Abstract
Background and Aims:
Current knowledge regarding the epidemiology of pouchitis is based on highly selected, mostly single-center, patient cohorts. Our objective was to prospectively determine the population-based incidence of pouchitis in patients with ulcerative colitis in the first 2 years after ileal pouch-anal anastomosis and analyze time trends of the incidence of pouchitis.
Methods:
Using national registries, we established a population-based cohort of all Danish patients undergoing proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis between 1996 and 2018. The primary outcome was the development of pouchitis within the first 2 years after surgery, evaluated by time period. We used Kaplan Meier and Cox Proportional Hazard modeling to evaluate time to development of pouchitis.
Results:
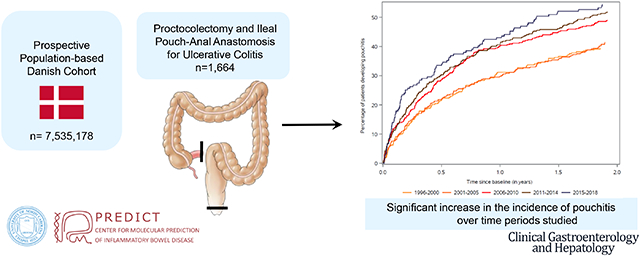
Overall, 1,664 patients underwent an ileal pouch-anal anastomosis. The cumulative incidence of pouchitis in the 2 years after ileal pouch-anal anastomosis increased throughout the study period, from 40% in 1996–2000, (95% CI:35%–46%) to 55% in 2015–2018, (95% CI:48%–63%). Patients undergoing surgery between 2015–2018 also demonstrated an increased risk of pouchitis compared to the earliest study period (1996–2000) after adjusting for sex, age, and socioeconomic status (Hazard Ratio 1.57, 95% CI:1.20–2.05).
Conclusions:
This first population-based study demonstrated a 15% absolute and 38% relative increase in the incidence of pouchitis among patients undergoing surgery between 1996 and 2018, with the greatest cumulative incidence of pouchitis demonstrated in the most recent era (2015–2018). The striking increase in the incidence of pouchitis highlights the need for further research into causes and prevention of pouchitis.
Keywords: epidemiology, ileal pouch-anal anastomosis, anti-tumor necrosis factor alpha, ulcerative colitis, colectomy
Graphical Abstract
INTRODUCTION
Although pouchitis is the most common complication after a colectomy with ileal pouch-anal anastomosis (IPAA) for ulcerative colitis (UC), our understanding of the epidemiology, disease course after IPAA, and risk factors for development of pouchitis is limited. In particular, much of our understanding regarding the natural history of pouch-related disorders has been generated from studies of selected populations.1–5
Approximately 40% of patients will develop pouchitis within the first year after IPAA,6 with up to 80% of patients developing pouchitis symptoms at some point in the disease course.3, 7 Prior studies have identified multiple associations,8–11 however there remains a lack of actionable factors for the identification of high-risk patients (where early intervention may be particularly useful). Recently, the preoperative use of anti-tumor necrosis factor alpha (anti-TNF) therapy has been associated with an increased risk for pouchitis.5, 12 Additionally, pediatric patients undergoing surgery in recent decades have demonstrated an increased risk for pouch failure.13 Given increasing trends in biologic use for the treatment of UC,14 understanding these risk factors in the broader epidemiologic trends of pouchitis is critical.
In this study, we aimed to analyze the incidence of pouchitis within the first 2 years after the final stage of an IPAA for UC across time periods, using prospectively collected data from the Danish health registers. Using a recently validated case-finding definition for pouchitis for administrative claims data,15 our primary objective was to evaluate any change in the incidence of pouchitis evaluating surgeries that occurred between 1996 and 2018. We also aimed to analyze the role of specific risk factors on the incidence of pouchitis, including pre-operative anti-TNF therapy, markers of UC severity, and extraintestinal manifestations (EIMs).
MATERIALS AND METHODS
Study Design and Population
We performed a nationwide cohort study using prospectively recorded data from the Danish National Patient Registry, The Danish National Prescription Registry, and the Danish Civil Registration System. Please see Supplemental Methods for further information regarding the Danish health registers.
We identified all patients who underwent a colectomy followed by an IPAA for UC between January 1, 1996 and December 31, 2018. Adult patients with UC were identified using previously published criteria,16 which rely on International Classification of Diseases (ICD) coding and have been validated using a pathology database as reference. Patients undergoing surgery were identified using Current Procedural Terminology (CPT) and/or The Nordic Medico-Statistical Committee Classification of Surgical Procedures (NCSP) coding for an ileal pouch-anal anastomosis, as defined in the Supplemental Methods. To ensure that the index date was the latest stage of IPAA surgery, we used the final “stage” of IPAA surgery, with follow-up beginning 30 days after this index date to avoid misclassification of the outcome.
