for light have been described in other organisms. For instance, female mosquitos prefer light during the day but not during the night (Baik et al., 2020). The degree of photophobicity displayed by drosophila larvae is highest early in day and lowest at dusk (Mazzoni et al., 2005). The fish parasite Argulus japonicus shows no preference for light or dark at 04:00, but then displays gradually strengthening phototaxis until 16:00 (Yoshizawa and Nogami, 2008), and both the marbled crayfish (Shiratori et al., 2017) and phyllosoma larvae of the Caribbean spiny lobster (Zirger et al., 2010) display phototaxis toward light during the night, and away from light during the day. Thus, for many species, the preference for light is linked to the time of day. Furthermore, these examples show that this innate visually guided behavior, while considered hard-wired, oscillates across the 24-h day/night cycle, and is therefore capable of being modulated.
Serotonin modulates the strength of the preference for light
We found that fluoxetine-exposed tadpoles displayed a stronger preference for light and that pCPA-exposed tadpoles displayed a weaker preference for light. These results indicate that the endogenous level of serotonin transmission during the day is intermediate—balanced somewhere between the experimentally enhanced and experimentally dampened levels—and that it generates the moderate preference for light displayed by control tadpoles during the day. Our findings also suggest that the observed weakening in the preference for light observed during the night (Figures 1B and 1C) is likely due to relatively low levels of serotonin release. We reasoned that if this is correct, the SSRI, which only boosts serotonin action once it is released, would not be as effective if its exposure was carried out in darkness. In this way, the SSRI was used as a tool to assay endogenous serotonin release. If normal serotonin release was absent or low, the SSRI would not be effective. Hence, that fluoxetine had little effect when its exposure was carried out in darkness suggests that endogenous serotonin release must be relatively low during the night. All together, these data suggest that the observed oscillation in the preference for light displayed by control tadpoles is manifested, at least in part, by a matched circadian-driven oscillation in serotonin release.
Serotonergic modulation of the preference for light is highly conserved
Serotonergic modulation of the preference for light has been reported across several different species of animals including Drosophila (Moncalvo and Campos, 2009), zebrafish (Cheng et al., 2016; Steenbergen et al., 2011; Burgess et al., 2010; Maximino et al., 2013), crabs (McPhee and Wilkens, 1989), and crayfish (Shiratori et al., 2017). In all these cases, serotonin activity was found to be positively correlated with the strength in the preference for light, suggesting a highly conserved, important form of modulation that adds flexibility to this innate, hard-wired behavior.
A similar study in zebrafish shows that increasing serotonin transmission by exposing zebrafish to an SSRI improved phototactic navigation to a source of light and that inhibiting serotonin using a pan serotonin receptor blocker inhibited navigation to the light (Burgess et al., 2010). While similar to our findings, there are also interesting differences in both the test design and the results. The zebrafish study is designed to study phototactic navigation while this study in tadpoles is designed to study long-term preferences which involve both phototaxing to the preferred region and then, once the preferred region has been reached, a different swimming pattern which prevents moving out of the preferred region (i.e. avoiding the dark). The results of the different behavioral assays lead to different interpretations about the action of serotonin. The navigation study concludes that fluoxetine strengthens specifically an ON visual circuit which promotes swimming forward toward the light but has no effect on an OFF circuit which works to initiate turns to avoid moving away from the light (Burgess et al., 2010). Our results of the open-field test, however, show that fluoxetine induces abnormally robust and frequent U-turns that prevent the tadpoles from moving out of the preferred light quadrant of the test dish, suggesting a strengthening of the OFF circuit. This discrepancy could be due to different effects of serotonin on visual systems of zebrafish and Xenopus tadpoles, or, more intriguingly and more likely, that the circuitry involved in phototactic navigation (i.e. swimming toward the preferred luminance) and those involved in swimming patterns that maintain the organism in the region of preferred luminance are distinct and are modulated by serotonin in different ways, but with the same overall outcome—an enhanced preference for light.
The results of this behavioral study give rise to several questions including what circuit underlies the innate preference for light and what is the mechanism by which serotonin regulates it. It is likely that the serotonin input that is responsible for regulating the preference for light originates from a group of serotonergic neurons residing in the raphe nucleus of tadpole hindbrain. This group of serotonergic neurons is known to be present by embryonic stages of development (Demarque and Spitzer, 2010; Sillar et al., 2006) and to send projections both caudally to the spinal cord and anteriorally to the optic tectum (van Mier et al., 1986; Zhao and Debski, 2005). In addition, our preliminary electrophysiological data show that 24-h exposure to fluoxetine strengthens the retinotectal projection, the glutamatergic synapse between the retinal ganglion cells (RGCs) in the eye and neurons of the optic tectum (unpublished data). However, because it is not yet known if the retinotectal projection underlies the innate preference for light, it cannot be concluded unequivocally that the change in behavior is generated by a change in this one visual projection, especially considering that fluoxetine exposure could be strengthening other visual circuits as well. Another possibility is that fluoxetine is enhancing the preference for light at the level of the retina. Subsets of serotonin-containing amacrine cells have been described in the tadpole retina (Huang and Moody, 1997), and serotonin receptors have been found to be expressed mostly in proliferating zones of the retina as well as post-mitotic cells of the inner plexiform layer (De Lucchini et al., 2003). While a role for serotonin in modulating how the retina processes information in the Xenopus tadpole has not been described, a study in the mouse retina shows functional synaptic connections between serotonin-containing amacrine cells and serotonin receptor-expressing RGCs, and, notably, that the expression of this serotonin receptor on the RGC dendrites is necessary for normal RGC responses to visual stimuli (Trakhtenberg et al., 2016). Therefore, it is possible that serotonin could be modulating the preference for light at the level of the retina.
Functional implications of an intermediate level of serotonin release
Our data suggest that during the day, the level of serotonin release is intermediate—neither high like that generated by fluoxetine nor low like that generated by pCPA. What may be the advantage, at the behavioral level, of maintaining an intermediate basal level of serotonin release? One advantage may be that an intermediate level would allow for small changes in serotonin release to generate rapid and tightly controlled changes in behavior, akin to the way in which neurons are known to balance their firing rates to be the most responsive to small changes in their input (Pratt and Aizenman, 2007). While this is only a theoretical prediction, there are many descriptions of rapid, transient serotonergic modulation of sensory-driven behaviors. For instance, in mice, a looming stimulus has been shown to inhibit serotonin release from serotonergic neurons in the dorsal raphe and this triggers an escape response. Once the looming stimulus is no longer in site, the inhibition of serotonin release ceases and so does the escape response (Huang et al., 2017). In zebrafish, optic flow-driven release of serotonin from serotonergic dorsal raphe neurons is perpetually modulating the strength of motor output (swimming) such that the zebrafish travels the desired distance (Kawashima et al., 2016). Thus, it seems reasonable that maintaining an intermediate level of serotonin transmission under basal conditions may optimize rapid, effective sensory-driven serotonergic modulation.
We have not yet determined, in tadpoles, what types of visual stimuli may transiently increase or decrease levels of serotonin release. The fact that we observed no acute effect of the SSRI on the preference for light, while somewhat surprising, could be because acute surges in serotonin transmission do not alter the long-term preference for light that we measure here. Furthermore, the SSRI only enhances endogenously released serotonin; it does not activate the serotonergic neurons. Future research will aim to identify specific visual stimuli that evoke or depress the release of serotonin. In addition, we will also determine how serotonin modulates other innate visually guided behaviors displayed by these tadpoles. Studying modulation of visual avoidance behaviors will be especially interesting given that we and others have recognized that Xenopus tadpoles perform significantly better on their visual avoidance test in the subjective morning compared to later in the afternoon. The visual avoidance test takes advantage of the tendency of tadpoles to avoid moving dots projected onto the floor of their test dish. This test is used to measure visual acuity (Dong et al., 2009). However, this test assumes that the tadpoles will strive to avoid the moving dots. In other words, a low score on this test is interpreted as poor visual acuity. Our data suggest that one reason that the tadpoles are performing worse in the afternoon is not due to worse visual acuity in the afternoon but because they are in their high serotonin, light-preferring, exploratory mode at that time and so may not fear the dots.