Outcome Measures
The primary outcome in this study was the development of pouchitis within the first 2 years after IPAA, evaluated by time periods. The 2-year time window was utilized given that pouchitis was defined using a previously developed case-finding definition for use in administrative claims data,12, 15 where patient turnover and loss to follow-up is much more common than in the Danish health registers. Patients with pouchitis were identified using the following critiera:15 1) Current Procedural Terminology (CPT)/The Nordic Medico-Statistical Committee Classification of Surgical Procedures (NCSP) coding for an IPAA, 2) a preoperative diagnosis of UC, 3) an ICD-10th Clinical Modification (ICD-10) diagnosis code for pouchitis (K91.850), NCSP code for pouchitis (DK528C), or a prescription for ciprofloxacin or metronidazole during the first 2 years after IPAA. Using this case-finding definition, we evaluated only the first diagnosis of pouchitis; recurrent episodes of pouchitis were not evaluated. Patients with a preoperative diagnosis of only Crohn’s disease (CD) were excluded; however, we evaluated the change in diagnosis to CD after IPAA, which was defined as an ICD-10 code for CD on at least 3 different occasions in the first 2 years after IPAA.12 We also evaluated the cumulative incidence of pouchitis over the entire follow-up period.
Exposures
The impact of year of surgery was evaluated by time period, categorized as follows: 1996–2000, 2001–2005, 2006–2010, 2011–2014, 2015–2018. In pre-specified analyses, we also examined the impact of specific factors on the incidence of pouchitis over time, including the preoperative use of anti-TNF therapy (Supplemental Table 2) and the impact of preoperative markers of severity and EIMs (Supplemental Tables 2 and 3) on the subsequent development of pouchitis. Preoperative anti-TNF use was defined as at least one prescription or infusion in the year prior to colectomy for UC, and was only analyzed in patients where surgery occurred between 2006 and 2018, given the lack of available anti-TNF therapies for UC prior to these time periods.
Covariates
Available patient demographics included sex, age at index date, year of IPAA, area socioeconomic index, and location. Area socioeconomic index was chosen as as a proxy for any inequalities in healthcare delivery and lifestyle factors that may exist despite a universal healthcare system present in Denmark. We also evaluated trends in IPAA surgeries by major institution/center in Denmark over the study period.
Statistical analysis
Continuous variables are reported as means with accompanying standard deviation (SD) while categorical variables are reported as raw values with percentages. Kaplan-Meier estimates and Cox-Proportional Hazard modeling were utilized in the evaluation of the time to development of pouchitis. In these analyses, patients were analyzed by the time period where surgery occurred, and the proportional hazards assumption was checked using the Schoenfeld residuals and graphic inspection of the log-transform of the cumulative hazards. For each patient who underwent a proctocolectomy with IPAA, the follow-up period began on the index date (30 days after the date of the final surgery). Patients were followed until the development of pouchitis, emigration, death, or the end of the study period (December 31, 2018), whichever came first.
In the examination of the relationship between time period of surgery and subsequent development of pouchitis, we initially constructed Cox Proportional Hazard models adjusted for sex, age, and socioeconomic index. We then performed a secondary analysis evaluating the relationship between anti-TNF use and pouchitis, restricting the study period to only those years where anti-TNF therapy was available prior to surgery. For all analyses, 2-sided p-values of 0.05 or less were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
In this national cohort of 7,535,178 persons, we identified 1,664 patients that underwent a colectomy with IPAA for UC between January 1, 1996 and December 31, 2018 (Figure 1). Among these, 46% were female and 51% were less than age 35 at the time of surgery (Table 1). The year of index date was relatively evenly distributed (Supplemental Table 4).
Figure 1.