Ethological implications
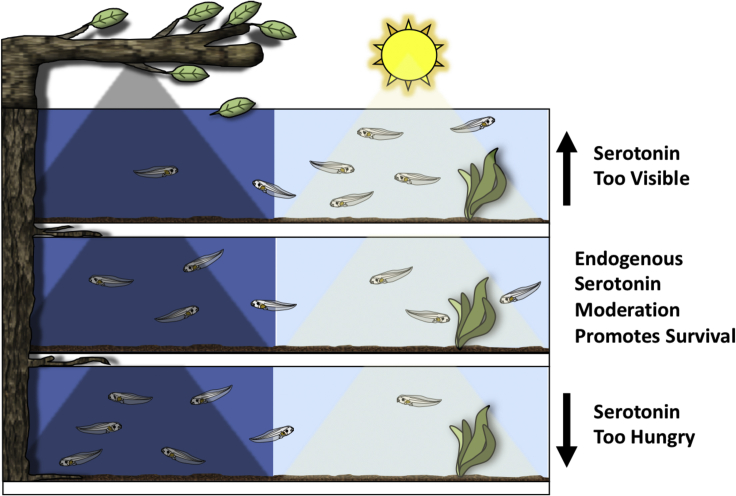
Here, using an open field test paradigm that measures long-term visual preferences of groups of freely swimming tadpoles, we found that tadpoles display a moderate preference for light over dark. In a previous study and using the same test paradigm, we found that tadpoles at these same developmental stages display a moderate preference for the color green, whether green was pitted against light or dark (Hunt et al., 2020). Thus, the overall ranking in preference displayed by normal tadpoles is green > light > dark. We also found across these two studies that pharmacologically enhancing serotonin release via exposing tadpoles to an SSRI rearranged the rank order of preferences to light ≫ green > dark. From an ethological standpoint, this marked alteration in visual preference order could be detrimental for survival in at least a couple of ways. First, it disrupts the normal innate preference for green (over light) which is thought to encourage tadpoles to reside near green aquatic plants, their source of shelter and food. Second, it leads to an abnormally strong preference for light which could render the tadpoles more predictable and visible to predators. Thus, the moderate nature of visual preferences displayed by control tadpoles, which is shaped by an intermediate level of serotonin release, may maximize the probability of survival (Figure 6). A moderate, as opposed to strong, preference is in accordance with a theoretical model of predator-prey interactions referred to as the shell game. This model predicts that survival of prey is maximized by being unpredictable (Mitchell and Lima, 2002). According to this model, displaying a strong preference for anything could render tadpoles, and especially groups of tadpoles, too easily found by predators, while a moderate preference would maximize being unpredictable.
Figure 6.
Ethological model
An ethological model suggested by the data. Normally (middle panel), during the day, tadpoles display a moderate yet significant preference for green over light, and a moderate yet significant preference for light over dark. Experimentally enhancing serotonin transmission by exposure to the SSRI fluoxetine (top panel), however, causes an abnormally strong preference for light over both dark and green wavelengths of light. In a natural setting, this could result in tadpoles residing for abnormally long periods of time in open, well-lit areas which may increase the chances of being detected by predators. Conversely, experimentally decreasing serotonin release (bottom panel) generates an abnormally weak preference for light. In a natural setting, this could impair normal exploration, foraging for food, and/or insufficient time spent in sun-warmed regions. Thus, the moderate preferences for green and light which are shaped by endogenous levels of serotonin release (middle panel) may optimize survival (Schematic by Harley Yerdon, Johnny Morris’ Wonders of Wildlife).
But if survival is optimized by being unpredictable, why display even moderate preferences? It may be important to spend time in well-lit regions during the day in order to explore and forage for food, or to reside near sun-warmed regions, and it may be important to reside near green plants which are a source of food and safety, but without ever being too obvious. These are ideas about how moderate behaviors, neither too strong nor too weak, may be optimal for survival and underscores the significance of the maintenance of an intermediate level of serotonin release during the day.
Limitations of this study
Individual vs group behavior
While it is most likely that the moderate preference displayed across groups of control tadpoles is due to each individual tadpole displaying a moderate preference, we do not track individual tadpoles in this study and therefore we cannot rule out the possibility of a polymorphism which would create a bimodal distribution of preferences displayed by individuals, i.e. any given group would consist of a subpopulation that strongly prefers light and subpopulation that avoids light. This sort of bimodal distribution of preference for light, however, is unlikely given that for each trial we choose at random 10 tadpoles from a clutch consisting of hundreds, and so the chance that the same ratio of (theoretical) light-preferring and light-avoiding tadpoles is consistently chosen to generate the incredibly consistent moderate preference seems unlikely. In summary, whether each individual tadpole shows a moderate preference for light, or whether a polymorphism creates a bimodal distribution in preferences for light, the population displays a stereotypically modest preference for light.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Fluoxetine | Sigma-Aldrich | Cat#F132 |
| 4-chloro-DL-phenylalanine methyl ester hydrocholoride (pCPA) | Sigma-Aldrich | Cat#C3635 |
| Experimental models: Organisms/strains | ||
| Xenopus laevis tadpoles | In-house mating of adult Xenopus frogs | NCBI:txid8355 |
| Software and algorithms | ||
| Excel | Microsoft | RRID:SCR_016137 |
| Prism9 | Graphpad | RRID:SCR_002798 |
| Powerpoint | Microsoft | N/A |
| iMovie | Apple | N/A |
| Quicktime Player | Apple | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Kara G. Pratt (kpratt4@uwyo.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
All animal husbandry and experimental procedures were approved by the University of Wyoming’s Institutional Animal Care and Use Committee (IACUC). X. laevis embryos were obtained from in-house mating of adult wild-type Xenopus frogs. Tadpoles were raised in Steinberg’s solution (100-150 tadpoles per 1.85 L glass bowls) and housed on a 12:12 light/dark cycle in white-walled incubators at 22°C. For most experiments, each group of 10 tadpoles was tested twice (i.e., 2 × 30-min trials) with a 1 to 2 h interval between tests. Any given group of tadpoles was tested either at developmental stage 48 (approximately 10 days post fertilization: dpf) or 49 (approximately 17 dpf) (i.e., not at both developmental time points). Developmental stages were identified according to Nieuwkoop and Faber (1994). The gender of the tadpoles was not determined because at these early stages of development there is no straightforward and reliable way to distinguish between male and female.
Method details
Pharmacological agents
To enhance endogenous serotonin transmission, tadpoles were exposed to the selective serotonin reuptake inhibitors (SSRI) Fluoxetine (Sigma–Aldrich, catalog #F132, 1μM dissolved in dimethyl sulfoxide (DMSO). To deplete serotonin, tadpoles were exposed to the tryptophan hydroxylase inhibitor 4-Chloro-DL-phenylalanine methyl ester hydrochloride (pCPA; Sigma–Aldrich, catalog C3635-1G, 5 μM). The concentrations of fluoxetine (1 μM) and pCPA (5 μM) used in this study were determined empirically by carrying out concentration-response curves to identify the highest non-lethal concentration of drug that did not alter normal swimming patterns. For this, developmental stage 48 tadpoles, in groups of 12, were exposed to a range of concentrations of a given pharmaceutical agent. After 24 h, swimming was assessed. Healthy developmental stage 48/49 tadpoles swim continually, in a head-down tail-up posture. The concentration of the drugs used in this study was the highest concentration in which 100% of the exposed tadpoles displayed this normal swimming pattern. In all experiments, exposure was achieved by adding the drugs to the tadpole’s rearing solution 24 h before testing.
Open-field group light preference assay
In Hunt et al., (2020) we created an assay to quantify tadpole color preference over time. We utilized the same assay to test tadpole preference for light vs. dark. Like the color preference test (Hunt et al., 2020), the light v. dark assay consists of a 14 cm-diameter circular Petri dish filled with 75 mls of Steinberg’s solution to create a depth of 6 mm. The dish was surrounded with black construction paper to obscure extraneous visual stimuli and placed on an LCD computer monitor (HP ZR22W or Dell E198FPf) with light projected onto 25% (i.e., 1/4th) of the dish’s floor. The remaining 3/4ths of the dish was dark (Figure 1A schematic). The light v. dark visual stimulus was created in PowerPoint, version 16.43. The light quadrant was made using the Grayscale Slider with brightness set at 100% and the remaining three dark quadrants at 0%. The intensity of the gray stimulus was set at 25%. Ten wild-type Xenopus tadpoles were placed into the dish and allowed to acclimate for 1 min. Their behavior was then recorded for 30-min with a digital video camera with a resolution of 60 frames per second (GoPro, San Mateo, CA) situated above the Petri dish then analyzed offline. For data analysis, Go Pro videos were downloaded using iMovie software (Apple) and viewed using Quicktime Player (Apple). During offline analysis, the number of tadpoles in the light were counted every minute on the minute. A tadpole was considered to be in the light if both of its eyes were in the light or if one eye and the majority of its head were in the light (Hunt et al., 2020). Otherwise, the tadpole was not included in the count. For the circadian experiments, the preference for light was tested at four time points across the 24-h day/night cycle: two times during the tadpoles' subjective daytime (10:00 and 16:00) and two times during their subjective nighttime (05:00 and 23:00). For the experiments that involved pharmacologically altering serotonin transmission, preference for light was always tested between 15:00 and 17:00, the time of day when tadpoles displayed the maximum strength in their preference for light. As a control, the effect of fluoxetine on preference for light was also tested at 23:00. For the dark-exposure experiments, normally reared tadpoles were exposed to fluoxetine 24 h before testing and then maintained in darkness until testing time.