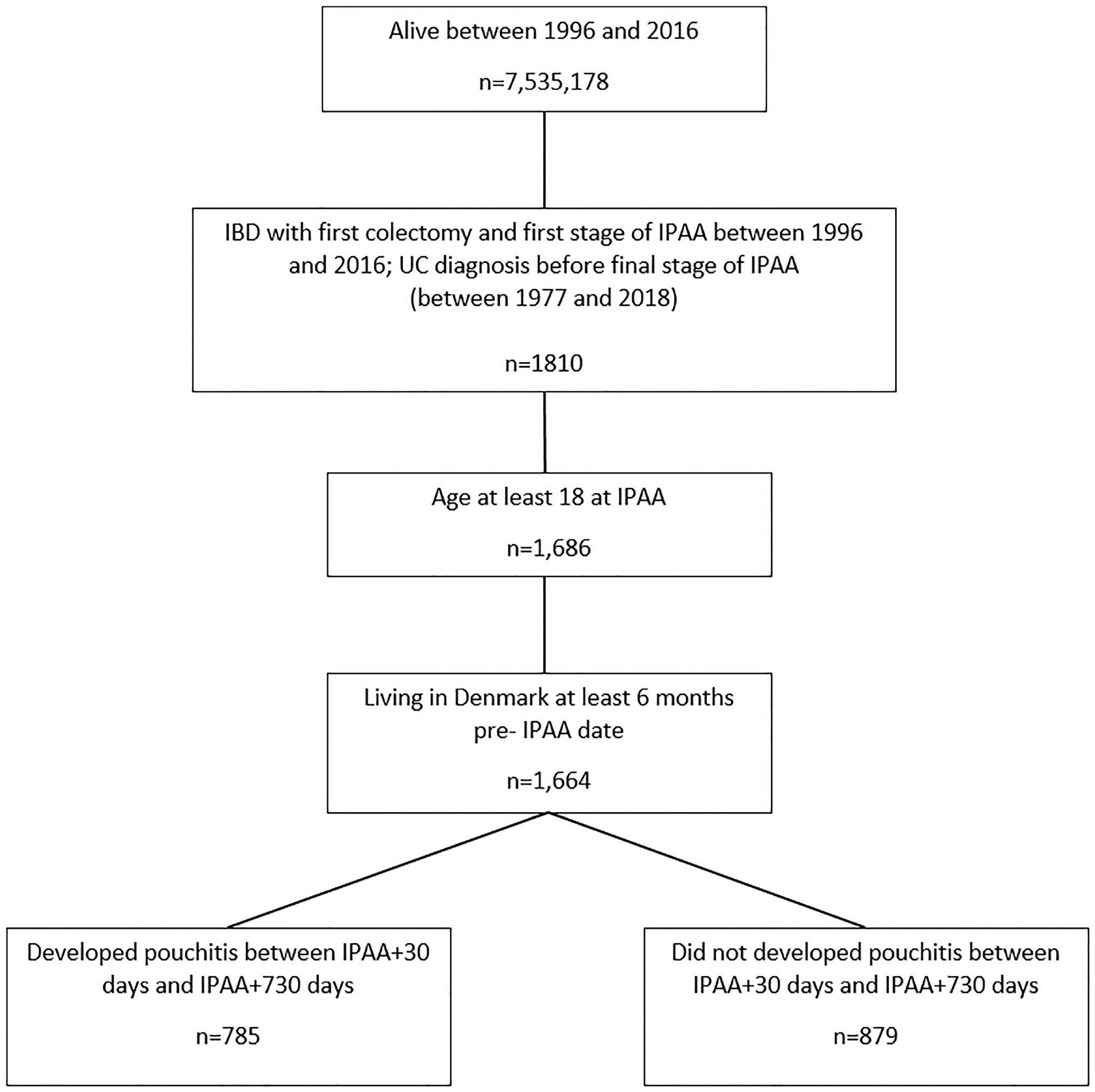
Flow chart of patient population
Table 1.
Clinical and demographic characteristics of patients undergoing proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis in Denmark, 1996–2018
| All patients n=1,664 | ||
|---|---|---|
| n | % | |
| Sex | ||
| Female | 768 | 46.2 |
| Male | 896 | 53.8 |
| Age at surgery (in years) | ||
| less than 35 | 847 | 50.9 |
| 35–64 | 817 | 49.1 |
| Year of surgery | ||
| 1996–2000 | 320 | 19.2 |
| 2001–2005 | 381 | 22.0 |
| 2006–2010 | 431 | 25.9 |
| 2011–2014 | 353 | 21.2 |
| 2015–2018 | 179 | 10.8 |
| Duration of ulcerative colitis prior to colectomy (median, quartile range) | 2.2 | 6.1 |
| Corticosteroid use in the year prior to IPAA | 623 | 37.4 |
| IBD-related hospitalization in the year prior to IPAA | 1,248 | 75.0 |
| Extraintestinal manifestations (in the 5 years prior to IPAA) | 159 | 9.6 |
Ileal pouch-anal anastomosis (IPAA)
The cumulative incidence of pouchitis in the first 2 years after IPAA increased over the study period (Table 2). For patients undergoing surgery between 1996–2000 and 2001–2005 the cumulative incidence of pouchitis at 2 years was 40% (95% CI 35%–46%) and 43% (95% CI 39%–49%) respectively. This rate increased for all subsequent study periods examined, peaking in 2015–2018 at 55% (95% CI 48%–63%, Figure 2). When analyzing the incidence of pouchitis over entire follow up period, the cumulative incidence was 88% (95% CI 85%–90%, Supplemental Figure 1), with a 5-year cumulative incidence rate of 62% (95% CI 60%–44%) and a 10-year cumulative incidence rate of 75% (95% CI 73%–78%). The cumulative incidence of CD in the first 2 years after IPAA was 7.5% (95% CI 6.2%–8.7%, Supplemental Table 5). Patients in the most recent time period (2015–2018) demonstrated the highest cumulative incidence of lower endoscopic procedures in the first 2 years after IPAA (62%, 95% CI 55%–69%, Supplemental Table 6).
Table 2.