Behavioral dynamics
To quantify tadpole swimming speed, we measured the distance a tadpole traveled from the time it enters to the time it exits the light quadrant of the test dish. For each of the videos analyzed, the first five border crossings into the light quadrant after the 15-min mark (the 27,000th frame of video) were recorded. All swimming distances were quantified in ImageJ, version 1.51m9 using the Measure function. To quantify how long a tadpole stayed in the light region of the test dish, we tracked the time in seconds from when they entered until they exited. We recorded the first 10 border crossings into the light quadrant after the 15-min mark for each of the videos analyzed. To quantify the frequency with which tadpoles crossed over into the light quadrant, we counted the number of tadpoles that entered the light quadrant from the 15 to 16-min mark of the video.
Quantification and statistical analysis
Data are reported as mean ± standard error of the mean (SEM). Several datasets were determined to follow a nonnormal distribution by Shapiro-Wilk test. Therefore, non-parametric tests were used for all analyses. All pairwise comparisons were performed with a non-parametric Mann-Whitney U test and the Kruskal–Wallis test was used for multiple-group comparisons unless otherwise noted. Error bars are SEM Statistical analyses were carried out using Excel and Graphpad. P-values < 0.05 were considered statistically significant. Details on the statistical analysis for individual experiments is included in the corresponding figure legends.
Acknowledgments
This work was supported by National Institute of General Medical Sciences (P20GM121310-01) and National Science Foundation (2212591) to K.G.P. U.G.U. was supported by National Institute of General Medical Sciences (P20GM103432). We thank the National Xenopus Resource RRID: SCR_013731 for providing protocols for Xenopus frog husbandry. We thank members of the Pratt lab and Drs. William “Trey” Todd and Carlos Aizenman for helpful comments on the manuscript.
Author contributions
J.R.B., J.E.H., U.G.U., and K.G.P. conceived and designed the experiments. J.R.B., U.G.U., P.O.O., and C.J.A. carried out and analyzed experiments. J.E.H. helped with experimental design and data analysis. J.G.L. carried out swimming pattern analysis and helped prepare figures. J.R.B., U.G.U., J.E.H., and K.G.P. prepared figures and wrote the paper.
Declaration of interests
The authors have no competing interests.
Published: November 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105375.
Supplemental information
Data and code availability
The datasets supporting this manuscript are available on request directed to the lead contact.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Asirim E.Z., Humberg T.H., Maier G.L., Sprecher S.G. Circadian and genetic modulation of visually-guided navigation in drosophila larvae. Sci. Rep. 2020;10:2752. doi: 10.1038/s41598-020-59614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik L.S., Nave C., Au D.D., Guda T., Chevez J.A., Holmes T.C. Circadian regulation of light-evoked attraction and avoidance behaviors in daytime-versus nighttime-biting mosquitos. Curr. Biol. 2020;30:3252–3259.e3. doi: 10.1016/j.cub.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D., Shomrat T., Nicolas C.L., Granata C., Levin M. A second-generation device for automated training and quantitative behavior analyses of molecularly-tractable model organisms. PLoS One. 2010;5:e14370. doi: 10.1371/journal.pone.0014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H.A., Schoch H., Granato M. Distinct retinal pathways drive spatial orientation behaviors in zebrafish navigation. Curr. Biol. 2010;20:381–386. doi: 10.1016/jcub.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R.-K., Krishan S., Jesuthasan S. Activation and inhibition of tph2 serotonergic neurons operate in tandem to influence larval zebrafish preference for light over darkness. Sci. Rep. 2016;6:20788. doi: 10.1038/srep20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucchini S., Ori M., Nardini M., Marracci S., Nardi I. Expression of 5-HT2B and 5-HT2C receptor genes is associated with the proliferative regions of the Xenopus developing brain and eye. Brain Res. Mol. Brain Res. 2003;115:196–201. doi: 10.1016/s0169-328x(03)00173-6. [DOI] [PubMed] [Google Scholar]
- Demarque M., Spitzer N.C. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron. 2010;67:321–334. doi: 10.1016/j.neuron.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Lee R.H., Xu H., Yang S., Pratt K.G., Cao V., Song Y.K., Nurmikko A., Aizenman C.D. Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J. Neurophysiol. 2009;101:803–815. doi: 10.1152/jn.90848.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza E.A. Does cognition have a role in plasticity of “innate behavior”? A perspective from drosophila. Front. Psychol. 2018 doi: 10.3389/fpsyg.2018.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza E.A., Colombe J., Brembs B. A decision underlies phototaxis in an insect. Open Biol. 2016;6:160229. doi: 10.1098/rsob.160229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Moody S.A. Three types of serotonin-containing amacrine cells in tadpole retina have distinct clonal origins. J. Comp. Neurol. 1997;387:42–52. [PubMed] [Google Scholar]
- Huang L., Yuan T., Tan M., Xi Y., Hu Y., Tao Q., Ahao Z., Zheng J., Han Y., Xu F., et al. A retinoraphe projection regulates serotonergic activity and looming-evoked defensive behavior. Nat. Commun. 2017;8:14908. doi: 10.1038/ncomms14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J.E., Bruno J.R., Pratt K.G. An innate preference displayed by Xenopus tadpoles is persistent and requires the tegmentum. Front. Behav. Neurosci. 2020;14:71. doi: 10.3389/fnbeh.2020.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger R.G., Hailman J.P. Ontogenetic shift of spectral phototactic preferences in anuran tadpoles. J. Comp. Physiol. Psychol. 1976;90:930–945. doi: 10.1037/h0077275. [DOI] [PubMed] [Google Scholar]
- Kawashima T., Zwart M.F., Yang C.T., Mensh B.D., Ahrens M.B. The serotonergic system tracks the outcomes of actions to mediate short-term motor learning. Cell. 2016;167:933–946.e20. doi: 10.1016/j.cell.2016.09.055. [DOI] [PubMed] [Google Scholar]
- Liu Z., Thakar A., Santoro S.W. Presenilin regulates retinotectal synapse formation through EphB2 receptor processing. Dev. Neurobiol. 2018;78:1171–1190. doi: 10.1002/dneu.22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C., Puty B., Benzecry R., Araujo J., Lima M.G., de Jesus Oliveira Batista E., de Matos Oliveira K.R., Crespo-Lopez M.E., Herculano A.M. Role of serotonin in zebrafish (Danio rerio) anxiety: relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and parachlorophenylalanine (pCPA) in two behavioral models. Neuropharmacology. 2013;71:83–97. doi: 10.1016/j.neuropharm.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Mazzoni E.O., Desplan C., Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293–300. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- McPhee M.J., Wilkens J.L. Serotonin, but not dopamine or octopamine, modifies locomotor and phototaxic behavior of the crab, Carcinus maenas. Can. J. Zool. 1989;67:391–393. [Google Scholar]