The cumulative incidence of pouchitis in the first 2 years after ileal pouch-anal anastomosis for ulcerative colitis, evaluated by time period of index surgery
| Time Period | Cumulative incidence at 1 year (95% CI) | Cumulative incidence at 2 years (95% CI) |
|---|---|---|
| 1996–2000 | 31% (26 – 37) | 40% (35 – 46) |
| 2001–2005 | 30% (25 – 35) | 43% (39 – 49) |
| 2006–2010 | 40% (36 – 45) | 50% (45 – 54) |
| 2011–2014 | 41% (36 – 46) | 52% (47 – 58) |
| 2015–2018 | 43% (36 – 51) | 55% (48 – 63) |
Figure 2.
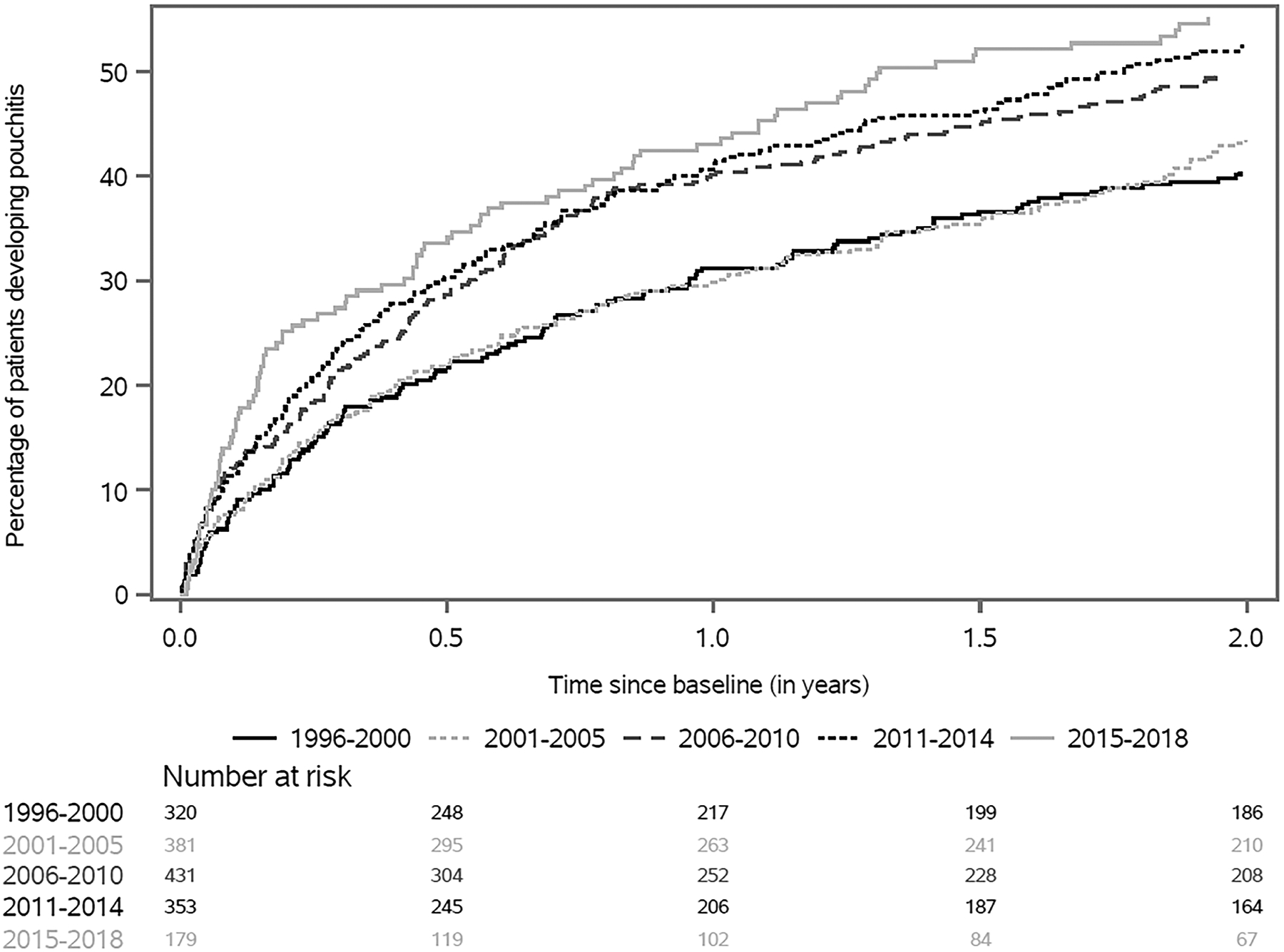
Cumulative incidence of pouchitis within the first two years after ileal pouch-anal anastomosis, analyzed by year of final stage of surgery
After adjusting for sex, age, and socioeconomic index, patients undergoing surgery in the time period 2015–2018 demonstrating the greatest risk for developing pouchitis (Hazard Ratio [HR] 1.57, 95% CI 1.20–2.05, Table 3). Patients undergoing surgery in 2006–2010 and 2011–2014 also demonstrated a statistically significantly increased risk for development of pouchitis in comparison to patients undergoing surgery in 1996–2000 (HRs 1.35, 95% CI 1.08–1.70 and 1.43, 95% CI 1.13–1.81 respectively). In a subsequent analysis stratifying by age at the time of surgery, similar patterns were demonstrated, however these were only statistically significant among patients aged 18–34 at the time of surgery (Supplemental Table 7). There were no significant changes in the criteria used to diagnosis pouchitis over the time periods analyzed (Supplemental Table 8).