- Mitchell W.A., Lima S.L. Predator-prey shell games: large scale movement and its implications for decision-making by prey. OIKOS. 2002;99:249–259. doi: 10.1034/j.1600-0706.2002.990205.x. [DOI] [Google Scholar]
- Moncalvo V.G.R., Campos A.R. Role of serotonergic neurons in the drosophila larval response to light. BMC Neurosci. 2009;10:66. doi: 10.1186/1471-2202-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T., Kito K., Miyashita Y., Asami K. Preference for background color of the Xenopus laevis tadpole. J. Exp. Zool. 1996;276:335–344. doi: 10.1002/(SICI)1097-010X(19961201)276:5<335. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N., Shettleworth S.J. Wavelength preferences and brightness cues in the water finding behavior of sea turtles. Beyond Behav. 1968;32:211–257. doi: 10.1163/156853968x00216. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P.D., Faber J. Routledge; 1994. Normal Table of Xenopus Laevis (Daudin) [Google Scholar]
- Park J.S., Ryu J.H., Choi T.I., Bae Y.K., Lee S., Kang H.J., Kim C.H. Innate color preference of zebrafish and its use in behavioral analyses. Mol. Cell. 2016;39:750–755. doi: 10.14348/molcells.2016.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt K.G., Aizenman C.D. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J. Neurosci. 2007;27:8268–8277. doi: 10.1523/JNEUROSCI.1738-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronych S.P., Souza K.A., Neff A.W., Wassersug R.J. Optomotor behavior in Xenopus laevis tadpoles as a measure of the effect of gravity on visual and vestibular neural integration. J. Exp. Biol. 1996;199:2689–2701. doi: 10.1242/jeb.199.2689. [DOI] [PubMed] [Google Scholar]
- Rothman G.R., Blackiston D.J., Levin M. Color and intensity discrimination in Xenopus laevis tadpoles. Anim. Cogn. 2016;19:911–919. doi: 10.1007/s10071-016-0990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Liu H.H., Schiapparelli L., McClatchy D., He H.Y., Yates J.R., 3rd, Cline H.T. Acute synthesis of CPEB is required for plasticity of visual avoidance behavior in Xenopus. Cell Rep. 2014;6:737–747. doi: 10.1016/j.celrep.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori C., Suzuki N., Momohara Y., Shiraishi K., Aonuma H., Nagayama T. Cyclic AMP-regulated opposing and parallel effects of serotonin and dopamine on phototaxis in the Marmorkrebs (marbled crayfish) Eur. J. Neurosci. 2017;46:1863–1874. doi: 10.1111/ejn.13632. [DOI] [PubMed] [Google Scholar]
- Sillar K.T., Wedderbrun J.F.S., Simmers A.J. Modulation of swimming rhythmicity by 5-hydroxytryptamine during postembryonic development in Xenopus laevis. Proc R. Soc. Lond. B. 1992;250:104–114. doi: 10.1098/rspb.1992.0137. [DOI] [PubMed] [Google Scholar]
- Sillar K.T., Reith C.A., McDearmid J.R. Development of aminergic neuromodulation of a spinal locomotor network controlling the swimming in Xenopus larvae. Ann. N. Y. Acad. Sci. 2006;860:318–332. doi: 10.1111/j.1749-6632.1998.tb09059.x. [DOI] [PubMed] [Google Scholar]
- Steenbergen P.J., Richardson M.K., Champgagne D.L. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: a pharmacological study. Behav. Brain Res. 2011;222:15–25. doi: 10.1016/j.bbr.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Trakhtenberg E.F., Pita-Thomas W., Fernandez S.G., Patel K.H., Venugopalan P., Shechter J.M., Morkin M.I., Galvao, Liu X., Dombrowski S.D., Goldberg J.L. Serotonin receptor 2C regulates neurite growth and is necessary for normal retinal processing of visual information. Dev. Neurobiol. 2016 doi: 10.1002/dneu.22391. [DOI] [PubMed] [Google Scholar]
- van Mier P., Joosten H.W.J., van Rheden R., Ten Donkelaar H.J. The development of serotonergic raphespinal projections in Xenopus laevis. Int. J. Devl. Neuroscience. 1986;4:465–475. doi: 10.1016/0736-5748(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Wassersug R.J. In: Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. Vial J.L., editor. University of Missouri Press; 1973. Aspects of social behavior of anuran larvae; pp. 273–297. [Google Scholar]
- Yoshizawa K., Nogami S. The first report of phototaxis of fish ectoparasite, Argulus japonicus. Res. Vet. Sci. 2008;85:128–130. doi: 10.1016/j.rvsc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Zhao B., Debski E.A. Serotonergic reticular formation cells in Rana pipiens: categorization, development, and tectal projections. J. Comp. Neurol. 2005;487:441–456. doi: 10.1002/cne.20593. [DOI] [PubMed] [Google Scholar]
- Zirgler T.A., Cohen J.H., Forward R.B. Proximate control of diel vertical migration in phyllosoma larvae of the Caribbean spiny lobster Panulirua argus. Biol. Bull. 2010;219:207–219. doi: 10.1086/BBLv219n3p207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this manuscript are available on request directed to the lead contact.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.