Table 3.
Development of pouchitis within the first two years after ileal pouch-anal anastomosis, evaluated by time period of index surgery
| Time Period | Unadjusted HR (95% CI) | Adjusted HR* (95% CI) |
|---|---|---|
| 1996–2000 | Reference | Reference |
| 2001–2005 | 1.05 (0.83 – 1.34) | 1.06 (0.84 – 1.34) |
| 2006–2010 | 1.36 (1.09 – 1.70) | 1.35 (1.08 – 1.70) |
| 2011–2014 | 1.46 (1.16 – 1.83) | 1.43 (1.13 – 1.81) |
| 2015–2018 | 1.61 (1.23 – 2.09) | 1.57 (1.20 – 2.05) |
Adjusted for sex, year of surgery, age and socioeconomic index
To evaluate the relationship between preoperative anti-TNF use and development of pouchitis, we restricted the study population to three periods: 2006–2010 (early anti-TNF), 2011–2014 (expanding anti-TNF), 2015–2018 (current anti-TNF). In this analysis, there was no statistically significant association between preoperative anti-TNF use and pouchitis within the first 2 years after IPAA, after adjusting for sex, age, and socioeconomic index (Table 4). Given the potential concern that patients may be undergoing colectomy later in the disease course of UC due to the introduction of anti-TNF therapy, we also evaluated the duration between UC diagnosis and colectomy, however there was no meaningful difference when examining the median duration between periods.
Table 4.
The relationship between preoperative anti-tumor necrosis factor alpha therapy and development of pouchitis within the first two years after ileal pouch-anal anastomosis, evaluated by time period of index surgery
| Adjusted HR (95% CI) | |
|---|---|
| All years (2006–2018) * | |
| Anti-TNF use | 1.14 (0.93 – 1.40) |
| No Anti-TNF use | Reference |
| 2006–2010 ** | |
| Anti-TNF use | 1.21 (0.85 – 1.70) |
| 2011–2014 ** | |
| Anti-TNF use | 1.03 (0.74 – 1.42) |
| 2015–2018 ** | |
| Anti-TNF use | 1.25 (0.82 – 1.87) |
Adjusted for sex, year of surgery, age and socioeconomic index
Adjusted for sex, age and socioeconomic index; no anti-TNF use is the reference for all comparisons presented above; p-value for interaction = 0.71
In an evaluation of clinical factors at the time of colectomy and the risk of subsequent development of pouchitis, patients with EIMs of IBD were at an increased risk for developing pouchitis (HR 1.40, 95% CI 1.11–1.74, Supplemental Table 9). Additionally, when excluding patients with primary sclerosing cholangitis (PSC) from the EIMs category, this relationship remained statistically significant (HR 1.46, 95% CI 1.13–1.86). The small number of patients with PSC prevented the evaluation of any association between PSC alone and the risk of pouchitis.
DISCUSSION
In this population-based, national cohort study of UC patients undergoing colectomy in Denmark, we demonstrated that the rates of pouchitis within the first 2 years after IPAA have significantly increased over the past 2 decades, using a validated case-finding definition for the study of pouchitis.15 These findings provide an important analysis of the epidemiology of pouchitis, given the ability to evaluate longitudinal outcomes and their association with clinical factors in an unselected patient population. Risk of pouchitis was increased in patients with EIMs, whereas pre-operative anti-TNF therapy and disease severity did not explain the increased risk. We do not believe that the increasing incidence is related strictly to improvements in diagnostic accuracy, however given that there was no significant change in the criteria used to diagnose pouchitis over the time periods examined. The case-finding definitions are based on either ICD coding for pouchitis or the use of the most common antibiotic treatments for pouchitis, which have been in use since the earliest study period.17 The increasing incidence of pouchitis over time should prompt further investigation into the underlying drivers of inflammatory disease after IPAA for UC, with the aim of improving risk stratification at the time of IPAA.
The majority of studies evaluating the epidemiology and natural disease course after IPAA have been performed in single-center studies and/or selected populations. Although these studies have laid the foundation for much of our understanding of pouchitis, there are limitations to the evaluations of these select populations, including issues related to loss of follow-up or intermittent survey responses in long-term longitudinal evaluations3, 18 and potential selection bias. The use of prospectively collected national registry data, where more complete data exist regarding the disease course of UC pre-colectomy and the development of pouchitis after IPAA is informative with regards trends in the pouchitis rates over time. Given the validated case-finding definition utilized in this study,15 we chose to estimate trends in the 2-year incidence rate of pouchitis. The Danish registries offer a unique opportunity for long-term follow up that is not possible in other data sources such as administrative claims data,12, 19 which allows for the confirmation of an overall high cumulative incidence rate of pouchitis (88%) that is similar to rates demonstrated in other populations.3, 7 However, we believe that the 2-year estimates provided in this study also provide valuable comparisons to the existing literature using these case-finding definitions and provide a foundation for future research.
Traditional estimates suggest that 40% of patients will develop pouchitis in the first year after IPAA.6, 20, 21 When examining the cumulative incidence of pouchitis in the first year after IPAA, the rates demonstrated in this study are similar, particularly when examining the study periods after 2006. Additionally, the 2-year incidence rates of pouchitis we demonstrated are similar to those identified in a recent study of patients in the United States with commercial insurance.12 However, the apparent stark increase in the 2-year cumulative incidence of pouchitis between 1996 and 2018 is concerning, with a 15% absolute increase and a 38% relative increase during this time period. These increases are particularly concerning given the significant impacts that pouch-related disorders have on patients, including increased disability and lower quality of life.7, 22
Use of anti-TNF therapies and other biologics for the treatment of UC has increased in the past 2 decades,14, 23 leading authors to speculate on the impact of anti-TNF exposure on postoperative outcomes in UC, including the development of pouchitis. In two recent evaluations, preoperative exposure to anti-TNF therapy has been associated with a significant increase in the risk of pouchitis.5, 12 Given these findings, we specifically analyzed the relationship between preoperative anti-TNF use and pouchitis between 2006 and 2018. Despite a higher rate of pouchitis during these time periods, in a multivariable model there was no significant association between pre-operative anti-TNF use and pouchitis.
Although the development of pouchitis is multifactorial,20, 21 the temporal relationships we demonstrated suggest that larger ecologic or epidemiologic shifts may be driving the increasing rate of pouchitis. Changes in surgical approach to IPAA have been suggested as a potential etiology of differences in outcomes, including the use of stapled anastomoses rather than hand-sewn anastomoses. However, in a meta-analysis comparing the two techniques, there was no difference in short-term complications (anastomotic leak or pelvic sepsis) or pouchitis.24 Additionally, double-stapling without mucosectomy has been the predominant surgical technique in Denmark since the mid-1990s.25 In a separate evaluation of Danish health registry data between 1996 and 2013, Mark-Christensen and colleagues demonstrated an increasing incidence of pelvic sepsis following IPAA for UC.25 Whether these immediate postoperative complications were due to an overall trend in the severity of disease at the time of colectomy/IPAA or other underlying factors remains unknown. We realize the likely shift in treatment patterns prior to colectomy during the study period, and the fact that patients undergoing proctocolectomy with IPAA during the current area may represent differences in disease management and severity.
Given these shifts in the rates of pouchitis, other potential etiologies for the increase in incidence rate must be considered. The impact of diet on both the microbiome and in particular the microbiota of the pouch (and subsequent risk for pouchitis) has generated interest recently.26 Although most studies have been performed in patients with an IPAA, and potentially those patients who have already developed inflammatory conditions of the pouch, the role of shifting dietary patterns or exposures over time should be considered in examining the increasing incidence rates of pouchitis. Recent studies have demonstrated a link between ultra-processed foods and increased risk of developing CD and UC.27 In prospective cohort studies, obesity has been associated with an increased risk of older-onset CD but not UC,28 and although obesity has been associated with short-term complications after IPAA, the effect on long-term functional outcomes including pouchitis is less clear.29 Our understanding of the environmental risk factors associated with pouchitis remains limited, and thus a dedicated consideration of shifts in the environmental exposures in the pre-, peri-, and immediate postoperative states is critical to understanding other potential drivers of the increasing rates of pouchitis.
In our evaluation, those patients with EIMs demonstrated an increased risk of developing pouchitis within the first 2 years after IPAA, an association previously demonstrated in a systematic review and meta-analysis by Hata et al.30 Although we were unable to analyze the presence of PSC as an independent risk factor for pouchitis, this diagnosis was included in the larger assessment of EIMs given the strong association between PSC and pouch-related inflammation.31 There is a potential that EIMs were under-recognized given the use diagnostic coding to identify EIMs in this study. However, we suspect that this undercoding would potentially even further magnify the relationship between EIMs and pouchitis.
Our study has multiple strengths including the nationwide prospective data collection, which minimizes the potential for selection bias, and the use of a validated case-finding definition for pouchitis, which minimizes misclassification bias. However our study does have limitations. We did not have information on potential environmental risk factors such as changes in dietary patterns or obesity that may aid in explaining the increasing incidence of pouchitis over time. These and other potential explanations for the increasing incidence of pouchitis should prompt future investigations into this relationship. Although the case-finding definitions provide important evaluations of the incidence of pouchitis over time, these were not designed to evaluate conditions such as chronic pouchitis or Crohn’s-like disease of the pouch. Additionally, although we evaluated the relationship between multiple clinical risk factors and the development of pouchitis, the evaluation of serologic and stool biomarkers for pouch-related conditions32 was not possible. We also could not evaluate granular disease-related data such as endoscopic data or direct measures of clinical disease activity.
In summary, using prospectively collected data from the nationwide Danish health registers, we demonstrated an increasing incidence of pouchitis among patients undergoing surgery between 1996 and 2018, with the greatest cumulative incidence of pouchitis demonstrated in the most recent era (2015–2018). Risk was related to co-occurrence of EIMs but not to anti-TNF treatment or markers of disease severity. Given the dramatic increase in the incidence of pouchitis, our findings should prompt future evaluations into the drivers of pouchitis in the current era to enable tailored, early intervention in high-risk populations.
Supplementary Material
WHAT YOU NEED TO KNOW.
BACKGROUND
Traditional estimates of the incidence of pouchitis after ileal pouch-anal anastomosis for ulcerative colitis have been performed in selected populations. We used new case-finding definitions for pouchitis to evaluate the incidence in a prospective, national cohort from Denmark.
FINDINGS
We identified a significant increase in the incidence of pouchitis in the first 2 years after ileal pouch-anal anastomosis when evaluated by the time period of surgery.
IMPLICATIONS FOR PATIENT CARE
The incidence of pouchitis appears to be increasing. This study should prompt diligence in the early identification of patients at risk for pouchitis and future research efforts to understand what is driving this increase to ultimately allow for early clinical intervention if applicable.
Funding:
This research was supported by grants from the American College of Gastroenterology, the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [K23DK127157-01], and the Danish National Research Foundation [DNRF148]. These funding sources had no role in the study design, collection, analysis and interpretation of the data or in the drafting of the manuscript.
Statement of Disclosure of Financial Conflicts of Interest:
Edward L. Barnes has served as a consultant for AbbVie, Gilead, Pfizer, and Target RWE. Hans H. Herfarth has served as a consultant for Alivio, AMAG, Finch, Gilead, Lycera, Merck, Otsuka, Pfizer, PureTech, Seres and research support from Pfizer and Artizan Biosciences Kristine H. Allin, Aske T. Iversen, and Tine Jess have no conflicts of interest.
Abbreviations:
- aOR
adjusted odds ratio
- anti-TNF
anti-tumor necrosis factor alpha
- CI
confidence interval
- CD
Crohn’s disease
- CPT
Current Procedural Terminology
- EIM
extraintestinal manifestations
- IPAA
ileal pouch-anal anastomosis
- IBD
inflammatory bowel disease
- ICD
International Classification of Diseases
- ICD-10
ICD-10th Clinical Modification
- NCSP
The Nordic Medico-Statistical Committee Classification of Surgical Procedures
- PSC
primary sclerosing cholangitis
- SD
standard deviation
- UC
ulcerative colitis
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Transparency Statement:
The data underling this article were provided from the Danish health registers by permission. Any requests regarding data availability and methods should be directed to the corresponding author.
REFERENCES
- 1.Fleshner P, Ippoliti A, Dubinsky M, et al. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol 2008;6:561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen B, Fazio VW, Remzi FH, et al. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:81–9. [DOI] [PubMed] [Google Scholar]
- 3.Lightner AL, Mathis KL, Dozois EJ, et al. Results at Up to 30 Years After Ileal Pouch-Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm Bowel Dis 2017;23:781–790. [DOI] [PubMed] [Google Scholar]
- 4.Kayal M, Plietz M, Rizvi A, et al. Inflammatory Pouch Conditions Are Common After Ileal Pouch Anal Anastomosis in Ulcerative Colitis Patients. Inflamm Bowel Dis 2020;26:1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertucci Zoccali M, Hyman NH, Skowron KB, et al. Exposure to Anti-tumor Necrosis Factor Medications Increases the Incidence of Pouchitis After Restorative Proctocolectomy in Patients With Ulcerative Colitis. Dis Colon Rectum 2019;62:1344–1351. [DOI] [PubMed] [Google Scholar]
- 6.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 2003;124:1202–9. [DOI] [PubMed] [Google Scholar]
- 7.Barnes EL, Herfarth HH, Sandler RS, et al. Pouch-Related Symptoms and Quality of Life in Patients with Ileal Pouch-Anal Anastomosis. Inflamm Bowel Dis 2017;23:1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen B Acute and chronic pouchitis--pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol 2012;9:323–33. [DOI] [PubMed] [Google Scholar]
- 9.Hoda KM, Collins JF, Knigge KL, et al. Predictors of pouchitis after ileal pouch-anal anastomosis: a retrospective review. Dis Colon Rectum 2008;51:554–60. [DOI] [PubMed] [Google Scholar]
- 10.Achkar JP, Al-Haddad M, Lashner B, et al. Differentiating Risk factors for Acute and Chronic Pouchitis. Clin Gastroenterol Hepatol 2005;3:60–66. [DOI] [PubMed] [Google Scholar]
- 11.Yanai H, Ben-Shachar S, Mlynarsky L, et al. The outcome of ulcerative colitis patients undergoing pouch surgery is determined by pre-surgical factors. Aliment Pharmacol Ther 2017;46:508–515. [DOI] [PubMed] [Google Scholar]
- 12.Barnes EL, Herfarth HH, Kappelman MD, et al. Incidence, Risk Factors, and Outcomes of Pouchitis and Pouch-Related Complications in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2021;19:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Runde J, Erondu A, Akiyama S, et al. Outcomes of Ileoanal Pouch Anastomosis in Pediatric Ulcerative Colitis Are Worse in the Modern Era: A Time Trend Analysis Outcomes Following Ileal Pouch-Anal Anastomosis in Pediatric Ulcerative Colitis. Inflamm Bowel Dis 2022. epublished January 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes EL, Jiang Y, Kappelman MD, et al. Decreasing Colectomy Rate for Ulcerative Colitis in the United States between 2007 and 2016: A Time Trend Analysis. Inflamm Bowel Dis 2020;26:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes EL, Kochar B, Herfarth HH, et al. Creation of a Case-Finding Definition for Identifying Patients with Acute Pouchitis in Administrative Claims Data. Clin Gastroenterol Hepatol 2021;19:842–844. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Andersen NN, Andersson M, et al. Comparison of Infliximab and Adalimumab in Biologic-Naive Patients With Ulcerative Colitis: A Nationwide Danish Cohort Study. Clin Gastroenterol Hepatol 2017;15:1218–1225 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst RD, Molinari M, Chung TP, et al. Prospective study of the incidence, timing and treatment of pouchitis in 104 consecutive patients after restorative proctocolectomy. Arch Surg 1996;131:497–500; discussion 501–2. [DOI] [PubMed] [Google Scholar]
- 18.Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg 2013;257:679–85. [DOI] [PubMed] [Google Scholar]
- 19.Long MD, Hutfless S, Kappelman MD, et al. Challenges in designing a national surveillance program for inflammatory bowel disease in the United States. Inflamm Bowel Dis 2014;20:398–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes EL, Lightner AL, Regueiro M. Peri-operative and Post-operative Management of Patients with Crohn’s Disease and Ulcerative Colitis. Clin Gastroenterol Hepatol 2020;18:1356–1366. [DOI] [PubMed] [Google Scholar]
- 21.Shen B Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol 2013;11:1538–49. [DOI] [PubMed] [Google Scholar]
- 22.Kayal M, Ungaro R, Riggs A, et al. Ileal Pouch Anal Anastomosis for the Management of Ulcerative Colitis is Associated with Significant Disability. Clin Gastroenterol Hepatol 2021. epublished May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut 2014;63:1607–16. [DOI] [PubMed] [Google Scholar]
- 24.Lovegrove RE, Constantinides VA, Heriot AG, et al. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg 2006;244:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mark-Christensen A, Kjaer MD, Ganesalingam S, et al. Increasing Incidence of Pelvic Sepsis Following Ileal Pouch-Anal Anastomosis for Ulcerative Colitis in Denmark: a Nationwide Cohort Study. Dis Colon Rectum 2019. [DOI] [PubMed] [Google Scholar]
- 26.Ardalan ZS, Yao CK, Sparrow MP, et al. Review article: the impact of diet on ileoanal pouch function and on the pathogenesis of pouchitis. Aliment Pharmacol Ther 2020;52:1323–1340. [DOI] [PubMed] [Google Scholar]
- 27.Narula N, Wong ECL, Dehghan M, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. Bmj 2021;374:n1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan SSM, Chen Y, Casey K, et al. Obesity is associated with increased risk of Crohn’s disease, but not ulcerative colitis: A pooled analysis of five prospective cohort studies. Clin Gastroenterol Hepatol 2021. epublished July 7. [DOI] [PubMed] [Google Scholar]
- 29.Emile SH, Khan SM, Wexner SD. A systematic review and meta-analysis of the outcome of ileal pouch anal anastomosis in patients with obesity. Surgery 2021;170:1629–1636. [DOI] [PubMed] [Google Scholar]
- 30.Hata K, Okada S, Shinagawa T, et al. Meta-analysis of the association of extraintestinal manifestations with the development of pouchitis in patients with ulcerative colitis. BJS Open 2019;3:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes EL, Holubar SD, Herfarth HH. Systematic Review and Meta-Analysis of Outcomes after Ileal Pouch-Anal Anastomosis in Primary Sclerosing Cholangitis-Ulcerative Colitis. J Crohns Colitis 2021;15:1272–1278. [DOI] [PubMed] [Google Scholar]
- 32.Singh S, Sharma PK, Loftus EV Jr., et al. Meta-analysis: serological markers and the risk of acute and chronic pouchitis. Aliment Pharmacol Ther 2013;37:867–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underling this article were provided from the Danish health registers by permission. Any requests regarding data availability and methods should be directed to the corresponding author.


